Abstract
Candida is a serious life-threatening pathogen, particularly with immunocompromised patients. Candida infections are considered as a major cause of morbidity and mortality in a broad range of immunocompromised patients. Candida infections are common in hospitalized patients and elderly people. The difficulty to eradicate Candida infections is owing to its unique switch between yeast and hyphae forms and more likely to biofilm formations that render resistance to antifungal therapy. Plants are known sources of natural medicines. Several plants show significant anti-Candida activities and some of them have lower minimum inhibitory concentration, making them promising candidates for anti-Candida therapy. However, none of these plant products is marketed for anti-Candida therapy because of lack of sufficient information about their efficacy, toxicity, and kinetics. This review revises major plants that have been tested for anti-Candida activities with recommendations for further use of some of these plants for more investigation and in vivo testing including the use of nanostructure lipid system.
Keywords: Anti-Candida, biofilm, Candida, natural products, plants
INTRODUCTION
Candida is a fungal pathogen[1] which is mostly known to cause high rate of mycotic infection to human worldwide.[2] Candida is known to cause mucosal and deep tissue infections. Candida infects mucosal tissues including mouth, esophagus, gut, and vagina.[3] Vaginal candidiasis continues to be a world health problem to women.[4] Candidal infections are common in hospitalized patients and elderly people, and are difficult to control.[5] About 50% of adults have Candida yeasts in their mouth and it is responsible for superficial easily treated infections. However, candidal infections can spread through the body and become life threatening, in particular with immunocompromised patients.[6,7] Candidiasis represents a major cause of death.[8] Candida can switch between two major forms, yeast and hyphae forms. The switch from yeast to hyphae is considered a major infectious agent of Candida.[9] In addition, Candida spp. produces biofilms on synthetic materials, which facilitates adhesion of the organisms to devices and renders the organism relatively resistant to antifungal therapy.[10] Catheter-associated Candida biofilms can lead to bloodstream infections.[11] Candida-infected catheters, in particular those associated with microbial biofilms, can represent 90% of infections among hospital-admitted patients and hence considered as a major cause of death.[11] Several synthetic drugs are established in the treatment regimens of candidal infections as indicated in Table 1, however drug resistance is developed.
Table 1.
Candida resistance to synthetic drugs
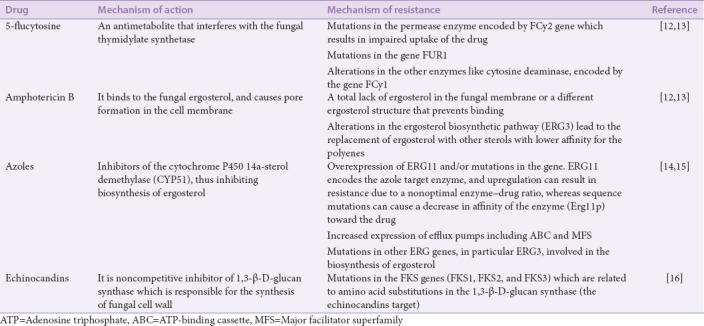
MECHANISMS OF CANDIDAL RESISTANCE TO SYNTHETIC DRUGS
The formation of biofilms in Candida and the transition from planktonic to sessile form are mainly associated with highly resistant phenotype. Other mechanisms of resistance include the expression of resistance genes, particularly those encoding efflux pumps, and the presence of persister cells.[17] Major synthetic drugs that develop candidal resistance include 5-flucytosin, amphotericin B, azoles, and echinocandins [Table 1].
PLANTS AS NATURAL SOURCES OF ANTI-CANDIDAL DRUGS
Plants are known for decades as the only source of medicines by traditional people.[18] Moreover, plants are still used as major remedies by several countries, particularly in Africa and Asia.[19] Several plant species showed effective anti-candidal activities [Table 2]. However, promoting a medicinal plant as an antimicrobial agent is challenging and requires more assessment including safety and efficacy prior to clinical study. Table 2 summarizes most of the reported plants tested for anti-candidal activities. Several of these plants showed promising minimum inhibitory concentration (MIC) such as peppermint (0.08 μg/mL), Thymus villosus (0.64 μg/mL), eucalyptus (0.05 μg/mL), lemongrass oil (0.06 μg/mL), Cinnamomum zeylanicum (0.01 μg/mL), ginger grass oil (0.08 μg/mL), and coriander (0.2 μg/mL), however they have never been deeply studied as anti-Candida drugs for the market use.
Table 2.
Natural anti-Candida products, their botanical sources, and minimum inhibitory concentration
This review article provides an overview of the reported natural anti-Candida products identified from plants and their mechanisms [Table 2]. Additionally, the current review article explores the possible biotechnological applications for the production of anti-Candida drugs and enhancing their activities.
MECHANISM OF ACTION OF ANTI-CANDIDA NATURAL PRODUCTS
The anti-Candida mechanisms of action initiated by plant natural products can involve inhibition of germination and biofilm formation, cell metabolism, cell wall integrity, cell membrane plasticity, or can involve induction of apoptosis [Figure 1].
Figure 1.
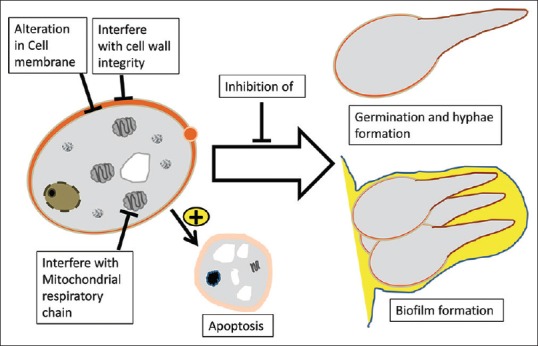
Representative drawing of the active sites and mechanisms of most tested plant anti-Candida agents
Inhibition of Candida biofilm formation and transition to hyphal form
The switch of Candida from yeast to hyphae is mainly accompanied by resistant biofilm formation. Candida biofilms are difficult to eradicate and are associated with resistance against many existing antifungals. Thymol which is a major constituent of thyme oil can interfere with biofilm metabolic activity and thus inhibits early and mature biofilm formation.[86] Anthraquinones isolated from Heterophyllaea pustulata showed significant activity against Candida tropicalis biofilm formation by interfering with the pro-oxidant–antioxidant balance leading to biofilm injury.[149] They also showed synergistic activity with amphotericin B. Geranium oil and its nanoemulsion showed antibiofilm activity against Candida albicans, C. tropicalis, and Candida glabrata. The smaller particle size of geranium nanoemulsion efficiently penetrates biofilms and hence damages the organism's cell membrane.[79] Similarly, cinnamic acid derivatives showed great antibiofilm activity against C. albicans at lower MIC compared to fluconazole. The most active cinnamic acid derivative is a hybrid of cinnamic acid with miconazole that leads to inhibition of biofilm at 2 μg/mL and reduction in metabolic activity of preformed biofilm at 8 μg/mL.[150,151] Furthermore, lemongrass oil and its major constituents exhibit strong inhibitory activity on Candida biofilm formation, germ tube formation (GTF), adherence, and candidal colonization.[130] Many terpenes including carvacrol, geraniol, and thymol showed strong activity in reducing the development of C. albicans biofilms. Carvacrol was able to inhibit Candida biofilm regardless of the tested species and of the biofilm maturation state.[152]
Inhibition of Candida germ tube formation
GTF is a transitional stage between yeast and hyphal cells which is an essential stage for Candida virulence activity.[153] GTF increases fungal adherence and penetration to infected tissues.[154] It has been shown that essential oil of oregano inhibits C. albicans GTF to a higher extent compared to other essential oils.[33] The inhibition of GTF is mainly related to the lipophilicity of the essential oils and their interaction with the Candida cell membrane, leading to changes and loss of the structural and enzymatic constituents of fungal cells including 1,3-β-D-glucan synthases, adenosine triphosphatase (ATPase), mannans, and chitin that are required in GTF.[155,156]
Alteration in Candida cell membrane
It has been reported that terpenes can cause alteration in Candida cell permeability by getting embedded between the fatty acyl chain in the membrane lipid bilayers and hence interrupting the lipid packing and consequently disturbing membrane structure and functions.[154] Geraniol increases the membrane fluidity by affecting the central part of the lipid bilayers.[157] Tea tree oil increases cell permeability and inhibits medium acidification.[114] Salvia sclarea oil and its major constituents, linalyl acetate and linalool, induce a significant increase in plasma membrane fluidity, which in turn induces cell apoptosis. Thymol affects cell membrane electrostatics and can create deviated membrane tension.[84] Coriander oil showed an increase in cell membrane permeability, loss of membrane potential, leakage of intracellular DNA, and damage of cytoplasmic membrane, thus causing impaired cellular functions.[84] Raphanus sativus antifungal peptide 2 (RsAFP2) is a plant defensin that can interact with the sphingolipid glucosylceramide (GlcCer) of susceptible fungal membranes but not with the human GlcCer, and hence can exhibit selective antifungal activity.[158,159] The RsAFP2–GlcCer interaction can lead to increase in the permeability, Ca2+ influx, and growth arrest.[160] Permeabilization due to RsAFP2 is mainly due to induction of many signaling pathways associated with the formation of reactive oxygen species (ROS), apoptosis, and caspase activation.[161] Geraniol oil derived from palmarosa oil, ninde oil, rose oil, and citronella oil can disturb the uniformity of cell membrane by interrupting sterol biosynthesis and inhibition of plasma membrane ATPase which is crucial for cell survival.[162] Taxodone is a diterpenoid compound isolated from Metasequoia glyptostroboides and Taxodium distichum, can cause loss of cell membrane integrity, and increases cell permeability, thus causing rapid loss of nucleic acid, ions, and some essential metabolites.[163]
