Abstract
The emergence and rapid development of seriously drug-resistant pathogens have created the greatest danger to public health and made the treatment of infectious diseases ineffective; to control the antibiotic-resistant microbes, the discovery of new effective antibacterials with new mechanisms of action against bacteria remains an urgent task to control the bacterial resistance. The paucity of infections in wild plants supports the role of innate defense system of plants. Many researchers nominate the natural extracts to act against bacterial resistance mechanisms, and the majority of them have now been focused on the combination of plant extracts and antibiotics to define the availability of resistance modification agents. Only very few numbers of natural products are successful to reach experiments circle beyond the in vitro assays. Phenols and phenolic acids could serve as good candidates to the natural antibacterial arsenal. The pyrogallol-based compounds are more potent than others such as catechol or resorcinol, gallic acid, and the hydroxycinnamic acid (ferulic acid) are destructing the bacterial cell wall of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, leading to leakage of cellular contents. These compounds have stronger activity against Gram-positive microorganisms, and some of them showed good synergism with antibiotics, for example, pentagalloylglucopyranose, is shown a synergism with penicillin G against methicillin-resistant S. aureus, another example is the interesting synergism between epicatechin gallate and oxacillin where the minimal inhibitory concentrations of oxacillin reduced around 500 times by the addition of epicatechin gallate to the antibiotic.
Keywords: Antibacterial, Escherichia coli, gallic acid, Staphylococcus aureus
INTRODUCTION
The discovery of penicillin was the start of antibiotics era which had a big victory to defeat pathogens for over 70 years; however, the emergence and rapid development of seriously drug-resistant pathogens have created the greatest danger to public health and made the treatment of infectious diseases ineffective.[1]
Bacteria can resist antibiotic/s by several means: (1) the modification of protein targets to antibiotics; (2) the production of enzymes which can degrade or modify the antibiotic structure rendering it unsuccessful in infections treatment; (3) the changes of cell wall permeability due to mutations; (4) the use of the pumping system to expel the antibiotics molecules.[2,3] Based on the various mechanisms of bacterial resistance listed above, resistance-modification agents (RMAs) which may address the resistance problem have been explored to modify the antibacterial resistances. The details of resistance modification mechanisms and RMAs were reviewed in detail by Abreu et al.[3] In this review, we tried to present the bacterial resistance problem in simple tailoring where the story has begun by presenting the resistance problem, mechanisms of resistance, and one of the solutions which is the possibility of using the phytomedicines in treatment protocols, specifically the in vitro assay of some phenols and phenolic acids, which are widely distributed in the plant kingdom. Searching for the credible information was accomplished using EBSCO as a searching engine to search for the most important in vitro experiments on phenols and phenolic acids during the last 20 years.
ANTIBACTERIAL RESISTANCE
The emergence of resistant strains to antibacterials was determined by the level of exposure of the pathogen to antibacterials, biochemical, and physiological effects of drugs on the microorganisms (pharmacodynamics) and the pharmacokinetic properties of the antibiotics.[4] The using of the antibacterials in a wrong dose and the general abuse of the antibacterials were the contributing factors of the emergence and dissemination of resistance.[5] The resistant microbes do not respond to the antibacterials due to either expression of resistance phenotype or inherent resistant genes.[6]
The multidrug resistance is more serious than the resistance to a single antibiotic. Examples are the methicillin-resistant Staphylococcus aureus (MRSA) and the toughest type of S. aureus which also resists vancomycin.[3] These kinds of bacteria cause around 25,000 dead cases and cost the European economy more than 1 billion euros.[2]
To control the antibiotic-resistant microbes, the discovery of new effective antibacterials with new mechanisms of action against bacteria remains an urgent task to control the bacterial resistance.[7] The WHO adopted several strategies to address the resistance problems such as using the antibiotics in the right dose and duration of treatment, enhancing healthy environment to control transmission of resistant strains, and supporting the research for finding new antibiotics.[8] The use of synergism between antibiotics is also an important strategy to control drug resistance by targeting more than one site of action and increases the bioavailability or the modification of resistance mechanisms.[9]
