Central Illustration
Key Words: cardiac lymphatic vessels, immune responses, interstitial edema, lymphangiogenesis, myocardial infarction, tissue fibrosis, vascular endothelial growth factor-C
Abbreviations and Acronyms: LYVE1, lymphatic vessel endothelial hyaluronan receptor 1; PDGFRβ, platelet-derived growth factor receptor β; PROX1, prospero hemeobox1; VEGF, vascular endothelial growth factor; VEGFR3, vascular endothelial growth factor receptor
Summary
The lymphatic vasculature plays a key role in regulating tissue fluid homeostasis, lipid transport, and immune surveillance throughout the body. Although it has been appreciated that the heart relies on lymphatic vessels to maintain fluid balance and that such balance must be tightly maintained to allow for normal cardiac output, it has only recently come to light that the lymphatic vasculature may serve as a therapeutic target with which to promote optimal healing following myocardial ischemia and infarction. This article reviews the subject of cardiac lymphatic vessels and highlights studies that imply targeting of lymphatic vessel development or transport using vascular endothelial growth factor-C therapy may serve as a promising avenue for future clinical application in the context of ischemic injury.
Hippocrates (460 to 370 bc) first described the colorless vessels that are now recognized as comprising the lymphatic vasculature; however, few studies focused on these specialized vessels in the ensuing 2,000-year period (1). Historically, the reason why this research has evolved so slowly is due to the technical difficulty in visualizing the colorless lymphatic vasculature. In the early 20th century, Florence Sabin injected ink into pig embryos and discovered that lymphatic vessels originated from out-sprouting of existing veins during development (2). Not until the end of the 20th century, when the lymphatic vasculature could be specifically visualized through the discovery of key genes that faithfully marked lymphatic endothelial cells, was it possible to truly observe and appreciate lymphatic anatomy 3, 4, 5. For this reason, the very presence of lymphatic vessels in some anatomic locations is still being described. For instance, in the central nervous system, lymphatic vessels in the meningeal membranes around the brain have only recently been discovered, where they function to facilitate immune cell and cerebral interstitial fluid drainage from the brain 6, 7, 8, 9. In the eye, a specialized structure referred to as Schlemm’s canal bears features of lymphatic vessels and regulates intraocular pressure 7, 9, 10. Novel functions of the lymphatic vasculature have also recently emerged, including a key role in preparing the developing lung for inflation at birth to avoid respiratory neonatal failure (11). Progress has also been made in defining a role for lymphatic vessels in transporting cholesterol from the artery wall to reduce risk of atherosclerosis. We have recently reviewed this subject, but we will not discuss it here (12).
Progress in lymphatic vessel research has also emerged in the heart. This review focuses on the current understanding of cardiac lymphatic biology. First, we describe the origin of the cardiac lymphatic vasculature during development and then review overall cardiac lymphatic anatomy and function. Finally, we discuss the potential of therapeutic strategies to target cardiac lymphatic vasculature.
Origin of the Systemic Lymphatic Vasculature
The lymphatic system includes lymphatic vessels, lymph nodes, and lymphoid organs (13). Different from the circulatory blood vessels, lymphatic vessels are structurally blind-ended at their distal segments. These blind ends form vessels that are designed to absorb interstitial fluid and immune cells and are called lymphatic capillaries. These vessels converge onto deeper lymphatic structures called collecting vessels that are equipped with valves and a muscular wall that promotes transport rather than absorption. Flow in the lymphatic vasculature as a whole is unidirectional and is designed to drain extravascular fluid, macromolecules, and immune cells back into the venous system to maintain interstitial fluid balance. The cells also transport absorbed lipids such as chylomicron particles from the gut, and throughout the body they function as a key structure in immune surveillance 12, 14. Despite much progress in all aspects of lymphatic vessel research, the organs from which the lymphatic vascular system originate is still under debate. As mentioned above, more than 100 years ago, lymphatic vessels were suggested to originate from lymph sacs by Florence Sabin (2). Sabin’s early work was found consistent with results shown later in mouse (15) and zebrafish (16).
An alternative proposed by Huntington and McClure (17) hypothesized that peripheral lymphatics arose from a nonvenous source through lymphangioblasts that gave rise to lymph sacs, suggesting that the origin of lymphatic vasculature is mixed. In addition to the undisputed venous origin of many lymphatic vessels, recent evidence also has emerged to argue in favor of the concept that lymphatic endothelial cells arise from other sources. In the lumbar and dorsal dermal layer of mouse embryonic skin, for example, approximately one-third of lymphatic vessels are derived from nonvenous sources (18). In zebrafish, lymphatic vessels indeed arise from the cardinal vein but derive from a subset of specialized angioblasts therein, where Wnt5b serves to induce lymphatic specification (19). Until recently, little has been defined regarding the origins of the lymphatic vasculature during organogenesis. In the last few years, however, work from different groups agree on the derivation of at least part of the intestinal lymphatic vasculature from nonvenous, local sources. These origins depend upon the prior formation of arteries (20) and appear to descend from hemogenic endothelium (21), a subset of endothelial cells in blood vessels serving as a source for hematopoietic progenitors during a narrow developmental window before liver and bone marrow become the major hematopoietic tissues (22).
