Abstract
Müller glia-derived progenitor cells (MGPCs) have the capability to regenerate neurons in the retinas of different vertebrate orders. The formation of MGPCs is regulated by a network of cell-signaling pathways. The purpose of this study was to investigate how BMP/Smad1/5/8- and TGFβ/Smad2/3-signaling are coordinated to influence the formation of MGPCs in the chick model system. We find that pSmad1/5/8 is selectively up-regulated in the nuclei of Müller glia following treatment with BMP4, FGF2 or NMDA-induced damage, and this up-regulation is blocked by a dorsomorphin analogue DMH1. By comparison, Smad2/3 is found in the nuclei of Müller glia in untreated retinas, and becomes localized to the cytoplasm following NMDA- or FGF2-treatment. These findings suggest a decrease in TGFβ- and increase in BMP-signaling when MGPCs are known to form. In both NMDA-damaged and FGF2-treated retinas, inhibition of BMP-signaling suppressed the proliferation of MGPCs, whereas inhibition of TGFβ-signaling stimulated the proliferation of MGPCs. Consistent with these findings, TGFβ2 suppressed the formation of MGPCs in NMDA-damaged retinas. Our findings indicate that BMP/TGFβ/Smad-signaling is recruited into the network of signaling pathways that controls the formation of proliferating MGPCs. We conclude that signaling through BMP4/Smad1/5/8 promotes the formation of MGPCs, whereas signaling through TGFβ/Smad2/3 suppresses the formation of MGPCs.
Keywords: Retina regeneration, Retinal glia
Introduction
Müller glia are the primary type of glial cell in the vertebrate retina and unlike astrocytes and microglia, are derived from neuroepthelial retinal stem cells (Jadhav et al. 2009). In a normal retina, Müller glia provide synaptic, structural, and metabolic support to neurons, yet maintain a significant overlap of transcriptional profiles with retinal progenitor cells (Blackshaw et al. 2004). Müller glia can be reprogrammed into proliferating retinal progenitors that can regenerate neurons in the zebrafish, chick, and mammalian retina (Fausett and Goldman 2006; Fischer and Reh 2001; Karl et al. 2008). Although Müller glia-derived progenitor cells (MGPCs) in the fish retina have a robust capacity to regenerate all types of neurons to restore vision, MGPCs in the chick retina have a limited ability to produce neurons, and this ability is further reduced in MGPCs in the rodent retina (reviewed by (Gallina et al. 2014a)).
Significant progress has been made in delineating the cell signaling pathways that orchestrate the transition of Müller glia into MGPCs. The zebrafish retina has an exceptional regenerative capacity that is regulated by a complex network of signaling pathways which includes Notch, Wnt, MAPK, Jak/Stat, PI3k/AKT, and TGFβ (Conner et al. 2014; Lenkowski et al. 2013; Nelson et al. 2012; Ramachandran et al. 2011; Wan et al. 2014; Zhao et al. 2014). In contrast, the chick retina has little or no capacity for functional regeneration, yet large numbers of proliferating MGPCs can be stimulated to form after neuronal cell death or FGF2-treatment without damage (Fischer and Reh 2001; Fischer et al. 2014b). Similar to the zebrafish, a network of cell-signaling pathways that influences MGPC-formation is being uncovered in the chick model system. The network of pathways that control the formation of MGPCs is known to include Notch-, Wnt/β-catenin-, MAPK-, Jak/Stat-, mTor-, Hedgehog-, and glucocorticoid-signaling (Fischer et al. 2009a; Fischer et al. 2009b; Gallina et al. 2015; Gallina et al. 2014b; Ghai et al. 2010; Hayes et al. 2007; Todd and Fischer 2015; Todd et al. 2016; Zelinka et al. 2016). In the mammalian retina, Müller glia have a severely attenuated capacity to become MGPCs and this process requires neuronal cell death followed by growth factor stimulation, or over-expression of the pro-neural transcription factor ascl1(Karl et al. 2008; Ooto et al. 2004; Ueki et al. 2015). Similar to the retinas of fish and birds, MAPK- and Wnt/β-catenin-signaling have been implicated in driving MGPC formation in the rodent retina (reviewed by (Hamon et al. 2016).
The purpose of this study was to investigate how BMP- and TGFβ-signaling influence the formation of proliferating MGPCs in the chick model system. BMPs signal through co-receptors BMPR-I and ALK2/3/6, to phosphorylate Smad1/5/8, whereas TGFβs signal through TGFβ-RII and ALK1/5 co-receptors to phosphorylate Smad2/3 (Brazil et al. 2015). pSmad2/3 and pSmad1/5/8 both interact and compete with Smad4 to translocate to the nucleus to effect transcription (reviewed by (Brazil et al. 2015); this provides a cell signaling-context wherein TGFβ- and BMP-signaling may act in opposition to regulate the formation of MGPCs. The Smad transcription factors play a crucial role in neural stem cell function during development and in postnatal neurogenic niches (Aigner and Bogdahn 2008; Bond et al. 2012). It has been shown that BMP4-signaling through Smad1/5/8 promotes the proliferation of Müller glia in rodent retinal explants (Ueki and Reh 2013). The involvement of BMP-signaling in reprogramming Müller glia into proliferating progenitors in vivo remains unexplored. However, TGFβ-signaling has been reported to suppress the proliferation of MGPCs in both zebrafish and rat retina (Close et al. 2005; Lenkowski et al. 2013). Herein, we explore how TGFβ- and BMP-signaling pathways are coordinated to influence the formation of MGPCs in the chick retina.
Methods and Materials
Animals
The use of animals in these experiments was in accordance with the guidelines established by the National Institutes of Health and the Ohio State University. Newly hatched wild type leghorn chickens (Gallus gallus domesticus) were obtained from Meyer Hatchery (Polk, Ohio). Postnatal chicks were kept on a cycle of 12 hours light, 12 hours dark (lights on at 8:00 AM). Chicks were housed in a stainless steel brooder at about 25°C and received water and Purinatm chick starter ad libitum.
Intraocular injections
Chickens were anesthetized via inhalation of 2.5% isoflurane in oxygen and intraocular injections performed as described previously (Fischer et al. 1998). For all experiments, the right eyes of chicks were injected with the “test” compound and the contra-lateral left eyes were injected with vehicle as a control. Compounds were injected in 20 μl sterile saline with 0.05 mg/ml bovine serum albumin added as a carrier. Compounds used in these studies included NMDA (38.5 or 154 μg/dose; Sigma-Aldrich), FGF2 (250 ng/dose; R&D systems), Smad3 inhibitor SIS3 (4μg/dose; Sigma-Aldrich), BMPR-inhibitor DMH1 (4μg/dose; Sigma-Aldrich), TGFβ-receptor (Alk5) inhibitor SB4315432 (2μg/dose; R&D Systems), recombinant human BMP4 (300ng/dose; R&D Systems), recombinant human TGFβ2 (300ng/dose; R&D Systems), IGF (400ng/dose; R&D Systems).Two μg of BrdU or EdU were injected to label proliferating cells. Injection paradigms are included in each figure.
Reverse transcriptase PCR
Individual retinas were placed in 1 ml of Trizol Reagent (Invitrogen) and total RNA was isolated according to the Trizol protocol and resuspended in 50μl RNAse free water. Genomic DNA was removed by using the DNA FREE kit provided by Ambion. cDNA was synthesized from mRNA by using Superscripttm III First Strand Synthesis System (Invitrogen) and oligodT primers according to the manufacturer’s protocol. Control reactions were performed using all components with the exception of the reverse transcriptase to exclude the possibility that primers were amplifying genomic DNA.
PCR primers were designed by using the Primer-BLAST primer design tool at NCBI (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). Primer sequences and predicted product sizes are listed in table 1. PCR reactions were performed by using standard protocols, PlatinumtmTaq (Invitrogen) and an Eppendorf thermal cycler. PCR products were run on an agarose gel to verify the predicted product sizes.
Table 1.
Antibodies, sources and working dilutions.
| Antigen | Working dilution | Host | Clone or catalog number | Source |
|---|---|---|---|---|
| pS6 | 1:750 | rabbit | #2215; Ser240/244 | Cell Signaling |
| Sox2 | 1:1000 | goat | Y-17 | Santa Cruz Immunochemicals |
| Egr1 | 1:1000 | goat | AF2818 | R&D Systems |
| BrdU | 1:200 | rat | OBT00030S | AbDSerotec Raleigh, NC |
| Phosphorylated-p38 MAPK | 1:400 | rabbit | 12F8 | Cell Signaling Technologies |
| TopAP | 1:100 | mouse | 2M6 | Dr. P. Linser University of Florida |
| Sox9 | 1:2000 | mouse | AB5535 | Chemicon |
| Pax6 | 1:50 | mouse | PAX6 | DSHB |
| Pax6 | 1:1000 | rabbit | PRB-278P | Covance |
| Klf4 | 1:50 | rabbit | ARP38430 | Aviva Systems Biology |
| pERK1/2 | 1:200 | rabbit | 137F5 | Cell Signaling Technologies |
| cFos | 1:400 | rabbit | K-25 | Santa Cruz Immunochemicals |
| pSmad1/5/9 | 1:200 | rabbit | D5B10 | Cell Signaling Technologies |
| Smad2 | 1:300 | rabbit | D43B4 | Cell Signaling Technologies |
| Glutamine Synthetase | 1:2000 | mouse | ab125724 | Abcam |
| CD45 | 1:200 | Mouse | HIS-C7 | Cedi Diagnostic |
| Nkx2.2 | 1:80 | Mouse | 74.5A5 | DSHB |
Fixation, sectioning and immunocytochemistry
Tissues were fixed, sectioned, and immunolabeled as described previously (Fischer et al. 2008; Fischer et al. 2009b). Working dilutions and sources of antibodies used in this study are listed in table 2. None of the observed labeling was due to non-specific labeling of secondary antibodies or autofluorescence because sections labeled with secondary antibodies alone were devoid of fluorescence. Secondary antibodies included donkey-anti-goat-Alexa488/568, goat-anti-rabbit-Alexa488/568/647, goat-anti-mouse-Alexa488/568/647, goat-anti-rat-Alexa488 (Life Technologies) diluted to 1:1000 in PBS plus 0.2% Triton X-100.