PLANTS AS SOURCES OF ANTIBACTERIALS
Plant extracts have been utilized from ancient times to treat various ailments, particularly in Asia. A major proportion of the world's population depends on traditional medicine for a healthy life.[10] In modern drug discovery and development processes, phytochemicals play a key role at the early stage of “lead” discovery, i.e. discovery of the bioactive (determined by various bioassays) natural molecule, which could be an ideal drug candidate itself or as a lead compound to develop for its structural analogs.[11,12]
Some of the phytochemicals are utilized by the plants themselves as a cover against many invaders, therefore playing a distinctive role as antibacterials.[13] The paucity of infections in wild plants supports the role of innate defense system of plants.[14] Many researchers nominate the natural extracts to act against bacterial resistance mechanisms, and the majority of them have now been focused on the combination of plant extracts and antibiotics to determine the existence and abundance of RMAs.[15,16]
Giant companies in pharmaceutical sector have invested a huge amount of money to synthesize chemical compounds without biological activities due to lack of special stereochemistry which natural products can offer; therefore, an increase in drug discovery from nature in the future will be expected.[17] Another important motive to discover natural products and produce alternatives to the limited or failed therapeutic protocols is the unlimited diversity of natural leads which are biologically active or ready for development and structure optimization strategies. In addition to that, the toxicity and low margin of safety of some antibacterials have promoted the interest in search for novel antibacterial products from other sources, particularly plant secondary metabolites (phytochemicals).[17]
There are different phytochemical approaches to follow for searching of natural antibacterials. The plant species are selected randomly and then their phytochemicals are categorized to different chemical groups. The second approach is the random selection of plant species followed by antibacterial assays; the third approach is to follow previous reports of researchers.
The most important approach is the follow-up of ethnomedical or traditional uses of plants against microbial infections.[18] Traditional medicines such as Ayurveda and Chinese medicine provide information about the medicinal use of plants. Information acquired from books, herbals, review articles, notes placed on voucher herbarium specimens, field work, and computer databases, such as NAPRALERT.[19]
The best way to find new antibacterial molecules with new mode of action is the following of an organized-bioguided extraction method. The starting of the bioguided extraction studies faced many challenges; one of those challenges is the authentication of the plant materials.[20] The authentication step is crucial for the following steps because inaccurate identification of the plant specimen leads definitely to false results. Another important challenge is the limited quantities of some active metabolites in the plant species, in addition to limitation of the collected plant quantity due to protection rights of some plants.[21]
The problem of shortage in medicinal plants is serious and must be put in the circle of care; therefore, the European Medicines Agency launched the controlled agricultural and collection code of practice to ensure a sustainable plants collection.[20] In addition to the previously mentioned challenges, only very few numbers of natural products are successful to reach experiments circle beyond the in vitro assays.
Drug discovery of compounds from natural sources is hindered by the complexity of the chemical structures of natural molecules which have complex stereochemistry and functional groups; sometimes, this makes their synthesis in laboratories, a tedious task compared to pharmaceutical compounds produced by medicinal chemistry;[22] however, the complex stereochemistry of natural compounds could be advantageous from another side of view.
Extraction of antibacterial natural products
To extract the phytochemicals, researchers can use open extraction system or closed (continuous) system like refluxing with Soxhlet or Clevenger apparatus. Extraction of phytochemicals is carried out using water or alcohol or other organic solvents such as hexane, methanol, or ethyl acetate under heat or at room temperature. Most of the antibacterials are extracted by an aqueous alcoholic solution which is later dried by rotary evaporator under vacuum.[23]
Antibacterial assay methods
The assays of antibacterials from plants are conducted with crude plant extract[24] and/or pure compounds.[25,26] Disk or agar well diffusion assay and broth micro- or macro-dilution assay are the most important assays used to evaluate the antibacterial activities.[27,28] The bioautographic method[29] is another way for the determination of antibacterial effects.
