Abstract
BACKGROUND
The authors previously reported that the c-kit–positive (c-kitPOS) cells isolated from slowly adhering (SA) but not from rapidly adhering (RA) fractions of cardiac mesenchymal cells (CMCs) are effective in preserving left ventricular (LV) function after myocardial infarction (MI).
OBJECTIVES
This study evaluated whether adherence to plastic alone, without c-kit sorting, was sufficient to isolate reparative CMCs.
METHODS
RA and SA CMCs were isolated from mouse hearts, expanded in vitro, characterized, and evaluated for therapeutic efficacy in mice subjected to MI.
RESULTS
Morphological and phenotypic analysis revealed that murine RA and SA CMCs are indistinguishable; nevertheless, transcriptome analysis showed that they possess fundamentally different gene expression profiles related to factors that regulate post-MI LV remodeling and repair. A similar population of SA CMCs was isolated from porcine endomyocardial biopsy samples. In mice given CMCs 2 days after MI, LV ejection fraction 28 days later was significantly increased in the SA CMC group (31.2 ± 1.0% vs. 24.7 ± 2.2% in vehicle-treated mice; p < 0.05) but not in the RA CMC group (24.1 ± 1.2%). Histological analysis showed reduced collagen deposition in the noninfarcted region in mice given SA CMCs (7.6 ± 1.5% vs. 14.5 ± 2.8% in vehicle-treated mice; p < 0.05) but not RA CMCs (11.7 ± 1.7%), which was associated with reduced infiltration of inflammatory cells (14.1 ± 1.6% vs. 21.3 ± 1.5% of total cells in vehicle and 19.3 ± 1.8% in RA CMCs; p < 0.05). Engraftment of SA CMCs was negligible, which implies a paracrine mechanism of action.
CONCLUSIONS
We identified a novel population of c-kit–negative reparative cardiac cells (SA CMCs) that can be isolated with a simple method based on adherence to plastic. SA CMCs exhibited robust reparative properties and offered numerous advantages, appearing to be more suitable than c-kitPOS cardiac progenitor cells for widespread clinical therapeutic application.
Keywords: fibrosis, inflammation, ischemic cardiomyopathy, myocardial infarction, repair
Transplantation of various types of adult stem or progenitor cells in infarcted myocardium improves left ventricular (LV) function despite the failure of transplanted cells to engraft, which suggests that the major mechanism of action is via paracrine actions (1–6). We have demonstrated that administration of c-kit–positive (c-kitPOS) cardiac progenitor cells (CPCs) after myocardial infarction (MI) alleviated LV dysfunction and remodeling in various preclinical models, including mice, rats, and pigs (7–14). Initial clinical data were also encouraging (15,16). As is the case for virtually all other cell types, the vast majority of transplanted c-kitPOS CPCs disappeared soon after transplantation (9–12,14,17). Interestingly, several studies suggested that c-kitPOS CPCs possess mesenchymal stromal characteristics and express classic mesenchymal stromal markers, including CD90, CD105, CD29, and CD73 (13,18–22), intimating that c-kitPOS CPCs might, in fact, be a subset of mesenchymal stromal cells. If this is the case, their reparative actions after MI might relate to the general properties of mesenchymal cells rather than to the expression of c-kit per se.
Although c-kitPOS CPCs are effective in repairing the infarcted heart, c-kit–based selection is prone to errors and controversy because of variability in c-kit expression levels and reagents (3,13). Furthermore, the expression of c-kit in culture appears to be labile. For example, we have found that in murine sorted c-kitPOS CPCs, the expression of c-kit decreased from 78% to <30% after 4 or 5 passages (13). Because c-kit expression can be lost easily during expansion in culture, we posit that many investigators are observing beneficial effects with cells that are no longer c-kitPOS at the time of transplantation despite selection for this marker. Recently, we discovered that the ability of myocardial cells to adhere to plastic enabled separation of a population of cells that maintained prominent c-kit positivity in vitro (13). Specifically, after cardiac digestion, we identified 2 cell populations with distinct properties: those that require more than 24 h to adhere to plastic, which we termed slowly adhering (SA), and those that adhere in < 24 h, which we termed rapidly adhering (RA). Because both SA and RA cells exhibit a mesenchymal phenotype, we have termed these cells cardiac mesenchymal cells (CMCs) (13). When sorted for c-kit, SA CMCs maintain relatively stable c-kit positivity (e.g., 51.8% at passage 6), whereas RA CMCs lose c-kit positivity relatively rapidly (e.g., 5.8% at passage 6). We have also found that SA c-kitPOS CMCs impart beneficial effects on myocardial function when transplanted into infarcted murine hearts, whereas RA CMCs do not (13).
What remains unresolved is whether the essential phenotypic feature that identifies this reparative SA c-kitPOS CMC population is the expression of c-kit or the slow adherence to plastic. In other words, is sorting for c-kit necessary to separate SA CMCs with reparative properties? The present study was undertaken to answer this critical question and determine whether slow adherence to plastic is sufficient to isolate reparative CMCs independent of c-kit sorting (and c-kit expression).
METHODS
Detailed methods are available in the Online Appendix.
RESULTS
We previously established a method to isolate a slowly adhering population of cells that stably express the c-kit receptor and exhibit reparative properties in a mouse model of post-MI cardiomyopathy (13). Additionally, we demonstrated that c-kit–sorted SA CMCs express higher levels of endothelial and cardiac markers and lower levels of fibroblast genes than c-kit–sorted RA CMCs. When administered 2 days after MI by percutaneous intra-ventricular injection, c-kit–sorted SA CMCs preserved LV function, reduced scar size, and increased angiogenesis, whereas c-kit–sorted RA CMCs were therapeutically ineffective (13).
On the basis of the aforementioned data, one could argue that the RA and SA segregation was more important therapeutically than c-kit sorting; in other words, sorting for c-kit may have been irrelevant for the cells’ reparative effects.
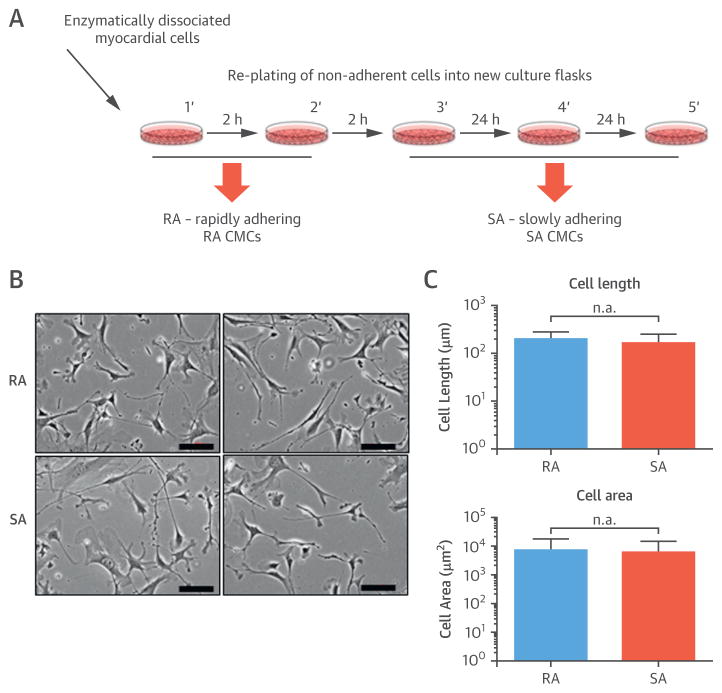
To address this issue, we isolated and characterized RA CMCs and SA CMCs without c-kit sorting (Figure 1A). Isolated RA CMCs and SA CMCs were expanded and analyzed between passages 3 and 8. Microscopic examination revealed that both cell types had a fibroblast-like morphology (Figure 1B). More detailed morphometric analysis indicated that cell length and area did not differ between RA CMCs and SA CMCs; the distribution of cell length and area suggested that both cell types were heterogeneous populations with a wide range of cell sizes (Figure 1C).