Interference with Candida mitochondrial respiratory chain
Respiration takes place in mitochondria that produce ATP required by all cells. The process is accompanied with the production of large amount of ROS such as hydrogen peroxide and hydroxyl radicals as by-products. ROS can cause damage to cell proteins, lipids, and DNA.[164] HsAFP1 is a plant defensin derived from Heuchera sanguinea that shows apoptotic action against C. albicans mainly due to accumulation of ROS leading to the induction of mitochondrion-dependent apoptosis.[165] Dill seed essential oil (DSEO) can inhibit mitochondrial dehydrogenases mainly due to the disruption of the citric acid cycle and thus the inhibition of ATP synthesis.[166] Furthermore, DSEO causes intracellular accumulation of ROS in C. albicans and hence has an antifungal activity.[166] In addition, amentoflavone derived from Selaginella tamariscina has been associated with the induction of mitochondrion-dependent apoptosis in C. albicans.[167] Lycopene is a carotenoid pigment mainly found in tomato that can cause accumulation of intracellular Ca2+ and interference with mitochondrial functions, such as cytochrome C release and mitochondrial depolarization, leading to caspase activation and ROS production and hence leads to mitochondrial dysfunction and apoptosis.[168]
Inhibition of Candida adherence
Essential oil of Rosmarinus officinalis showed anti-adherent activity of C. albicans. The biological activity of R. officinalis is mainly associated with its main chemical components, including cineole, limonene, and cymene.[91] Schinus terebinthifolius and Croton urucurana have also showed strong anti-adherent activity of C. albicans that is associated with the presence of apigenin. Apigenin can modulate gene expression and reduce the formation of glucan, leading to biofilm inhibition activity.[169]
Induction of Candida apoptosis
Baicalein is a flavonoid isolated from the roots of Scutellaria baicalensis Georgi and shows potent activity against fluconazole-resistant C. albicans. Baicalein mainly inhibits C. albicans by inducing programmed cell death (apoptosis) and reduction of drug extrusion out of the yeast cells.[170] Silibinin, a natural product extracted from Silybum marianum (milk thistle), can cause Candida apoptosis through interference with mitochondrial Ca2+ signaling. Ca2+ signaling plays an important role in physiological processes and it is associated with stress responses in fungi.[171]
Interference with Candida cell metabolism
Allicin isolated from Allium sativum (garlic) shows a strong anti-Candida activity mainly by inhibition of thiol-containing amino acids and proteins, therefore interfering with cell metabolism.[172] Human cells contain glutathione which can bind to allicin preventing cell damage whereas glutathione is lacking in Candida that makes allicin as selective and effective candidate in anti-Candida therapy.[173]
Interference with Candida cell wall integrity
Cell wall integrity is very important during growth and morphogenesis of Candida cells and in the face of external challenges that cause cell wall stress. Several natural products have showed interference effects with Candida cell wall integrity. For example, RsAFP2 defensin interacts with Candida cell wall GlcCers and hence damages cell wall integrity. Furthermore, it can disrupt the localization of septins and blocks the switch from yeast to hypha. The black tea polyphenols including catechins and theaflavins can cause Candida cell wall damage.[144] Similarly, casuarinin isolated from Plinia cauliflora can target C. albicans cell wall, leading to significant changes in the cell wall architecture including the outer glycoprotein layer and cell wall porosity.[174]
RESISTANCE OF CANDIDA TO PLANT NATURAL PRODUCTS
Candida strains lacking GlcCer in their membranes, either because of nonfunctional synthase enzyme or its complete absence (as in Saccharomyces cerevisiae or C. glabrata), are resistant to RsAFP2 and hence protected from cell permeabilization.[160] C. tropicalis shows resistance against Uncaria tomentosa, mainly due to the enhanced ability of Candida to form biofilms.[175]
TOXICITY OF NATURAL ANTI-CANDIDA PRODUCTS
The cytotoxic activities of anti-Candida natural products are rarely investigated and only few products have been tested. For example, the toxicity of geraniol oil was measured by hemolytic assay on human erythrocytes. Geraniol oil caused only 1% cell lysis at 5 μg/mL MIC compared to 10% lysis by amphotericin B or fluconazole at same tested concentrations, suggesting the safety of geraniol.[162] The cytotoxicity of Morinda royoc L extract was also investigated on vero cells (African green monkey kidney cells). M. royoc L extract showed no toxic activities according to criteria established by the American National Cancer Institute (IC50 ≥200 mcg/mL).[176] Furthermore, oral administration of M. royoc in rats showed no toxic effects, suggesting that M. royoc is a good anti-Candida product.[177]
IN VIVO INVESTIGATION OF NATURAL ANTI-CANDIDA AGENTS
The anti-candidal activities of suppositories made from saponins derived from Solanum chrysotrichum were investigated in vulvovaginal candidiasis mice model. S. chrysotrichum treatment showed no significant difference in clinical effectiveness compared to ketoconazole.[178] On the other hand, garlic tablets (Garcin) showed similar activity to fluconazole on Candida vaginitis in women admitted to a health-care center in Iran, suggesting that garlic could be an alternative to fluconazole in the treatment of Candida infection.[179] U. tomentosa extract was clinically investigated in fifty patients with denture stomatitis. U. tomentosa is effective as miconazole on C. albicans, C. tropicalis, C. glabrata, and C. krusei; however, C. tropicalis showed resistance due to its ability to biofilm formation.[175] The anti-candidal activity of Cassia fistula seeds was tested in mice model. The seed extract showed 6-fold decrease in C. albicans in blood samples and kidneys of the tested animals.[73,180]
FUTURE PROSPECTIVE AND BIOTECHNOLOGY ADVANCES IN THE PRODUCTION OF ANTI-CANDIDA-ACTIVE PLANTS
The need for new anti-Candida agents is increasing, especially with the emergence of resistant Candida strains. The effectiveness of natural agents against different strains of fungi, particularly Candida, is confirmed in several publications. It has been reported that many patencies are using natural products as anti-Candida. For example, Indigo naturalis or indigo-producing plant extract has been used in the topical treatment of candidiasis.[181] A patent made from oral herbal preparation developed by Piramal Life Sciences showed efficient activity against oral candidiasis.[182] Pharmalp developed an anti-candidal formula derived from Epilobium parviflorum for the use in the prevention and/or treatment of Candida infection.[183]
The screening for anti-Candida natural active products increased significantly during the past two decades. Several investigations have assessed the anti-Candida activities of natural products of plants from different geographical regions in the world. For example, Duarte et al. examined the anti-Candida activities of extracts of 258 Brazilian medicinal plant species.[184] However, other regions are still in the preliminary investigation stages such as the Arabian deserts. Desert plants of the arid/hyperarid climates of the Arab Gulf region are exposed to several environmental stresses, such as heat, drought, and salinity. Such stresses may provide new active compounds which might have effective and unique anti-Candida activities.