Only recently have data emerged regarding the development and origins of lymphatic vasculature in the heart (23). Lymphatic vessels emerge within the developing heart at approximately embryonic day 12.5 (E12.5) and are first visualized in association with the ventral outflow tract. At E14.5, LYVE1+PROX1+VEGFR3+ lymphatics sprout from the sinus venosus and extend to cover the dorsal surface of the left and right ventricles by E16.5. During late gestation and early post-natal development, the lymphatic vasculature expands to cover the entire epicardial surface of heart (including dorsal and ventral surfaces) and appears to mature by 2 weeks of age. Beginning at E15.5, lymphatic vessels are found in close proximity to developing coronary veins and remain associated with venous vasculature thereafter. Although interconnections between lymphatic and venous endothelial cells are observed at early time points, these connections are lost as the lymphatic vasculature matures. Intriguingly, lineage tracing studies using Tie2-Cre and Pdgfrβ-CreERT2 mice (carrying markers of vascular endothelial cells) revealed that cardiac lymphatics were derived from a mixed pool of progenitors including venous and putative hemogenic endothelial origins.
Flow Patterns of Cardiac Lymph
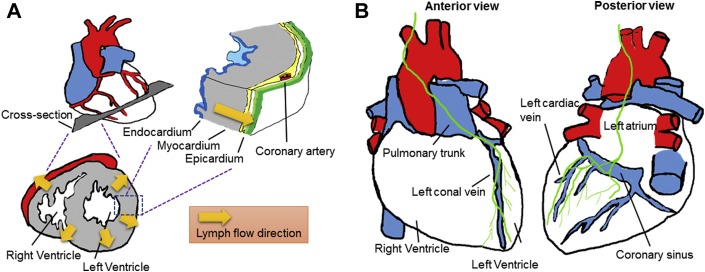
The cardiac lymphatic vasculature was first described in the 17th century by Rudbeck, discussed in Bradham and Parker (24). Patek (25), publishing in 1939, revealed that the mammalian cardiac lymphatic vasculature invested all layers of the heart: the subepicardium, the myocardium, and the subendocardium. The flow and composition of cardiac lymph in dog were analyzed 26, 27. That work revealed that lymph flow passed from the endocardium, after interstitial fluid entered lymphatic capillaries therein, to the epicardium where collecting lymphatic vessels with activity capable of propelling lymph flow forward was observed (Figure 1A). Two main collecting lymphatic vessels emerged, including one that runs along the left conal vein toward the left side of the pulmonary trunk and further upward to the mediastinum to drain the right and left ventricles and the other, which can be seen on the atrial surface, that runs along the left cardiac vein (corresponding to the left marginal vein in humans), coronary sinus, and the left atrium upward to the mediastinum (Figure 1B) 25, 28, 29. Lymphatic valves, operating similarly as venous valves, maintain lymph flow unidirectionally, with the greatest number of valves appearing in the subepicardial collecting lymphatic vessels. The ventricles are invested with more lymphatic vessels than the atria (25). Lymphatic vessels appear in the cardiac valves, potentially playing important roles. When cardiac lymphatic drainage is impaired, the lymphatic capillary network in the mitral valve appears increased (30). Dogs and pigs have lymphatic vessels in both atrioventricular valves, including tricuspid and mitral valves, but humans have lymphatic vessels only in the mitral valve (31).
Figure 1.
Cardiac Lymphatic Drainage of an Adult Mouse Heart
(A) Cross-section of a heart demonstrates the direction of cardiac lymph drainage from endocardium, myocardium to epicardium (flow indicated by arrows). (B) Two major collecting vessels (green) on the epicardium then assist to drain lymph, originally derived from the endocardium, from apex to base: one collecting vessel (left) runs along the left conal vein upward to the pulmonary trunk and mediastinum; the other vessel (right) runs along the left cardiac vein and passes the coronary sinus and left atrium upward to the mediastinum.
Function of Cardiac Lymphatic Vessels: Historical and New Concepts
As delineated later, the concept that lymphatic function is critical to maintain cardiac function has been clear for some time. However, only recently has the idea emerged that targeting the lymphatic vasculature might provide therapeutic benefit. Specifically, new evidence suggests that an expanded lymphatic vasculature, brought about by lymphangiogenesis, and improved lymphatic function augment tissue repair and prevent adverse remodeling in the injured heart. We trace the development of this area through its history.