FIGURE 1. RA CMC and SA CMC Phenotype.
(A) This schematic displays the protocol for isolating rapidly adhering (RA) cardiac mesenchymal cells (CMC) and slowly adhering (SA) CMCs, shown as (B) representative images. (C) There were no significant differences in cell length or area between the groups. Values are mean ± SEM.
Next, we evaluated the proliferative potential of the cells. We found that RA CMCs proliferated slightly more slowly and had a longer population doubling time (32.3 ± 2.8 h vs. 26.7 ± 1.2 h for SA CMCs; p < 0.05). After performing clonogenicity assays or colony-forming unit of fibroblast assays, which estimate the number of proliferative CMCs in the isolated cell populations, we found that SA CMCs were more clonogenic than RA CMCs (colony-forming unit of fibroblast assay: 8.0 ± 1.6% vs. 4.7 ± 1.6%, respectively; p < 0.05) (Online Figure 1).
To gain more information about the cell phenotype, we performed flow cytometric analysis of mesenchymal, endothelial, and hematopoietic markers (Table 1, Online Figure 2). Both cell lines expressed surface markers of mesenchymal lineage: CD90, CD29, CD73, CD105, and CD44. However, expression of CD90 was lower in SA than in RA CMCs (52.4% vs. 83.6%). CD90 might be a marker of detrimental profibrotic and proinflammatory actions of CMCs (23). Conversely, the expression of CD73 was higher in the SA than RA population (79.5% vs. 62.0%). CD73 is an ectonuclease involved in the breakdown of extracellular adenosine triphosphate to adenosine; as such, it promotes an anti-inflammatory response. Expression of endothelial markers (FLK1, CD31, and CD34) was negligible in both cell types. Furthermore, both cells were negative for the hematopoietic marker CD45 and the pericyte marker CD146. Other mesenchymal markers, such as CD106, CD9, and CD13, were expressed, whereas CD271 and CD166 were not. Expression of the stem cell marker c-kit was low (11.1% vs. 7.1%), and most of the cells were positive for Sca-1 (73.6% vs. 63.1%).
TABLE 1.
Flow Cytometric Analysis
| Marker, % | RA CMCs | SA CMCs |
|---|---|---|
| CD90 | 83.6 ± 8.8 | 52.4 ± 35.4 |
| CD29 | 98.5 ± 0.2 | 99.4 ± 0.2 |
| CD105 | 96.8 ± 3.5 | 95.4 ± 1.8 |
| CD73 | 62.0 ± 15.4 | 79.5 ± 10.4 |
| CD44 | 99.3 ± 0.4 | 99.5 ± 0.4 |
| CD39 | 17.0 ± 11.3 | 12.9 ± 17.9 |
| CD106 | 90.4 ± 9.6 | 69.1 ± 32.7 |
| CD9 | 95.6 ± 3.2 | 97.1 ± 3.5 |
| CD271 | 0.3 ± 0.2 | 0.3 ± 2.0 |
| CD166 | 0.2 ± 0.1 | 3.5 ± 5.6 |
| CD146 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| CD13 | 0.4 ± 0.1 | 0.4 ± 0.2 |
| FLK1 | 1.0 ± 0.9 | 0.9 ± 0.6 |
| CD31 | 1.8 ± 0.7 | 1.5 ± 0.6 |
| CD34 | 8.6 ± 10.2 | 18.1 ± 26.5 |
| c-kit | 11.1 ± 5.9 | 7.1 ± 3.8 |
| Sca-1 | 71.6 ± 16.8 | 63.1 ± 14.6 |
| CD45 | 5.7 ± 2.3 | 2.9 ± 1.2 |
| CD11b | 1.4 ± 1.0 | 1.1 ± 0.3 |
| MHCI | 34.4 ± 25.4 | 16.9 ± 16.8 |
| MHCII | 0.8 ± 0.5 | 0.5 ± 0.1 |
Values are mean ± SEM.
CMC = cardiac mesenchymal cell; RA = rapidly adhering; SA = slowly adhering.
Most (>95%) c-kitPOS CPCs injected into infarcted hearts do not engraft, and their contribution to myocytes or endothelium is negligible (e.g., <1% of myocytes) (7–12,14). Despite this, c-kitPOS CPC administration consistently has improved cardiac function (7–12,14). Similarly, in the current study, we found virtually no cell engraftment in the myocardium 28 days after cell injection (vide infra), which indicates that these cells contributed to cardiac repair by paracrine mechanisms, that is, by releasing factors that activate endogenous mechanisms of repair (3). Paracrine factors include proteins, bioactive lipids, and extracellular vesicles composed of lipids, proteins, and nucleic acids (1–3,6,24–30). Consequently, we evaluated the RA CMC and SA CMC secretome by analyzing gene array profiles at the ribonucleic acid (RNA) level.