On the other hand, modern biotechnology techniques can improve the activity of plant extracts including anti-Candida; for example, the development of nanostructure lipid system. Nanostructure lipid system can improve the antimicrobial activity of plant extract, reduce the required doses, and reduce side effects. Nanostructure lipid system improves the anti-Candida activity of aqueous ethanol extract of stems and leaves of Astronium sp.[185] The nanostructure lipid system can reduce the MIC of the plant extract ~ 9 times. Nanostructure lipid system can efficiently compartmentalize specific active components and modify their properties and behavior of plant extracts in a biological environment.[125] Moreover, recent advances in metabolomics and engineering of target pathways may provide an optimized commercial production of the natural compounds and enhancement of their activity. Usually, metabolomics using various bioanalytical tools such as nuclear magnetic resonance, liquid chromatography-mass spectrometry (MS), and gas chromatography-MS can be done to identify the potential anti-Candida compounds. Once these compounds are identified and their biosynthetic pathways are assigned, candidate genes can be identified in silico [Figure 2]. Consequently, target pathways can be engineered with overexpression of the desired transcription factors and genes or silencing of the undesired competitive genes and pathways to enhance their production levels [Figure 2].
Figure 2.
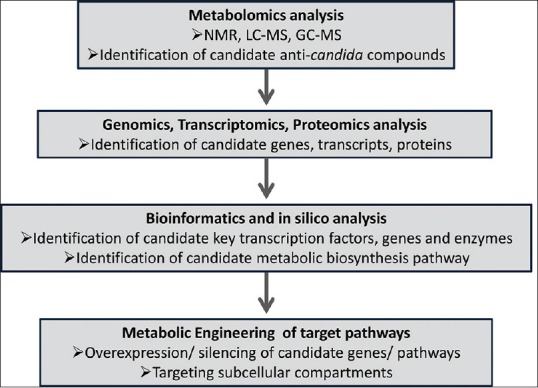
Simplified flow chart showing the utilization of different biotechnological approaches for identifying, developing, and enhancing the production levels of anti-Candida candidate compounds from a plant source
CONCLUSION
As concluding remarks, several plant natural products have been tested for anti-Candida activities. Several of these plant products can target critical processes in Candida biological activities including cell wall integrity, cell membrane plasticity, cell metabolism, respiratory chain, adherence to host cell, germination and biofilm formation, or induction of apoptosis. Despite these great anti-Candida activities of plant products compared to controls, only few have been tested in vivo and none of them have ever been clinically used as anti-Candida. On the other hand, although some of these products including garlic, probiotics, peppermint, cinnamon, ginger, and propolis are present in the pharmaceutical market for other medical purposes, they have never been used as anti-Candida. The need for new anti-Candida is urgent since Candida is known as a serious resistant microbe, and hence promotion of some of the selected plant products for clinical testing will be beneficial.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.d’Enfert C. Hidden killers: Persistence of opportunistic fungal pathogens in the human host. Curr Opin Microbiol. 2009;12:358–64. doi: 10.1016/j.mib.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL. The epidemiological features of invasive mycotic infections in the San Francisco Bay area 1992-1993: Results of population-based laboratory active surveillance. Clin Infect Dis. 1998;27:1138–47. [PubMed] [Google Scholar]
- 3.Schulze J, Sonnenborn U. Yeasts in the gut: From commensals to infectious agents. Dtsch Arztebl Int. 2009;106:837–42. doi: 10.3238/arztebl.2009.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamad M, Kazandji N, Awadallah S, Allam H. Prevalence and epidemiological characteristics of vaginal candidiasis in the UAE. Mycoses. 2014;57:184–90. doi: 10.1111/myc.12141. [DOI] [PubMed] [Google Scholar]
- 5.Sydnor ER, Perl TM. Hospital epidemiology and infection control in acute-care settings. Clin Microbiol Rev. 2011;24:141–73. doi: 10.1128/CMR.00027-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elewski BE. Onychomycosis: Pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415–29. doi: 10.1128/cmr.11.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tada H, Nemoto E, Shimauchi H, Watanabe T, Mikami T, Matsumoto T, et al. Saccharomyces cerevisiae - and Candida albicans -derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol Immunol. 2002;46:503–12. doi: 10.1111/j.1348-0421.2002.tb02727.x. [DOI] [PubMed] [Google Scholar]
- 8.Edwards JE, Jr, Lehrer RI, Stiehm ER, Fischer TJ, Young LS. Severe candidal infections: Clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978;89:91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- 9.Brand A. Hyphal growth in human fungal pathogens and its role in virulence. Int J Microbiol 2012. 2012 doi: 10.1155/2012/517529. 517529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–67. doi: 10.1128/CMR.17.2.255-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiDone L, Oga D, Krysan DJ. A novel assay of biofilm antifungal activity reveals that amphotericin B and caspofungin lyse Candida albicans cells in biofilms. Yeast. 2011;28:561–8. doi: 10.1002/yea.1860. [DOI] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff A. Mechanisms of resistance to antifungal agents: Yeasts and filamentous fungi. Rev Iberoam Micol. 2008;25:101–6. doi: 10.1016/s1130-1406(08)70027-5. [DOI] [PubMed] [Google Scholar]
- 13.Sanglard D, Kuchler K, Ischer F, Pagani JL, Monod M, Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–86. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White T. Mechanisms of resistance to antifungal drugs. In: Murray PR, Jorgensen JH, et al., editors. Manual of Clinical Microbiology. 9th ed. Washington DC, USA: ASM Press; 2007. pp. 1961–71. [Google Scholar]
- 15.Morace G, Perdoni F, Borghi E. Antifungal drug resistance in Candida species. J Glob Antimicrob Resist. 2014;2:254–9. doi: 10.1016/j.jgar.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Eidi S, Azadi HG, Rahbar N, Mehmannavaz HR. Evaluation of antifungal activity of hydroalcoholic extracts of Citrullus colocynthis fruit. J Herb Med. 2015;5:36–40. [Google Scholar]
- 17.Braga PC, Culici M, Alfieri M, Dal Sasso M. Thymol inhibits Candida albicans biofilm formation and mature biofilm. Int J Antimicrob Agents. 2008;31:472–7. doi: 10.1016/j.ijantimicag.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 18.Humber JM. The role of complementary and alternative medicine: Accommodating pluralism. JAMA. 2002;288:1655–6. [Google Scholar]
- 19.Bishop FL, Lewith GT. Who Uses CAM? A narrative review of demographic characteristics and health factors associated with CAM use. Evid Based Complement Alternat Med. 2010;7:11–28. doi: 10.1093/ecam/nen023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanih NF, Ndip RN. Evaluation of the acetone and aqueous extracts of mature stem bark of Sclerocarya birrea for antioxidant and antimicrobial properties. Evid Based Complement Alternat Med 2012. 2012 doi: 10.1155/2012/834156. 834156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ertürk Ö. Antibacterial and antifungal activity of ethanolic extracts from eleven spice plants. Biologia. 2006;61:275–8. [Google Scholar]
- 22.Tatsadjieu LN, Essia Ngang JJ, Ngassoum MB, Etoa FX. Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloides and Zanthoxylum leprieurii from Cameroon. Fitoterapia. 2003;74:469–72. doi: 10.1016/s0367-326x(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 23.Kalidindi N, Thimmaiah NV, Jagadeesh NV, Nandeep R, Swetha S, Kalidindi B. Antifungal and antioxidant activities of organic and aqueous extracts of Annona squamosa Linn. leaves. J Food Drug Anal. 2015;23:795–802. doi: 10.1016/j.jfda.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrade EH, Zoghbi MD, Maia JG, Fabricius H, Marx F. Chemical characterization of the fruit of Annona squamosa L. occurring in the Amazon. J Food Comp Anal. 2001;14:227–32. [Google Scholar]