Similar to other organs, the heart relies on cardiac lymphatics to drain tissue fluid in order to maintain the steady-state interstitial fluid equilibrium. Myocardial contractions help to propel cardiac lymphatic flow. During diastole, when the heart muscle relaxes, the ventricles fill with blood, and the resultant increase in chamber pressure drives the flow of lymph from the subendocardial to the myocardial lymphatics. Subsequently, during systole, ventricular contraction supplies the necessary force to propel lymph from the myocardial to the subepicardial lymphatics (32). If flow rate becomes reduced, increased myocardial interstitial edema, which may contain necrotic products or toxins, occurs, leading to increased interstitial pressure and to compromised cardiac functions under both acute and chronic conditions (33), due to increased ventricular stiffness in diastole and reduced cardiac performance 33, 34. Indeed, a very small change in interstitial pressure in the heart can have extreme effects on cardiac output 33, 34, 35.
Osmotic forces are also at play in regulating fluid balance in the heart. Under normal conditions, the protein concentration of interstitial fluid (and lymph) is much lower than that of plasma. This imbalance creates a scenario that favors water movement back into the bloodstream as it flows toward higher protein concentration. However, the impact of osmotic forces is diminished by the fact that smaller proteins are more osmotically active than larger proteins and that smaller proteins leak across inflamed vasculature, resulting in increased interstitial protein concentration. As a consequence, osmotic forces that would normally drive fluid movement from the interstitial space into lymphatics and eventually the venous system are severely blunted and resultant tissue edema ensues (36).
Cardiac surgery often produces damage to the lymphatic vasculature that can lead to acute or occasionally chronic edema (37). Thus, experiments have been undertaken to describe the impact of surgical lymphatic obstruction. Acute cardiac lymphatic obstruction causes interstitial myocardial edema, which in turn degrades ventricle performance, characterized by decreased contractility and increased diastolic stiffness 38, 39. Chronic lymphatic vessel blockade, surgically induced for experimental purposes, leads to cardiac fibrosis 40, 41. Fibrosis in turn increases interstitial resistance and compounds the problem of fluid flow through the heart. This can be due to collagen deposition (42) or possibly deposition of other extracellular matrix components.
There are still many outstanding questions in the field to be answered, and it is a “black box” in understanding the detailed mechanisms of lymphatic drainage through the heart. Are lymphatic capillaries in the myocardium understood at the cellular level? How do cardiac lymphatic capillaries take up fluid when pressures (>100 mm Hg) are generated every second? Myocardial contractions help to propel cardiac lymphatic flow, but is the lymphatic flow solely dependent upon cardiac contraction? If not, how is it regulated? Clearly, much remains to be learned, and future investigation is needed to answer those questions.
Connections Among Interstitial Edema, Immune Responses, and Lymphangiogenesis: Lessons Learned From the Skin
As indicated above, interstitial edema contributes to tissue fibrosis, which reinforces sluggish or rerouted interstitial flow. In skin, lymphedema and accompanying fibrosis are also associated with elevated infiltrated immune cells including macrophages and lymphocytes 43, 44, 45. At least in some scenarios, the inhibition of cyclooxygenase 2 reduces the severity of lymphedema, suggesting the potential role of inflammation in maintaining lymphedema (46). Stimulation of lymphangiogenesis, again at least in some settings, can act effectively to inhibit chronic skin inflammation and immune cell accumulation and increase lymphatic drainage 47, 48. However, whether lymphangiogenesis is necessary to support additional lymph flow, as is often assumed, is unclear, as a reasonable counterargument can be made that the presence of lymphatic vessels at their basal density is more than sufficient to cover the physiological and even pathological needs to cope with problems of accumulated fluid (13). In addition, lymphangiogenesis combined with the formation of tertiary lymphoid organs under certain circumstances may sustain immune responses by facilitating the transport of antigens as well as immune cells (13). Inactivation of lymphatic vessel has been suggested to serve as a novel therapy to reduce cardiac allograft rejection and arteriosclerosis (49).
During the initial phase of edema after injury, M2 polarized macrophages, the major cell expressing vascular endothelial growth factor (VEGF)-C (50), regulate the formation of collateral lymphatic capillaries. By contrast, accumulation of CD4+ T cells in the later stage inhibits lymphangiogenesis and induces sclerosis of collecting lymphatics that leads to interstitial fibrosis and edema (51). Loss of CD4+ T but not CD8+ T or CD25+ T cells decreases lymphedema, inflammation and fibrosis and increases lymphangiogenesis (43), suggesting that lymphatic growth is a highly coordinated process. Th2 T cells and their cytokines interleukin (IL)-4 and IL-13 negatively regulate lymphangiogenesis and lymphatic function (52); anti-IL-4 and anti-IL-13 antibodies reduce lymphedema, suggesting the significant roles of Th2 T cells in the development of interstitial fibrosis 52, 53. In contrast, excessive generation of immature lymphatic vessels through Th1 and Th17 CD4+ inflammatory T cells can drive the pathogenesis of lymphedema (54). In sum, skin lymphedema induces inflammation that includes recruited immune cells that in turn potentially cause the formation of fibrotic tissues. Targeting lymphangiogenesis may be a therapy to repair the skin lesions and possibly those in other organs. However, in order for this approach to move forward, it is critical to understand the mechanisms by which lymphangiogenesis improves, but sometimes worsens, edema and in particular to understand if the main purpose of inflammatory lymphangiogenesis is to promote fluid or cell clearance.