GENE EXPRESSION PROFILE
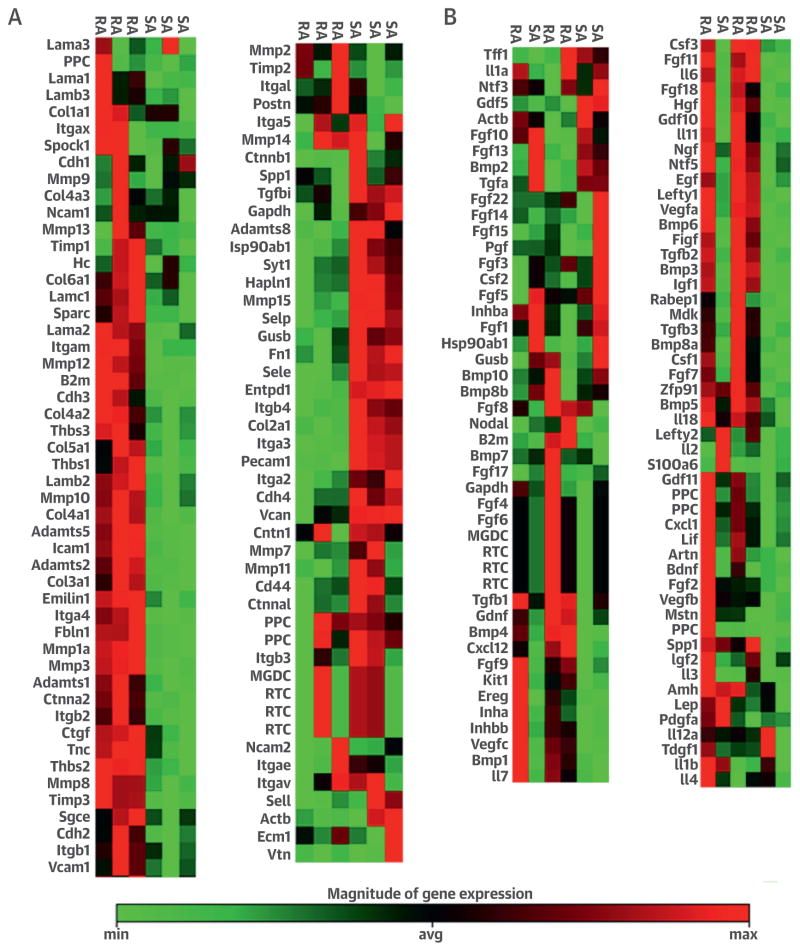
In principle, CMCs transplanted in the acute phase after MI could facilitate collagen deposition, limit scar expansion, and promote resolution of inflammation. To investigate these potential mechanisms, we performed gene arrays to compare the expression profile of extracellular matrix (ECM) genes. Heat map analysis showed that the expression of ECM remodeling genes was higher in RA CMCs than SA CMCs, which suggests that RA CMCs might represent a subset of activated CMCs (Figure 2A, Online Figure 3). Consistent with this, the expression of ECM genes, such as Col4a1, Col4a2, Col3a1, Adamts2, Adamts5, Mpp13, Mmp12, and Mmp8, was higher in RA than in SA cells, which suggests that RA CMCs are more actively involved in ECM remodeling (Figure 2A, Online Figure 3).
FIGURE 2. Real-Time PCR Gene Array.
Total ribonucleic acid from RA and SA CMCs was isolated and subjected to real-time gene array for extracellular matrix remodeling targets (A) and growth factors (B). In each panel, the cell type to which the column refers is indicated at the top. Avg =average; max = maximum; min = minimum; PCR = polymerase chain reaction; other abbreviations as in Figure 1.
We also performed real-time polymerase chain reaction gene arrays for cytokines. Analysis of the heat maps indicated that in general, cytokine gene expression was more robust in RA CMCs than in SA CMCs, which again suggests that RA CMCs are a sub-population of activated cells. The only cytokines significantly elevated in SA CMCs were Fgf5 and Fgf13, both of which exert pro-proliferative and prosurvival actions (Figure 2B, Online Figure 3). Interestingly, Fgf5 gene transfer has been shown to enhance blood flow and myocardial contractile function as a result of increased angiogenesis (31). Additionally, the expression of detrimental proinflammatory (Il6 and Csf1) and profibrotic (Tgfb1 and Tgfb2) cytokines (32–38) was lower in SA CMCs (Figure 2B, Online Figure 3). The distinctive pattern of cytokine gene expression by SA CMCs (i.e., increased expression of pro-proliferative and prosurvival factors [Fgf5 and Fgf13] and reduced expression of proinflammatory [Il6 and ] and profibrotic [Il6 and Csf1] factors) might result in a reduction in inflammation and in promotion of repair by SA CMCs in vivo.
To further characterize RA and SA CMCs, we performed microRNA profiling and found that 14 micro-RNAs were exclusively expressed in the RA CMCs and 12 in the SA CMCs (Online Figure 4). Among 271 microRNAs expressed in both cells, 53 were down-regulated in SA CMCs and 12 were upregulated in SA CMCs compared with RA CMCs (Online Figure 4). These data further support the notion that these 2 cell populations are fundamentally different and could have a different effect on myocardial repair. To test this possibility, we used our murine model of post-MI cardiomyopathy.
At 48 h after MI, vehicle, RA CMCs, or SA CMCs (1 × 105) were injected into the border zone of the infarct. Three days after transplantation (5 days after reperfusion), the degree of LV dysfunction and dilatation was similar among the 3 groups, which suggests that the injury after ischemia-reperfusion was comparable (Figure 3).
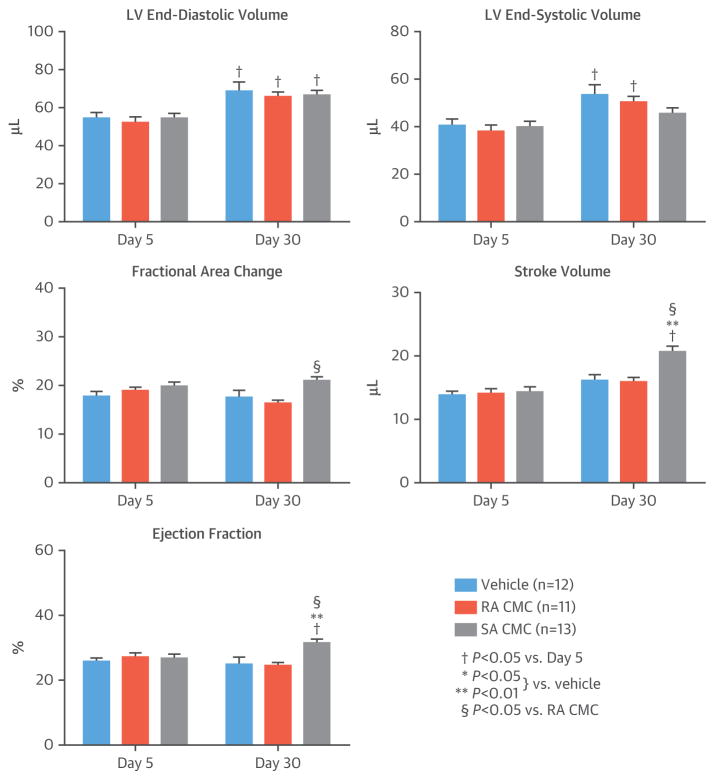
FIGURE 3. Echocardiographic Analysis.
Echocardiographic studies performed 3 and then 28 days after vehicle or cell administration, corresponding to day 5 and then day 30 after MI, showed varying changes in left ventricular (LV) volumes and other parameters. Values are mean ± SEM. Abbreviations as in Figure 1.
The final echocardiographic analysis was performed 30 days after MI (28 days after vehicle or cell treatment) (Figure 3). As expected, LV end-diastolic volume increased in vehicle-treated mice on day 30 compared with day 5 after MI; similar changes were observed in the RA and SA CMC–treated groups, so that on day 30 after MI, there was no difference among groups. LV end-systolic volume also increased significantly in vehicle- and RA CMC–treated mice on day 30; in contrast, the increase was not statistically significant in SA CMC–treated mice (Figure 3). Load-dependent indexes of LV performance (fractional area change, stroke volume, and LV ejection fraction [LVEF]) did not change appreciably between day 5 and day 30 in vehicle- and RA CMC–treated mice but increased in SA CMC–treated animals, so that on day 30, these variables were significantly greater in the SA CMC group. For example, on day 30, LVEF was 31.2 ± 1.0% in the SA CMC group versus 24.7 ± 2.2% in the vehicle group (p < 0.05) and 24.1 ± 1.2% in the RA CMC group (Figure 3). These data demonstrated that SA CMCs but not RA CMCs were effective in preserving LV function in this murine model of ischemic cardiomyopathy.