- 25.Gbago O, Zhang H, Mlyuka ES, Song Y. Chemical composition, nutritional properties and antioxidant activity of monkey apple (Anisophyllea laurina R. Br. ex Sabine) Scand J Food Nutr. 2014;2:281–7. [Google Scholar]
- 26.Onivogui G, Diaby M, Chen X, Zhang H, Kargbo MR, Song Y. Antibacterial and antifungal activities of various solvent extracts from the leaves and stem bark of Anisophyllea laurina R. Br ex Sabine used as traditional medicine in Guinea. J Ethnopharmacol. 2015;20(168):287–90. doi: 10.1016/j.jep.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Madhumitha G, Saral AM. Preliminary phytochemical analysis, antibacterial, antifungal and anticandidal activities of successive extracts of Crossandra infundibuliformis. Asian Pac J Trop Med. 2011;4:192–5. doi: 10.1016/S1995-7645(11)60067-9. [DOI] [PubMed] [Google Scholar]
- 28.Sule A, Ahmed QU, Latip J, Samah OA, Omar MN, Umar A, et al. Antifungal activity of Andrographis paniculata extracts and active principles against skin pathogenic fungal strains in vitro. Pharm Biol. 2012;50:850–6. doi: 10.3109/13880209.2011.641021. [DOI] [PubMed] [Google Scholar]
- 29.Webster D, Taschereau P, Belland RJ, Sand C, Rennie RP. Antifungal activity of medicinal plant extracts; preliminary screening studies. J Ethnopharmacol. 2008;115:140–6. doi: 10.1016/j.jep.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Sharma V, Singh I, Chaudhary P. Acorus calamus (The Healing Plant): A review on its medicinal potential, micropropagation and conservation. Nat Prod Res. 2014;28:1454–66. doi: 10.1080/14786419.2014.915827. [DOI] [PubMed] [Google Scholar]
- 31.Shamim S, Ahmed SW, Azhar I. Antifungal activity of Allium, Aloe and Solanum species. Pharm Biol. 2004;42:491–8. [Google Scholar]
- 32.Price KR, Rhodes MJ. Analysis of the major flavonol glycosides present in four varieties of onion (Allium cepa) and changes in composition resulting from autolysis. J Sci Food Agric. 1997;74:331–9. [Google Scholar]
- 33.Silva F, Ferreira S, Duarte A, Mendonça DI, Domingues FC. Antifungal activity of Coriandrum sativum essential oil, its mode of action against Candida species and potential synergism with amphotericin B. Phytomedicine. 2011;19:42–7. doi: 10.1016/j.phymed.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 34.Begnami AF, Duarte MC, Furletti V, Rehder VL. Antimicrobial potential of Coriandrum sativum L. against different Candida species in vitro. Food Chem. 2010;118:74–7. [Google Scholar]
- 35.Naeini A, Naderi NJ, Shokri H. Analysis and in vitro anti-Candida antifungal activity of Cuminum cyminum and Salvadora persica herbs extracts against pathogenic Candida strains. J Mycol Med. 2014;24:13–8. doi: 10.1016/j.mycmed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Castro RD, Lima EO. Anti-Candida activity and chemical composition of Cinnamomum zeylanicum blume essential oil. Braz Arch Biol Technol. 2013;56:749–55. [Google Scholar]
- 37.Sadeghi Nejad B, Rajabi M, Zarei Mamoudabadi A, Zarrin M. In Vitro Anti-Candida activity of the hydroalcoholic extracts of Heracleum persicum fruit against? phatogenic Candida species. Jundishapur J Microbiol. 2014;7:e8703. doi: 10.5812/jjm.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amvam Zollo PH, Abondo R, Biyiti L, Menut C, Bessière JM. Aromatic plants of tropical central Africa xxxviii: Chemical composition of the essential oils from four Aframomum species collected in Cameroon. J Essent Oil Res. 2002;14:95–8. [Google Scholar]
- 39.Watcho P, Kamtchouing P, Sokeng S, Moundipa PF, Tantchou J, Essame JL, et al. Reversible antispermatogenic and antifertility activities of Mondia whitei L. in male albino rat. Phytother Res. 2001;15:26–9. doi: 10.1002/1099-1573(200102)15:1<26::aid-ptr679>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 40.Adekunle AA, Ikumapayi AM. Antifungal property and phytochemical screening of the crude extracts of Funtumia elastica and Mallotus oppositifolius. West Indian Med J. 2006;55:219–23. doi: 10.1590/s0043-31442006000400003. [DOI] [PubMed] [Google Scholar]
- 41.Ogbolu DO, Oni AA, Daini OA, Oloko AP. In vitro antimicrobial properties of coconut oil on Candida species in Ibadan, Nigeria. J Med Food. 2007;10:384–7. doi: 10.1089/jmf.2006.1209. [DOI] [PubMed] [Google Scholar]
- 42.Borah B, Phukon P, Hazarika MP, Ahmed R, Sarmah DK, Wann SB, et al. Calamus leptospadix Griff. a high saponin yielding plant with antimicrobial property. Ind Crops Prod. 2016;82:127–32. [Google Scholar]
- 43.Khosravi AR, Shokri H, Kermani S, Dakhili M, Madani M, Parsa S. Antifungal properties of Artemisia sieberi and Origanum vulgare essential oils against Candida glabrata isolates obtained from patients with vulvovaginal candidiasis. J Med Mycol. 2011;21:93–9. [Google Scholar]
- 44.Lubian CT, Teixeira JM, Lund RG, Nascente PS, Del Pino FA. Atividade antifúngica do extrato aquoso de Arctium minus (Hill) Bernh. (Asteraceae) sobre espécies orais de Candida. Rev Bras Plantas Med. 2010;12:157–62. [Google Scholar]
- 45.Erdemoglu N, Turan NN, Akkol EK, Sener B, Abacioglu N. Estimation of anti-inflammatory, antinociceptive and antioxidant activities of Arctium minus (Hill) Bernh. ssp. minus. J Ethnopharmacol. 2009;121:318–23. doi: 10.1016/j.jep.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Woods-Panzaru S, Nelson D, McCollum G, Ballard LM, Millar BC, Maeda Y, et al. An examination of antibacterial and antifungal properties of constituents described in traditional Ulster cures and remedies. Ulster Med J. 2009;78:13–5. [PMC free article] [PubMed] [Google Scholar]
- 47.Nchu F, Aderogba MA, Mdee LK, Eloff JN. Isolation of anti-Candida albicans compounds from Markhamia obtusifolia (Baker) Sprague (Bignoniaceae) S Afr J Bot. 2010;76:54–7. [Google Scholar]
- 48.Pereira AM, Hernandes C, Pereira SI, Bertoni BW, França SC, Pereira PS, et al. Evaluation of anticandidal and antioxidant activities of phenolic compounds from Pyrostegia venusta (Ker Gawl.). Miers. Chem Biol Interact. 2014;224:136–41. doi: 10.1016/j.cbi.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Mafioleti L, da Silva Junior IF, Colodel EM, Flach A, Martins DT. Evaluation of the toxicity and antimicrobial activity of hydroethanolic extract of Arrabidaea chica (Humb. and Bonpl.) B. Verl. J Ethnopharmacol. 2013;150:576–82. doi: 10.1016/j.jep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Yamashita M, Kaneko M, Tokuda H, Nishimura K, Kumeda Y, Iida A. Synthesis and evaluation of bioactive naphthoquinones from the Brazilian medicinal plant Tabebuia avellanedae. Bioorg Med Chem. 2009;17:6286–91. doi: 10.1016/j.bmc.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 51.Kouokam JC, Jahns T, Becker H. Antimicrobial activity of the essential oil and some isolated sulfur-rich compounds from Scorodophloeus zenkeri. Planta Med. 2002;68:1082–7. doi: 10.1055/s-2002-36341. [DOI] [PubMed] [Google Scholar]
- 52.Mbosso Teinkela JE, Assob Nguedia JC, Meyer F, Vouffo Donfack E, Lenta Ndjakou B, Ngouela S, et al. In vitro antimicrobial and anti-proliferative activities of plant extracts from Spathodea campanulata, Ficus bubu and Carica papaya. Pharm Biol. 2016;54:1086–95. doi: 10.3109/13880209.2015.1103273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giordani R, Siepaio M, Moulin-Traffort J, Régli P. Antifungal action of Carica papaya latex: Isolation of fungal cell wall hydrolysing enzymes. Mycoses. 1991;34:469–77. doi: 10.1111/j.1439-0507.1991.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 54.Teodoro GR, Brighenti FL, Delbem AC, Delbem ÁC, Khouri S, Gontijo AV, et al. Antifungal activity of extracts and isolated compounds from Buchenavia tomentosa on Candida albicans and non-albicans. Future Microbiol. 2015;10:917–27. doi: 10.2217/fmb.15.20. [DOI] [PubMed] [Google Scholar]
- 55.Masoko P, Picard J, Howard RL, Mampuru LJ, Eloff JN. In vivo antifungal effect of Combretum and Terminalia species extracts on cutaneous wound healing in immunosuppressed rats. Pharm Biol. 2010;48:621–32. doi: 10.3109/13880200903229080. [DOI] [PubMed] [Google Scholar]
