Abstract
Filter-based toxicology studies are conducted to establish the biological plausibility of the well-established health impacts associated with fine particulate matter (PM2.5) exposure. Ambient PM2.5 collected on filters is extracted into solution for toxicology applications, but frequently, characterization is nonexistent or only performed on filter-based PM2.5, without consideration of compositional differences that occur during the extraction processes. To date, the impact of making associations to measured components in ambient instead of extracted PM2.5 has not been investigated. Filter-based PM2.5 was collected at locations (n = 5) and detailed characterization of both ambient and extracted PM2.5 was performed. Alveolar macrophages (AMJ2-C11) were exposed (3, 24, and 48 h) to PM2.5 and the pro-inflammatory cytokine interleukin (IL)-6 was measured. IL-6 release differed significantly between PM2.5 collected from different locations; surprisingly, IL-6 release was highest following treatment with PM2.5 from the lowest ambient concentration location. IL-6 was negatively correlated with the sum of ambient metals analyzed, as well as with concentrations of specific constituents which have been previously associated with respiratory health effects. However, positive correlations of IL-6 with extracted concentrations indicated that the negative associations between IL-6 and ambient concentrations do not accurately represent the relationship between inflammation and PM2.5 exposure. Additionally, seven organic compounds had significant associations with IL-6 release when considering ambient concentrations, but they were not detected in the extracted solution. Basing inflammatory associations on ambient concentrations that are not necessarily representative of in vitro exposures creates misleading results; this study highlights the importance of characterizing extraction solutions to conduct accurate health impact research.
Keywords: Filter-based particulate matter, Particulate matter toxicity, Filter extraction, Toxicity bias
1 Introduction
Exposure to ambient fine particulate matter (PM2.5) has been associated with morbidity and mortality related to respiratory inflammation (Abbey et al. 1995; Dominici et al. 2006; Tecer et al. 2008; Ostro et al. 2009). Associations have also been found between adverse respiratory health outcomes and specific constituents of PM2.5, such as metallic and organic species, independent of total PM2.5 concentration (Mar et al. 2000; Ostro et al. 2007; Ostro et al. 2009). To identify the mechanisms of ambient PM2.5-induced respiratory illnesses and establish the biological plausibility of epidemiological findings, toxicology studies have employed filter-based collection of ambient PM2.5 (Choi et al. 2004; Sawyer et al. 2010; Kumar et al. 2015).
Through the collection of ambient PM2.5 on filters, toxicology studies can be conducted to identify associations between PM2.5 components and biological outcomes. This practice can identify specific components that are most impactful through investigation of the genotoxicity (Dellinger et al. 2001; Topinka et al. 2011; Wang et al. 2011), mutagenicity (de Kok et al. 2005; Skarek et al. 2007; de Brito et al. 2013), oxidative potential (Godri et al. 2011; Gualtieri et al. 2012; Janssen et al. 2014), and inflammatory impacts (Happo et al. 2010; Riva et al. 2011; Akhtar et al. 2014; Kumar et al. 2015). In these previous studies, the complex mixture of PM2.5 was characterized prior to complete preparation for use in the toxicology studies, and this characterization data was used to make associations to biological responses. The practice of basing association of components present on the ambient filter and not on the extraction solution disregards compositional changes that may occur during the extraction process to prepare filter-based PM2.5 for toxicology research.
Recently, complications of extraction have been demonstrated, where total PM2.5 mass extracted can remain high (80%), while less efficient extraction and even complete loss of health-relevant components of PM2.5 (i.e., benzo[a]pyrene, Ni, pyrene) can occur (Roper et al. 2015). Methods for extraction vary between research groups as detailed in Roper et al. (2015). These differences in methods implemented along with the characteristics of collected PM2.5 (i.e., source contributions, mass loadings) can impact extraction efficiency (Bein and Wexler 2014; Roper et al. 2015). The lack of a standardized extraction protocol is of further concern, as it results in methods that vary between research groups and further inhibit cross-study comparisons.
While growing research has begun to characterize extracted PM2.5 components for associations to oxidative potential (Verma et al. 2012) and genotoxic (Danielsen et al. 2011), mutagenic (Cavanagh et al. 2009), and inflammatory (Van Winkle et al. 2015) outcomes, associations are still routinely made to ambient values. Additionally, the resultant discrepancies in associations between using ambient or extracted values has yet to be evaluated. Enhanced understanding of how ambient samples translate to extracted solutions—and how this can impact interpretation of biological outcomes—will ultimately enable the identification of the causal components of PM2.5-associated health effects.
In this study, the release of the pro-inflammatory cytokine, interleukin (IL)-6, was assessed in alveolar macrophages (AM) following exposure to PM2.5 samples that were extracted from ambient filters. Importantly, this study was designed to establish the need for well-characterized extraction solutions when identifying causal components. Associations between IL-6 release and PM2.5 were made using both ambient and corresponding extracted PM2.5 measurements to determine if findings were dependent on the stage at which PM2.5 was characterized.
2 Methods
2.1 PM2.5 Collection
Sampling and extraction methods have been described in detail previously (Roper et al. 2015). Briefly, portable ambient air samplers were deployed approximately 3 m above ground level on metal utility poles for seven consecutive days. Four sampling locations were distributed throughout downtown Pittsburgh, PA, and one additional site was located in a park 14.5 km upwind of the urban area. Harvard Impactors (HI) and cyclone adapted HIs (Air Diagnostics and Engineering Inc., Harrison, ME) served as size segregators, collecting PM2.5 on 37-mm-diameter Teflon™ (PTFE) filters and 37-mm quartz filters, respectively (Pall Corporation, Ann Arbor, MI). Four samplers were co-located at each location: two filters (one PTFE, one quartz) were collected for the characterization of ambient PM2.5, and two filters (both PTFE) were collected for extraction and subsequent toxicology research with in vitro exposures.
2.2 PM2.5 Extraction
Following sampling, PTFE filters collected for in vitro exposures underwent gravimetric analysis on an ultra-microbalance (model XP2U; Mettler Toledo, Columbus, OH) following a 48-h equilibration in a temperature and humidity controlled chamber (20.0 °C and 35% humidity). Filters were weighed pre- and post-sampling to determine ambient PM2.5 mass collected. Using two PTFE filters per sampling location to ensure adequate mass for exposures, samples were sonicated in a 9:1 solution of methanol in sterile Milli-Q water. The resultant PM2.5 suspensions were concentrated through lyophilization to dryness without filtration and stored at −20 °C. PTFE filters underwent gravimetric analysis following sonication to determine the mass removed from each filter. In preparation for in vitro analysis, PM2.5 from each sampling location was re-suspended in serum-free Dulbecco’s Modified Eagle Medium (DMEM). In order to maintain both concentration and compositional differences between locations, PM2.5 from each sampling location was re-suspended in an equal volume of DMEM, which was the volume required in order to translate PM2.5 mass from the lowest ambient concentration to an extraction concentration of 70 μg/mL. This concentration has been previously established to induce the release of cytokines (Becker et al. 2003; Sawyer et al. 2010); all extracted sample concentrations remained lower than thresholds previously observed to induce cell death (Schins et al. 2002). Aliquots of extracted samples were re-deposited onto PTFE and quartz filters (Roper et al. 2015) and were characterized according to the same procedures as ambient PM2.5, described below.