Myocardial Ischemia-Induced Tissue Fibrosis Caused by Immune Cells Affects Lymphatic Drainage
Myocardial ischemia, the lack of blood supply to the heart, causes rapid death of cardiac myocytes. At the same time, fluid accumulates in the cardiac interstitial space, leading to the formation of myocardial edema due to increased myocardial microvascular permeability and the filtration rate exceeding the lymph flow rate 33, 55. Similar to skin lymphedema, myocardial edema can trigger cardiac fibrosis 33, 56 as shown by increased collagen synthesis including types I and III by fibroblasts 57, 58. In the infarct zone, inflammatory signals recruit neutrophils within 1 day and, thereafter, monocyte/macrophages arrive. During the first 3 days post-injury, Ly-6Chi monocytes infiltrate and scavenge necrotic debris, and when resolution begins, they differentiate into Ly-6Clo macrophages that promote healing 59, 60. During this process, cytokines are produced and promote the activation of collagen-secreting fibroblasts (61), including secretion of anti-inflammatory cytokines such as IL-10 (62) and IL-13 (63). Although resultant fibrotic tissue can compensate for the loss of cardiomyocytes and provide structural support following myocardial infarction to protect against cardiac rupture, myocardial fibrosis also has negative consequences including hindering interstitial fluid drainage, impairing cardiac function, and contributing to adverse ventricular remodeling. Intriguingly, lymphatic drainage through the myocardium can be recovered by remodeling collateral circulation (64), and this alleviates inflammatory responses orchestrated by chemokines and recruited leukocytes elicited following ischemic tissue injury (65).
Many concepts, including flow and lymphangiogenic response to inflammation, are borrowed from the lesson learned from the skin. Although those concepts may apply to the heart as well, they are also likely to be organ-specific mechanisms. Clearly, there will be many new connections to be discovered as we explore the lymphatic field in full.
Lymphangiogenesis as a Target to Promote Heart Function After Injury
After myocardial infarction, when much tissue is destroyed, the restoration of even the basal density of lymphatic vessels is needed to restore interstitial flow. The basal lymphangiogenic response is quite robust in humans 66, 67 and also in animals 23, 68. The increases in lymphatic density are sustained during the healing phase, longer than that of blood vessels (66). Perhaps there is a prolonged need for lymphatic vessels during the healing phase, possibly to drain excessive proteins and fluid. When lymphatics fail to remove accumulated fluid, expansion of the interstitial fluid compartment increases the diffusion distance for oxygen, exacerbates the hypoxic state, and increases the scale of infarct development (69).