- 56.Masoko P, Mdee LK, Mampuru LJ, Eloff JN. Biological activity of two related triterpenes isolated from Combretum nelsonii (Combretaceae) leaves. Nat Prod Res. 2008;22:1074–84. doi: 10.1080/14786410802267494. [DOI] [PubMed] [Google Scholar]
- 57.Katerere DR, Gray AI, Nash RJ, Waigh RD. Antimicrobial activity of pentacyclic triterpenes isolated from African Combretaceae. Phytochemistry. 2003;63:81–8. doi: 10.1016/s0031-9422(02)00726-4. [DOI] [PubMed] [Google Scholar]
- 58.Dawe A, Pierre S, Tsala DE, Habtemariam S. Phytochemical constituents of Combretum Loefl (Combretaceae) Pharm Crop. 2013;4:38–59. [Google Scholar]
- 59.Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Christensen SB, Sittie A, et al. New anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J Nat Prod. 1997;60:739–42. doi: 10.1021/np970010m. [DOI] [PubMed] [Google Scholar]
- 60.Shai LJ, McGaw LJ, Eloff JN. Extracts of the leaves and twigs of the threatened tree Curtisia dentata (Cornaceae) are more active against Candida albicans and other microorganisms than the stem bark extract. S Afr J Bot. 2009;75:363–6. [Google Scholar]
- 61.Oyedemi SO, Oyedemi BO, Arowosegbe S, Afolayan AJ. Phytochemicals analysis and medicinal potentials of hydroalcoholic extract from Curtisia dentata (Burm.f) C.A. Sm stem bark. Int J Mol Sci. 2012;13:6189–203. doi: 10.3390/ijms13056189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dzoyem JP, Kechia FA, Kuete V, Pieme AC, Akak CM, Tangmouo JG, et al. Phytotoxic, antifungal activities and acute toxicity studies of the crude extract and compounds from Diospyros canaliculata. Nat Prod Res. 2011;25:741–9. doi: 10.1080/14786419.2010.531392. [DOI] [PubMed] [Google Scholar]
- 63.Dzoyem JP, Tangmouo JG, Lontsi D, Etoa FX, Lohoue PJ. In vitro antifungal activity of extract and plumbagin from the stem bark of Diospyros crassiflora Hiern (Ebenaceae) Phytother Res. 2007;21:671–4. doi: 10.1002/ptr.2140. [DOI] [PubMed] [Google Scholar]
- 64.Parsaeimehr A, Sargsyan E, Javidnia K. A comparative study of the antibacterial, antifungal and antioxidant activity and total content of phenolic compounds of cell cultures and wild plants of three endemic species of Ephedra. Molecules. 2010;15:1668–78. doi: 10.3390/molecules15031668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Araújo MG, Hilário F, Nogueira LG, Vilegas W, Santos LC, Bauab TM. Chemical constituents of the methanolic extract of leaves of Leiothrix spiralis Ruhland and their antimicrobial activity. Molecules. 2011;16:10479–90. doi: 10.3390/molecules161210479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajeh MA, Zuraini Z, Sasidharan S, Latha LY, Amutha S. Assessment of Euphorbia hirta L. leaf, flower, stem and root extracts for their antibacterial and antifungal activity and brine shrimp lethality. Molecules. 2010;15:6008. doi: 10.3390/molecules15096008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alviano WS, Mendonça-Filho RR, Alviano DS, Bizzo HR, Souto-Padrón T, Rodrigues ML, et al. Antimicrobial activity of Croton cajucara Benth linalool-rich essential oil on artificial biofilms and planktonic microorganisms. Oral Microbiol Immunol. 2005;20:101–5. doi: 10.1111/j.1399-302X.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 68.Jain R, Jindal KC, Singh S, Datta A. Inventors: Pharmaceutical compositions comprising an extract of Euphorbia prostrata. New Delhi, India: Assignee: Panacea Biotec Limited; 2008. [Google Scholar]
- 69.Achi O. Composition and antibacterial activities of Tetrapleura tetraptera Taub. pod extracts. Res J Microbiol. 2010;5:1138–44. [Google Scholar]
- 70.das Neves MV, da Silva TM, Lima Ede O, da Cunha EV, Oliveira Ede J. Isoflavone formononetin from red propolis acts as a fungicide against Candida sp. Braz J Microbiol. 2016;47:159–66. doi: 10.1016/j.bjm.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joycharat N, Boonma C, Thammavong S, Yingyongnarongkul BE, Limsuwan S, Voravuthikunchai SP, et al. Chemical constituents and biological activities of Albizia myriophylla wood. Pharm Biol. 2016;54:62–73. doi: 10.3109/13880209.2015.1014920. [DOI] [PubMed] [Google Scholar]
- 72.Favre-Godal Q, Dorsaz S, Queiroz EF, Marcourt L, Ebrahimi SN, Allard PM, et al. Anti-Candida cassane-type diterpenoids from the root bark of Swartzia simplex. J Nat Prod. 2015;78:2994–3004. doi: 10.1021/acs.jnatprod.5b00744. [DOI] [PubMed] [Google Scholar]
- 73.Jothy SL, Zakariah Z, Chen Y, Sasidharan S. In vitro, in situ and in vivo studies on the anticandidal activity of Cassia fistula seed extract. Molecules. 2012;17:6997–7009. doi: 10.3390/molecules17066997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lachumy SJ, Zuraini Z, Sasidharan S. Antimicrobial activity and toxicity of methanol extract of Cassia fistula seeds. Res J Pharm Biol Chem Sci. 2010;1:391–8. [Google Scholar]
- 75.Martins N, Ferreira IC, Henriques M, Silva S. In vitro anti-Candida activity of Glycyrrhiza glabra L. Ind Crops Prod. 2016;83:81–5. [Google Scholar]
- 76.Timothy S, Wazis C, Adati R, Maspalma I. Antifungal activity of aqueous and ethanolic leaf extracts of Cassia Alata Linn. JAPS. 2012;2:1–3. [Google Scholar]
- 77.Wang J, Lou J, Luo C, Zhou L, Wang M, Wang L. Phenolic compounds from Halimodendron halodendron (Pall.). voss and their antimicrobial and antioxidant activities. Int J Mol Sci. 2012;13:11349–64. doi: 10.3390/ijms130911349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siler B, Zivkovic S, Banjanac T, Cvetkovic J, Nestorovic Živkovic J, Ciric A, et al. Centauries as underestimated food additives: Antioxidant and antimicrobial potential. Food Chem. 2014;147:367–76. doi: 10.1016/j.foodchem.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 79.Giongo JL, de Almeida Vaucher R, Fausto VP, Quatrin PM, Lopes LQ, Santos RC, et al. Anti-Candida activity assessment of Pelargonium graveolens oil free and nanoemulsion in biofilm formation in hospital medical supplies. Microb Pathog. 2016;100:170–8. doi: 10.1016/j.micpath.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 80.Krisch J, Ördögh L, Galgóczy L, Papp T, Vágvölgyi C. Anticandidal effect of berry juices and extracts from Ribes species. Open Life Sci. 2009;4:86–9. [Google Scholar]
- 81.Noumi E, Snoussi M, Hajlaoui H, Valentin E, Bakhrouf A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur J Clin Microbiol Infect Dis. 2010;29:81–8. doi: 10.1007/s10096-009-0824-3. [DOI] [PubMed] [Google Scholar]
- 82.Lu Y, Foo LY. Polyphenolics of Salvia – A review. Phytochemistry. 2002;59:117–40. doi: 10.1016/s0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 83.Martins N, Barros L, Santos-Buelga C, Henriques M, Silva S, Ferreira IC. Evaluation of bioactive properties and phenolic compounds in different extracts prepared from Salvia officinalis L. Food Chem. 2015;170:378–85. doi: 10.1016/j.foodchem.2014.08.096. [DOI] [PubMed] [Google Scholar]
- 84.Pina-Vaz C, Gonçalves Rodrigues A, Pinto E, Costa-de-Oliveira S, Tavares C, Salgueiro L, et al. Antifungal activity of Thymus oils and their major compounds. J Eur Acad Dermatol Venereol. 2004;18:73–8. doi: 10.1111/j.1468-3083.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 85.Naeini A, Khosravi AR, Chitsaz M, Shokri H, Kamlnejad M. Anti-Candida albicans activity of some Iranian plants used in traditional medicine. J Med Mycol. 2009;19:168–72. [Google Scholar]
- 86.Pinto E, Gonçalves MJ, Hrimpeng K, Pinto J, Vaz S, Vale-Silva LA, et al. Antifungal activity of the essential oil of Thymus villosus subsp. lusitanicus against Candida, Cryptococcus, Aspergillus and dermatophyte species. Ind Crop Prod. 2013;51:93–9. [Google Scholar]
- 87.Teixeira B, Marques A, Ramos C, Serrano C, Matos O, Neng NR, et al. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J Sci Food Agric. 2013;93:2707–14. doi: 10.1002/jsfa.6089. [DOI] [PubMed] [Google Scholar]