2.3 PM2.5 Characterization
PM2.5 concentrations were calculated via gravimetric analysis of PTFE filters pre- and post-deployment. Ambient and extracted PM2.5 composition was determined via x-ray fluorescence (XRF) analysis of inorganic species (from PTFE filter) and thermal desorption gas chromatography mass spectrometry (TD-GC-MS) analysis of organics (from quartz filter), at Desert Research Institutes, DRI (Reno, NV). Inorganic species, referred to hereafter as “metals” (n = 51), and organics (n = 34) analyzed are listed in Table 1. A schematic of characterization of ambient and extracted samples is provided in Supplementary Figure 1.
Table 1.
List of constituents analyzed
| Metals | Organics | ||||
|---|---|---|---|---|---|
| Ag | Cu | Na | Sn | 1-Methylphenanthrene (1MP) | Dibenzo[a,h]anthracene (DBahA) |
| Al | Eu | Nb | Sr | 2-Methylphenanthrene (2MP) | Dibenzothiophene (DBT) |
| As | Fe | Ni | Ta | 9-Fluorenone (9Flo) | Fluoranthene (Flu) |
| Au | Ga | P | Tb | Acenapthene (Ace) | Fluorine (F) |
| Ba | Hf | Pb | Ti | Acenaphthylene (Acy) | Hopanes (n = 10) |
| Br | Hg | Pd | Tl | Benzo[a]anthracene (BaAnt) | Indeno[1,2,3-cd]pyrene (Ipyr) |
| Ca | In | Rb | U | Benzo[a]pyrene (BaPyr) | Phenanthrene (P) |
| Cd | Ir | S | V | Benzo[b]fluoranthene (BbFl) | Pyrene (Pyr) |
| Ce | K | Sb | W | Benzo[e]pyrene (BePyr) | Steranes (n = 4) |
| Cl | La | Sc | Y | Benzo(ghi)fluoranthene (BghiFl) | |
| Co | Mg | Se | Zn | Benzo[ghi]perylene (BghiPer) | |
| Cr | Mn | Si | Zr | Benzo(jk)fluoranthene (BjkFl) | |
| Cs | Mo | Sm | Chrysene (Chr) | ||
2.4 In Vitro PM2.5 Exposure
Mouse alveolar macrophage cells, AMJ2-C11 (American Type Culture Collection, ATCC, Rockville, MD) were cultured in DMEM supplemented with 5% fetal bovine serum (FBS), 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 5 mM HEPES, 50 U/mL penicillin, and 50 μg/mL streptomycin, following the ATCC protocol.
Cells were seeded at 2.5 × 105 onto six-well plates and underwent a 2-h starvation at 37 °C in a 5% CO2/95% air atmosphere. Immediately following starvation, all wells were exposed to PM2.5 suspended in serum-free DMEM or controls for 3, 24, or 48 h. Control cells received media alone, media containing lipopolysaccharide (LPS) to control for potential endotoxin contamination (Tager et al. 2010), or media containing extracts of unexposed filters to control for filter material released during the extraction procedures. LPS contamination of PM2.5 samples was quantified using a chromogenic endotoxin quantitation kit (Pierce LAL Chromogenic Endotoxin Quantitation Kit; Thermo Scientific, Pittsburgh, PA) and LPS-control cells were treated with the highest LPS concentration detected from previous samples collected in Pittsburgh, PA (0.174 EU/mL). Duplicate wells for each sample/control were exposed at each time point.
2.5 Post-Exposure Analysis
Following exposure (3, 24, or 48 h), plates were vigorously shaken and cell media was collected. Total cell counts (Z1 Coulter Particle Counter; Beckman Coulter Inc., Brea, CA) were recorded; media was then centrifuged at 14,000×g for 5 min. Supernatants were collected and stored at −80 °C until analysis.
IL-6 concentrations were measured in duplicate for all cell supernatants following the manufacturer’s instructions for an enzyme-linked immunosorbent assay (ELISA) specific for mouse IL-6 (R&D Systems, Minneapolis, MN).
2.6 Statistical Analysis
Statistical analysis for all data was performed with StataSE 13 (StataCorp, LP, College Station, TX) and Prism 6.0 (GraphPad Software, Inc., San Diego, CA). All data are reported as a mean ± standard error of the mean (SEM). Data obtained for IL-6 concentrations between treatments and controls was analyzed using one-way analysis of variance (ANOVA) with Bonferroni’s test for multiple post hoc comparisons where appropriate. Pearson’s correlation coefficients were calculated for IL-6 concentrations to both ambient and extracted components of PM2.5. Differences with p values <0.05 were considered significant; statistically significant findings were only observed for 24 and 48 h post-exposure.
3 Results
3.1 IL-6 Release Following PM2.5 Exposure
Sampling locations (sites 1 to 5) were ordered lowest to highest with respect to ambient PM2.5 concentration, without consideration of composition or extracted concentration. IL-6 release from AMs following exposure to PM2.5 from each location was measured relative to media controls at 3, 24, and 48 h (Fig. 1). Equipment failure during the sampling period resulted in reduced collection of PM2.5 at site 4, and IL-6 was only evaluated at 3 h post-exposure. IL-6 release following treatment with PM2.5 from sites with the highest ambient concentrations (sites 3–5) was not significantly different from the control at any time point. In contrast, PM2.5 from the site with the lowest ambient concentration (site 1) induced a significant increase in IL-6 release at 24 and 48 h. IL-6 release in response to PM2.5 from site 2 was significantly higher than both the control and PM2.5 from other sampling locations following 24 h of exposure. A significant prolonged release of IL-6 (up to 48 h) was only observed following exposure to PM2.5 from site 1.
Fig. 1.
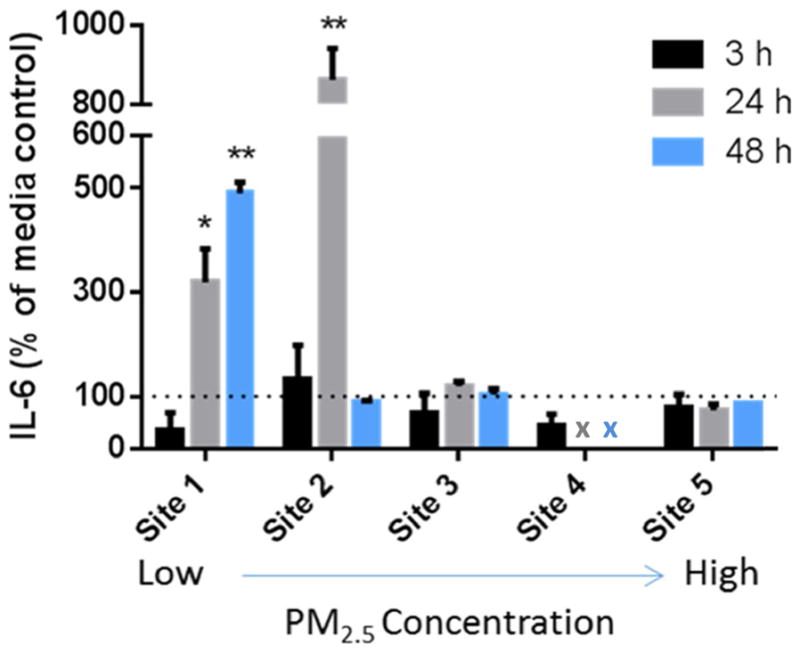
IL-6 concentrations (% of media alone control) measured by ELISA method in AMJ2-C11 cell supernatants following PM2.5 exposure of 3, 24, and 48 h from varying ambient samples (n = 2/site and time). Sampling locations are ordered from low to high (1 to 5) ambient PM2.5 concentrations. Doses (μg/mL) for cell exposure were 71.9 (site 1), 125.1 (site 2), 121.4 (site 3), 122.6 (site 4), and 151.2 (site 5). Results are presented as mean ± SEM with * and ** indicating a statistically significant difference from control (p < 0.05 and 0.001, respectively). “x” indicates time points missing from site 4 due to equipment failure during ambient PM2.5 collection
In order to determine the impact of ambient LPS present in PM2.5 on the release of IL-6, the percent change between LPS-induced IL-6 and concentrations from cells treated with PM2.5 was calculated for each time point. At 3 h, all PM2.5-induced expression of IL-6 was below the response observed in the LPS-treated cells (expression levels ranged between sampling locations from 18.3 to 78.8% below the LPS control). At 24 and 48 h following PM2.5, all sampling locations resulted in elevated levels of IL-6 compared to the LPS control (6.8 to 998.9% above at 24 h and 40.6 to 364.6% above at 48 h). The initial impact of LPS was evident in the pro-inflammatory release at 3 h but following a prolonged exposure (24 and 48 h), all PM2.5 samples induced expression of IL-6 above levels due to LPS treatment alone. Sonicated blank filters that acted as an extraction methods control did not show a significant difference in IL-6 release from media controls at any of the time points.