Table 2.
Forward and reverse primer sequences (5′ – 3′) and predicted product sizes (in brackets).
| Gene name | Forward | Reverse | Product size (bp) |
|---|---|---|---|
| BMP2 | TCA GGC CGT TGT TAG TGA CG | ACG CTG TTT GTG TTT CGC TT | (89) |
| BMP4 | GGC AGG AAG AAA GTC GCA GA | CTG GGG ATG ACG GCT GAT TT | (155) |
| BMP7 | CTT GGG GTT GAT GCT TTG CC | GAG CAC CCA GGA AGG GAT TC | (187) |
| TGF• 1 | TGG ATC CAC GAA CCC AAA GG | CAG GCA CGG ACC ACC ATA TT | (233) |
| TGF• 2 | GTC ACG CTG TTT CTG GGG TA | GAG CCC CTC ACC ATC CTC TA | (114) |
| TGF• 3 | GTG GCT GTC CTT CGA TGT CA | CTG ATT TCC AGG CCG AGG TT | (78) |
| Gapdh | CAT CCA AGG AGT GAG CCA AG | TGG AGG AAG AAA TTG GAG GA | (161) |
Labeling for EdU or BrdU
For BrdU-labeling, immunolabeled sections were fixed in 2% paraformaldehyde for 10 minutes, washed for 5 minutes in PBS, washed for 8 minutes in 4N HCl, washed 3x times in PBS, and processed for immunolabeling with antibodies to BrdU.
For EdU-labeling, immunolabeled sections were fixed in 4% formaldehyde in PBS for 5 minutes at room temperature, washed for 5 minutes with PBS, permeabilized with 0.5% Triton X-100 in PBS for 1 minute at room temperature, and washed twice for 5 minutes in PBS. Sections were incubated for 30 minutes at room temperature in 2M Tris, 50 mM CuSO4, Alexa Fluor 568 Azide (Thermo Fisher Scientific), and 0.5M ascorbic acid in dH2O.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
We used the TUNEL method to to identify dying cells that contained fragmented DNA. We used an In Situ Cell Death Kit (TMR red; Roche Applied Science), as per the manufacturer’s instructions.
Photography, measurements, cell counts and statistics
Photomicrographs were obtained using a Leica DM5000B microscope equipped with epifluorescence and Leica DC500 digital camera. Confocal images were obtained using a Leica SP8 imaging system at the Hunt-Curtis Imaging Facility at The Ohio State University. Images were optimized for color, brightness and contrast, multiple channels overlaid and figures constructed by using Adobe Photoshop. Cell counts were performed on representative images. To avoid the possibility of region-specific differences within the retina, cell counts were consistently made from the same region of retina for each data set.
Similar to previous reports (Ghai et al. 2009; Stanke et al. 2010), immunofluorescence was quantified by using ImagePro6.2 (Media Cybernetics, Bethesda, MD, USA). Identical illumination, microscope, and camera settings were used to obtain images for quantification. Retinal areas were sampled from 5.4 MP digital images. These areas were randomly sampled over the inner nuclear layer (INL) where the nuclei of the bipolar and amacrine neurons were observed. Measurement for content in the nuclei of Müller glia/MGPCs were made by selecting the total area of pixel values ≥70 for Sox2 or Sox9 immunofluorescence (in the red channel), and copying nuclear Smad2/3, pSMAD1/5/8, KLF4, or Pax6 (in the green channel). This copied data was pasted into a separate file for quantification or onto 70% grayscale background for figures. Measurements were made for regions containing pixels with intensity values of 68 or greater (0 = black and 255 = saturated); a threshold that included labeling in the bipolar or amacrine neurons. The total area was calculated for regions with pixel intensities > 68. The average pixel intensity was calculated for all pixels within threshold regions. The density sum was calculated as the total of pixel values for all pixels within threshold regions. These calculations were determined for retinal regions sampled from six different retinas for each experimental condition. The mean area, intensity, and density sum was calculated for the pixels within threshold regions from ≥4 retinas for each experimental condition.
In Figures 1c–e, determination of the percentage of Smad2 in Sox2+ nuclei in the INL was determined as follows, similar to previous descriptions (Gallina et al. 2015; Todd and Fischer 2015). Images were cropped to fixed areas of the INL. By using ImagePro 6.02, immunofluorescence was measured as the summation of pixel values (density sum) above threshold (68 in the green channel) within each cropped area. Then the area occupied by Sox2-labeling was selected in the red channel (pixel value of 180 ± 75) and the selected areas in the green channel (Smad2) were cut and pasted into a separate image for quantification, as described above. For each individual image (n=6) the percentage of Smad2 (above threshold)present in Sox2-labeled nuclei over the total density sum of Smad2 within cropped regions of the INL was calculated and averaged for control and NMDA-treated retinas.
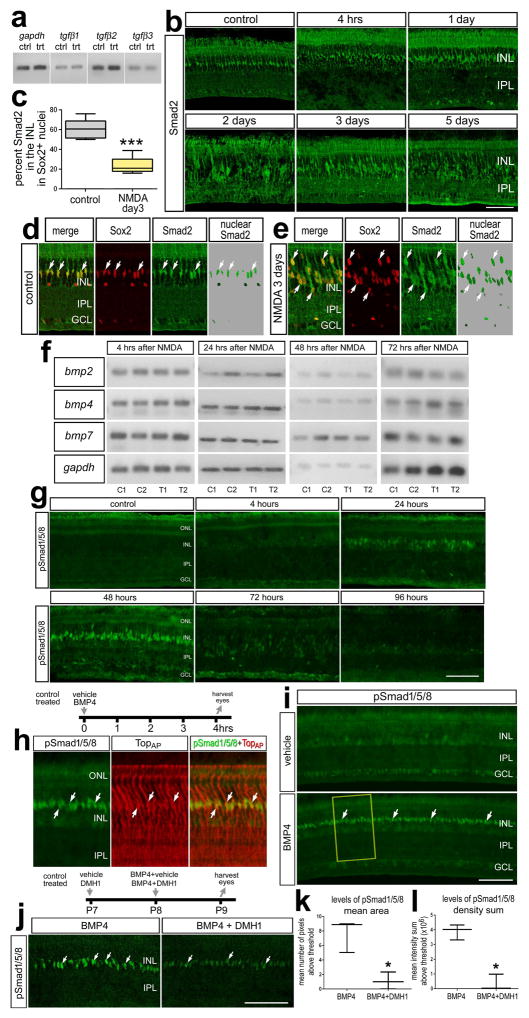
Figure 1.
TGFβs, BMPs and Smad-signaling in Müller glia and MGPCs in NMDA-damaged retinas. Eyes were injected with saline or 500 nmol NMDA and harvested at different times after treatment. a; RT-PCR was used to detect gapdh, tgfβ1, tgfβ2 and tgfβ3 in control and NMDA-damaged retinas at 2 days after treatment. b; Sections of the retina were labeled with antibodies to Smad2. Retinas were obtained at 4hrs, 1 day, 2 days, 3 days and 5 days after NMDA-treatment. c: The mean (±SD; n=6) percentage of Smad2 in INL that overlaps with Sox2+ nuclei of Müller glia. d and e: Confocal optical sections of control and NMDA (day 3) treated retinas demonstrating the overlap of Smad2 in Sox2+ nuclei of Müller glia or MGPCs. Significance of difference (***p<0.001) was determined by using a two-tailed paired student’s t-test. f: RT-PCR was used to detect mRNA to bmp2, bmp4, bmp7 and gapdh in control and NMDA-treated retinas at 4, 24, 48, 72 hrs after treatment. g,h,i and j; Retinas were labeled with antibodies to pSmad1/5/8 (green) and TopAP (red). Retinas were obtained for eyes that injected with saline (vehicle) or BMP4 and harvested 4 hrs later (h and i), or eyes were injected with 30% DMSO in saline (control) or DMH1 (treated) at P7, BMP4 (control) or BMP4 + DMH1 (treated) at P8, and eyes harvested P9. k and l: mean area (±SD) and mean density sum (±SD) of pSmad1/5/8-immunofluorescence in the INL of retinas treated with BMP4 vs BMP4+DMH1. Significance of difference (*p<0.05,) was determined by using a Mann-Whitney U test. Arrows indicate the nuclei of MGPCs. The calibration bar (50 μm) in panel b applies to b alone, the bar in g applies to g alone, the bar in i applies to i alone, and the bar in j applies to d,e and j. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ONL – outer nuclear layer.