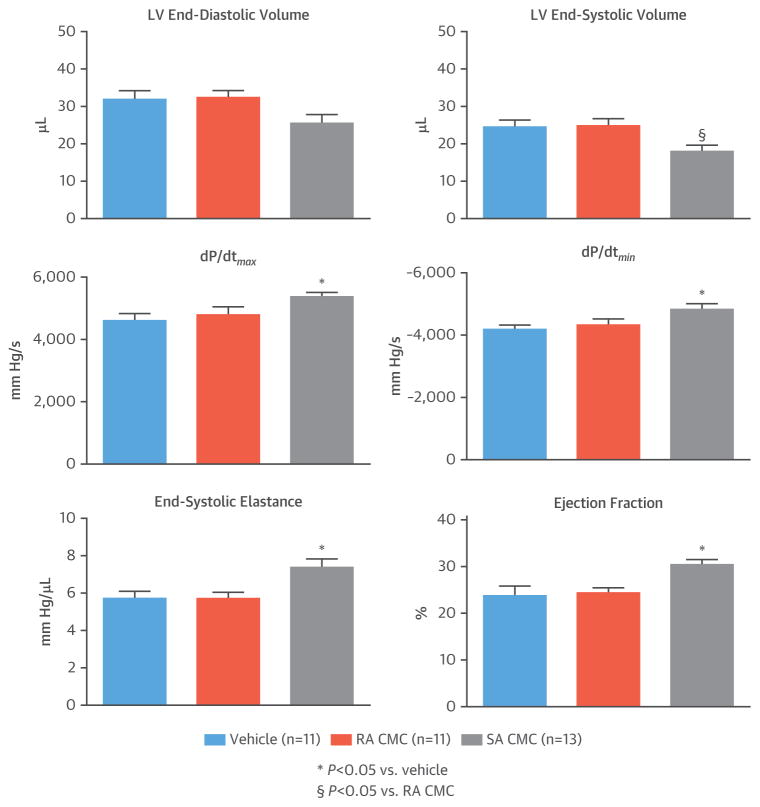
Two days after the final echocardiographic analysis, just before euthanasia (day 32 after MI), we performed hemodynamic assessment of LV function using a pressure-volume (Millar, Inc., Houston, Texas) catheter. Consistent with the echocardiographic data, SA CMCs but not RA CMCs were effective in preserving LV function compared with vehicle, without significantly affecting end-diastolic volumes (Figure 4). Importantly, this was the case not only for load-dependent parameters (LVEF and LV dP/dt) but also for load-independent (end-systolic elastance) parameters (Figure 4). Thus, 2 independent methods for assessing myocardial function demonstrated that SA CMCs alleviated post-MI LV dysfunction whereas RA CMCs did not.
FIGURE 4. Hemodynamic Assessment of LV Function.
Hemodynamic studies performed at 30 days after vehicle or cell administration (32 days after MI) and just before euthanasia showed significant changes in SA CMCs in most parameters. Values are mean ± SEM. Abbreviations as in Figures 1 and 3.
CMC EFFECTS AND MECHANISMS
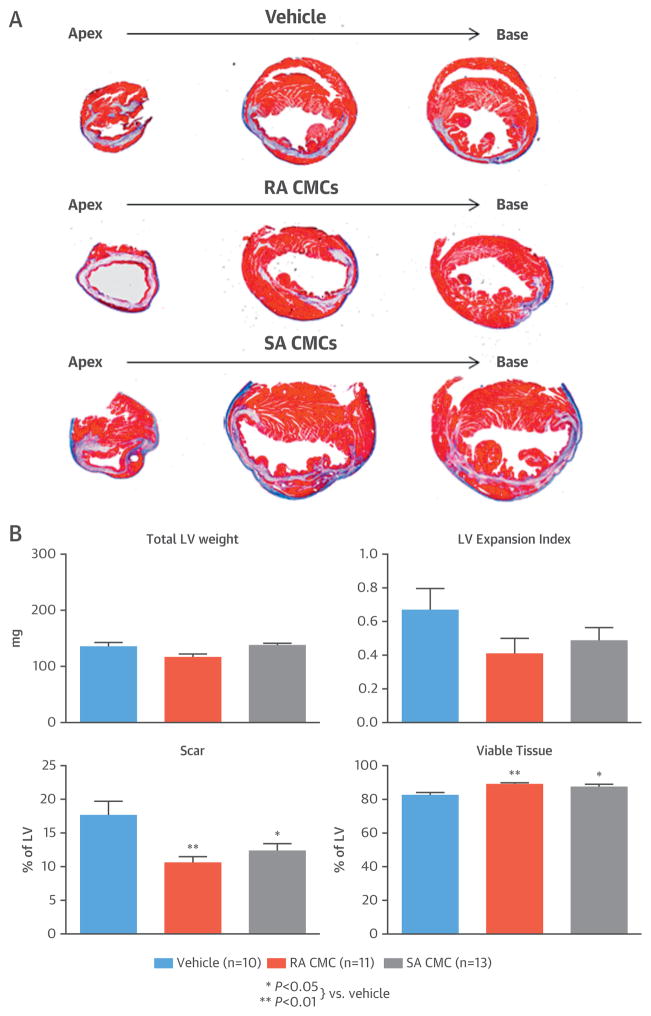
The effects of CMCs on LV remodeling were assessed by Masson’s trichrome and picrosirius red staining. Each heart was cut at 2-mm intervals starting from the apex, and a total of 4 blocks were generated, sectioned, and stained. Morphometric analysis demonstrated that compared with vehicle-treated hearts, scar size was reduced and viable tissue was increased in both RA and SA CMC–treated hearts (Figure 5).
FIGURE 5. Morphometric Analysis.
(A) Representative Masson’s trichrome-stained heart sections are shown from mice euthanized 30 days after administration of vehicle or SA CMCs. (B) An analysis of various LV parameters demonstrated significantly reduced scar tissue and increased viable tissue. Values are mean ± SEM. Abbreviations as in Figures 1 and 3.