Total cells increased for all treatments and controls over time with no visible indication of cell death except for a duplicate of AMs exposed to PM2.5 from site 2 for 48 h. This duplicate was observed to have a reduced cell count, solely at this time point, and visual signs of cell death while the complementary sample had consistent cell counts to all other groups, IL-6 was only measured in the site 2 sample without reduced cell counts. This decision was made based on the change in cell counts from this sample being solely at the 48 h time point with no indication of death at earlier measurements of the same sample and irreproducibility, suggesting contamination of the specific well of cells treated.
3.2 Ambient Versus Extracted Comparisons
Detailed comparisons of the composition of ambient and corresponding extracted PM2.5 have been previously reported (Roper et al. 2015) for the samples used in this study. Briefly, total percentage of PM2.5 mass recovery was high while a significant loss of metals and organics was observed for all sampling locations, with highest losses observed as ambient PM2.5 concentration increased. Ambient concentrations for sites 1–5 were 7.78, 13.73, 13.80, 15.01, and 16.35 μg/m3, respectively. Ambient and extracted values for select metals (Table 2) and organics (Table 3) display the differences in contributions of components of PM2.5 following extraction from filters. Ratios of ambient/extracted concentrations varied across locations for total measured metals (sites 1–5 = 1.18, 1.26, 1.53, 1.84, and 5.55, respectively) as well as organics (sites 1–5 = 2.03, 2.51, 8.64, 6.48, and 2.69, respectively). Loss of the contribution to PM2.5 mass of components occurred across all sampling sites in the extracted samples compared to ambient samples for all components measured except Al, Ce, Na, 1MP, Ipyr, and BghiPer. These six components had observed mass ratios that increased at one or more sampling locations following extraction; a hypothesized rationale for these unexpected findings was that the recovery rate of these components was elevated at specific locations or in comparison to other constituents (Roper et al. 2015) and therefore these components had a greater contribution to extracted samples.
Table 2.
Ambient and extracted metals (ng/μg PM2.5) for each sampling location
| Site | Al | Cd | Ce | Cl | Cr | Cu | Fe | Mn | Na | Ni | Pb | S | Sr | Zn | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Amb | 0.50 | 0.00 | 0.71 | 8.88 | 0.15 | 0.21 | 5.79 | 0.24 | 79.06 | 0.04 | 0.32 | 73.89 | 0.05 | 1.75 | 189.73 |
| Ext | 0.60 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 2.49 | 0.06 | 43.78 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 161.18 | |
| 2 | Amb | 2.93 | 0.00 | 0.32 | 38.92 | 0.11 | 0.44 | 15.53 | 0.99 | 48.49 | 0.04 | 0.39 | 45.53 | 0.05 | 2.75 | 151.71 |
| Ext | 3.61 | 0.00 | 0.52 | 19.03 | 0.00 | 0.15 | 7.44 | 0.42 | 67.52 | 0.04 | 0.13 | 2.06 | 0.01 | 0.91 | 120.21 | |
| 3 | Amb | 3.99 | 0.05 | 0.01 | 41.96 | 0.10 | 0.42 | 13.36 | 0.91 | 44.66 | 0.04 | 0.52 | 45.70 | 0.06 | 2.32 | 170.37 |
| Ext | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 5.37 | 0.25 | 57.30 | 0.01 | 0.13 | 0.00 | 0.00 | 0.06 | 111.37 | |
| 4 | Amb | 3.65 | 0.00 | 0.17 | 28.50 | 0.14 | 0.32 | 13.68 | 1.37 | 41.11 | 0.04 | 0.28 | 39.37 | 0.04 | 2.32 | 144.26 |
| Ext | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 3.01 | 0.22 | 68.39 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 78.41 | |
| 5 | Amb | 4.09 | 0.03 | 0.31 | 50.81 | 0.11 | 0.44 | 16.18 | 1.19 | 54.68 | 0.04 | 0.40 | 46.83 | 0.05 | 2.51 | 196.23 |
| Ext | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.06 | 4.71 | 0.25 | 0.00 | 0.01 | 0.06 | 0.00 | 0.00 | 0.03 | 35.38 | |
Ambient (“Amb”) and extracted (“Ext”) metals (ng/μg PM2.5) determined through gravimetric analysis of PM2.5 mass and metal characterization by XRF. Ambient values measured directly from the ambient collected filter and extracted from the extraction solution that was re-deposited onto a filter. “Total” refers to sum of all components analyzed (n = 51)
Table 3.