Central retina was defined as the region within a 3 mm radius of the posterior pole of the eye, and peripheral retina was defined as an annular region between 3 mm and 0.5mm from the CMZ. Cell counts were consistently counted in peripheral regions of the retina. The identity of BrdU-labeled cells was determined based on previous findings that 100% of the proliferating cells in the chick retina are comprised of Sox2/9+ Müller glia in the INL/ONL, Sox2/9/Nkx2.2+ Non-Astrocytic Inner Retinal Glia (NIRG) cells in the IPL, GCL and NFL (the NIRG cells do not migrate distally into the retina), and CD45+ (Sox2/9-) microglia (Fischer et al. 2010; Zelinka et al. 2012). NIRG cells are a unique type of glial cell that has been described in the chick retina (Fischer et al. 2010; Rompani and Cepko 2010), and possibly the retinas of reptiles (Todd et al. 2015), that migrate into the retina from the optic nerve (Rompani and Cepko 2010). Sox2+ nuclei in the INL were identified as Müller glia based on their large size and fusiform shape which was distinctly different from the Sox2+ nuclei of cholinergic amacrine cells which are small and round (Fischer et al. 2010).
GraphPad Prism 6 was used for statistical analyses. Where significance of difference was determined between two treatment groups accounting for inter-individual variability (means of treated-control values) we performed a two-tailed, paired t-test. Where significance of difference was determined between two treatment groups we performed a two-tailed, unpaired t-test.
Results
BMP- and TGFβ-signaling are modulated following NMDA-damage
We first examined whether TGFβ ligands were expressed in normal and NMDA-damaged chick retinas by using RT-PCR. We found tgfβ1, tgfβ2 and tgfβ3 were present in normal retinas, with PCR products for tgfβ2 suggesting highest levels of expression (Fig. 1a). In NMDA-damaged retinas, tgfβ ligands were detected with no obvious changes in levels of expression (Fig. 1a). We next sought to characterize the TGFβ-associated transcription factor, Smad2, in NMDA-damaged retinas. Intraocular injections of NMDA cause excitotoxic death of inner retinal neurons and the formation of proliferating MGPCs (Fischer and Reh 2001). Antibodies to pSmad2 (Cell Signaling Technologies; #3108) failed to produce convincing labeling in normal retinas or in response to intraocular injection of TGFβ2 (data not shown). Thus, we relied upon patterns of labeling and sub-cellular distribution of Smad2 as readouts of signaling for TGFβ/Smad2. We assumed that Smad2 that was localized within nuclei indicated cells with active TGFβ-signaling; whereas Smad2 distributed throughout the cytoplasm indicated cells with the capacity to respond to TGFβ-signaling but were inactive (Brazil et al. 2015). In normal retina, we found most (~60%) of the Smad2-immunofluorescence was found in the Sox2+ nuclei of Müller glia (Figs. 1b–d), suggesting that TGFβ-signaling is active in normal Müller glia. By comparison, at two and three days after NMDA-treatment, Smad2 immunofluorescence was distributed throughout the cytoplasm, whereas about 20% of the total Smad2-immunofluorescence was localized to the nuclei of Müller glia (Figs. 1b–e). This suggests signaling through TGFβ/Smad2 was decreased at the time MGPCs are known to form.
We next examined BMP ligand expression in normal and NMDA-damaged retinas by using RT-PCR. We observed that bmp2, bmp4 and bmp7 were present in normal and NMDA-damaged retinas at 4h, 24h, 48h, and 72h after treatment (Fig. 1f). There were no obvious differences in levels of PCR products in control and treated retinas. To determine whether BMP-signaling is active in normal or damaged retinas, retinal sections were immunolabeled for pSmad1/5/8 following NMDA-treatment. Upon BMPR activation, Smad1/5/8 is phosphorylated and then translocates to the nucleus to act as a transcription factor. Thus, the accumulation of pSmad1/5/8 in the nucleus is a direct readout of active BMP-signaling. Undamaged retinas did not have detectable levels of pSmad1/5/8 (Fig. 1g). Following NMDA-treatment, levels of pSmad1/5/8 remained low at 4 hrs after damage and increased in the nuclei of Müller glia at 24 hrs after damage (Fig. 1g). Levels of pSmad1/5/8 appeared increased in the nuclei of presumptive Müller glia at 48 hrs, decreased at 72 hrs, and was below levels of detection at 96 hrs after treatment (Fig. 1g). Collectively, these findings indicate that in normal retinas TGFβ/Smad2 signaling is relatively high and BMP/Smad1/5/8 activity is low in Müller glia, whereas following NMDA-treatment, signaling for TGFβ/Smad2 is transiently decreased and signaling for BMP/Smad1/5/8 is transiently increased in Müller glia.
To examine the specificity of the pSmad1/5/8 antibody we tested if immunolabeling was induced by exogenous BMP4. We found that a single intraocular injection of BMP4 induced pSmad1/5/8 in TopAP-positive Müller glia within 4hrs of treatment (Figs. 1h,i). Co-application of a BMPR1 receptor inhibitor DMH1, a dorsomorphin analog, with BMP4 significantly reduced the up-regulation of pSmad1/5/8 in Müller glia (Figs. 1j–l). Collectively, these findings suggest that BMP/Smad1/5/8-signaling is selectively activated in Müller glia by exogenous BMP4.
Inhibition of BMPR1 attenuates MGPC formation in the damaged retina
In the chick retina, MGPCs are defined as Müller glia that: (i) de-differentiate (i.e. down-regulated glutamine synthetase), (ii) up-regulate progenitor factors (such as Klf4, Six3,Pax6, Chx10, Egr1, nestin/transitin, ascl1a), and (iii) re-enter the cell cycle (up-regulate PCNA, pHisH3 and accumulate BrdU/EdU) (reviewed by Gallina et al 2014 and Fischer and Bongini 2010). The Müller glia appear to uniformly activate cell signaling pathways such as MAPK, mTor, Wnt/β-catenin, Jak/Stat and pSmad1/5/8 before acquisition of progenitor phenotype (Fischer et al. 2009b; Gallina et al. 2015; Todd et al. 2016; Zelinka et al. 2016). Thus, we use proliferation as a defining characteristic of MGPCs.
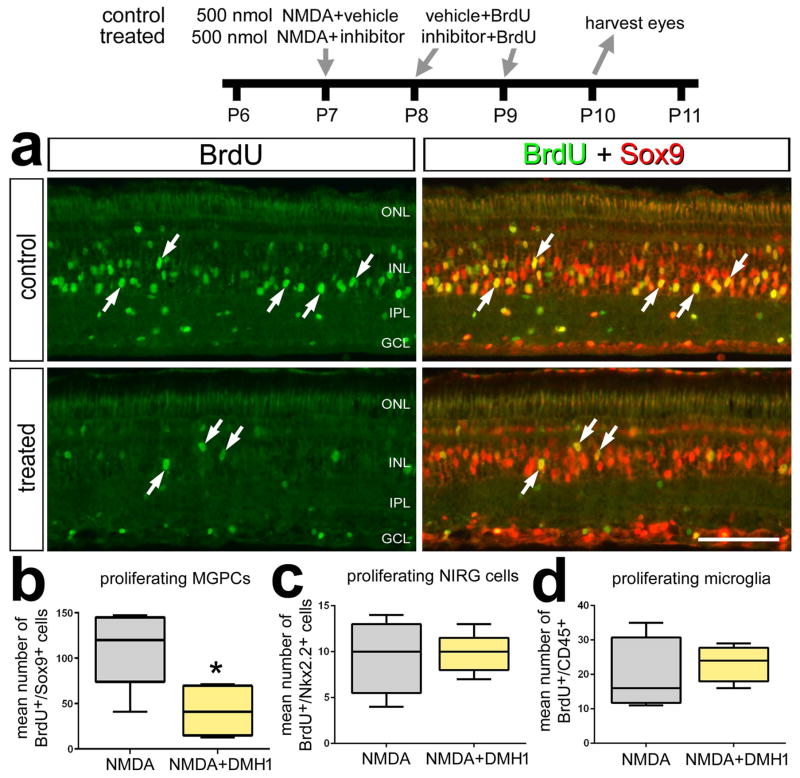
To determine whether BMP-signaling is involved in the formation of MGPCs, we inhibited the BMPR1 receptor with DMH1 in NMDA-damaged retinas. Treatment of NMDA-damaged retinas with DMH1 significantly decreased the number of BrdU+/Sox9+ MGPCs (Figs. 2a,b). By comparison, DMH1-treatment had no effect upon numbers of proliferating NIRG cells and microglia (Fig. 2b). DMH1-treatment of NMDA-damaged retinas failed to influence levels of stem cell-related factors including Pax6, Klf4, cFos, nuclear β-catenin, and pS6 (a read-out of mTor-signaling; not shown).
Figure 2.
Inhibition of BMP-signaling suppresses the formation of proliferating MGPCs in damaged retinas. Eyes were injected with 500 nmol NMDA+vehicle (control) or NMDA+DMH1 (treated) at P7, vehicle+BrdU or DMH1+BrdU at P8 and P9, and retinas were harvested at P10. Retinal sections were labeled with antibodies to BrdU (green) and Sox9 (red). The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile. Arrows indicate the nuclei of MGPCs and the calibration bar in panel a represents 50 μm. Significance of difference (*p<0.05, ***p<0.001) was determined by using a two-tailed paired student’s t-test. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ONL – outer nuclear layer.