- 88.Ličina BZ, Stefanović OD, Vasić SM, Radojević ID, Dekić MS, Čomić LR. Biological activities of the extracts from wild growing Origanum vulgare L. Food Control. 2013;33:498–504. [Google Scholar]
- 89.Palmeira-de-Oliveira A, Gaspar C, Palmeira-de-Oliveira R, Silva-Dias A, Salgueiro L, Cavaleiro C, et al. The anti-Candida activity of Thymbra capitata essential oil: Effect upon pre-formed biofilm. J Ethnopharmacol. 2012;140:379–83. doi: 10.1016/j.jep.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 90.Khan A, Ahmad A, Akhtar F, Yousuf S, Xess I, Khan LA, et al. Ocimum sanctum essential oil and its active principles exert their antifungal activity by disrupting ergosterol biosynthesis and membrane integrity. Res Microbiol. 2010;161:816–23. doi: 10.1016/j.resmic.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 91.Cavalcanti YW, Almeida LD, Padilha WW. Anti-adherent activity of Rosmarinus officinalis essential oil on Candida albicans: An SEM analysis. Rev Odonto Ciênc. 2011;26:139–44. [Google Scholar]
- 92.Ozcan MM, Chalchat JC. Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.). oil from Turkey. Int J Food Sci Nutr. 2008;59:691–8. doi: 10.1080/09637480701777944. [DOI] [PubMed] [Google Scholar]
- 93.Gazim ZC, Amorim AC, Hovell AM, Rezende CM, Nascimento IA, Ferreira GA, et al. Seasonal variation, chemical composition, and analgesic and antimicrobial activities of the essential oil from leaves of Tetradenia riparia (Hochst.). Codd in Southern Brazil. Molecules. 2010;15:5509–24. doi: 10.3390/molecules15085509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Džamić AM, Soković MD, Novaković M, Jadranin M, Ristić MS, Tešević V, et al. Composition, antifungal and antioxidant properties of Hyssopus officinalis L. subsp. pilifer (Pant.) Murb. essential oil and deodorized extracts. Ind Crop Prod. 2013;51:401–7. [Google Scholar]
- 95.Li YC, Liang HC, Chen HM, Tan LR, Yi YY, Qin Z, et al. Anti-Candida albicans activity and pharmacokinetics of pogostone isolated from Pogostemonis Herba. Phytomedicine. 2012;20:77–83. doi: 10.1016/j.phymed.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 96.Ünver A, Arslan D, Çetýnkaya Z, Özcan MM. Antimycotic activity of methanol extracts of sage (Salvia officinalis L.), laurel (Laurus nobilis L.) and thyme (Thymbra spicata L.) J Essent Oil Bear Pl. 2008;11(1):90–5. [Google Scholar]
- 97.Wong KS, Tsang WK. In vitro antifungal activity of the aqueous extract of Scutellaria baicalensis Georgi root against Candida albicans. Int J Antimicrob Agents. 2009;34:284–5. doi: 10.1016/j.ijantimicag.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 98.Modaressi M, Shahsavari R, Ahmadi F, Rahimi-Nasrabadi M, Abiri R, Mikaeli A, et al. The evaluation of antibacterial, antifungal and antioxidant activity of methanolic extract of Mindium laevigatum (vent.). rech. F. from central part of Iran. Jundishapur J Nat Pharm Prod. 2013;8:34–40. [PMC free article] [PubMed] [Google Scholar]
- 99.Ren B, Xia B, Li W, Wu J, Zhang H. Two novel phenolic compounds from Stenoloma chusanum and their antifungal activity. Chem Nat Compd. 2009;45:182–6. [Google Scholar]
- 100.Unlu M, Ergene E, Unlu GV, Zeytinoglu HS, Vural N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae) Food Chem Toxicol. 2010;48:3274–80. doi: 10.1016/j.fct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 101.Oro D, Heissler A, Rossi EM, Scapin D, da Silva Malheiros P, Boff E. Antifungal activity of natural compounds against Candida species isolated from HIV-positive patients. Asian Pac J Trop Biomed. 2015;5:781–4. [Google Scholar]
- 102.Anibal PC, Peixoto IT, Foglio MA, Höfling JF. Antifungal activity of the ethanolic extracts of Punica granatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Braz J Microbiol. 2013;44:839–48. doi: 10.1590/S1517-83822013005000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Endo EH, Cortez DA, Ueda-Nakamura T, Nakamura CV, Dias Filho BP. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res Microbiol. 2010;161:534–40. doi: 10.1016/j.resmic.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 104.Saad S, Taher M, Susanti D, Qaralleh H, Awang AF. In vitro antimicrobial activity of mangrove plant Sonneratia alba. Asian Pac J Trop Biomed. 2012;2:427–9. doi: 10.1016/S2221-1691(12)60069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alshami I, Alharbi AE. Hibiscus sabdariffa extract inhibits in vitro biofilm formation capacity of Candida albicans isolated from recurrent urinary tract infections. Asian Pac J Trop Biomed. 2014;4:104–8. doi: 10.1016/S2221-1691(14)60217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang H, Gao A, Li F, Zhang G, Ho HI, Liao W. Mechanism of action of tetrandrine, a natural inhibitor of Candida albicans drug efflux pumps. Yakugaku Zasshi. 2009;129:623–30. doi: 10.1248/yakushi.129.623. [DOI] [PubMed] [Google Scholar]
- 107.Ngameni B, Kuete V, Simo IK, Mbaveng AT, Awoussong PK, Patnam R, et al. Antibacterial and antifungal activities of the crude extract and compounds from Dorstenia turbinata (Moraceae) S Afr J Bot. 2009;75:256–61. [Google Scholar]
- 108.Jean ET, Louis MK, Jules CA, Franck M, Diane CS, Bruno NL, et al. In vitro evaluation of antimicrobial and antiproliferative activities for compounds isolated from the Ficus bubu Warb. (Moraceae) fruits: Chemotaxonomic significance. Drug Deliv Lett. 2015;5:122–31. [Google Scholar]
- 109.Yessoufou K, Elansary HO, Mahmoud EA, Skalicka-Woźniak K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind Crop Prod. 2015;74:752–8. [Google Scholar]
- 110.Bidarigh S, Khoshkholgh PM, Massiha A, Issazadeh K. In vitro anti-Candida activity of Ficus lyrata L. ethyl acetate latex extract and nystatin on clinical isolates and standard strains of Candida albicans Int Conf Biotechnol Environ Manage. 2011;18:115–9. [Google Scholar]
- 111.Safaei-Ghomi J, Ahd AA. Antimicrobial and antifungal properties of the essential oil and methanol extracts of Eucalyptus largiflorens and Eucalyptus intertexta. Pharmacogn Mag. 2010;6:172–5. doi: 10.4103/0973-1296.66930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tyagi AK, Malik A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 2011;126:228–35. [Google Scholar]
- 113.Prabhakar K, Kumar LS, Rajendran S, Chandrasekaran M, Bhaskar K, Sajit Khan AK. Antifungal activity of plant extracts against Candida species from oral lesions. Indian J Pharm Sci. 2008;70:801–3. doi: 10.4103/0250-474X.49128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hammer KA, Carson CF, Riley TV. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J Antimicrob Chemother. 2004;53:1081–5. doi: 10.1093/jac/dkh243. [DOI] [PubMed] [Google Scholar]
- 115.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (Tea Tree) oil: A review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hammer KA, Carson CF, Riley TV. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J Appl Microbiol. 2003;95:853–60. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 117.Oliveira GF, Furtado NA, Silva Filho AA, Martins CH, Bastos JK, Cunha WR, et al. Antimicrobial activity of Syzygium cumini (Myrtaceae) leaves extract. Braz J Microbiol. 2007;38:381–4. [Google Scholar]
- 118.Ashour HM. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol Ther. 2008;7:399–403. doi: 10.4161/cbt.7.3.5367. [DOI] [PubMed] [Google Scholar]
- 119.Nenaah G. Antibacterial and antifungal activities of (beta)-carboline alkaloids of Peganum harmala (L) seeds and their combination effects. Fitoterapia. 2010;81:779–82. doi: 10.1016/j.fitote.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 120.Diba K, Geramishoar M, Sharbatkhori M, Hosseinpur L. Antifungal activity of alcoholic extract of Peganum harmala, in vitro. Urmia Med J. 2010;20:271–7. [Google Scholar]