Ambient and extracted organics (pg/μg PM2.5) for each sampling location
| Site | Acy | Pyr | 1MP | Chr | BbFl | BjkFl | BaAnt | BePyr | BaPyr | Ipyr | BghiPer | Hopanes | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Amb | 955.96 | 20.61 | 3.83 | 31.54 | 30.81 | 69.50 | 13.93 | 16.55 | 11.16 | 11.52 | 54.06 | 3.95 | 1300.76 |
| Ext | 658.20 | 0.00 | 5.75 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 52.48 | 0.00 | 641.26 | |
| 2 | Amb | 784.48 | 25.81 | 2.56 | 49.39 | 33.94 | 65.41 | 24.86 | 25.62 | 18.74 | 18.69 | 73.68 | 20.33 | 1230.16 |
| Ext | 430.50 | 0.00 | 2.67 | 0.00 | 14.69 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 89.79 | 0.00 | 489.91 | |
| 3 | Amb | 1464.59 | 23.12 | 2.45 | 39.25 | 34.55 | 53.51 | 19.76 | 19.41 | 14.65 | 13.69 | 51.63 | 16.07 | 1827.10 |
| Ext | 140.95 | 0.00 | 2.43 | 0.00 | 18.71 | 0.00 | 0.00 | 0.00 | 0.00 | 15.79 | 89.52 | 0.00 | 211.39 | |
| 4 | Amb | 914.71 | 13.49 | 1.58 | 33.45 | 30.83 | 48.22 | 16.16 | 18.20 | 10.80 | 16.10 | 70.76 | 14.77 | 1231.76 |
| Ext | 194.88 | 0.00 | 2.87 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 37.66 | 0.00 | 190.08 | |
| 5 | Amb | 1189.79 | 0.00 | 2.36 | 32.47 | 31.32 | 43.89 | 18.24 | 16.02 | 11.38 | 8.90 | 42.13 | 19.42 | 1476.44 |
| Ext | 489.52 | 0.00 | 2.82 | 0.00 | 10.55 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 74.54 | 0.00 | 548.96 | |
Ambient (“Amb”) and extracted (“Ext”) metals (ng/μg PM2.5) determined through gravimetric analysis of PM2.5 mass and organics characterization by TD-GC-MS. Ambient values measured directly from the ambient collected filter and extracted from the extraction solution that was re-deposited onto a filter. “Total” refers to sum of all organic components analyzed (n = 34)
Release of IL-6 was correlated to both mass as well as specific constituents. The inflammatory marker was poorly correlated with total PM2.5 (<0.184) across all sampling locations at 3 and 24 h for both ambient and extracted concentrations. After 48 h of exposure, IL-6 was negatively correlated with ambient (−0.592) and extracted (−0.581) concentrations (p = 0.043 and 0.048, respectively). The increased IL-6 concentrations following exposure to PM2.5 from sampling site 1, discussed above, are consistent with these results.
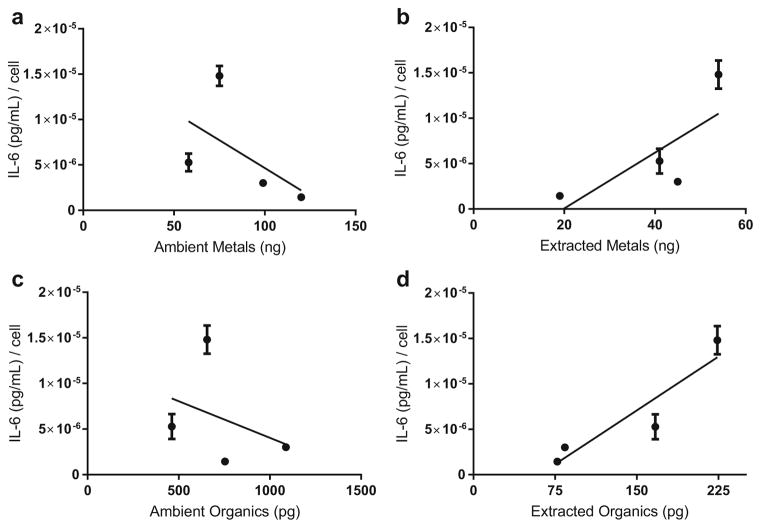
The trends between IL-6 release and sums of total metals (Table 4) and organics (Table 5) analyzed are reported for both ambient and extracted measurements at all time points. At 3 h, no correlation was observed for total metals or organics in either ambient or extracted samples. At 24 h, there was a negative correlation between total ambient metals and IL-6 concentrations (p = .006), whereas no such relationship was seen for total extracted metals. Ambient metals and organics were both observed to have a negative relationship with IL-6 release. Conversely, extracted metals and organics both showed a positive relationship with IL-6 release (Fig. 2). At 48 h, total metals were slightly positively correlated to inflammation for both ambient and extracted measurements. Total organics correlations again differed, with a slight negative relationship with ambient and a slight positive relationship with extracted measurements.
Table 4.
Pearson’s correlations of metals to IL-6 release
| 3 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Am | Ex | Am | Ex | Am | Ex | |
| Al | 0.075 | 0.385 | −0.016 | 0.961▲ | −0.586* | −0.091 |
| Ca | 0.195 | −0.118 | −0.070 | −0.346 | −0.543 | 0.594* |
| Cd | −0.057 | 0.007 | −0.519 | −0.372 | −0.395 | −0.218 |
| Ce | −0.034 | 0.404 | −0.025 | 0.945▲ | 0.532 | −0.215 |
| Cl | 0.231 | 0.404 | 0.068 | 0.945▲ | −0.598* | −0.215 |
| Cr | −0.226 | 0.007 | −0.089 | −0.372 | 0.574* | −0.218 |
| Cs | 0.404 | 0.239 | 0.945* | 0.891▲ | −0.215 | −0.325 |
| Cu | 0.323 | 0.323 | 0.242 | 0.852* | −0.611* | −0.281 |
| Fe | 0.282 | 0.429 | 0.269 | 0.642* | −0.602* | −0.540 |
| Mg | −0.226 | −0.254 | −0.749* | −0.239 | 0.465 | 0.610* |
| Mn | 0.072 | 0.419 | 0.158 | 0.634* | −0.599* | −0.544 |
| Mo | 0.035 | −0.223 | 0.129 | −0.206 | 0.547 | 0.579* |
| Na | −0.121 | 0.066 | −0.259 | 0.603 | 0.585* | −0.002 |
| Ni | −0.242 | 0.437 | −0.856* | 0.857* | 0.378 | −0.381 |
| Pb | 0.119 | 0.245 | −0.133 | 0.534 | −0.456 | −0.057 |
| S | −0.145 | 0.404 | −0.246 | 0.945▲ | 0.608* | −0.215 |
| Sn | −0.166 | −0.007 | −0.489 | −0.372 | 0.482 | −0.218 |
| Sr | −0.010 | 0.286 | −0.590 | 0.739* | −0.147 | 0.292 |
| Y | 0.100 | −0.208 | 0.299 | −0.226 | 0.513 | 0.609* |
| Zn | 0.386 | 0.409 | 0.490 | 0.934▲ | −0.576* | −0.252 |
| Total | −0.123 | −0.015 | −0.796* | 0.198 | 0.323 | 0.470 |
Correlations of IL-6 release at 3, 24, and 48 h to both ambient (Am) and extracted (Ex) metal constituents. “Total” refers to the sum of all metals (n = 51) analyzed in Table 1.
indicate a statistically significant correlation with p < 0.05 and <0.001, respectively
Table 5.