TGFβ-signaling negatively regulates Müller glia proliferation in damaged retinas
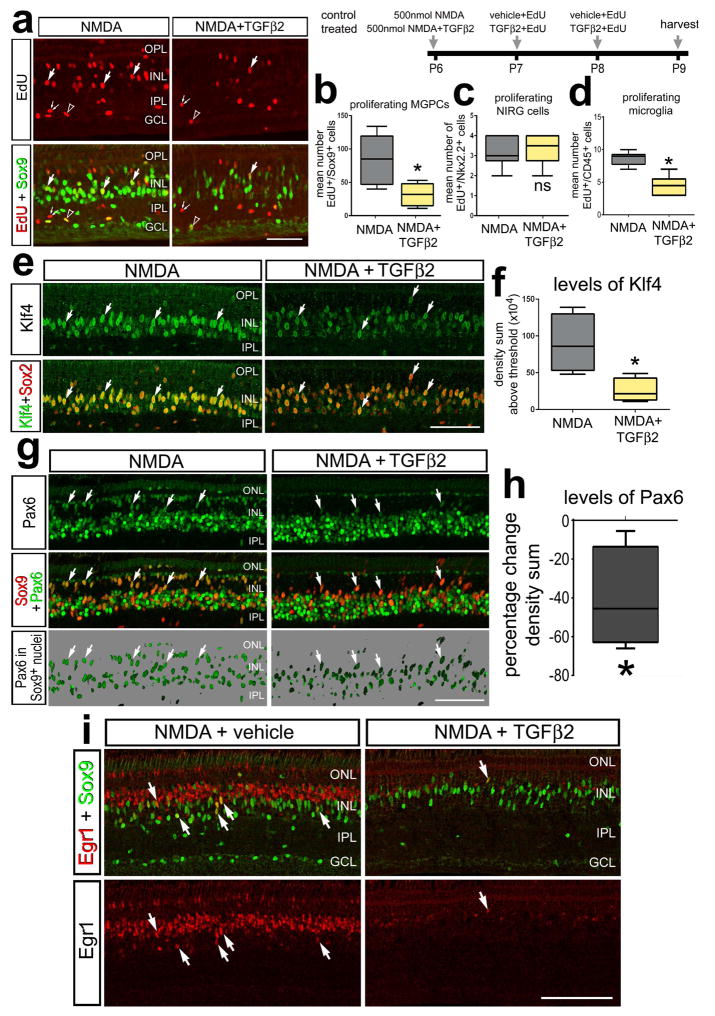
TGFβ-signaling inhibits the proliferation of Müller glia and/or MGPCs in zebrafish and rodent retinas (Close et al. 2005; Lenkowski et al. 2013). To test whether TGFβ-signaling influences the formation of MGPCs in the avian retina, we injected TGFβ2 into NMDA-treated eyes. We chose TGFβ2 because it was the most abundantly expressed TGFβ-ligand in the chick retina (see Fig. 1a) and is the most highly expressed TGFβ-isoform in the retina and brain of rodents (Close et al. 2005; Constam et al. 1994). We found that TGFβ2 significantly suppressed proliferating MGPCs in damaged retinas (Figs. 3a,b). TGFβ2 did not affect the proliferation of NIRG cells but did inhibit microglia proliferation (Figs. 3c,d). In addition to a decrease in proliferation of Müller glia, TGFβ2 reduced levels of the retinal stem cell factor Pax6, in MGPCs (Figs. 3g,h). Pax6 is a transcription factor required for proper function of retinal progenitor cells during development and regeneration in the retina (Ashery-Padan and Gruss 2001; Thummel et al. 2010). Similarly, there were reduced levels of the stem cell factor Klf4 in TGFβ2-treated MGPCs in damaged retinas (Figs. 3e,f). Egr1, an immediate early gene known to be expressed by MGPCs and progenitors at the retinal margin (Fischer et al. 2009b) was also reduced by TGFβ2 in damaged retinas (Fig. 3i); however, Egr1 was down-regulated in both MGPCs and presumptive bipolar cells (Fig. 3i). Taken together, these data suggests TGFβ2 negatively influences the capacity for Müller glia to transition into proliferating progenitors in damaged retinas.
Figure 3.
TGFβ2 inhibits the formation of proliferating MGPCs in NMDA-damaged retinas. Eyes were injected with 500 nmol NMDA+vehicle (control) or NMDA+TGFβ2 (treated) at P6, vehicle+EdU or TGFβ2+EdU at P7 and P8, and retinas harvested at P9. Sections of the retina were labeled for EdU (red) and antibodies to Sox9 (green; a), Klf4 (green) and Sox2 (red; e), Pax6 (green) and Sox9 (red; g), or Sox9 (green) and Egr1 (red; i). The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile. The plots illustrate the number of proliferating MGPCs (b), NIRG cells (c), and microglia (d), levels of Klf4 in the nuclei of Müller glia/MGPCs (f), and percent change in the nuclear levels of Pax6 in Müller glia/MGPCs (h). Significance of difference (*p<0.05) was determined by using a two-tailed paired student’s t-test. Arrows indicate the nuclei of MGPCs. The calibration bar in each panel represents 50 μm. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ONL – outer nuclear layer.
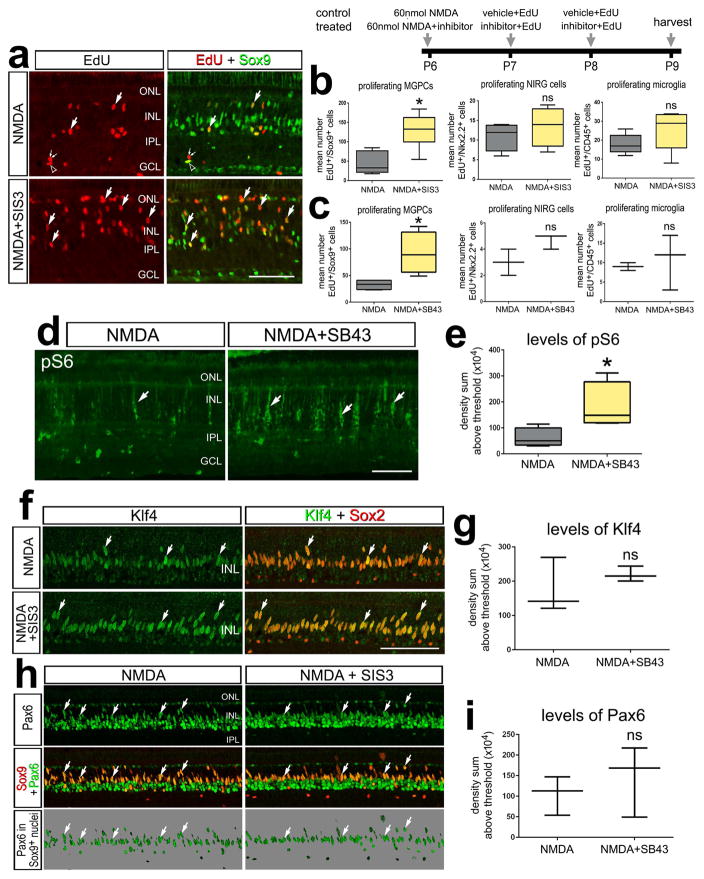
Since TGFβ2 suppresses the formation of MGPCs, we wanted to test whether inhibition of TGFβ-signaling would lead to an increase in the generation of MGPCs in damaged retinas. We applied SIS3, a specific inhibitor of Smad3, a TGFβ-associated transcription factor (Jinnin et al. 2006), following retinal damage and observed a significant increase in the amount of proliferating Müller glia (Figs. 4a,b). The pro-mitogenic effect of Smad3 inhibition was specific to Müller glia, as proliferation of NIRG cells and microglia were unaffected (Fig. 4b). To further test whether inhibition of TGFβ-signaling influences the proliferation of MGPCs, we utilized SB431542, a small molecule inhibitor that selectively inhibits the TGFβ type 1 receptor (TGFβ-R1), and the activin receptor-like kinases (ALK) 4,5 and 7 (Inman et al. 2002). We found that inhibition at the level of the TGFβ receptor significantly increased numbers of proliferating MGPCs in NMDA-damaged retinas (Fig. 4c). The proliferation of NIRG cells and microglia were unaffected by TGFβ-R1 inhibition (Fig. 4c). There was an increase in expression of pS6, a read-out of mTor, following inhibition of TGFβ (Figs. 4d,e). However, expression levels Pax6 and Klf4 were unchanged following SIS3-treatment in NMDA-damaged retinas (Figs. 4, f–i).
Figure 4.
Inhibition of TGFβ-signaling stimulates the formation of proliferating MGPCs in damaged retinas. Eyes were injected with 60 nmol NMDA alone (control) or 60 nmol NMDA + inhibitor (SIS3 or SB431542) at P6, EdU alone or EdU+inhibitor at P7 and P8, and retinas harvested at P9. Sections of the retina were labeled for EdU (red) and antibodies to Sox9 (green; a), Klf4 (green) and Sox2 (red; f), a read-out of mTor-signaling pS6 (green; d), and Pax6 (green) and Sox9 (red; h).The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile. The plots illustrate the abundance of proliferating MGPCs, NIRG cells, and microglia (b,c), and levels of pS6, Klf4 and Pax6 in Müller glia/MGPCs (e,g,i). Significance of difference (*p<0.05, ***p<0.001) was determined by using a two-tailed paired student’s t-test. Arrows indicate the nuclei of MGPCs. The calibration bars in a, d, e and g indicate 50 μm. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ONL – outer nuclear layer.