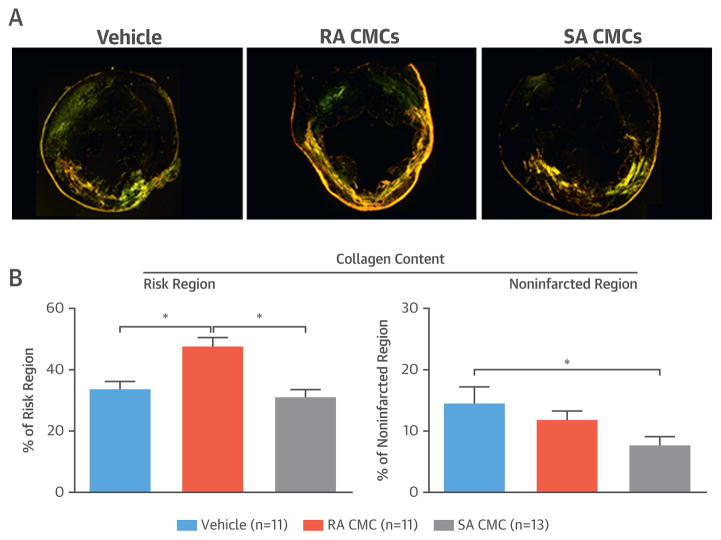
Heart function after MI relies on the contractile performance of the remaining viable tissue in the noninfarcted region. Excessive collagen deposition in this region contributes to LV remodeling and impaired LV function (39–41). We evaluated collagen deposition in LV sections stained with picrosirius red and imaged with polarized light. Collagen content was evaluated with pixel density analysis and expressed as a percentage of total myocardial area (Figure 6). In the risk region, collagen content did not differ between SA CMC– and vehicle-treated groups, but it was significantly increased in RA CMC–treated mice. In the noninfarcted region, however, collagen content was significantly reduced in the group treated with SA CMCs compared with vehicle-treated mice (7.6 ± 1.5% vs. 14.5 ± 2.8%, respectively; p < 0.05) (Figure 6); in contrast, collagen content in RA CMC–treated hearts did not differ significantly from that in vehicle-treated hearts (Figure 6). These observations are consistent with the hypothesis that the improvement in LV function effected by SA CMCs might be due in part to a reduction of collagen deposition in the noninfarcted regions of the LV.
FIGURE 6. Myocardial Collagen Content.
(A) Representative microscopic images of LV sections stained with picrosirius red from a vehicle-treated, an RA CMC–treated, and an SA CMC–treated mouse were acquired with polarized light. (B) Quantitative analysis of polarized light microscopic images showed significant differences in collagen content as a percentage of the risk and noninfarcted regions. Values are mean ± SEM. *p < 0.05. Abbreviations as in Figure 1.
In all groups, capillary density (assessed with isolectin B4 staining) was lower in the infarcted than the noninfarcted region. There was no statistical difference in capillary density among the 3 groups in either the infarcted or noninfarcted region (Online Figure 5A and 5B).
Myocyte hypertrophy was evaluated with wheat germ agglutinin staining and measurement of myocyte cross-sectional area. There was no difference in myocyte cross-sectional area between SA CMC–treated and vehicle-treated hearts in the border zone, infarcted region, and noninfarcted region (Online Figure 5C). RA CMC–treated hearts exhibited larger cross-sectional area compared with the 2 other groups in the border zone and infarcted region.
In this study, green fluorescent protein (GFP)–positive (GFPPOS) male CMCs were injected into female mice. The number of GFPPOS cells remaining at 28 days after transplantation was negligible (averaging < 2 cells per LV section) in both RA and SA CMC–treated hearts, which indicates minimal engraftment of transplanted CMCs (data not shown). To assess formation of new myocytes, mice were given bromodeoxyuridine continuously from the day of treatment to euthanasia. The number of newly formed myocytes (alpha-sarcomeric actin–positive and bromodeoxyuridine-positive cells) was also negligible (<0.5% of myocytes) in all groups (data not shown). Thus, formation of new myocytes could not explain LV function improvement after SA CMC treatment.
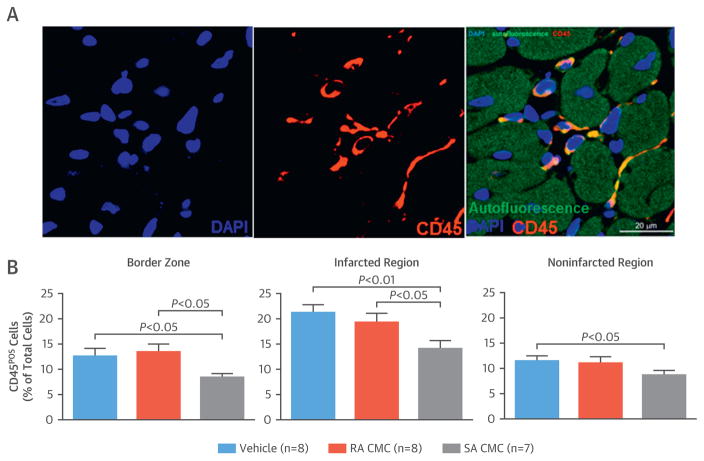
Because the immune system plays an important role not only in post-MI infarct healing but also in adverse remodeling and progression of heart failure, we evaluated the myocardial content of immune cells with CD45 staining (Figure 7A). In all groups, the infarcted region accumulated more CD45POS cells than the border zone or the non-infarcted region (Figure 7B). Administration of SA but not RA CMCs significantly reduced the content of immune cells in all LV regions compared with vehicle (Figure 7B).
FIGURE 7. Myocardial Content of Inflammatory Cells.
(A) Representative microscopic images of left ventricular sections were stained for CD45 and deoxyribonucleic acid (with 4′,6-diamidino-2-phenylindole [DAPI]). (B) Quantitative analysis of CD45-positive cells was performed in the border zone and infarcted and noninfarcted regions of the left ventricular sections. Values are mean ± SEM. Abbreviations as in Figure 1.
To further explore the mechanism of CMCs on immune cells, bone marrow macrophages were activated with proinflammatory stimuli (lipopolysaccharide and interferon-γ) in the presence of RA or SA CMCs. Lipopolysaccharide and interferon-γ stimulation produced a robust activation of proinflammatory cytokines (Tnfa, Il12b, Il1b, and Il6) measured at the messenger RNA level using quantitative polymerase chain reaction. Presence of CMCs blunted this proinflammatory response, because Tnfa, Il12b, and Il6 levels were significantly lower, and the expression of Il1b tended to decrease in CMC-treated macrophages, although the change did not reach statistical significance. SA CMCs were more effective than RA CMCs, because the macrophage expression of Tnfa and Il12b was lower in the SA CMC–treated group (Online Figure 6).