Pearson’s correlations of organics to IL-6 release
| 3 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Am | Ex | Am | Ex | Am | Ex | |
| Acy | −0.153 | 0.064 | −0.678* | 0.001 | −0.266 | 0.453 |
| Ace | 0.404 | - | 0.945▲ | - | −0.215 | - |
| F | 0.323 | - | 0.279 | - | −0.590* | - |
| P | 0.158 | - | 0.193 | - | 0.516 | - |
| Flu | 0.279 | - | 0.722* | - | −0.001 | - |
| Pyr | 0.146 | - | 0.514 | - | 0.109 | - |
| 9Flo | −0.161 | - | −0.236 | - | 0.608* | - |
| DBT | −0.063 | - | −0.295 | - | −0.267 | - |
| 1MP | −0.008 | −0.215 | −0.145 | −0.220 | 0.607* | 0.603* |
| 2MP | 0.296 | - | 0.536 | - | −0.502 | - |
| Chr | 0.401 | - | 0.825* | - | −0.389 | - |
| BbFl | 0.271 | 0.280 | 0.410 | 0.232 | −0.447 | −0.557 |
| BjkFl | 0.145 | - | 0.412 | - | 0.461 | - |
| BaAnt | 0.432 | - | 0.687* | - | −0.519 | - |
| BePyr | 0.381 | - | 0.881▲ | - | −0.317 | - |
| BaPyr | 0.400 | - | 0.814* | - | −0.387 | - |
| Ipyr | 0.235 | −0.063 | 0.866* | −0.295 | −0.211 | −0.267 |
| DBahA | 0.218 | - | 0.709* | - | −0.406 | - |
| BghiPer | 0.172 | 0.329 | 0.923▲ | 0.386 | −0.032 | −0.569 |
| BghiFl | 0.369 | - | 0.667* | - | −0.528 | - |
| Hopanes | 0.337 | - | 0.335 | - | −0.603* | - |
| Steranes | 0.247 | - | 0.203 | - | −0.509 | - |
| Total | −0.085 | 0.124 | −0.533 | 0.046 | −0.303 | 0.405 |
Correlations of IL-6 release at 3, 24, and 48 h to both ambient (Am) and extracted (Ex) organic constituents. “Total” refers to the sum of all organics (n = 34) analyzed in Table 1. – indicates a missing value due to the constituent not being present in the extracted solution.
indicate a statistically significant correlation with p < 0.05 and <0.001, respectively
Fig. 2.
Scatterplots of IL-6 release from each sampling location by constituents following 24 h of PM2.5 exposure. IL-6 [(pg/mL)/cell] release per total ambient (a) and extracted (b) metals (ng) or total ambient (c) and extracted (d) organics (pg). Totals refer to the sum of all constituents measured (Table 1), metals (n = 51) and organics (n = 34). Results are presented as mean ± SEM (n = 2/site)
The correlations between IL-6 concentrations and the proportional mass of individual metals (ng/μg PM2.5, Table 4) and organics (pg/μg PM2.5, Table 5) were calculated for all sampling locations at each time point following exposure using both ambient and extracted values. Normalization to total PM2.5 mass was utilized to eliminate the impact of differing doses between sampling locations (70–151 μg/mL).
At 3 h, specific extracted metals were positively related to IL-6 releases, including Ce, Mn, Ni, and Zn, and these relationships were not observed when using ambient measurements. Interestingly, at 24 h, significant correlations between Ni and IL-6 were observed, with inverse relationships when using ambient (− .856, p = 0.002) and extracted (0.857, p = 0.002) measurements. Positive relationships were present with extracted measurements of Al, Ce, Cl, Cs, S, and Zn (p = 0.001) and Cu, Fe, and Mn (p = 0.002, 0.045, and 0.049, respectively). These relationships were not observed in the ambient metals, with the exception of Cs (p = 0.001). At 48 h, negative relationships of specific ambient metals were observed: Al, Cl, Cu, Fe, Mn, and Zn (p = 0.045, 0.040, 0.035, 0.039, 0.040, and 0.050, respectively). Positive correlations were present for the extracted metals, Ca, Mg, Mo, and Y (p = 0.042, 0.035, 0.049, and 0.036, respectively).
At 3 h, positive correlations were observed for individual ambient organic components: Ace, Chr, BaAnt, and BaPyr. However, none of these constituents were present in extracted samples; Acy, 1MP, BbFl, Ipyr, and BghiPer were the only organics detected following extraction. At 24 h, individual ambient organics had positive correlations with IL-6 including Ace (p = 0.001), Flu (p = 0.018), Chr (p = 0.003), BaAnt (p = 0.028), BePyr (p = 0.001), BaPyr (p = 0.004), DBahA (p = 0.022), BghiPer (p = 0.001), and BghiFl (p = 0.035). These correlations were not observed for extracted organics, and a majority of the compounds were not even present in extracted samples with the primary hypothesized reason for loss due to the volatility of the compounds and loss throughout the multistep extraction process (Roper et al. 2015). In contrast to ambient associations, extracted Ipyr had a slightly negative association with IL-6. The negative association between IL-6 and ambient Acy was not observed in the extracted samples where nearly no correlation was observed. At 48 h, negative relationships were observed with ambient measurements of fluorine and hopanes (p = 0.043 and 0.038, respectively), while these compounds were not detected in the extracted samples and therefore no correlation was observed.
4 Discussion
IL-6 release following exposure to ambient PM2.5 has been well documented in cells involved in the respiratory inflammatory response: macrophages (Becker et al. 2005; Jalava et al. 2006), alveolar epithelial cells (Hetland et al. 2004; Shang et al. 2013), and bronchial epithelial cells (Watterson et al. 2007; Lauer et al. 2009). Our findings corroborate previous time-course experiments in which significant release of IL-6 after PM2.5 exposure was observed at longer time points (more than 3 h) (Watterson et al. 2012). Interestingly, not all sampling locations induced release of IL-6 above controls and the locations which had significant increases in IL-6 were from sites with the lowest ambient concentrations. Based on previous epidemiology research of the impact of varying PM2.5 concentrations on inflammatory-associated health outcomes (Ruckerl et al. 2006; Anderson et al. 2012; Villeneuve et al. 2015), these low concentration locations would be hypothesized to induce comparably reduced responses in vitro. While elevated release of a pro-inflammatory mediator with increasing ambient concentrations was not observed in this study, previous in vitro exposures have found similar results. IL-6 was not observed to have a significant increase compared to controls following exposure to PM2.5 from an urban sampling location with high vehicular traffic (Perrone et al. 2010). Findings of mutagenic effects of PM2.5 have been similarly unexpected: for example, Zhao et al. (2002) found that the sampling location selected as the “clean” control site, free of any major industrial sources, caused elevated mutagenic potency compared to sampling locations with vehicular and industrial sources. When interpreting these findings, an important factor to consider is that conclusions are drawn from ambient concentrations prior to extraction, and the differences in PM2.5 following extraction are a potential cause for discrepancies with epidemiology research.
The inconsistency of these results with current epidemiological findings was also observed for specific constituents of PM2.5. Metals established to be associated with health effects, including Ni, S, and Sr (Bernstein et al. 2004; Schwarze et al. 2006), had negative correlations with IL-6 expression when using ambient values but significant positive correlations when using extracted PM2.5 values, highlighting the potential for misinterpretation of biological outcomes in filter-based toxicology research. Of the organics analyzed, seven were found to have significant correlations to IL-6 expression, but were not detectable in the extracted PM2.5. Significant or complete losses in both metals (i.e., Cu, Cr, Fe, Ni, Zn) and organics (i.e., benzo[a]pyrene, benzo[e]pyrene, benzo[ghi]perylene, pyrene) occur when preparing ambient PM2.5 using the extraction methods detailed (Roper et al. 2015). Ambient values of these constituents have previously been used to make associations to inflammation (Huang et al. 2003; Akhtar et al. 2014) and oxidative potential (Valavanidis et al. 2005; Janssen et al. 2014). This study demonstrated that based solely on ambient measurements, the associations between IL-6 and PM2.5 constituents made are inaccurate and may indicate health relevancy of compounds, while associations made using data from extracted PM2.5 do not support this conclusion. When correlating IL-6 expression to extracted values, positive correlations were observed for both total metals and total organics concentrations, as anticipated from established epidemiology research (Schaumann et al. 2004; Delfino et al. 2010). Characterization of extracted PM2.5 solution allows for measurements that are representative of the exposure and avoids inaccurate associations between constituents and inflammatory responses.