To detect the antibacterial action, the researchers can use the disk diffusion method, in which a paper disk contained the natural extract or pure compound is laid on top of an inoculated agar plate. The volume or quantity of the natural material deposited on the paper disks, the thickness of the agar layer, and whether a solvent is used can affect the results considerably between studies. In the agar well test, when many extracts are placed for assay or plenty of bacteria need to be tested, the material assayed for antibacterial activity is introduced into wells cut of agar plate.[30,31]
In broth dilution method, the antibacterial activity parameter such as the minimal inhibitory concentration (MIC) is quantified by detecting the optical density (OD) using an automated spectrophotometer or by counting the viable colonies left after incubating the inoculum in the existence of the antibacterial materials for a defined period of time. Although this method is accurate, it is a tedious process and requires a longer period of time than OD measurement. Resazurin (Alamar blue) can be used in microplate assay method as a highly sensitive indicator, accurately reflect the activity of the bacterial cells.[32] The Alamar blue assay method is sensitive enough to be compared with OD measurement and viable colonies counting, and it is more sensitive than the agar dilution method.[33]
The time-kill assay is used to define the time required to reach a bactericidal or bacteriostatic level. The time-kill kinetic graph is presented by plotting the number of viable cells remaining in the broth after addition of the natural extract versus time. The destruction of the bacterial cell by antibacterial agent and loss of cellular contents can be studied by scanning electron microscopy.[34,35]
IN VITRO ANTIBACTERIAL ACTIVITIES OF SOME PHENOLS AND PHENOLIC ACIDS
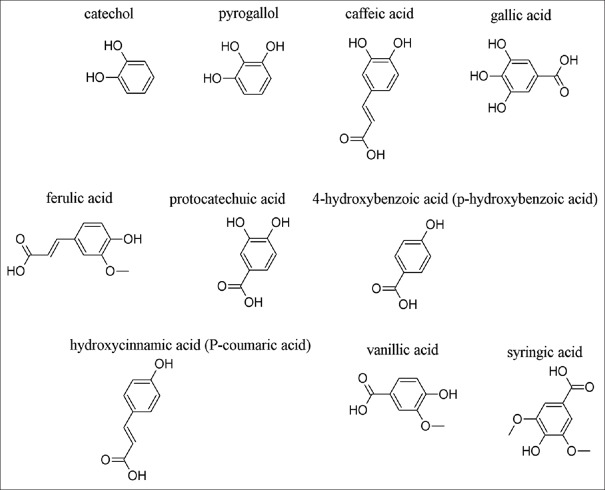
Phenols such as catechol and pyrogallol showed activity against some microorganisms. For example, pyrogallol showed MIC 2.4–2500 μg/ml and pyrocatechol MIC range: 4.9–312.5 μg/ml against several microorganisms causing periodontitis.[36] Taguri et al. recorded the antibacterial activities of 22 polyphenolic structures against several Gram-positive and Gram-negative bacteria; the results revealed that the pyrogallol-based compounds were more potent than others such as catechol or resorcinol.[37] There was evidence that increased hydroxylation of those phenols results in increased toxicity or antibacterial activities.[38] The mechanisms of action of simple phenols are probably proceed through interaction with sulfhydryl groups in microbial enzymes, leading to inhibition of those enzymes or due to nonspecific proteins interactions.[39] The structures of some phenols and phenolic acids are shown in Figure 1.
Figure 1.