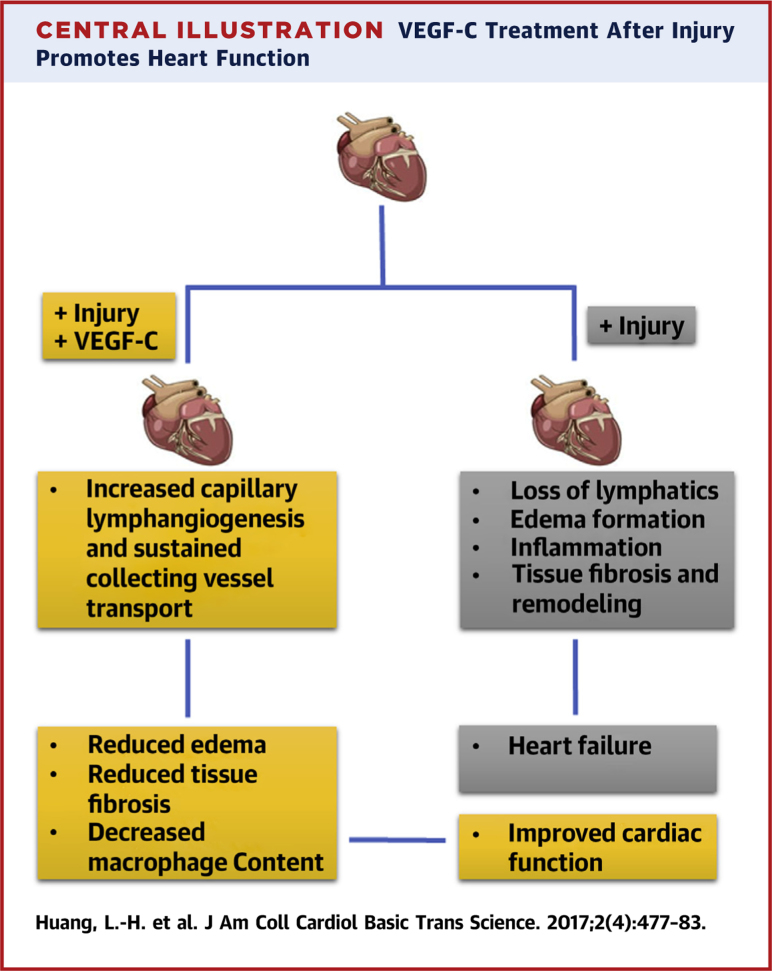
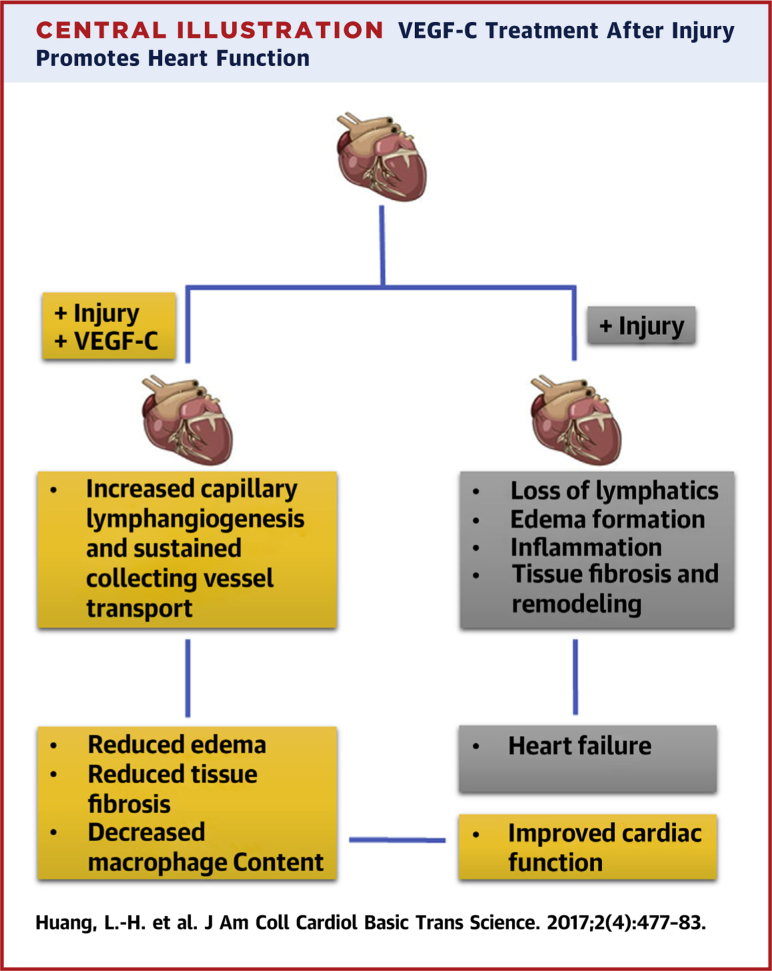
Given the role of VEGF-C in wound repair to induce lymphangiogenesis, its use therapeutically benefits lymphedema (70). To investigate the influence of lymphangiogenesis on cardiac function, VEGF-C therapy was applied after myocardial infarction and found to improve cardiac function post-myocardial infarction while limiting fibrosis and lingering inflammation 23, 68. Although 1 of these 2 studies pointed solely to the impact of VEGF-C on increasing local lymphangiogenesis, Henri et al. (68) observed that local lymphangiogenesis was quite robust in the untreated cohort but that nonetheless VEGF-C therapy was beneficial. The study went on to find that lymphatic capillary remodeling was far from the whole story, that indeed the major issue appeared to be adverse remodeling of the collecting lymphatics that negatively impacted fluid transport from infarcted and adjacent uninfarcted areas (68). Thus, it is possible that, although lymphangiogenesis of the small, blind-ended lymphatic capillaries garners attention most readily because this change is most obvious (23), the most relevant therapeutic target may be sustaining function of the deeper collecting lymphatic vessels (68). Collectively, these 2 studies raise a promising new approach to restoring the heart after myocardial infarction and highlight the potential that additional work on the lymphatic vasculature in the cardiac system may yield in treating dysfunction of the heart (Central Illustration).
Central Illustration.
VEGF-C Treatment After Injury Promotes Heart Function
Increased lymphangiogenesis of lymphatic capillaries and pre-collecting and collecting vessels remodeling after treating the infarct heart with VEGF-C improves cardiac functions in the infarcted heart. VEGF = vascular endothelial growth factor.
Conclusions
Although the lymphatic vasculature in the heart has been studied for some time, recent studies in the field have introduced for the first time the possibility that heart conditions such as ischemia and infarction may be treated by direct targeting of the lymphatic vasculature. In particular, the therapeutic administration of VEGF-C in 2 different studies improved the rate and quality of cardiac healing in experimental myocardial infarction in mice and rats. The positive outcome may be attributed to increased lymphangiogenesis of lymphatic capillaries in the heart but may otherwise also relate to sustained functionality of the deeper lymphatic collecting vessels. There is much work to be done in considering lymphatic transport as a means to combat adverse events in the heart. The lymphatic vessels of the heart deserve increased attention.