Porcine CMCs were isolated from endomyocardial biopsy samples (EMBs) and characterized with methods similar to those used in mice. EMBs were collected from either the right or the left ventricle (average total weight of tissue: 55.8 ± 13.3 mg; n-4). After enzymatic digestion, single-cell suspensions underwent differential plating to obtain the RA and SA CMC fraction, as described for the mouse CMCs. Brightfield images were collected at passages 3 to 6 (Online Figure 7A). As was the case with murine cells, cell morphology was indistinguishable between RA and SA CMCs, and cells had a typical fibroblast-like morphology. The average cell size (105 ± 11 μm and 108 ± 12 μm) and area (1,748 ± 131 μm2 and 1,860 ± 148 μm2) were not significantly different between RA and SA CMCs, respectively. When these cells were plated at limiting dilution, small clones were obtained from single CMCs, which indicates that they possess clonogenic and self-renewing properties; slightly (but not significantly) higher clonogenicity was observed in the SA CMCs (0.15 ± 0.03% and 0.17 ± 0.06% in RA and SA CMCs, respectively). Flow cytometric analysis confirmed expression of mesenchymal markers (CD90, CD105, CD29, CD73, and CD44) and lack of endothelial and hematopoietic markers (CD31 and CD45, respectively) (Online Figure 7A). Despite the small amount of starting tissue, we were able to grow, on average, 2 × 107 and 1.2 × 108 CMCs by passages 3 and 4, respectively. These data suggest that the EMB-based method can be applied to isolate reparative CMCs from small myocardial biopsy samples in humans and enable production of clinically relevant numbers of cells.
DISCUSSION
In this study, we characterized the cardiac reparative capacity of distinct CMC populations phenotypically stratified on the basis of differential adherence properties in vitro. Despite markedly different propensities for plastic adherence, RA CMCs and SA CMCs are morphologically indistinguishable, with minor differences in their surface marker expression. SA CMCs are more clonogenic and have a shorter population doubling time than RA CMCs; furthermore, the expression profile of genes related to ECM remodeling and cytokines is fundamentally different between the 2 populations. When injected into infarcted hearts, SA but not RA CMCs improved LV function, limited collagen deposition in the non-infarcted regions, and reduced inflammatory cell infiltration (Central Illustration). Thus, the simplified isolation method described herein was sufficient to isolate cardiac cells that are beneficial in ischemic cardiomyopathy. Previous studies have described various heart-derived cells with therapeutic properties (42–47). However, to the best of our knowledge, this is the first report to describe a population of c-kit–negative cardiac cells that was identified by slow adherence to plastic and possessed robust reparative properties in vivo. As elaborated later, these SA CMCs offer several advantages and appear to be more suitable for widespread clinical use than c-kitPOS CPCs.
CENTRAL ILLUSTRATION. CMCs Isolated Based on Adherence.
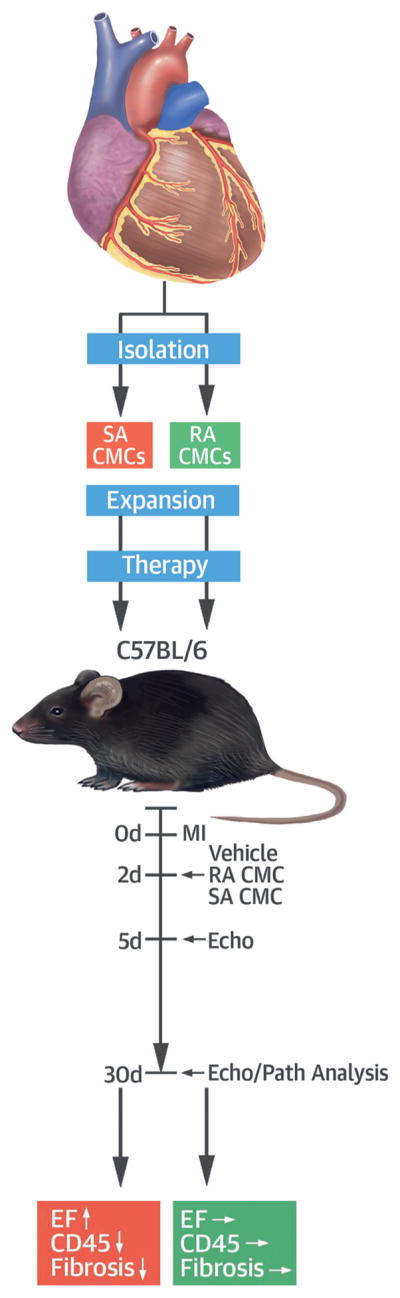
This study evaluated the effectiveness of slowly adhering (SA) versus rapidly adhering (RA) cardiac mesenchymal cells (CMCs) in preserving left ventricular function after myocardial infarction (MI). The CMCs were isolated from mouse hearts, expanded, and injected in mice 2 days post MI along with vehicle treatment. At 30 days post MI, SA but not RA CMCs increased left ventricular ejection fraction (EF), reduced collagen deposition in noninfarcted regions, and reduced the content of CD45-positive cells, providing robust cardiac reparative properties. ↓ = decrease; ↑ = increase; → = no change; Echo = echocardiographic analysis; Path = pathological analysis.
Although the precise mechanism whereby cell therapy improved LV function after MI remains unclear, only a small number of transplanted cells survived in the heart, and an even smaller number differentiated toward myocytes, which implies that they promoted cardiac repair by producing shortlived paracrine factors (3,4,14,17). Therefore, transplanted cells did not have to have stem or progenitor characteristics to be reparative. Among the most studied cell types for the treatment of heart failure are the c-kitPOS, lineage-negative cardiac cells. Although they were initially thought to be multi-potent stem cells with trilineage differentiation potential and ability to differentiate into myocytes (42), subsequent studies from our laboratory have not supported this concept, indicating instead that these cells acted via paracrine mechanisms (3,4,7–12,14). Genetic fate-mapping studies showed that endogenous cardiac c-kitPOS cells contributed minimally to myocytes; instead, they differentiated into endothelium or mesenchyma (48). Moreover, c-kit–sorted cardiac cells also express mesenchymal markers, and some studies showed that these cells have properties similar to bone marrow mesenchymal cells, with ability to differentiate into bone, cartilage, and adipose cells (10,13,18,19,21,22). Thus, considerable evidence suggests that c-kitPOS cardiac cells might be a population of CMCs (3). In a recent study, we demonstrated that compared with c-kitPOS cells sorted from the RA CMC fraction of the heart, c-kitPOS cells sorted from the SA CMC fraction exhibited a more proangiogenic gene profile and a greater ability to promote endogenous repair after MI (13). Accordingly, in the current study, we tested the hypothesis that RA and SA segregation was sufficient to isolate reparative CMCs without c-kit sorting.
We found that both RA CMCs and SA CMCs have morphology (Figure 1) and surface marker expression (Online Figure 2, Table 1) typical of fibroblasts and that these features were not appreciably different between the 2 cell populations. Similar to other mesenchymal cells, expression of endothelial markers (CD31, FLK1), hematopoietic markers (CD45), and c-kit was negligible in both cells (Online Figure 2, Table 1). Thus, both cell types exhibited a typical mesenchymal morphology and phenotype. However, the expression profile of cytokine genes and genes regulating remodeling was different in these 2 cell populations (Figure 2), which suggests fundamental differences. Higher expression of profibrotic factors in RA CMCs versus SA CMCs would suggest that these cells are a form of activated fibroblasts and that after transplantation into the infarcted heart, they might play an active role in detrimental remodeling. This concept was supported by the in vivo studies. When injected into infarcted hearts, SA but not RA CMCs improved cardiac function, evaluated by both echocardiography and hemodynamic studies (Figures 3 and 4), which confirms our hypothesis that CMC isolation based on adherence to plastic dishes is effective in isolating reparative cells. The effects of CMCs observed in this study cannot be compared with those observed in our previous study of c-kit–sorted RA CMCs and SA CMCs (13) because of the differences in mouse models (transient vs. permanent coronary occlusion) and route of cell administration (intramyocardial vs. intraventricular).