Inhibition of BMPRI suppresses FGF2-mediated cell signaling in Müller glia and suppresses the proliferation of FGF2-induced MGPCs
FGF2-MAPK signaling plays a prominent role in MGPC formation in the avian retina and is known to interact with Notch-, Wnt-, MAPK-, JAK/Stat-, mTor-, Hedgehog-, and glucocorticoid-signaling (Fischer et al. 2009b; Gallina et al. 2015; Gallina et al. 2014b; Ghai et al. 2010; Todd and Fischer 2015; Todd et al. 2016; Zelinka et al. 2016). Thus, we investigated whether the FGF2/MAPK-pathway interacts with BMP/Smad-signaling. We found that two consecutive daily doses of FGF2 induced expression of pSmad1/5/8 in the nuclei of Müller glia (Fig. 5a). Application of DMH1 with FGF2 suppressed the up-regulation of pSmad1/5/8 in Müller glia (Fig. 5a). We next examined whether inhibition of BMPR1 with DMH1 influenced other read-outs of cell signaling pathways. Although DMH1 treatment had no effect upon FGF2-induced up-regulation of pERK1/2 (not shown), inhibition of BMPRI suppressed FGF2-induced increases in cFos and pS6 expression in Müller glia (Figs. 5b–e). This suggests inhibition of BMP-signaling disrupts FGF-signaling by down-regulating readouts of FGF’s targets, including MAPK-signaling and mTor- signaling.
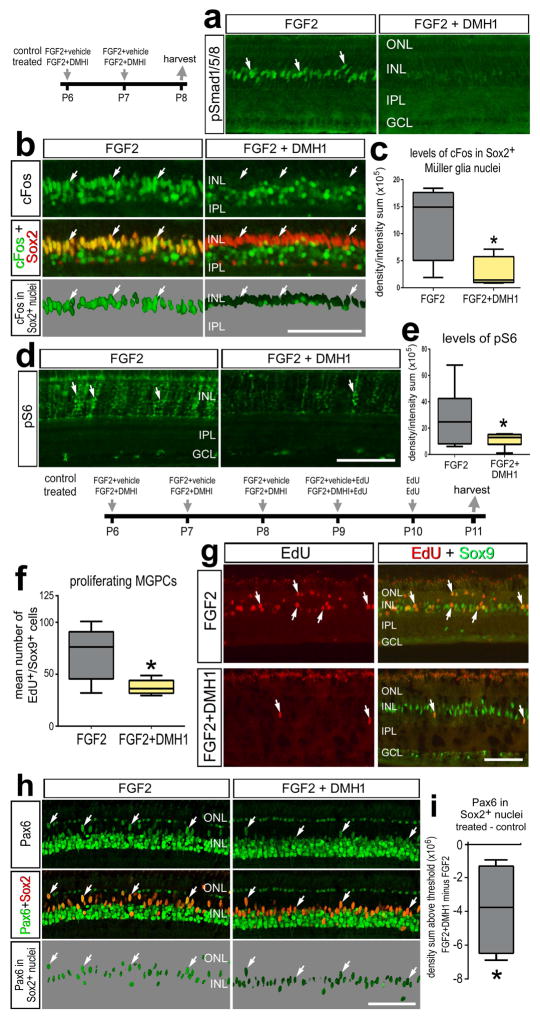
Figure 5.
Smad1/5/8-signaling is part of the cell-signaling network initiated by FGF2/MAPK that stimulates the proliferation of MGPCs in FGF2-treated retinas. a–e; Eyes were injected with FGF2+vehicle (control) or FGF2+DMH1 (treated) at P6 and P7, and retinas harvested at P8. f–i; eyes were injected daily with FGF2+vehicle (control) or FGF2+DMH1 (treated) at P6-P9, EdU alone at P10, and retinas harvested at P11. Sections of the retina were labeled with antibodies to pSmad1/5/8 (a), cFos (green) and Sox2 (red; b), or pS6 (d). The panels with a 70% grayscale background display cFos or Pax6 localized within the Sox9+ or Sox2+ nuclei of Müller glia (b), or Pax6 localized within the Sox2+ nuclei of Müller glia (h). The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile. The plots illustrates the control and treated levels (intensity sum) of cFos (c), levels of pS6 (e), or Pax6 (i) in Müller glia/MGPCs. The plot in f illustrates the abundance of proliferating MGPCs in control and treated retinas. Significance of difference (*p<0.05) was determined by using a two-tailed paired student’s t-test. Arrows indicate the nuclei of MGPCs. The calibration bar (50 μm) in panel b applies to a and b, the bar in d applies to d alone, the bar in g applies to g alone, and the bar in h applies to h alone. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ONL – outer nuclear layer.
Since inhibition of BMPR1 interferes with downstream FGF-signaling, we hypothesized that BMP-receptor inhibition would suppress the proliferation of FGF2 induced MGPCs. We have found that four consecutive daily intraocular injections of FGF2 stimulates the formation of proliferating MGPCs in the absence of retinal damage (Fischer et al. 2014a). We found that inhibition of BMPR1 attenuated the proliferation of FGF2-induced MGPCs (Figs. 5f,g). Further, inhibition of BMPR1 resulted in a significant reduction of Pax6 expression in MGPCs (Figs. 5h,i), whereas levels of Klf4 were not affected (not shown). Collectively, these data suggests that BMP signaling is a critical downstream component of MAPK signaling in FGF2-mediated MGPC formation.
Inhibition of SMAD3 enhances FGF2-mediated cell signaling in Müller glia and the proliferation of FGF2-induced MGPCs
We next investigated whether TGFβ2 and signaling through Smad3 influences the formation of MGPCs in the absence of damage. We found inhibition of Smad3 with the small molecule inhibitor SIS3 had no effect upon Müller glia in normal retinas. A single intraocular injection of SIS3 failed to influence cell signaling in Müller glia; levels of pERK1/2, pS6, or pStat3 were unaffected (data not shown). We combined SIS3 with FGF2 to examine whether inhibition of Smad3 would facilitate FGF2 signaling. SIS3 was co-applied with FGF2 for two consecutive days resulted in a significant increase in cFos in Müller glia, whereas levels of ps6 were unaffected (Fig. 6a–d). With four consecutive daily intraocular injections of FGF2, which are known to stimulate the formation of proliferating MGPCs (Fischer et al., 2014), the sub-cellular distribution of Smad2 appeared to shift out of nuclei and into the cytoplasm of Müller glia (Fig. 6e), suggesting that signaling through TGFβ/Smad2/3 was decreased in FGF2-treated retinas. With three consecutive days of treatment, application of SIS3 with FGF2 resulted in significant increase in the number of proliferating MGPCs compared to numbers seen in retinas treated with FGF2 alone (Figs. 6f,g).
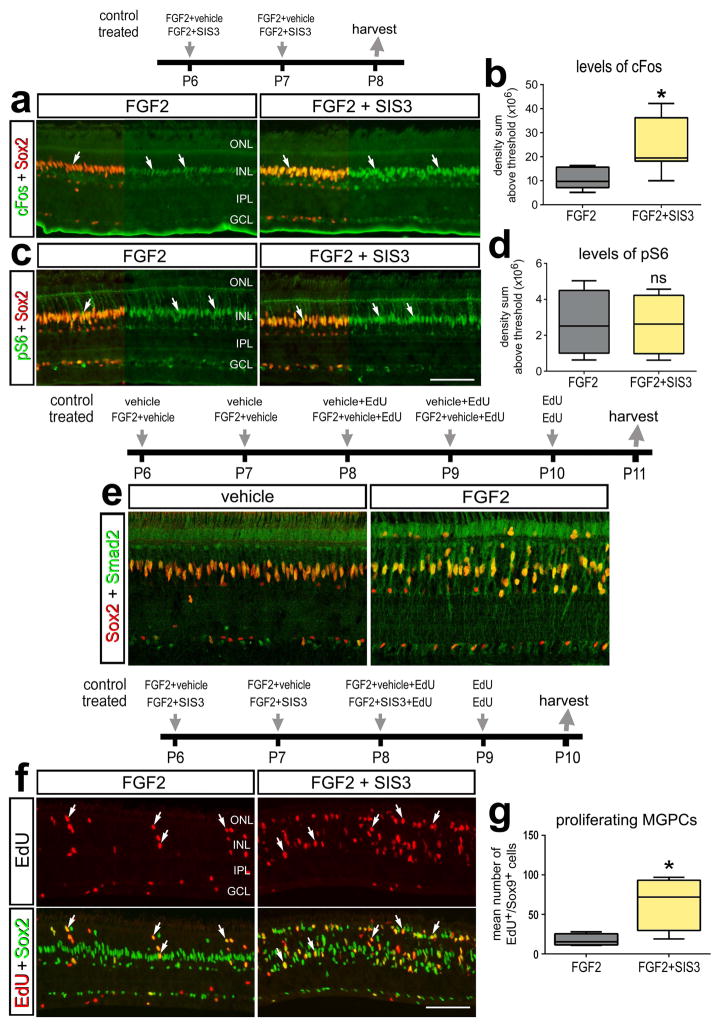
Figure 6.