- 121.Ayandele AA, Adebiyi AO. The phytochemical analysis and antimicrobial screening of extracts of Olax subscorpioïdea. Afr J Biotech. 2007;6:868–70. [Google Scholar]
- 122.Picerno P, Mencherini T, Sansone F, Del Gaudio P, Granata I, Porta A, et al. Screening of a polar extract of Paeonia rockii: Composition and antioxidant and antifungal activities. J Ethnopharmacol. 2011;138:705–12. doi: 10.1016/j.jep.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 123.Sharma H, Yunus GY, Agrawal R, Kalra M, Verma S, Bhattar S. Antifungal efficacy of three medicinal plants Glycyrrhiza glabra, Ficus religiosa and Plantago major against oral Candida albicans Acomparative analysis. Indian J Dent Res. 2016;27:433–6. doi: 10.4103/0970-9290.191895. [DOI] [PubMed] [Google Scholar]
- 124.de Paiva SR, Figueiredo MR, Aragão TV, Kaplan MA. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Mem Inst Oswaldo Cruz. 2003;98:959–61. doi: 10.1590/s0074-02762003000700017. [DOI] [PubMed] [Google Scholar]
- 125.Carlini EA, Duarte-Almeida JM, Tabach R. Assessment of the toxicity of the Brazilian pepper trees Schinus terebinthifolius raddi (Aroeira-da-praia) and Myracrodruon urundeuva allemão (Aroeira-do-sertão) Phytother Res. 2013;27:692–8. doi: 10.1002/ptr.4767. [DOI] [PubMed] [Google Scholar]
- 126.Tangarife-Castaño V, Correa-Royero JB, Roa-Linares VC, Pino-Benitez N, Betancur-Galvis LA, Durán DC, et al. Anti-dermatophyte, anti-Fusarium and cytotoxic activity of essential oils and plant extracts of Piper genus. J Essent Oil Res. 2014;26:221–7. [Google Scholar]
- 127.Pessini GL, Dias Filho BP, Nakamura CV, Cortez DA. Antifungal activity of the extracts and neolignans from Piper regnellii (Miq.). C. DC. var. pallescens (C. DC.) Yunck. J Braz Chem Soc. 2005;16:1130–3. [Google Scholar]
- 128.Gbewonyo WS, Candy DJ. Chromatographic isolation of insecticidal amides from Piper guineense roots. J Chromatogr A. 1992;607:105–11. [Google Scholar]
- 129.Rajeswara Rao BR, Rajput DK, Patel RP. Essential oil profiles of different parts of palmarosa (Cymbopogon martinii (Roxb.). wats. var. motia burk.) J Essent Oil Res. 2009;21:519–21. [Google Scholar]
- 130.Taweechaisupapong S, Aieamsaard J, Chitropas P, Khunkitti W. Inhibitory effect of lemongrass oil and its major constituents on Candida biofilm and germ tube formation. S Afr J Bot. 2012;81:95–102. [Google Scholar]
- 131.Gbenou JD, Ahounou JF, Akakpo HB, Laleye A, Yayi E, Gbaguidi F, et al. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol Biol Rep. 2013;40:1127–34. doi: 10.1007/s11033-012-2155-1. [DOI] [PubMed] [Google Scholar]
- 132.Inderjit, Dakshini KM. Investigations on some aspects of chemical ecology of cogongrass Imperata cylindrica (L.) Beauv. J Chem Ecol. 1991;17:343–52. doi: 10.1007/BF00994337. [DOI] [PubMed] [Google Scholar]
- 133.Ibraheim ZZ, Ahmed AS, Gouda YG. Phytochemical and biological studies of Adiantum capillus-veneris L. Saudi Pharm J. 2011;19:65–74. doi: 10.1016/j.jsps.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ishaq MS, Hussain MM, Afridi MS, Ali G, Khattak M, Ahmad S, et al. In vitro phytochemical, antibacterial, and antifungal activities of leaf, stem, and root extracts of Adiantum capillus veneris. ScientificWorldJournal. 2014;2014:269793. doi: 10.1155/2014/269793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bita A, Rosu AF, Calina D, Rosu L, Zlatian O, Dindere C, et al. An alternative treatment for Candida infections with Nigella sativa extracts. Eur J Hosp Pharm Sci Pract. 2012;19:162–3. [Google Scholar]
- 136.García-Sosa K, Villarreal-Alvarez N, Lübben P, Peña-Rodríguez LM. Chrysophanol, an antimicrobial anthraquinone from the root extract of Colubrina greggii. J Mex Chem Soc. 2006;50:76–8. [Google Scholar]
- 137.Jainkittivong A, Butsarakamruha T, Langlais RP. Antifungal activity of Morinda citrifolia fruit extract against Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:394–8. doi: 10.1016/j.tripleo.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 138.Toure A, Bahi C, Ouattara K, Djama JA, Coulibaly A. Phytochemical screening and in vitro antifungal activities of extracts of leaves of Morinda morindoides (Morinda, Rubiaceae) J Med Plant Res. 2011;5:6780–6. [Google Scholar]
- 139.Tsuzuki JK, Svidzinski TI, Shinobu CS, Silva LF, Rodrigues-Filho E, Cortez DA, et al. Antifungal activity of the extracts and saponins from Sapindus saponaria L. An Acad Bras Cienc. 2007;79:577–83. doi: 10.1590/s0001-37652007000400002. [DOI] [PubMed] [Google Scholar]
- 140.Basile A, Rigano D, Conte B, Bruno M, Rosselli S, Sorbo S. Antibacterial and antifungal activities of acetonic extract from Paullinia cupana Mart. seeds. Nat Prod Res. 2013;27:2084–90. doi: 10.1080/14786419.2013.784868. [DOI] [PubMed] [Google Scholar]
- 141.Nordin MA, Wan Harun WH, Abdul Razak F. Antifungal susceptibility and growth inhibitory response of oral Candida species to Brucea javanica Linn. extract. BMC Complement Altern Med. 2013;13:342. doi: 10.1186/1472-6882-13-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Das J, Lahan JP, Srivastava RB. Solanum melongena: A potential source of antifungal agent. Indian J Microbiol. 2010;50(Suppl 1):62–9. doi: 10.1007/s12088-010-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee DG, Park Y, Kim MR, Jung HJ, Seu YB, Hahm KS, et al. Anti-fungal effects of phenolic amides isolated from the root bark of Lycium chinense. Biotechnol Lett. 2004;26:1125–30. doi: 10.1023/B:BILE.0000035483.85790.f7. [DOI] [PubMed] [Google Scholar]
- 144.Sitheeque MA, Panagoda GJ, Yau J, Amarakoon AM, Udagama UR, Samaranayake LP. Antifungal activity of black tea polyphenols (catechins and theaflavins) against Candida species. Chemotherapy. 2009;55:189–96. doi: 10.1159/000216836. [DOI] [PubMed] [Google Scholar]
- 145.Salgueiro LR, Cavaleiro C, Gonçalves MJ, Proença da Cunha A. Antimicrobial activity and chemical composition of the essential oil of Lippia graveolens from Guatemala. Planta Med. 2003;69:80–3. doi: 10.1055/s-2003-37032. [DOI] [PubMed] [Google Scholar]
- 146.Oliveira DR, Leitão GG, Bizzo HR, Lopes Ds, Alviano DS, Alviano CS, et al. Chemical and antimicrobial analyses of essential oil of Lippia origanoides H.B.K. Food Chem. 2007;101:236–40. [Google Scholar]
- 147.Neelofar K, Shreaz S, Rimple B, Muralidhar S, Nikhat M, Khan LA. Curcumin as a promising anticandidal of clinical interest. Can J Microbiol. 2011;57:204–10. doi: 10.1139/W10-117. [DOI] [PubMed] [Google Scholar]
- 148.Ghasemzadeh A, Jaafar HZ, Rahmat A. Identification and concentration of some flavonoid components in Malaysian young ginger (Zingiber officinale Roscoe) varieties by a high performance liquid chromatography method. Molecules. 2010;15:6231–43. doi: 10.3390/molecules15096231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Marioni J, da Silva MA, Cabrera JL, Montoya SC, Paraje MG. The anthraquinones rubiadin and its 1-methyl ether isolated from Heterophyllaea pustulata reduces Candida tropicalis biofilms formation. Phytomedicine. 2016;23:1321–8. doi: 10.1016/j.phymed.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 150.De Vita D, Simonetti G, Pandolfi F, Costi R, Di Santo R, D’Auria FD, et al. Exploring the anti-biofilm activity of cinnamic acid derivatives in Candida albicans. Bioorg Med Chem Lett. 2016;26:5931–5. doi: 10.1016/j.bmcl.2016.10.091. [DOI] [PubMed] [Google Scholar]
- 151.Munin E, Giroldo LM, Alves LP, Costa MS. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT) J Photochem Photobiol B. 2007;88:16–20. doi: 10.1016/j.jphotobiol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 152.Dalleau S, Cateau E, Bergès T, Berjeaud JM, Imbert C. In vitro activity of terpenes against Candida biofilms. Int J Antimicrob Agents. 2008;31:572–6. doi: 10.1016/j.ijantimicag.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 153.Cutler JE. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991;45:187–218. doi: 10.1146/annurev.mi.45.100191.001155. [DOI] [PubMed] [Google Scholar]