Structures of some phenols and phenolic acids
Some phenolic acids such as caffeic acid have antibacterial activities. Caffeic acid showed MIC equal to 1600 μg/ml against S. aureus and Escherichia coli compared to ampicillin which showed MIC 0.1 and 3.2 μg/ml, respectively.[40] Gallic acid is a phenolic acid showing an effect against Enterococcus faecalis.[41] It also showed the greatest effect, among several phenolic compounds, against a group of bacterial strains including Pseudomonas aeruginosa, S. aureus, Moraxella catarrhalis, E. faecalis, Streptococcus agalactiae, and Streptococcus pneumonia.[42] Gallic acid showed good activity against Campylobacter and produced bactericidal activity against two Campylobacter coli strains with MIC equal to 61.5–125 μg/ml.[43]
In another study, the antibacterial activities of gallic acid and ferulic acid were detected against several bacterial isolates. Gallic acid showed MIC equal to 500 μg/ml against P. aeruginosa, 1500 μg/ml against E. coli, 1750 μg/ml against S. aureus, and 2000 μg/ml against Listeria monocytogenes; MIC of ferulic acid was 100 μg/ml against E. coli and P. aeruginosa and 1100 μg/ml and 1250 μg/ml against S. aureus and L. monocytogenes, respectively. Minimal bactericidal concentrations for both compounds against all microorganisms were 2500–5500 μg/ml.[44]
Gallic acid and ferulic acid affect the bacterial cell wall of S. aureus, E. coli, and P. aeruginosa, producing local damage and leakage of cellular materials.[44]
Wild polish mushrooms, which contain protocatechuic acid, 4-hydroxybenzoic acid, vanillic acid, syringic acid, caffeic acids, and p-coumaric and ferulic acids, showed intermediate antibacterial activities with MIC ranging from 156 to 5000 μg/ml, against a range of Gram-positive and Gram-negative bacteria with a stronger activity against Gram-positive microorganisms.[45]
Methyl gallate, a major metabolite from Galla rhois, an Asian plant collected in Korea, showed anti-Salmonella behavior against several Salmonella strains with MIC ranging from 3.9 to 125 μg/ml.[46] The in vivo therapeutic activity of methyl gallate was well proved by the absence of lethargy and liver problems, the two common consequences of Salmonella in treated mice.[46] Methyl gallate demonstrated good antibacterial effect against E. coli and Shigella flexneri with MIC of 30 μg/ml.[47]
Three Potentilla species were found to be rich in hyperoside, (+)-catechin, caffeic acid, ferulic acid, rutin, and ellagic acid, which were tested for their antibacterial activities. P. fruticosa had the highest effect against Gram-positive bacteria, Gram-negative bacterium (P. aeruginosa), and a fungus Candida albicans with MIC values of 0.78–6.25 mg/ml.[48]
Plant polyphenols interact with each other to improve the antibacterial actions.[49] Polyphenolic compounds present in a Cistus salviifolius extract showed synergism. Flavonoids in combination with ellagitannins in certain percentage showed inhibition against the growth of S. aureus.[49]
The combination of isoquercitrin (10 μg/ml) with gallic acid (10 μg/ml) was successful in inhibition the growth of S. aureus, while their MIC separately was 10 times more.[50] The ethyl acetate fraction from the ethanol extract of Searsia chirindensis was the most active antibacterial fraction, which contained methyl gallate, myricetin-3-O-arabinopyranoside, myricetin-3-O-rhamnoside, kaempferol-3-O-rhamnoside, and quercetin-3-O-arabinofuranoside. The MICs of these compounds ranged from 30 to 250 μg/ml against Campylobacter jejuni, E. coli, S. flexneri, and S. aureus.[47]
The seed kernels of Mangifera indica L. consisted of the following phenolic acids: gallic acid, methyl gallate, and pentagalloylglucopyranose. The extract inhibited the growth of MRSA, due to the major phenolic principle, pentagalloylglucopyranose, which showed a synergism with penicillin G against MRSA.[51]