TGFβ/Smad2-signaling is part of the network that inhibits the proliferation of MGPCs in FGF2-treated retinas. a–d; Eyes were injected with FGF2+vehicle (control) or FGF2+SIS3 (treated) at P6 and P7, and retinas harvested at P8. e; Eyes were injected with vehicle (control) or FGF2 (treated) at P6-P10 and retinas were harvested at P11. f–g; Eyes were injected with FGF2+vehicle (control) or FGF2+SIS3 (treated) at P6, P7 and P8, EdU at P9, and retinas were harvested at P10. Sections of the retina were labeled with antibodies to cFos (green) and Sox2 (red) (a), ps6 (green) and Sox2 (red) (b), Smad2 (green) and Sox2 (red) (e), and EdU (red) and Sox2 (green) (f). The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile. The plots illustrates the control and treated levels (intensity sum) of cFos (b) and levels of pS6 (d). The plot in (g) represents the amount of proliferating Müller glia/MGPCs. Significance of difference (*p<0.05, ***p<0.001) was determined by using a two-tailed paired student’s t-test. Arrows indicate the nuclei of MGPCs. The calibration bar (50 μm) in panel a applies to a, b, and f. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ONL – outer nuclear layer.
In the majority of eyes (6 out of 8), the combination of TGFβ2 and FGF2 resulted in significant retinal damage. We detected significant numbers of TUNEL-positive cells in the INL and GCL of retinas treated with TGFβ2 and FGF2 (not shown). Thus, we were unable to determine whether TGFβ2 influenced the formation of MGPCs in FGF2-treated eyes in the absence of damage. The causes underlying the retinal pathology remains uncertain, but may involve changes in vascular integrity given that blood was observed within the vitreous chamber of eyes treated with TGFβ2 and FGF2 (not shown). Similarly, the combination of FGF2 and IGF1, but not either factor alone, results in cell death in the retina, but this results from angle closure and acute elevation of IOP (Ritchey et al. 2012).
Proliferation of retinal progenitors in the circumferential marginal zone (CMZ)
To examine whether signaling through Smad3 influences the proliferation of progenitor cells other than MGPCs, we tested whether the proliferation of retinal progenitors in the circumferential marginal zone (CMZ) was affected by the inhibitor SIS3. The eyes of post-hatch chicks are known to contain a CMZ at the far peripheral edge of the retina (Fischer et al. 2002; Fischer and Reh 2000; Ghai et al. 2008). Intraocular injections of SIS3 alone had no effect upon the proliferation of CMZ progenitors (not shown). Thus, we examined if co-application of SIS3 with insulin-like growth factor 1 (IGF1), which is known to stimulate the proliferation of CMZ progenitors (Fischer and Reh, 2000), influenced CMZ progenitors. We found that application of SIS3 with IGF1 nearly doubled the number of newly generated cells added to the retina from proliferating CMZ progenitors (Figs. 7a–c). These findings suggest that CMZ progenitors which proliferate in response to IGF1 may be suppressed by signaling through Smad3, but under normal conditions, signaling through Smad3 is not required to keep these progenitors quiescent.
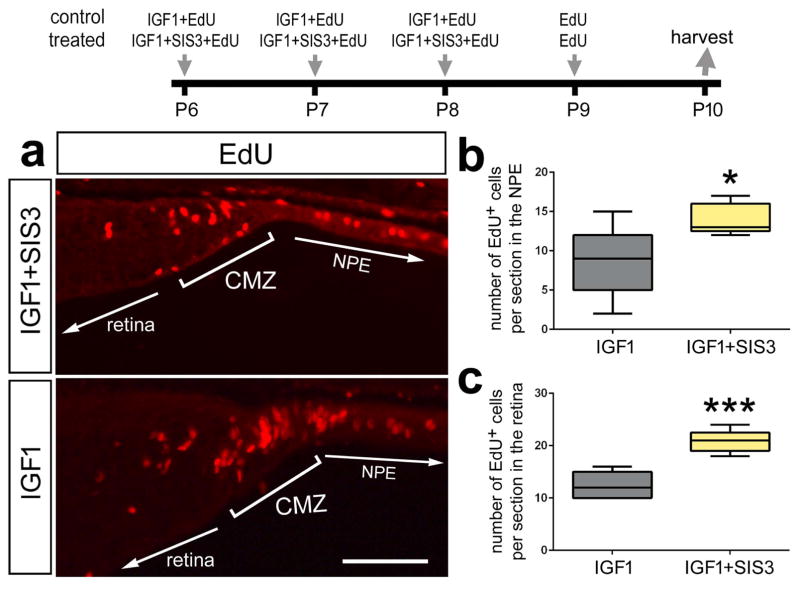
Figure 7.
Inhibition of Smad3 stimulates the proliferation of progenitors at the retinal margin. Eyes were injected with IGF1 (control) or IGF1+SIS3 (Smad3-inhibitor; treated) at P6, P7, P8, EdU alone at P9 and retinas were harvested at P10. Sections of the retina were labeled for EdU (a). The box plots illustrate the mean, upper extreme, lower extreme, upper quartile and lower quartile. The plots represent the numbers of proliferating cells in the non pigmented epithelium (200 μm anterior to the peripheral edge of the retina; b) and retinal margin (CMZ + retina; c). Significance of difference (*p<0.05, ***p<0.001) was determined by using a two-tailed paired student’s t-test. The calibration bar in panel a represents 50 μm. Abbreviations: CMZ – ciliary marginal zone, NPE - Non-pigmented epithelium.
Previous studies have shown that proliferating CMZ progenitors normally add new neurons to the peripheral edge of the retina (Fischer and Reh, 2000, Dev Biol). Further, treatment with IGF1/insulin not only stimulates the proliferation of CMZ progenitors, but includes progeny that differentiate as neurons (Fischer et al., 2002, Development). Thus, we tested whether inhibition of TGFβ-signaling, with SIS3, combined with IGF1 influenced the number of progeny that differentiate as neurons. We found that co-application of SIS3 and IGF1 had no significant effect upon the percentage of EdU-labeled cells that were positive for the neuronal markers HuD or Otx2 (data not shown).
Discussion
Our findings suggest BMP/Smad1/5/8-signaling is part of the network of cell-signaling pathways that drives the formation of proliferating MGPCs (Fig. 8). These data add another component to a complex regulatory network of signaling pathways which regulate the formation of MGPCs in the avian retina (Gallina et al. 2014a). We found that the Bmp2, -4, and -7 are present in normal and NMDA-damaged retinas. Similarly, it has been reported that Bmp2, -4, and -7 are present in the embryonic chick retina (Belecky-Adams and Adler 2001). In the developing chick eye, Noggin-soaked beads reduced proliferation in vivo and BMP4 increased proliferation in dissociated retinal progenitor cells (Trousse et al. 2001). Over-expression of BMPR1 also induces proliferation during early stages of embryonic chick retinal regeneration (Haynes et al. 2007). Collectively, these findings, suggest that the proliferation of retinal progenitors is stimulated by BMP-signaling.
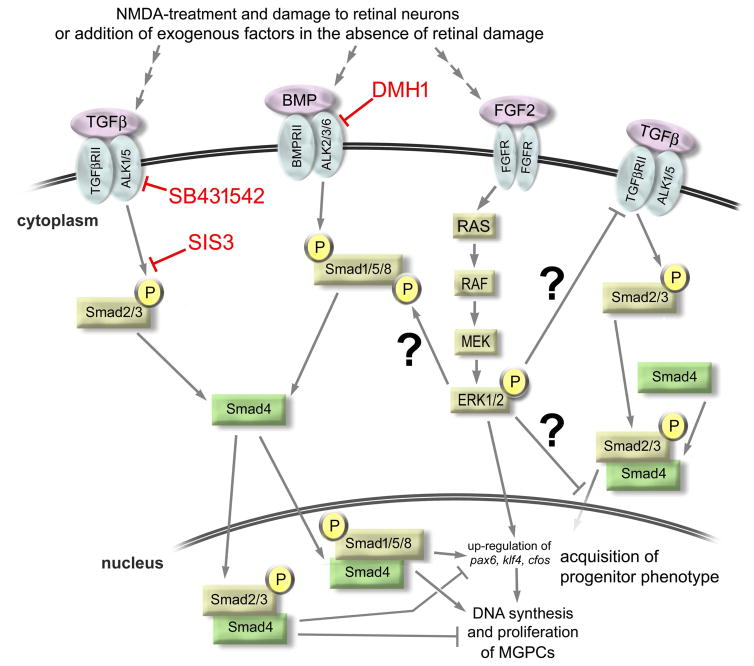
Figure 8.
Schematic summary of cell-signaling in Müller glia and during the formation of proliferating Müller glia-derived progenitors. The schematic indicates possible cross-talk between TGFβ/Smad2-, BMP/Smad1/5/8- and FGF2/MAPK-signaling pathways, and cites of action of different compounds used in the current study. Red lines and text indicate the different small molecule inhibitors used in the current study. Question marks indicate unconfirmed interactions.
BMP-signaling may also promote reactive phenotypes in mature retinal glia. For example, astrocytes and the MIO-M1 Müller cell line up-regulate genes associated with reactive gliosis in response to BMP7 treatment, which is consistent with findings that Müller glia normally express BMPR1 (Dharmarajan et al. 2014). In contrast, treatment with BMP4 did not lead to any increase in genes associated with gliosis in MIO-M1 Müller glia (Dharmarajan et al. 2014). Additionally, NMDA-treatment and light-induced damage stimulates Bmp2, -4 and -7 in the mouse retina (Ueki and Reh 2012). In human proliferative vitreoretinopathy (PVR), there is aberrant proliferation of Müller glia and this correlates with up-regulation of Bmp4 and Smad1 (Hollborn et al. 2005). Taken together, these data suggests up-regulation of BMP-signaling may be a response common to different types of retinal injury across species and may promote proliferation of retinal glia.