Our results demonstrated that a simple method based on differential adhesion to plastic is effective in isolating reparative CMCs. This method offers several advantages. Because it does not require surface marker–based sorting with antibodies, it enables isolation of SA CMCs from virtually any species. Indeed, as shown above, we have used this method to isolate and characterize porcine SA CMCs that are phenotypically similar to murine SA CMCs (Online Figure 7), which indicates that these cells are not unique to the mouse. Additionally, we have conducted preliminary studies in which we isolated a similar cell population from rat and human tissue. This method was particularly advantageous when isolating cells from small tissue samples (e.g., EMBs); because the sorting step is omitted, larger amounts of cells can be grown in a shorter period, which reduces the cost of cell production and the number of passages, resulting in the use of younger, potentially more effcacious cells. Indeed, our studies with porcine EMBs demonstrated that clinically relevant cell numbers (~1 × 108) can be generated by passage 4 from only ~50 mg of tissue.
Furthermore, because this method allows separation of reparative from nonreparative cells, the nonreparative cells can be used as an ideal control for studying the functional properties of SA CMCs, which should facilitate identification of molecular mechanisms of repair. Dermal fibroblasts have been used as control cells for studies of cardiac cells (49,50); however, because of the different tissue of origin, comparisons of these 2 cell populations might not necessarily help identify the mechanisms. Because they are ineffective and originate from the myocardium, RA CMCs appear to be a more suitable cell control than dermal fibroblasts.
Finally, the method described herein generated cells in a more reproducible manner because it did not require any specialized reagents for isolation such as antibodies. Antibody-based techniques are well established, but the presence of significant lot or manufacturer differences can result in non-reproducible results. This is especially problematic when the same procedure must be used to isolate cells from different species (e.g., pigs or humans), because successful cell isolation will depend on the availability of specific antibodies and because one must assume that the same surface marker will be expressed on homologous cell types in different species. In summary, this new CMC isolation method was easy, inexpensive, and reproducible, and it enabled production of large quantities of cells even from small amounts of tissue such as EMBs.
As is the case for most cell types studied heretofore (4), the mechanisms underlying the salubrious effects of SA CMCs in vivo remain unclear. We found no evidence of increased angiogenesis (Online Figure 5) or new cardiomyocyte formation, judging from analysis of bromodeoxyuridine positivity and myocyte cross-sectional area (Online Figure 5). Histological analysis showed that both RA CMCs and SA CMCs significantly reduced scar size and increased total LV viable tissue (Figure 5). This suggested that reducing scar size alone was insufficient to ameliorate LV function. Therefore, we assessed collagen deposition in the infarcted and noninfarcted regions. Although neither cell type reduced collagen content in the risk region, hearts treated with SA CMCs exhibited significantly less collagen in the noninfarcted region (Figure 6). Because the overall contractile performance of the LV after MI depends primarily on the surviving noninfarcted regions, these observations can partially explain the fact that myocardial function was improved by SA but not RA CMCs.
Recent reports showed that immune cells, mainly macrophages, accumulate in the noninfarcted regions of the failing heart and contribute to adverse LV remodeling and progressive dysfunction (41,51). Therefore, we evaluated the immune cell content of the myocardium by staining heart sections for CD45. We found that administration of SA but not RA CMCs significantly reduced the content of CD45POS cells in both the infarcted and noninfarcted regions of the ventricle (Figure 7). This profound anti-infiammatory action of SA CMCs correlated with the reduced collagen content in the noninfarcted regions of the heart and might be responsible in part for attenuating contractile dysfunction. The observations made in this initial evaluation of SA CMCs are hypothesis generating; considerable additional work will be necessary to elucidate the mechanism(s) whereby SA CMCs modulate fibrosis and infiammation and to clarify the functional consequences of these actions.
STUDY LIMITATIONS
Several aspects of the methodology used in this study merit comment. We performed a 60-min coronary occlusion to ensure that infarcts would be large and reproducible and would result in severe depression of LV function (LVEF <35%). We studied reperfused infarcts (as opposed to a permanent coronary occlusion) because clinically, reperfusion occurs in most patients with MI, either spontaneously or iatrogenically. We injected CMCs intramyocardially to maximize cell retention; furthermore, intracoronary infusion of CMCs could be complicated by differential homing of RA and SA CMCs, which would complicate data interpretation. We used cells isolated from male GFP transgenic mice so that we could track them with GFP. To ensure similar LV dysfunction among the groups, we performed echocardiographic analysis 5 days after MI (3 days after cell or vehicle injection) and included in the study only mice with substantial depression of LV function (LVEF <35%). Both surgeons and sonographers were blinded to cell and vehicle group assignments; microscopists performing histological analysis were also blinded to group assignment.
CONCLUSIONS
We described a new population of c-kitNEG CMCs that have considerable therapeutic potential and offer several advantages. Compared with c-kitPOS CPCs, in vitro expansion of CMCs is easier, faster, cheaper, and more reproducible, because it obviates the need to sort cells with c-kit antibodies. Furthermore, the starting number of CMCs in EMBs is much greater than that of c-kitPOS CPCs, which makes it possible to generate clinically relevant numbers of cells with fewer in vitro passages and thus less senescence. CMCs might be more suitable than CPCs for widespread clinical use and provide more reproducible results in preclinical studies.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE
The salutary effects of transplanted cells in patients with heart failure are determined more by paracrine function than stem cell properties, because improvement in LV function occurs in the absence of cell engraftment. CMCs outnumber progenitor cells, are simpler to isolate, and can facilitate myocardial repair after ischemic injury.
TRANSLATIONAL OUTLOOK
The clinical utility of reproducible CMCs isolated from small samples, such as endomyocardial biopsy samples, should be investigated in patients with MI or heart failure.
Acknowledgments
This work was supported by National Institutes of Health grants P20 GM103492, P01 HL078825 (to Drs. Wysoczynski and Bolli), and UM1 HL113530 (to Dr. Bolli), and an American Heart Association scientist development grant 13SDG14560005 (to Dr. Wysoczynski).
ABBREVIATIONS AND ACRONYMS
- CMC
cardiac mesenchymal cell
- CPC
cardiac progenitor cell
- ECM
extracellular matrix
- EMB
endomyocardial biopsy
- GFP
green fluorescent protein
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- RA
rapidly adhering
- RNA
ribonucleic acid
- SA
slowly adhering
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For an expanded Methods section, please see the online version of this paper.