Some phenolic compounds such as hydroquinone, thymol, carvacrol, butylated hydroxyanisole, and octyl gallate were assayed against S. aureus. As a result, octyl gallate had MIC equal to 21 μg/ml; hydroquinone had MIC 103 μg/ml, and carvacrol and thymol showed MICs of 413 μg/ml.[52]
Panduratin A, a natural chalcone compound extracted from Kaempferia pandurata Roxb, had potent activity against S. aureus with MIC equal to 0.06–2 μg/ml. An interesting study of synergism between phenolic materials and penicillins demonstrated the lowering MIC of oxacillin around 500 times by epicatechin gallate.[53,54]
Acylphloroglucinols (Olympicins A–E) isolated from the aerial parts of the plant Hypericum olympicum L. cf. uniflorum were evaluated as antibacterial agents against MRSA and multidrug-resistant S. aureus. Olympicin A had MIC of 0.5–1 μg/ml; others had MIC between 84 and 128 μg/ml against several S. aureus isolates.[55] Arzanol, a very strong anti-staphylococcal drug from Helichrysum italicum ssp. Microphyllum, had MIC equal to 1–4 μg/ml against different S. aureus strains.[56] Potent fraction isolated from Hypericum beanie contained two acylphloroglucinols, 1,5-dihydroxy-2-(2’-methylpropionyl)-3-methoxy-6-methylbenzene, and 1,5-dihydroxy-2-(2’-methylbutanoyl)-3-methoxy-6-methylbenzene. This mixture showed MIC equal to 16–32 μg/ml against multidrug-resistant S. aureus.[57]
Catechin gallate, epicatechin gallate, and epigallocatechin gallate showed β-lactamase and penicillin-binding protein 2a (PBP2a) inhibition to potentiate the antibiotic activity against S. aureus and MRSA.[58,59,60] Using epigallocatechin gallate (25 μg/ml) can restore MIC of imipenem to <4 μg/ml against around 18 MRSA isolates out of 24 isolates.[59] Epigallocatechin gallate (50 μg/ml) inhibited Tet (K) pump and increased intracellular concentration of tetracycline and ultimately its activity.[60] Epicatechin gallate decreased the MIC of norfloxacin 4 times against S. aureus with NorA.[61]
Ethyl gallate and other alky gallate caused cell wall disruption of the methicillin-sensitive S. aureus and MRSA and potentiated the antibacterial activities of β-lactam antibiotics, probably by inhibition of PBP2a.[62] Gallic acid showed synergism with sulfamethoxazole or tetracycline against several P. aeruginosa isolates[63] and with streptomycin against the same Gram-negative isolate[64] by disruptions of cell wall integrity.
CONCLUSION
Phenols and phenolic acids could play a positive role in the treatment of infections caused by resistant bacteria since they have the abilities to link with and disable some bacterial enzymes essential for bacterial cell wall synthesis in a way, which may modify the course of resistance of certain kinds of bacteria such as S. aureus. The combination of those phenolics with certain antibiotics is indeed better than claiming the use of those weak to moderate active antibacterial agents as a sole therapy; however, we must not forget that some local communities still use plant extracts containing phenols and phenolic acids for the treatment of some ailments such as bacterial infections, and the in vitro results of the antibacterial activities support those ethnomedical use.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Oluwatuyi M, Kaatz GW, Gibbons S. Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry. 2004;65:3249–54. doi: 10.1016/j.phytochem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13:42–51. doi: 10.1038/nrmicro3380. [DOI] [PubMed] [Google Scholar]
- 3.Abreu AC, McBain AJ, Simões M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat Prod Rep. 2012;29:1007–21. doi: 10.1039/c2np20035j. [DOI] [PubMed] [Google Scholar]
- 4.Andersson DI, Hughes D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat Rev Microbiol. 2010;8:260–71. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 5.Maisnier-Patin S, Andersson DI. Adaptation to the deleterious effects of antimicrobial drug resistance mutations by compensatory evolution. Res Microbiol. 2004;155:360–9. doi: 10.1016/j.resmic.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert P, Maira-Litran T, McBain AJ, Rickard AH, Whyte FW. The physiology and collective recalcitrance of microbial biofilm communities. Adv Microb Physiol. 2002;46:202–56. [PubMed] [Google Scholar]