- 154.Sikkema J, de Bont JA, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–22. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kurtz MB, Douglas CM. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35:79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 156.Inouye S, Watanabe M, Nishiyama Y, Takeo K, Akao M, Yamaguchi H. Antisporulating and respiration-inhibitory effects of essential oils on filamentous fungi. Mycoses. 1998;41:403–10. doi: 10.1111/j.1439-0507.1998.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 157.Bard M, Albrecht MR, Gupta N, Guynn CJ, Stillwell W. Geraniol interferes with membrane functions in strains of Candida and Saccharomyces. Lipids. 1988;23:534–8. doi: 10.1007/BF02535593. [DOI] [PubMed] [Google Scholar]
- 158.Thevissen K, Warnecke DC, François IE, Leipelt M, Heinz E, Ott C, et al. Defensins from insects and plants interact with fungal glucosylceramides. J Biol Chem. 2004;279:3900–5. doi: 10.1074/jbc.M311165200. [DOI] [PubMed] [Google Scholar]
- 159.Leipelt M, Warnecke D, Zähringer U, Ott C, Müller F, Hube B, et al. Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. J Biol Chem. 2001;276:33621–9. doi: 10.1074/jbc.M104952200. [DOI] [PubMed] [Google Scholar]
- 160.Thevissen K, Ghazi A, De Samblanx GW, Brownlee C, Osborn RW, Broekaert WF. Fungal membrane responses induced by plant defensins and thionins. J Biol Chem. 1996;271:15018–25. doi: 10.1074/jbc.271.25.15018. [DOI] [PubMed] [Google Scholar]
- 161.Aerts AM, Carmona-Gutierrez D, Lefevre S, Govaert G, François IE, Madeo F, et al. The antifungal plant defensin RsAFP2 from radish induces apoptosis in a metacaspase independent way in Candida, albicans. FEBS Lett. 2009;583:2513–6. doi: 10.1016/j.febslet.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 162.Sharma Y, Khan LA, Manzoor N. Anti-Candida activity of geraniol involves disruption of cell membrane integrity and function. J Mycol Med. 2016;26:244–54. doi: 10.1016/j.mycmed.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 163.Bajpai VK, Park YH, Kang SC. A diterpenoid taxodone from Metasequoia glyptostroboides with antimycotic potential against clinical isolates of Candida species. J Mycol Med. 2015;25:e31–8. doi: 10.1016/j.mycmed.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 164.Batandier C, Fontaine E, Kériel C, Leverve XM. Determination of mitochondrial reactive oxygen species: Methodological aspects. J Cell Mol Med. 2002;6:175–87. doi: 10.1111/j.1582-4934.2002.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Aerts AM, Bammens L, Govaert G, Carmona-Gutierrez D, Madeo F, Cammue BP, et al. The antifungal plant defensin HsAFP1 from Heuchera sanguinea induces apoptosis in Candida albicans. Front Microbiol. 2011;2:47. doi: 10.3389/fmicb.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen Y, Zeng H, Tian J, Ban X, Ma B, Wang Y. Antifungal mechanism of essential oil from Anethum graveolens seeds against Candida albicans. J Med Microbiol. 2013;62(Pt 8):1175–83. doi: 10.1099/jmm.0.055467-0. [DOI] [PubMed] [Google Scholar]
- 167.Hwang IS, Lee J, Jin HG, Woo ER, Lee DG. Amentoflavone stimulates mitochondrial dysfunction and induces apoptotic cell death in Candida albicans. Mycopathologia. 2012;173:207–18. doi: 10.1007/s11046-011-9503-x. [DOI] [PubMed] [Google Scholar]
- 168.Choi H, Lee DG. Lycopene induces apoptosis in Candida albicans through reactive oxygen species production and mitochondrial dysfunction. Biochimie. 2015;115:108–15. doi: 10.1016/j.biochi.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 169.Barbieri DS, Tonial F, Lopez PV, Sales Maia BH, Santos GD, Ribas MO, et al. Antiadherent activity of Schinus terebinthifolius and Croton urucurana extracts on in vitro biofilm formation of Candida albicans and Streptococcus mutans. Arch Oral Biol. 2014;59:887–96. doi: 10.1016/j.archoralbio.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 170.Serpa R, França EJ, Furlaneto-Maia L, Andrade CG, Diniz A, Furlaneto MC. In vitro antifungal activity of the flavonoid baicalein against Candida species. J Med Microbiol. 2012;61(Pt 12):1704–8. doi: 10.1099/jmm.0.047852-0. [DOI] [PubMed] [Google Scholar]
- 171.Yun DG, Lee DG. Silibinin triggers yeast apoptosis related to mitochondrial Ca2+ influx in Candida albicans. Int J Biochem Cell Biol. 2016;80:1–9. doi: 10.1016/j.biocel.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 172.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–9. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 173.Davis SR. An overview of the antifungal properties of allicin and its breakdown products – The possibility of a safe and effective antifungal prophylactic. Mycoses. 2005;48:95–100. doi: 10.1111/j.1439-0507.2004.01076.x. [DOI] [PubMed] [Google Scholar]
- 174.Souza-Moreira TM, Severi JA, Lee K, Preechasuth K, Santos E, Gow NA, et al. Anti-Candida targets and cytotoxicity of casuarinin isolated from Plinia cauliflora leaves in a bioactivity-guided study. Molecules. 2013;18:8095–108. doi: 10.3390/molecules18078095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tay LY, Jorge JH, Herrera DR, Campanha NH, Gomes BP, Andre Dos Santos F. Evaluation of different treatment methods against denture stomatitis: A randomized clinical study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:72–7. doi: 10.1016/j.oooo.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 176.Tangarife-Castaño V, Correa-Royero J, Zapata-Londoño B, Durán C, Stanshenko E, Mesa-Arango AC. Anti-Candida albicans activity, cytotoxicity and interaction with antifungal drugs of essential oils and extracts from aromatic and medicinal plants. Infectio. 2011;15:160–7. [Google Scholar]
- 177.González Torres Y, Scull Campos I, Bada Barro AM, González Navarro B, Fuentes Morales D, Arteaga Pérez ME, et al. Ensayo de toxicidad a dosis repetidas del extracto acuoso de Morinda royoc L. en ratas Cenp: SPRD. Rev Cubana Plant Med. 2003;8 [Epub ahead of print] [Google Scholar]
- 178.Herrera-Arellano A, López-Villegas EO, Rodríguez-Tovar AV, Zamilpa A, Jiménez-Ferrer E, Tortoriello J, et al. Use of antifungal saponin SC-2 of Solanum chrysotrichum for the treatment of vulvovaginal candidiasis In vitro studies and clinical experiences. Afr J Tradit Complement Altern Med. 2013;10:410–7. [PMC free article] [PubMed] [Google Scholar]
- 179.Ebrahimy F, Dolatian M, Moatar F, Majd HA. Comparison of the therapeutic effects of Garcin(®) and fluconazole on Candida vaginitis. Singapore Med J. 2015;56:567–72. doi: 10.11622/smedj.2015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Irshad Shreaz S, Manzoor N, Khan LA, Rizvi MM. Anticandidal activity of Cassia fistula and its effect on ergosterol biosynthesis. Pharm Biol. 2011;49:727–33. doi: 10.3109/13880209.2010.544318. [DOI] [PubMed] [Google Scholar]
- 181.Andres P, Chantalat L, Lin YK. Treatment of Candidiasis with Indigo Naturalis or Indigo-Producing Plant Extract. Google Patents. 2016 [Google Scholar]
- 182.Chauhan VS, Salkar KS. Oral Herbal Composition for the Treatment of Oral Candidiasis. Google Patents. 2013 [Google Scholar]
- 183.Meuwly P, Abbet C, Schnyder B, Denis JM, Simonnet X. Anti-Candida Compositions and Uses Thereof. Google Patents. 2015 [Google Scholar]
- 184.Duarte MC, Figueira GM, Sartoratto A, Rehder VL, Delarmelina C. Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol. 2005;97:305–11. doi: 10.1016/j.jep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 185.Bonifácio BV, Ramos MA, da Silva PB, Negri KM, de Oliveira Lopes É, de Souza LP, et al. Nanostructured lipid system as a strategy to improve the anti-Candida albicans activity of Astronium sp. Int J Nanomedicine. 2015;10:5081–92. doi: 10.2147/IJN.S79684. [DOI] [PMC free article] [PubMed] [Google Scholar]