We found that inhibition of BMPR1 with small molecule inhibitor DMH1 attenuated the proliferation of Müller glia in NMDA-damaged and FGF2-stimulated retinas. DMH1 is a BMPRI/ALK2 inhibitor that is based on the structure of Dorsomorphin, which is known to inhibit BMP4 mediated Smad1/5/8 phosphorylation (reviewed by (Brazil et al. 2015)). Consistent with a previous report in the mouse retina (Ueki and Reh 2012), we observed pSmad1/5/8 activity predominantly in Müller glia cells after NMDA-induced retinal damage. In mouse retinal explants, Dorsomorphin and Noggin both suppressed EGF-induced proliferation of Müller glia (Ueki and Reh 2013). Similarly, Noggin suppresses FGF2-mediated retinal regeneration in the embryonic chick (Haynes et al. 2007). In contrast with the embryonic chick retina (Haynes et al. 2007), we found that the combination of BMP4 with FGF2 did not increase numbers of proliferating MGPCs compared to numbers seen in retinas treated with FGF2 alone. We propose that signaling through Smad1/5/8 may be saturated in FGF2-treated retinas, since we detected pSmad1/5/8 in the nuclei of FGF2-treated Müller glia and this was not increased by co-application of BMP4. Therefore, the combination of BMP4 and FGF2 did not potentiate the formation of MGPCs. In mouse retinal explants, treatment with EGF stimulates pSmad1/5/8, Bmp7 expression, and Müller glia proliferation (Ueki and Reh 2013). Activation of BMP-signaling alone failed to stimulate Müller glia proliferation, suggesting BMP/Smad1/5/8-signaling is required, but not sufficient for Müller glia proliferation in the mouse retina (Ueki and Reh 2013). Collectively, these findings indicate that BMP/Smad1/5/8-signaling is a necessary, but not sufficient, component of the network of signaling pathways that promote the proliferation of Müller glia and/or MGPCs in warm-blooded vertebrates.
The formation of proliferating MGPCs may involve a push-pull antagonism between TGFβ/Smad2/3- and BMP/Smad1/5/8-signaling, wherein TGFβ/Smad2/3-inhibits and BMP/Smad1/5/8-signaling promotes Müller glia reprogramming (Fig. 8). We found TGFβ/Smad2/3-signaling is down-regulated in response to retinal damage. We found that activation of TGFβ/Smad2/3 signaling with recombinant TGFβ2 suppressed MGPC formation, whereas inhibition of TGFβ/Smad2/3 with small molecule inhibitors increased MGPC proliferation. Furthermore, we observed an increase in proliferation of retinal progenitors in the CMZ in response to Smad3 inhibition. Similarly, TGFβ/Smad2/3-signaling is known to suppress the formation of MGPCs and proliferation of late-stage progenitors and/or Müller glia in the retinas of zebrafish and rodents, respectively (Close et al. 2005; Lenkowski et al. 2013). Taken together, these findings suggest that inhibiting the repressive role of TGFβ/Smad2/3-signaling is a necessary step in promoting the formation of proliferating MGPCs in the vertebrate retina.
Consistent with findings that TGFβ/Smad2/3-signaling suppresses the reprogramming of mature Müller glia into proliferating MGPCs, this pathway may additionally suppress proliferation of late-stage progenitors and promote glial maturation during retinal development. The loss of one allele of TGFβ-RII (loss-of-function) or inhibitory Smad7 (gain-of-function) resulted in no significant proliferation or reactivity of Müller glia in NMDA-induced damaged retinas (Kugler et al. 2015). By comparison, in postnatal rat retina, TGFβ-signaling potently suppresses the proliferation of progenitors during the later stages of retinal development (Close et al. 2005), when there is a gradual acquisition of Müller glial phenotype coupled with a diminished ability of progenitors to proliferate (Nelson et al. 2011). TGFβ2 is expressed by inner retinal neurons and is the most highly expressed TGFβ ligand, compared to TGFβ1 and TGFβ3, in the postnatal rat retina (Close et al. 2005). This implies that TGFβ-ligand produced by maturing inner retinal neurons acts on late-stage retinal progenitors and Müller glia to promote the end of retinal histogenesis. Collectively, these findings suggest that during late stages of retinal development, TGFβ-signaling may suppress proliferation and promote acquisition of glial character.
During neural development, BMP-signaling is known to influence the differentiation of neurons and glia, depending on the developmental context (Guillemot 2007). In cultures of early-stage progenitors, BMP-signaling promotes neurogenesis, whereas in cultures of late-stage progenitors, BMP-signaling increases astrocyte differentiation (Gross et al. 1996; Li et al. 1998; Nakashima et al. 2001). In the adult subventricular zone, BMP-signaling influences the neurogenic niche to suppress neurogenesis from stem cells (Lim et al. 2000). We found that modulation of BMP/Smad1/5/8-signaling had no effect upon the number of progeny derived from MGPCs that differentiated as neurons. Similarly, in the developing chick embryo, data suggest that BMP-signaling does not influence the differentiation of retinal neurons (Huang et al. 2015; Muller et al. 2007; Steinfeld et al. 2013). Thus, the retina may be different from other regions of the CNS in that BMP/Smad1/5/8-signaling does not influence specification of neuronal and glial cells types.
Conclusions
We conclude that the formation of proliferating MGPCs in damaged or FGF2-treated retinas is mediated by the opposing effects of TGFβ/Smad2/3- and BMP/Smad1/5/8-signaling. We conclude that TGFβ/Smad2/3- and BMP/Smad1/5/8-signaling are part of a large network of signaling pathways that regulate the formation of MGPCs. TGFβ/Smad2/3- and BMP/Smad1/5/8-signaling can be activated in Müller glia down-stream of FGF/MAPK-signaling. However, the neuronal differentiation of MGPC progeny was not influenced by signaling through TGFβ/Smad2/3 and BMP/Smad1/5/8. Collectively, these data suggests that activation of the BMP pathway combined with inhibition of the TGFβ pathway is a promising strategy to promote Müller glia reprogramming in higher vertebrates.
Main Points.
Using the chick model system to study the retina in vivo, we find that cell signaling through BMP4/Smad1/5/8 stimulates the formation of proliferating Müller glia-derived progenitor cells, whereas TGFβ2/Smad2/3 suppresses this process.
Acknowledgments
The antibodies to Nkx2.2 (developed by Drs. T.M Jessell and S. Brenner-Morton), and Pax6 (developed by Dr. A. Kawakami) were obtained from the Developmental Studies Hybridoma Bank developed under auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. This work was supported by a grant (EY022030-5) from the National Eye Institute, National Institutes of Health. The authors declare no competing financial interests.