Frequently, biological assays are performed using PM2.5 that has not been characterized in either ambient or extracted form (Akhtar et al. 2010; Deng et al. 2013; Happo et al. 2013; Jeong et al. 2014). This practice avoids misidentification of causal constituents but disregards compositional variability altogether, despite the establishment of PM2.5 composition as a driver of health effects, independent of concentration (Mar et al. 2000; Ostro et al. 2010). Furthermore, the use of uncharacterized PM2.5 hinders the ultimate goal of identifying key constituents responsible for health effects. Limited toxicology studies using filter-based extraction methods have characterized samples following partial or complete extraction procedures (Gerlofs-Nijland et al. 2007; Jalava et al. 2009; Huang et al. 2014; Mirowsky et al. 2015). These studies allow for connections to be made to components or groups of components in PM2.5 that are the predominant factors in PM2.5-associated health outcomes. Awareness of the need for research to base associations on extracted values of PM2.5 will facilitate accurate determination of associations to corroborate epidemiological findings and ultimately to identify the mechanisms of PM2.5-related health effects.
While this preliminary research raised potential issues of not using properly characterized PM2.5, there are a number of limitations that must be considered for future research. First, the dose (μg PM2.5/mL) varied between sampling locations and cells were therefore exposed to varying concentrations of ambient PM2.5. All concentrations were within a range commonly utilized for in vitro studies, and while direct measurements of cell death were not recorded, cell counts increased over time at all locations indicating proliferation continued and was more substantial than cell death. These doses were selected to preserve both compositional and concentration differences between sampling locations for in vitro exposures. In all data analysis of specific PM2.5 constituents, values were normalized to total PM2.5 to allow for comparison of constituent effects independent of PM2.5 mass. While this study explored effects on a single inflammatory marker, PM2.5 has been shown to induce a robust release of numerous cytokines and markers of oxidative stress (Mitschik et al. 2008; Araujo 2010; Anderson et al. 2012). Additionally, the macrophage cell line utilized is reflective of responses specific to that line, and as such is not predictive of the response by other cell types or systemic responses. Future studies using equivalent doses of PM2.5 in other cells essential to the inflammatory response to PM2.5 (epithelial cells or co-culture systems) or in vivo models with the measurement of multiple inflammatory markers would provide further information on the inflammatory response to PM2.5 as well as further demonstrate the importance of well-characterized samples when making associations with these cell types.
5 Conclusions
PM2.5 toxicology research has previously made associations between biological responses and ambient measurements, disregarding subsequent changes due to extraction methods. This study has shown that associations made to ambient PM2.5 are not comparable to those made to extracted PM2.5 for the methods and marker analyzed. This misrepresentation is due to compositional alterations in PM2.5 that occur during extraction of PM2.5 from a filter. Recent studies have investigated the potential consequences of different filter extraction methods on biological impacts of PM2.5; marked differences in inflammatory gene expression were observed and found to be dependent on the extraction method utilized (Van Winkle et al. 2015). In conjunction with the current study, these results demonstrate the importance of extraction methods as well as the importance of characterizing PM2.5 composition when studying toxicological outcomes. Increased understanding of the impacts of PM2.5 on inflammatory responses is essential to create targeted interventions relevant to human health; it is imperative that such interventions be based on accurate associations made using well-characterized PM2.5 following extraction.
Acknowledgments
The authors would like to thank members of Dr. Luis Ortiz’s laboratory at the University of Pittsburgh for assistance with in vitro studies. Funding for this project was provided from departmental funds from the University of Pittsburgh’s Environmental and Occupational Health Department.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11270-016-3219-y) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Conflict of Interest The authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Disclaimer The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH). Mention of company names or products does not constitute endorsement by NIOSH.
Contributor Information
Courtney Roper, Department of Environmental and Occupational Health, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA.
Lauren G. Chubb, Department of Environmental and Occupational Health, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA, Office of Mine Safety and Health Research, National Institute for Occupational Safety and Health, Pittsburgh, PA, USA
Leah Cambal, Department of Environmental and Occupational Health, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA.
Brett Tunno, Department of Environmental and Occupational Health, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA.
Jane E. Clougherty, Department of Environmental and Occupational Health, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
Cheryl Fattman, Department of Environmental and Occupational Health, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA.
Steven E. Mischler, Department of Environmental and Occupational Health, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA, Office of Mine Safety and Health Research, National Institute for Occupational Safety and Health, Pittsburgh, PA, USA
References
- Abbey DE, Ostro BE, Petersen F, Burchette RJ. Chronic respiratory symptoms associated with estimated long-term ambient concentrations of fine particulates less than 2.5 microns in aerodynamic diameter (PM2.5) and other air pollutants. Journal of exposure analysis and environmental epidemiology. 1995;5(2):137–159. [PubMed] [Google Scholar]
- Akhtar US, McWhinney RD, Rastogi N, Abbatt JP, Evans GJ, Scott JA. Cytotoxic and proinflammatory effects of ambient and source-related particulate matter (PM) in relation to the production of reactive oxygen species (ROS) and cytokine adsorption by particles. Inhalation Toxicology. 2010;22(Suppl 2):37–47. doi: 10.3109/08958378.2010.518377. [DOI] [PubMed] [Google Scholar]
- Akhtar US, Ratstogi N, McWhinney RD, Urch B, Chow C, Evans GJ, Scott JA. The combined effects of physicochemical properties of size-fractionated ambient particulate matter on in vitro toxicity in human A549 lung epithelial cells. Toxicology Reports. 2014;1:145–156. doi: 10.1016/j.toxrep.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. Journal of medical toxicology : official journal of the American College of Medical Toxicology. 2012;8(2):166–175. doi: 10.1007/s13181-011-0203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JA. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air quality, Atmosphere, Health. 2010;4(1):79–93. doi: 10.1007/s11869-010-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicology and Applied Pharmacology. 2005;207(2 Suppl):269–275. doi: 10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Sioutas C, Cassee FR. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Experimental Lung Research. 2003;29(1):29–44. doi: 10.1080/01902140303762. [DOI] [PubMed] [Google Scholar]