Footnotes
Drs. Randolph and Huang were supported by National Institutes of Health (NIH) (R01 HL118206). Dr. Lavine was supported by funding from NIH (K08 HL123519), Children’s Discovery Institute of Washington University, St. Louis Children’s Hospital (CHII2015-462 CH-II-2017-628), Foundation of Barnes-Jewish Hospital (8038-88), Burroughs Foundation Welcome Fund, and Longer Life Foundation (2016-004).
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Li-Hao Huang, Email: paul.huang@wustl.edu.
Gwendalyn J. Randolph, Email: gjrandolph@wuslt.edu.
References
- 1.Lord R.S. The white veins: conceptual difficulties in the history of the lymphatics. Med Hist. 1968;12:174–184. doi: 10.1017/s0025727300013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabin F.R. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. American Journal of Anatomy. 1902;1:367–389. [Google Scholar]
- 3.Wigle J.T., Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 4.Breiteneder-Geleff S., Soleiman A., Kowalski H. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerji S., Ni J., Wang S.-X. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aspelund A., Antila S., Proulx S.T. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park D.-Y., Lee J., Park I. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J Clin Invest. 2014;124:3960–3974. doi: 10.1172/JCI75392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kizhatil K., Ryan M., Marchant J.K., Henrich S., John S.W. Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014;12:e1001912. doi: 10.1371/journal.pbio.1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomson B.R., Heinen S., Jeansson M. A lymphatic defect causes ocular hypertension and glaucoma in mice. J Clin Invest. 2014;124:4320–4324. doi: 10.1172/JCI77162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aspelund A., Tammela T., Antila S. The Schlemm’s canal is a VEGF-C/VEGFR-3–responsive lymphatic-like vessel. J Clin Invest. 2014;124:3975–3986. doi: 10.1172/JCI75395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakus Z., Gleghorn J.P., Enis D.R. Lymphatic function is required prenatally for lung inflation at birth. J Exp Med. 2014;211:815–826. doi: 10.1084/jem.20132308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L.-H., Elvington A., Randolph G.J. The role of the lymphatic system in cholesterol transport. Front Pharmacol. 2015;6:182. doi: 10.3389/fphar.2015.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randolph G.J., Ivanov S., Zinselmeyer B.H., Scallan J.P. The lymphatic system: integral roles in immunity. Annu Rev Immunol. 2017;35:31–52. doi: 10.1146/annurev-immunol-041015-055354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randolph G.J., Miller N.E. Lymphatic transport of high-density lipoproteins and chylomicrons. J Clin Invest. 2014;124:929–935. doi: 10.1172/JCI71610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan R.S., Dillard M.E., Lagutin O.V. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaniv K., Isogai S., Castranova D., Dye L., Hitomi J., Weinstein B.M. Live imaging of lymphatic development in the zebrafish. Nat Med. 2006;12:711–716. doi: 10.1038/nm1427. [DOI] [PubMed] [Google Scholar]
- 17.Huntington G.S., McClure C.F. The anatomy and development of the jugular lymph sacs in the domestic cat (Felis domestica) Am J Anat. 1910;10:177–312. [Google Scholar]
- 18.Martinez-Corral I., Ulvmar M.H., Stanczuk L. Nonvenous origin of dermal lymphatic vasculature. Circ Res. 2015;116:1649–1654. doi: 10.1161/CIRCRESAHA.116.306170. [DOI] [PubMed] [Google Scholar]
- 19.Nicenboim J., Malkinson G., Lupo T. Lymphatic vessels arise from specialized angioblasts within a venous niche. Nature. 2015;522:56–61. doi: 10.1038/nature14425. [DOI] [PubMed] [Google Scholar]
- 20.Mahadevan A., Welsh Ian C., Sivakumar A. The left-right Pitx2 pathway drives organ-specific arterial and lymphatic development in the intestine. Dev Cell. 2014;31:690–706. doi: 10.1016/j.devcel.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanczuk L., Martinez-Corral I., Ulvmar M.H. cKit Lineage hemogenic endothelium-derived cells contribute to mesenteric lymphatic vessels. Cell Rep. 2015;10:1708–1721. doi: 10.1016/j.celrep.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 22.Lancrin C., Sroczynska P., Stephenson C., Allen T., Kouskoff V., Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klotz L., Norman S., Vieira J.M. Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature. 2015;522:62–67. doi: 10.1038/nature14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradham R.R., Parker E.F. The cardiac lymphatics. Ann Thorac Surg. 1973;15:526–535. doi: 10.1016/s0003-4975(10)65339-8. [DOI] [PubMed] [Google Scholar]