References
- 1.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res. 2016;118:95–107. doi: 10.1161/CIRCRESAHA.115.305373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keith MC, Bolli R. “String theory” of c-kitpos cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results [published correction appears in Circ Res 2015;116:e133] Circ Res. 2015;116:1216–30. doi: 10.1161/CIRCRESAHA.116.305557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–34. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loughran JH, Chugh AR, Ismail I, Bolli R. Stem cell therapy: promising treatment in heart failure? Curr Heart Fail Rep. 2013;10:73–80. doi: 10.1007/s11897-012-0128-2. [DOI] [PubMed] [Google Scholar]
- 6.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–9. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Guo Y, Ou Q, et al. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849–64. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang XL, Rokosh G, Sanganalmath SK, et al. Effects of intracoronary infusion of escalating doses of cardiac stem cells in rats with acute myocardial infarction. Circ Heart Fail. 2015;8:757–65. doi: 10.1161/CIRCHEARTFAILURE.115.002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang XL, Li Q, Rokosh G, et al. Long-term outcome of administration of c-kit(POS) cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res. 2016;118:1091–105. doi: 10.1161/CIRCRESAHA.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tokita Y, Tang XL, Li Q, et al. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res. 2016;119:635–51. doi: 10.1161/CIRCRESAHA.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolli R, Tang XL, Sanganalmath SK, et al. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–31. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang XL, Rokosh G, Sanganalmath SK, et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wysoczynski M, Dassanayaka S, Zafir A, et al. A new method to stabilize c-kit expression in reparative cardiac mesenchymal cells. Front Cell Dev Biol. 2016;4:78. doi: 10.3389/fcell.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong KU, Guo Y, Li QH, et al. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolli R, Chugh AR, D’Amario D, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Chugh AR, Beache GM, Loughran JH, et al. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong KU, Li QH, Guo Y, et al. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itzhaki-Alfia A, Leor J, Raanani E, et al. Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation. 2009;120:2559–66. doi: 10.1161/CIRCULATIONAHA.109.849588. [DOI] [PubMed] [Google Scholar]
- 19.Matuszczak S, Czapla J, Jarosz-Biej M, et al. Characteristic of c-Kit+ progenitor cells in explanted human hearts. Clin Res Cardiol. 2014;103:711–8. doi: 10.1007/s00392-014-0705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Meglio F, Castaldo C, Nurzynska D, et al. Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J Mol Cell Cardiol. 2010;49:719–27. doi: 10.1016/j.yjmcc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 21.Salabei JK, Lorkiewicz PK, Mehra P, et al. Type 2 diabetes dysregulates glucose metabolism in cardiac progenitor cells. J Biol Chem. 2016;291:13634–48. doi: 10.1074/jbc.M116.722496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zafir A, Bradley JA, Long BW, et al. O-GlcNAcylation negatively regulates cardiomyogenic fate in adult mouse cardiac mesenchymal stromal cells. PLoS One. 2015;10:e0142939. doi: 10.1371/journal.pone.0142939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng K, Ibrahim A, Hensley MT, et al. Relative roles of CD90 and c-kit to the regenerative efficacy of cardiosphere-derived cells in humans and in a mouse model of myocardial infarction. J Am Heart Assoc. 2014;3:e001260. doi: 10.1161/JAHA.114.001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quijada P, Sussman MA. Making it stick: chasing the optimal stem cells for cardiac regeneration. Expert Rev Cardiovasc Ther. 2014;12:1275–88. doi: 10.1586/14779072.2014.972941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatzistergos KE, Hare JM. Cell therapy: targeting endogenous repair versus remuscularization. Circ Res. 2015;117:659–61. doi: 10.1161/CIRCRESAHA.115.307346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–30. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanina C, Hare JM. Mesenchymal stem cells as a biological drug for heart disease: where are we with cardiac cell-based therapy? Circ Res. 2015;117:229–33. doi: 10.1161/CIRCRESAHA.117.306306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishore R, Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res. 2016;118:330–43. doi: 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emanueli C, Shearn AI, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul Pharmacol. 2015;71:24–30. doi: 10.1016/j.vph.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–44. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 31.Giordano FJ, Ping P, McKirnan MD, et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischemic region of the heart. Nat Med. 1996;2:534–9. doi: 10.1038/nm0596-534. [DOI] [PubMed] [Google Scholar]
- 32.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen B, Frangogiannis NG. Immune cells in repair of the infarcted myocardium. Microcirculation. 2017;24:e12305. doi: 10.1111/micc.12305. [DOI] [PubMed] [Google Scholar]
- 34.Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest. 2013;43:986–95. doi: 10.1111/eci.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frangogiannis NG. The infiammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frangogiannis NG. The immune system and the remodeling infarcted heart: cell biological insights and therapeutic opportunities. J Cardiovasc Pharmacol. 2014;63:185–95. doi: 10.1097/FJC.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frangogiannis NG. Inflammation in cardiac injury, repair and regeneration. Curr Opin Cardiol. 2015;30:240–5. doi: 10.1097/HCO.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burchfield JS, Xie M, Hill JA. Pathological ventricular remodeling: mechanisms: part 1 of 2. Circulation. 2013;128:388–400. doi: 10.1161/CIRCULATIONAHA.113.001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Sager HB, Hulsmans M, Lavine KJ, et al. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res. 2016;119:853–64. doi: 10.1161/CIRCRESAHA.116.309001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 43.Uchida S, De Gaspari P, Kostin S, et al. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports. 2013;1:397–410. doi: 10.1016/j.stemcr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ott HC, Matthiesen TS, Brechtken J, et al. The adult human heart as a source for stem cells: repair strategies with embryonic-like progenitor cells. Nat Clin Pract Cardiovasc Med. 2007;4:S27–39. doi: 10.1038/ncpcardio0771. [DOI] [PubMed] [Google Scholar]
- 45.Ling L, Bai J, Gu R, et al. Sca-1+ cardiac progenitor cell therapy with cells overexpressing integrin-linked kinase improves cardiac function after myocardial infarction. Transplantation. 2013;95:1187–96. doi: 10.1097/TP.0b013e31828a9423. [DOI] [PubMed] [Google Scholar]
- 46.Rossini A, Frati C, Lagrasta C, et al. Human cardiac and bone marrow stromal cells exhibit distinctive properties related to their origin. Cardiovasc Res. 2011;89:650–60. doi: 10.1093/cvr/cvq290. [DOI] [PubMed] [Google Scholar]
- 47.Chimenti I, Smith RR, Li TS, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res. 2010;106:971–80. doi: 10.1161/CIRCRESAHA.109.210682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Berlo JH, Kanisicak O, Maillet M, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–41. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ibrahim AG, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports. 2014;2:606–19. doi: 10.1016/j.stemcr.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tseliou E, Fouad J, Reich H, et al. Fibroblasts rendered antifibrotic, antiapoptotic, and angiogenic by priming with cardiosphere-derived extracellular membrane vesicles. J Am Coll Cardiol. 2015;66:599–611. doi: 10.1016/j.jacc.2015.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ismahil MA, Hamid T, Bansal SS, Patel B, Kingery JR, Prabhu SD. Remodeling of the mono-nuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ Res. 2014;114:266–82. doi: 10.1161/CIRCRESAHA.113.301720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.