- Bein KJ, Wexler AS. A high-efficiency, low-bias method for extracting particulate matter from filter and impactor substrates. Atmospheric Environment. 2014;90:87–95. [Google Scholar]
- Bernstein JA, Alexis N, Barnes C, Bernstein IL, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB. Health effects of air pollution. The Journal of Allergy and Clinical Immunology. 2004;114(5):1116–1123. doi: 10.1016/j.jaci.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Cavanagh JA, Trought K, Brown L, Duggan S. Exploratory investigation of the chemical characteristics and relative toxicity of ambient air particulates from two New Zealand cities. The Science of the Total Environment. 2009;407(18):5007–5018. doi: 10.1016/j.scitotenv.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Choi JH, Kim JS, Kim YC, Kim YS, Chung NH, Cho MH. Comparative study of PM2.5- and PM10-induced oxidative stress in rat lung epithelial cells. Journal of Veterinary Science. 2004;5(1):11–18. [PubMed] [Google Scholar]
- Danielsen PH, Moller P, Jensen KA, Sharma AK, Wallin H, Bossi R, Autrup H, Molhave L, Ravanat JL, Briede JJ, de Kok TM, Loft S. Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chemical Research in Toxicology. 2011;24(2):168–184. doi: 10.1021/tx100407m. [DOI] [PubMed] [Google Scholar]
- de Brito KC, de Lemos CT, Rocha JA, Mielli AC, Matzenbacher C, Vargas VM. Comparative genotoxicity of airborne particulate matter (PM2.5) using Salmonella, plants and mammalian cells. Ecotoxicology and Environmental Safety. 2013;94:14–20. doi: 10.1016/j.ecoenv.2013.04.014. [DOI] [PubMed] [Google Scholar]
- de Kok TM, Hogervorst JG, Briede JJ, van Herwijnen MH, Maas LM, Moonen EJ, Driece HA, Kleinjans JC. Genotoxicity and physicochemical characteristics of traffic-related ambient particulate matter. Environmental and Molecular Mutagenesis. 2005;46(2):71–80. doi: 10.1002/em.20133. [DOI] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Arhami M, Polidori A, Gillen DL, George SC, Shafer MM, Schauer JJ, Sioutas C. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology. 2010;21(6):892–902. doi: 10.1097/EDE.0b013e3181f20e6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chemical Research in Toxicology. 2001;14(10):1371–1377. doi: 10.1021/tx010050x. [DOI] [PubMed] [Google Scholar]
- Deng X, Rui W, Zhang F, Ding W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biology and Toxicology. 2013;29(3):143–157. doi: 10.1007/s10565-013-9242-5. [DOI] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA : the journal of the American Medical Association. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, Dormans JA, Bloemen HJ, Leseman DL, John A, Boere F, Kelly FJ, Mudway IS, Jimenez AA, Donaldson K, Guastadisegni C, Janssen NA, Brunekreef B, Sandstrom T, van Bree L, Cassee FR. Toxicity of coarse and fine particulate matter from sites with contrasting traffic profiles. Inhalation Toxicology. 2007;19(13):1055–1069. doi: 10.1080/08958370701626261. [DOI] [PubMed] [Google Scholar]
- Godri KJ, Harrison RM, Evans T, Baker T, Dunster C, Mudway IS, Kelly FJ. Increased oxidative burden associated with traffic component of ambient particulate matter at roadside and urban background schools sites in London. PloS One. 2011;6(7):e21961. doi: 10.1371/journal.pone.0021961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri M, Longhin E, Mattioli M, Mantecca P, Tinaglia V, Mangano E, Proverbio MC, Bestetti G, Camatini M, Battaglia C. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicology Letters. 2012;209(2):136–145. doi: 10.1016/j.toxlet.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Happo M, Markkanen A, Markkanen P, Jalava P, Kuuspalo K, Leskinen A, Sippula O, Lehtinen K, Jokiniemi J, Hirvonen MR. Seasonal variation in the toxicological properties of size-segregated indoor and outdoor air particulate matter. Toxicology in vitro : an international journal published in association with BIBRA. 2013;27(5):1550–1561. doi: 10.1016/j.tiv.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Happo MS, Hirvonen MR, Halinen AI, Jalava PI, Pennanen AS, Sillanpaa M, Hillamo R, Salonen RO. Seasonal variation in chemical composition of size-segregated urban air particles and the inflammatory activity in the mouse lung. Inhalation Toxicology. 2010;22(1):17–32. doi: 10.3109/08958370902862426. [DOI] [PubMed] [Google Scholar]
- Hetland RB, Cassee FR, Refsnes M, Schwarze PE, Lag M, Boere AJ, Dybing E. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicology in vitro : an international journal published in association with BIBRA. 2004;18(2):203–212. doi: 10.1016/s0887-2333(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhang J, Peng S, Tian M, Chen J, Shen H. Effects of water soluble PM2.5 extracts exposure on human lung epithelial cells (A549): a proteomic study. Journal of Applied Toxicology : JAT. 2014;34(6):675–687. doi: 10.1002/jat.2910. [DOI] [PubMed] [Google Scholar]
- Huang SL, Hsu MK, Chan CC. Effects of submicrometer particle compositions on cytokine production and lipid peroxidation of human bronchial epithelial cells. Environmental Health Perspectives. 2003;111(4):478–482. doi: 10.1289/ehp.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalava PI, Hirvonen MR, Sillanpaa M, Pennanen AS, Happo MS, Hillamo R, Cassee FR, Gerlofs-Nijland M, Borm PJ, Schins RP, Janssen NA, Salonen RO. Associations of urban air particulate composition with inflammatory and cytotoxic responses in RAW 246.7 cell line. Inhalation Toxicology. 2009;21(12):994–1006. doi: 10.1080/08958370802695710. [DOI] [PubMed] [Google Scholar]
- Jalava PI, Salonen RO, Halinen AI, Penttinen P, Pennanen AS, Sillanpaa M, Sandell E, Hillamo R, Hirvonen MR. In vitro inflammatory and cytotoxic effects of size-segregated particulate samples collected during long-range transport of wildfire smoke to Helsinki. Toxicology and Applied Pharmacology. 2006;215(3):341–353. doi: 10.1016/j.taap.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Janssen NA, Yang A, Strak M, Steenhof M, Hellack B, Gerlofs-Nijland ME, Kuhlbusch T, Kelly F, Harrison R, Brunekreef B, Hoek G, Cassee F. Oxidative potential of particulate matter collected at sites with different source characteristics. The Science of the Total Environment. 2014;472:572–581. doi: 10.1016/j.scitotenv.2013.11.099. [DOI] [PubMed] [Google Scholar]
- Jeong SC, Shin CY, Song MK, Cho Y, Ryu JC. Gene expression profiling of human alveolar epithelial cells (A549 cells) exposed to atmospheric particulate matter 2.5 collected from Seoul, Korea. Molecular and Cellular Toxicology. 2014;10:361–368. [Google Scholar]
- Kumar RK, Shadie AM, Bucknall MP, Rutlidge H, Garthwaite L, Herbert C, Halliburton B, Parsons KS, Wark PA. Differential injurious effects of ambient and traffic-derived particulate matter on airway epithelial cells. Respirology. 2015;20(1):73–79. doi: 10.1111/resp.12381. [DOI] [PubMed] [Google Scholar]