- 25.Patek P.R. The morphology of the lymphactics of the mammalian heart. Am J Anat. 1939;64:203–249. [Google Scholar]
- 26.Drinker C.K., Warren M.F., Maurer F.W., McCarrell J.D. The flow, pressure, and composition of cardiac lymph. Am J Physiol-Legacy Content. 1940;130:43–55. [Google Scholar]
- 27.Miller A.J., Ellis A., Katz L.N. Cardiac lymph: flow rates and composition in dogs. Am J Physiol-Legacy Content. 1964;206:63–66. doi: 10.1152/ajplegacy.1964.206.1.63. [DOI] [PubMed] [Google Scholar]
- 28.Flaht-Zabost A., Gula G., Ciszek B. Cardiac mouse lymphatics: developmental and anatomical update. Anat Rec (Hoboken) 2014;297:1115–1130. doi: 10.1002/ar.22912. [DOI] [PubMed] [Google Scholar]
- 29.Ratajska A., Gula G., Flaht-Zabost A. Comparative and developmental anatomy of cardiac lymphatics. ScientificWorldJournal. 2014;2014:183170. doi: 10.1155/2014/183170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller A.J., Pick R., Katz L.N. Lymphatics of the mitral valve of the dog demonstration and discussion of the possible significance. Circ Res. 1961;9:1005–1009. doi: 10.1161/01.res.9.5.1005. [DOI] [PubMed] [Google Scholar]
- 31.Johnson R.A., Blake T.M. Lymphatics of the heart. Circulation. 1966;33:137–142. doi: 10.1161/01.cir.33.1.137. [DOI] [PubMed] [Google Scholar]
- 32.Cui Y. Impact of lymphatic vessels on the Heart. Thorac Cardiovasc Surg. 2010;58:1–7. doi: 10.1055/s-0029-1240553. [DOI] [PubMed] [Google Scholar]
- 33.Laine G., Allen S. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ Res. 1991;68:1713–1721. doi: 10.1161/01.res.68.6.1713. [DOI] [PubMed] [Google Scholar]
- 34.Pogatsa G., Dubecz E., Gabor G. The role of myocardial edema in the left ventricular diastolic stiffness. Basic Res Cardiol. 1976;71:263–269. doi: 10.1007/BF01906451. [DOI] [PubMed] [Google Scholar]
- 35.Dongaonkar R.M., Stewart R.H., Geissler H.J., Laine G.A. Myocardial microvascular permeability, interstitial edema, and compromised cardiac function. Cardiovasc Res. 2010;11:145. doi: 10.1093/cvr/cvq145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stewart R.H., Rohn D.A., Mehlhorn U., Davis K.L., Allen S.J., Laine G.A. Regulation of microvascular filtration in the myocardium by interstitial fluid pressure. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1465–R1469. doi: 10.1152/ajpregu.1996.271.6.R1465. [DOI] [PubMed] [Google Scholar]
- 37.Mehlhorn U., Geissler H.J., Laine G.A., Allen S.J. Myocardial fluid balance. Eur J Cardiothorac Surg. 2001;20:1220–1230. doi: 10.1016/s1010-7940(01)01031-4. [DOI] [PubMed] [Google Scholar]
- 38.Ludwig L.L., Schertel E.R., Pratt J.W. Impairment of left ventricular function by acute cardiac lymphatic obstruction. Cardiovasc Res. 1997;33:164–171. doi: 10.1016/s0008-6363(96)00177-0. [DOI] [PubMed] [Google Scholar]
- 39.Sun S., Lie J. Cardiac lymphatic obstruction: ultrastructure of acute-phase myocardial injury in dogs. Mayo Clinic Proc. 1977:785–792. [PubMed] [Google Scholar]
- 40.Miller A.J., Pick R., Katz L.N., Jones C., Rodgers J. Ventricular endomyocardial changes after impairment of cardiac lymph flow in dogs. Brit Heart J. 1963;25:182. doi: 10.1136/hrt.25.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kluge T., Ullal S.R. Pathology of the heart following chronic cardiac lymphatic obstruction. Acta Pathologica Microbiologica Scandinavica Section A Pathology. 1972;80:150–158. doi: 10.1111/j.1699-0463.1972.tb02159.x. [DOI] [PubMed] [Google Scholar]
- 42.Stewart R.H., Geissler H.J., Allen S.J., Laine G.A. Protein washdown as a defense mechanism against myocardial edema. Am J Physiol Heart Circ Physiol. 2000;279:H1864–H1868. doi: 10.1152/ajpheart.2000.279.4.H1864. [DOI] [PubMed] [Google Scholar]
- 43.Zampell J.C., Yan A., Elhadad S., Avraham T., Weitman E., Mehrara B.J. CD4+ cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS One. 2012;7:e49940. doi: 10.1371/journal.pone.0049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rockson S.G. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- 45.Gousopoulos E., Proulx S.T., Scholl J., Uecker M., Detmar M. Prominent lymphatic vessel hyperplasia with progressive dysfunction and distinct immune cell infiltration in lymphedema. Am J Pathol. 2016;186:2193–2203. doi: 10.1016/j.ajpath.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Kashiwagi S., Hosono K., Suzuki T., Takeda A., Uchinuma E., Majima M. Role of COX-2 in lymphangiogenesis and restoration of lymphatic flow in secondary lymphedema. Lab Invest. 2011;91:1314–1325. doi: 10.1038/labinvest.2011.84. [DOI] [PubMed] [Google Scholar]