Footnotes
Author Contributions: LT designed and executed experiments, gathered data, constructed figures and contributed to writing the manuscript, IP, NS, NM, executed experiments, gathered data, and contributed to writing the manuscript, AJF designed experiments, constructed figures and contributed to writing the manuscript
References
- Aigner L, Bogdahn U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell Tissue Res. 2008;331:225–41. doi: 10.1007/s00441-007-0466-7. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Curr Opin Cell Biol. 2001;13:706–14. doi: 10.1016/s0955-0674(00)00274-x. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams T, Adler R. Developmental expression patterns of bone morphogenetic proteins, receptors, and binding proteins in the chick retina. J Comp Neurol. 2001;430:562–72. [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, et al. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AM, Bhalala OG, Kessler JA. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev Neurobiol. 2012;72:1068–84. doi: 10.1002/dneu.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazil DP, Church RH, Surae S, Godson C, Martin F. BMP signalling: agony and antagony in the family. Trends Cell Biol. 2015;25:249–64. doi: 10.1016/j.tcb.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development. 2005;132:3015–26. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- Conner C, Ackerman KM, Lahne M, Hobgood JS, Hyde DR. Repressing Notch Signaling and Expressing TNFalpha Are Sufficient to Mimic Retinal Regeneration by Inducing Muller Glial Proliferation to Generate Committed Progenitor Cells. J Neurosci. 2014;34:14403–19. doi: 10.1523/JNEUROSCI.0498-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constam DB, Schmid P, Aguzzi A, Schachner M, Fontana A. Transient production of TGF-beta 2 by postnatal cerebellar neurons and its effect on neuroblast proliferation. Eur J Neurosci. 1994;6:766–78. doi: 10.1111/j.1460-9568.1994.tb00988.x. [DOI] [PubMed] [Google Scholar]
- Dharmarajan S, Gurel Z, Wang S, Sorenson CM, Sheibani N, Belecky-Adams TL. Bone morphogenetic protein 7 regulates reactive gliosis in retinal astrocytes and Muller glia. Mol Vis. 2014;20:1085–108. [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–13. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002;129:2283–91. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–52. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev Biol. 2008;317:196–212. doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Ritchey ER, Sherwood P. Mitogen-activated protein kinase-signaling regulates the ability of Müller glia to proliferate and protect retinal neurons against excitotoxicity. Glia. 2009a;57:1538–1552. doi: 10.1002/glia.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Tuten W. Mitogen-activated protein kinase-signaling stimulates Muller glia to proliferate in acutely damaged chicken retina. Glia. 2009b;57:166–81. doi: 10.1002/glia.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Scott MA, Zelinka C, Sherwood P. A novel type of glial cell in the retina is stimulated by insulin-like growth factor 1 and may exacerbate damage to neurons and Muller glia. Glia. 2010;58:633–49. doi: 10.1002/glia.20950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J Comp Neurol. 1998;393:1–15. [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Gallina D, Scott MA, Todd L. Reactive microglia and macrophage facilitate the formation of Muller glia-derived retinal progenitors. Glia. 2014a;62:1608–1628. doi: 10.1002/glia.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Zelinka C, Gallina D, Scott MA, Todd L. Reactive microglia and macrophage facilitate the formation of Muller glia-derived retinal progenitors. Glia. 2014b;62:1608–28. doi: 10.1002/glia.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Palazzo I, Steffenson L, Todd L, Fischer AJ. Wnt/betacatenin-signaling and the formation of Muller glia-derived progenitors in the chick retina. Dev Neurobiol. 2015 doi: 10.1002/dneu.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Todd L, Fischer AJ. A comparative analysis of Muller glia-mediated regeneration in the vertebrate retina. Exp Eye Res. 2014a;123:121–130. doi: 10.1016/j.exer.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina D, Zelinka C, Fischer AJ. Glucocorticoid receptors in the retina, Muller glia and the formation of Muller glia-derived progenitors. Development. 2014b;141:3340–51. doi: 10.1242/dev.109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Stanke JJ, Fischer AJ. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res. 2008;1192:76–89. doi: 10.1016/j.brainres.2007.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Serotonin released from amacrine neurons is scavenged and degraded in bipolar neurons in the retina. J Neurochem. 2009;111:1–14. doi: 10.1111/j.1471-4159.2009.06270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Zelinka C, Fischer AJ. Notch signaling influences neuroprotective and proliferative properties of mature Muller glia. J Neurosci. 2010;30:3101–12. doi: 10.1523/JNEUROSCI.4919-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Hamon A, Roger JE, Yang XJ, Perron M. Muller glial cell-dependent regeneration of the neural retina: An overview across vertebrate model systems. Dev Dyn. 2016;245:727–38. doi: 10.1002/dvdy.24375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312:300–11. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes T, Gutierrez C, Aycinena JC, Tsonis PA, Del Rio-Tsonis K. BMP signaling mediates stem/progenitor cell-induced retina regeneration. Proc Natl Acad Sci U S A. 2007;104:20380–5. doi: 10.1073/pnas.0707202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollborn M, Tenckhoff S, Jahn K, Iandiev I, Biedermann B, Schnurrbusch UE, Limb GA, Reichenbach A, Wolf S, Wiedemann P, et al. Changes in retinal gene expression in proliferative vitreoretinopathy: glial cell expression of HB-EGF. Mol Vis. 2005;11:397–413. [PubMed] [Google Scholar]
- Huang J, Liu Y, Oltean A, Beebe DC. Bmp4 from the optic vesicle specifies murine retina formation. Dev Biol. 2015;402:119–26. doi: 10.1016/j.ydbio.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman GJ, Nicolas FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Jadhav AP, Roesch K, Cepko CL. Development and neurogenic potential of Muller glial cells in the vertebrate retina. Prog Retin Eye Res. 2009;28:249–62. doi: 10.1016/j.preteyeres.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69:597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- Karl MO, Hayes S, Nelson BR, Tan K, Buckingham B, Reh TA. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci U S A. 2008;105:19508–13. doi: 10.1073/pnas.0807453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugler M, Schlecht A, Fuchshofer R, Kleiter I, Aigner L, Tamm ER, Braunger BM. Heterozygous modulation of TGF-beta signaling does not influence Muller glia cell reactivity or proliferation following NMDA-induced damage. Histochem Cell Biol. 2015;144:443–55. doi: 10.1007/s00418-015-1354-y. [DOI] [PubMed] [Google Scholar]
- Lenkowski JR, Qin Z, Sifuentes CJ, Thummel R, Soto CM, Moens CB, Raymond PA. Retinal regeneration in adult zebrafish requires regulation of TGFbeta signaling. Glia. 2013;61:1687–97. doi: 10.1002/glia.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cogswell CA, LoTurco JJ. Neuronal differentiation of precursors in the neocortical ventricular zone is triggered by BMP. J Neurosci. 1998;18:8853–62. doi: 10.1523/JNEUROSCI.18-21-08853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–26. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Muller F, Rohrer H, Vogel-Hopker A. Bone morphogenetic proteins specify the retinal pigment epithelium in the chick embryo. Development. 2007;134:3483–93. doi: 10.1242/dev.02884. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Takizawa T, Ochiai W, Yanagisawa M, Hisatsune T, Nakafuku M, Miyazono K, Kishimoto T, Kageyama R, Taga T. BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl Acad Sci U S A. 2001;98:5868–73. doi: 10.1073/pnas.101109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, Lamba DA, Reh TA. Genome-wide analysis of Muller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One. 2011;6:e22817. doi: 10.1371/journal.pone.0022817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Gorsuch RA, Bailey TJ, Ackerman KM, Kassen SC, Hyde DR. Stat3 defines three populations of Muller glia and is required for initiating maximal muller glia proliferation in the regenerating zebrafish retina. J Comp Neurol. 2012;520:4294–311. doi: 10.1002/cne.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–9. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Zhao XF, Goldman D. Ascl1a/Dkk/beta-catenin signaling pathway is necessary and glycogen synthase kinase-3beta inhibition is sufficient for zebrafish retina regeneration. Proc Natl Acad Sci U S A. 2011;108:15858–63. doi: 10.1073/pnas.1107220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey ER, Zelinka CP, Tang J, Liu J, Fischer AJ. The combination of IGF1 and FGF2 and the induction of excessive ocular growth and extreme myopia. Exp Eye Res. 2012;99:1–16. doi: 10.1016/j.exer.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompani SB, Cepko CL. A common progenitor for retinal astrocytes and oligodendrocytes. J Neurosci. 2010;30:4970–80. doi: 10.1523/JNEUROSCI.3456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanke J, Moose HE, El-Hodiri HM, Fischer AJ. Comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J Comp Neurol. 2010;518:2316–33. doi: 10.1002/cne.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld J, Steinfeld I, Coronato N, Hampel ML, Layer PG, Araki M, Vogel-Hopker A. RPE specification in the chick is mediated by surface ectoderm-derived BMP and Wnt signalling. Development. 2013;140:4959–69. doi: 10.1242/dev.096990. [DOI] [PubMed] [Google Scholar]
- Thummel R, Enright JM, Kassen SC, Montgomery JE, Bailey TJ, Hyde DR. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90:572–82. doi: 10.1016/j.exer.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Fischer AJ. Hedgehog-signaling stimulates the formation of proliferating Müller glia-derived progenitor cells in the retina. Development. 2015;142:2610–2622. doi: 10.1242/dev.121616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Squires N, Suarez L, Fischer AJ. Jak/Stat signaling regulates the proliferation and neurogenic potential of Muller glia-derived progenitor cells in the avian retina. Sci Rep. 2016;6:35703. doi: 10.1038/srep35703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd L, Suarez L, Squires N, Zelinka CP, Gribbins K, Fischer AJ. Comparative analysis of glucagonergic cells, glia and the circumferential marginal zone in the reptilian retina. J Comp Neurol. 2015 doi: 10.1002/cne.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trousse F, Esteve P, Bovolenta P. Bmp4 mediates apoptotic cell death in the developing chick eye. J Neurosci. 2001;21:1292–301. doi: 10.1523/JNEUROSCI.21-04-01292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Reh TA. Activation of BMP-Smad1/5/8 signaling promotes survival of retinal ganglion cells after damage in vivo. PLoS One. 2012;7:e38690. doi: 10.1371/journal.pone.0038690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Reh TA. EGF stimulates Muller glial proliferation via a BMP-dependent mechanism. Glia. 2013;61:778–89. doi: 10.1002/glia.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki Y, Wilken MS, Cox KE, Chipman L, Jorstad N, Sternhagen K, Simic M, Ullom K, Nakafuku M, Reh TA. Transgenic expression of the proneural transcription factor Ascl1 in Muller glia stimulates retinal regeneration in young mice. Proc Natl Acad Sci U S A. 2015;112:13717–22. doi: 10.1073/pnas.1510595112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Zhao XF, Vojtek A, Goldman D. Retinal Injury, Growth Factors, and Cytokines Converge on beta-Catenin and pStat3 Signaling to Stimulate Retina Regeneration. Cell Rep. 2014;9:285–97. doi: 10.1016/j.celrep.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinka CP, Scott MA, Volkov L, Fischer AJ. The Reactivity, Distribution and Abundance of Non-Astrocytic Inner Retinal Glial (NIRG) Cells Are Regulated by Microglia, Acute Damage, and IGF1. PLoS One. 2012;7:e44477. doi: 10.1371/journal.pone.0044477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinka CP, Volkov L, Goodman ZA, Todd L, Palazzo I, Bishop WA, Fischer AJ. mTor signaling is required for the formation of proliferating Muller glia-derived progenitor cells in the chick retina. Development. 2016;143:1859–73. doi: 10.1242/dev.133215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XF, Wan J, Powell C, Ramachandran R, Myers MG, Jr, Goldman D. Leptin and IL-6 family cytokines synergize to stimulate muller glia reprogramming and retina regeneration. Cell Rep. 2014;9:272–84. doi: 10.1016/j.celrep.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]