- Lauer FT, Mitchell LA, Bedrick E, McDonald JD, Lee WY, Li WW, Olvera H, Amaya MA, Berwick M, Gonzales M, Currey R, Pingitore NE, Burchiel SW. Temporal-spatial analysis of US-Mexico border environmental fine and coarse PM air sample extract activity in human bronchial epithelial cells. Toxicology and Applied Pharmacology. 2009;238(1):1–10. doi: 10.1016/j.taap.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in Phoenix, 1995–1997. Environmental Health Perspectives. 2000;108(4):347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowsky JE, Jin L, Thurston G, Lighthall D, Tyner T, Horton L, Galdanes K, Chillrud S, Ross J, Pinkerton KE, Chen LC, Lippmann M, Gordon T. In vitro and in vivo toxicity of urban and rural particulate matter from California. Atmospheric Environment. 2015;103:256–262. doi: 10.1016/j.atmosenv.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitschik S, Schierl R, Nowak D, Jorres RA. Effects of particulate matter on cytokine production in vitro: a comparative analysis of published studies. Inhalation Toxicology. 2008;20(4):399–414. doi: 10.1080/08958370801903784. [DOI] [PubMed] [Google Scholar]
- Ostro B, Feng WY, Broadwin R, Green S, Lipsett M. The effects of components of fine particulate air pollution on mortality in California: results from CALFINE. Environmental Health Perspectives. 2007;115(1):13–19. doi: 10.1289/ehp.9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, Henderson KD, Bernstein L. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environmental Health Perspectives. 2010;118(3):363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, Roth L, Malig B, Marty M. The effects of fine particle components on respiratory hospital admissions in children. Environmental Health Perspectives. 2009;117(3):475–480. doi: 10.1289/ehp.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone MG, Gualtieri M, Ferrero L, Lo Porto C, Udisti R, Bolzacchini E, Camatini M. Seasonal variations in chemical composition and in vitro biological effects of fine PM from Milan. Chemosphere. 2010;78(11):1368–1377. doi: 10.1016/j.chemosphere.2009.12.071. [DOI] [PubMed] [Google Scholar]
- Riva DR, Magalhaes CB, Lopes AA, Lancas T, Mauad T, Malm O, Valenca SS, Saldiva PH, Faffe DS, Zin WA. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhalation Toxicology. 2011;23(5):257–267. doi: 10.3109/08958378.2011.566290. [DOI] [PubMed] [Google Scholar]
- Roper C, Chubb LG, Cambal L, Tunno B, Clougherty JE, Mischler SE. Characterization of ambient and extracted PM2.5 collected on filters for toxicology applications. Inhalation Toxicology. 2015;27:673–681. doi: 10.3109/08958378.2015.1092185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckerl R, Ibald-Mulli A, Koenig W, Schneider A, Woelke G, Cyrys J, Heinrich J, Marder V, Frampton M, Wichmann HE, Peters A. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. American Journal of Respiratory and Critical Care Medicine. 2006;173(4):432–441. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- Sawyer K, Mundandhara S, Ghio AJ, Madden MC. The effects of ambient particulate matter on human alveolar macrophage oxidative and inflammatory responses. Journal of Toxicology and Environmental Health Part A. 2010;73(1):41–57. doi: 10.1080/15287390903248901. [DOI] [PubMed] [Google Scholar]
- Schaumann F, Borm PJ, Herbrich A, Knoch J, Pitz M, Schins RP, Luettig B, Hohlfeld JM, Heinrich J, Krug N. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. American Journal of Respiratory and Critical Care Medicine. 2004;170(8):898–903. doi: 10.1164/rccm.200403-423OC. [DOI] [PubMed] [Google Scholar]
- Schins R, Knaapen A, Weishaupt C, Winzer A, Borm P. Cytotoxic and inflammatory effects of coarse and fine particulate matter in macrophages and epithelial cells. The Annals of Occupational Hygiene. 2002;46:203–206. [Google Scholar]
- Schwarze PE, Ovrevik J, Lag M, Refsnes M, Nafstad P, Hetland RB, Dybing E. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Human & Experimental Toxicology. 2006;25(10):559–579. doi: 10.1177/096032706072520. [DOI] [PubMed] [Google Scholar]
- Shang Y, Fan L, Feng J, Lv S, Wu M, Li B, Zang YS. Genotoxic and inflammatory effects of organic extracts from traffic-related particulate matter in human lung epithelial A549 cells: the role of quinones. Toxicology In Vitro : an international journal published in association with BIBRA. 2013;27(2):922–931. doi: 10.1016/j.tiv.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Skarek M, Janosek J, Cupr P, Kohoutek J, Novotna-Rychetska A, Holoubek I. Evaluation of genotoxic and non-genotoxic effects of organic air pollution using in vitro bioassays. Environment International. 2007;33(7):859–866. doi: 10.1016/j.envint.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Tager IB, Lurmann FW, Haight T, Alcorn S, Penfold B, Hammond SK. Temporal and spatial patterns of ambient endotoxin concentrations in Fresno, California. Environmental Health Perspectives. 2010;118(10):1490–1496. doi: 10.1289/ehp.0901602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecer LH, Alagha O, Karaca F, Tuncel G, Eldes N. Particulate matter (PM(2.5), PM(10–2.5), and PM(10)) and children’s hospital admissions for asthma and respiratory diseases: a bidirectional case-crossover study. Journal of toxicology and environmental health Part A. 2008;71(8):512–520. doi: 10.1080/15287390801907459. [DOI] [PubMed] [Google Scholar]
- Topinka J, Rossner P, Jr, Milcova A, Schmuczerova J, Svecova V, Sram RJ. DNA adducts and oxidative DNA damage induced by organic extracts from PM2.5 in an acellular assay. Toxicology Letters. 2011;202(3):186–192. doi: 10.1016/j.toxlet.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlahoyianni T, Fiotakis K. Comparative study of the formation of oxidative damage marker 8-hydroxy-2'-deoxyguanosine (8-OHdG) adduct from the nucleoside 2'-deoxyguanosine by transition metals and suspensions of particulate matter in relation to metal content and redox reactivity. Free Radical Research. 2005;39(10):1071–1081. doi: 10.1080/10715760500188671. [DOI] [PubMed] [Google Scholar]
- Van Winkle LS, Bein K, Anderson D, Pinkerton KE, Tablin F, Wilson D, Wexler AS. Biological dose response to PM2.5: effect of particle extraction method on platelet and lung responses. Toxicological Sciences : an official journal of the Society of Toxicology. 2015;143(2):349–35. doi: 10.1093/toxsci/kfu230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V, Rico-Martinez R, Kotra N, King L, Liu J, Snell TW, Weber RJ. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fine ambient aerosols. Environmental Science & Technology. 2012;46(20):11384–11392. doi: 10.1021/es302484r. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Weichenthal SA, Crouse D, Miller AB, To T, Martin RV, van Donkelaar A, Wall C, Burnett RT. Long-term exposure to fine particulate matter air pollution and mortality among Canadian women. Epidemiology. 2015;26(4):536–545. doi: 10.1097/EDE.0000000000000294. [DOI] [PubMed] [Google Scholar]
- Wang W, Jariyasopit N, Schrlau J, Jia Y, Tao S, Yu TW, Dashwood RH, Zhang W, Wang X, Simonich SL. Concentration and photochemistry of PAHs, NPAHs, and OPAHs and toxicity of PM2.5 during the Beijing Olympic Games. Environmental Science & Technology. 2011;45(16):6887–6895. doi: 10.1021/es201443z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson TL, Hamilton B, Martin RS, Coulombe RA., Jr Urban particulate matter activates Akt in human lung cells. Archives of Toxicology. 2012;86(1):121–135. doi: 10.1007/s00204-011-0739-5. [DOI] [PubMed] [Google Scholar]
- Watterson TL, Sorensen J, Martin R, Coulombe RA., Jr Effects of PM2.5 collected from Cache Valley Utah on genes associated with the inflammatory response in human lung cells. Journal of Toxicology and Environmental Health Part A. 2007;70(20):1731–1744. doi: 10.1080/15287390701457746. [DOI] [PubMed] [Google Scholar]
- Zhao X, Wan Z, Chen G, Zhu H, Jiang S, Yao J. Genotoxic activity of extractable organic matter from urban airborne particles in Shanghai, China. Mutation Research. 2002;514(1–2):177–192. doi: 10.1016/s1383-5718(01)00338-2. [DOI] [PubMed] [Google Scholar]