- 47.Huggenberger R., Ullmann S., Proulx S.T., Pytowski B., Alitalo K., Detmar M. Stimulation of lymphangiogenesis via VEGFR-3 inhibits chronic skin inflammation. J Exp Med. 2010;207:2255–2269. doi: 10.1084/jem.20100559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim H., Kataru R.P., Koh G.Y. Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest. 2014;124:936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nykänen A.I., Sandelin H., Krebs R. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010;121:1413–1422. doi: 10.1161/CIRCULATIONAHA.109.910703. [DOI] [PubMed] [Google Scholar]
- 50.Ghanta S., Cuzzone D.A., Torrisi J.S. Regulation of inflammation and fibrosis by macrophages in lymphedema. Am J Physiol Heart Circ Physiol. 2015;308:H1065–H1077. doi: 10.1152/ajpheart.00598.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gardenier J.C., Hespe G.E., Kataru R.P. Diphtheria toxin-mediated ablation of lymphatic endothelial cells results in progressive lymphedema. JCI Insight. 2016;1:e84095. doi: 10.1172/jci.insight.84095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin K., Kataru R.P., Park H.J. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat Comm. 2015;6:6196. doi: 10.1038/ncomms7196. [DOI] [PubMed] [Google Scholar]
- 53.Avraham T., Zampell J.C., Yan A. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114–1126. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogata F., Fujiu K., Matsumoto S. Excess lymphangiogenesis cooperatively induced by macrophages and CD4+ T cells drives the pathogenesis of lymphedema. J Invest Dermatol. 2016;136:706–714. doi: 10.1016/j.jid.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Mehlhorn U., Davis K.L., Laine G.A., Geissler H.J., Allen S.J. Myocardial fluid balance in acute hypertension. Microcirculation. 1996;3:371–378. doi: 10.3109/10739689609148309. [DOI] [PubMed] [Google Scholar]
- 56.Laine G.A., Granger H.J. Microvascular, interstitial, and lymphatic interactions in normal heart. Am J Physiol Heart Circ Physiol. 1985;249:H834–H842. doi: 10.1152/ajpheart.1985.249.4.H834. [DOI] [PubMed] [Google Scholar]
- 57.Davis K., Laine G., Geissler H., Mehlhorn U., Brennan M., Allen S. Effects of myocardial edema on the development of myocardial interstitial fibrosis. Microcirculation. 2000;7:269–280. [PubMed] [Google Scholar]
- 58.Kong D., Kong X., Wang L. Effect of cardiac lymph flow obstruction on cardiac collagen synthesis and interstitial fibrosis. Physiol Res. 2006;55:253. doi: 10.33549/physiolres.930727. [DOI] [PubMed] [Google Scholar]
- 59.Hilgendorf I., Gerhardt L.M., Tan T.C. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–1622. doi: 10.1161/CIRCRESAHA.114.303204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Epelman S., Lavine K.J., Beaudin A.E. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hofmann U., Beyersdorf N., Weirather J. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 62.Dobaczewski M., Xia Y., Bujak M., Gonzalez-Quesada C., Frangogiannis N.G. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–2187. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wynn T. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos A.C., de Lima J.J.P., Botelho M.F. Cardiac lymphatic dynamics after ischemia and reperfusion—experimental model. Nucl Med Biol. 1998;25:685–688. doi: 10.1016/s0969-8051(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 65.Frangogiannis N.G. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738. [PubMed] [Google Scholar]
- 66.Ishikawa Y., Akishima-Fukasawa Y., Ito K. Lymphangiogenesis in myocardial remodelling after infarction. Histopathology. 2007;51:345–353. doi: 10.1111/j.1365-2559.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nakamura K., Rockson S.G. The role of the lymphatic circulation in the natural history and expression of cardiovascular disease. Int J Cardiol. 2008;129:309–317. doi: 10.1016/j.ijcard.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Henri O., Pouehe C., Houssari M. Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction. Circulation. 2016;133:1484–1497. doi: 10.1161/CIRCULATIONAHA.115.020143. [DOI] [PubMed] [Google Scholar]
- 69.Dellsperger K.C., Clothier J.L., Hartnett J.A., Haun L.M., Marcus M.L. Acceleration of the wavefront of myocardial necrosis by chronic hypertension and left ventricular hypertrophy in dogs. Circ Res. 1988;63:87–96. doi: 10.1161/01.res.63.1.87. [DOI] [PubMed] [Google Scholar]
- 70.Szuba A., Skobe M., Karkkainen M.J. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 2002;16:1985–1987. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]