- 7.Theuretzbacher U. Resistance drives antibacterial drug development. Curr Opin Pharmacol. 2011;11:433–8. doi: 10.1016/j.coph.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 8.WHO. European strategic action plan on antibiotic resistance. Baku, Azerbaijan: WHO; 2011. [Google Scholar]
- 9.Wagner H, Ulrich-Merzenich G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16:97–110. doi: 10.1016/j.phymed.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Prabuseenivasan S, Jayakumar M, Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement Altern Med. 2006;6:39. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarker SD. Chemistry for Pharmacy Students: General, Organic and Natural Product Chemistry. The Atrium, Southern Gate, Chichester, West Sussex PO19 8SQ, England: John Wiley & Sons Ltd; 2007. [Google Scholar]
- 12.Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simões M, Bennett RN, Rosa EA. Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep. 2009;26:746–57. doi: 10.1039/b821648g. [DOI] [PubMed] [Google Scholar]
- 14.Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15:639–52. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Chlez Z, Hohmann J, Molnar J. A source of complementary therapeutics. In: Chattopadhyay D, editor. Ethnomedicine. 1st ed. India: Research Signpost; 2010. pp. 179–201. [Google Scholar]
- 16.Sibanda T, Okoh AI. The challenges of overcoming antibiotic resistance: Plant extracts as potential sources of antimicrobial and resistance modifying agents. Afr J Biotechnol. 2007;6:2886–96. [Google Scholar]
- 17.Gibbons S. Phytochemicals for bacterial resistance – Strengths, weaknesses and opportunities. Planta Med. 2008;74:594–602. doi: 10.1055/s-2008-1074518. [DOI] [PubMed] [Google Scholar]
- 18.Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(Suppl 1):69–75. doi: 10.1289/ehp.01109s169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farnsworth N. Natural Products Alert (NAPRALERT) [Homepage of University of Illinois at Chicago, Chicago, U.S.A.] [Last accessed on 2016 Mar 09]. Available from: https://www.napralert.org/
- 20.Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, Linder T, Wawrosch C, Uhrin P, et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv. 2015;33:1582–614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David B, Wolfender J, Dias DA. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem Rev. 2015;14:299–315. [Google Scholar]
- 22.Henrich CJ, Beutler JA. Matching the power of high throughput screening to the chemical diversity of natural products. Nat Prod Rep. 2013;30:1284–98. doi: 10.1039/c3np70052f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor RS, Edel F, Manandhar NP, Towers GH. Antimicrobial activities of Southern Nepalese medicinal plants. J Ethnopharmacol. 1996;50:97–102. doi: 10.1016/0378-8741(95)01335-0. [DOI] [PubMed] [Google Scholar]
- 24.Silva O, Duarte A, Cabrita J, Pimentel M, Diniz A, Gomes E. Antimicrobial activity of Guinea-Bissau traditional remedies. J Ethnopharmacol. 1996;50:55–9. doi: 10.1016/0378-8741(95)01323-7. [DOI] [PubMed] [Google Scholar]
- 25.Afolayan AJ, Meyer JJ. The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J Ethnopharmacol. 1997;57:177–81. doi: 10.1016/s0378-8741(97)00065-2. [DOI] [PubMed] [Google Scholar]
- 26.Batista O, Duarte A, Nascimento J, Simões MF, de la Torre MC, Rodríguez B. Structure and antimicrobial activity of diterpenes from the roots of Plectranthus hereroensis. J Nat Prod. 1994;57:858–61. doi: 10.1021/np50108a031. [DOI] [PubMed] [Google Scholar]
- 27.Ayafor JF, Tchuendem MH, Nyasse B, Tillequin F, Anke H. Novel bioactive diterpenoids from Aframomum aulacocarpos. J Nat Prod. 1994;57:917–23. doi: 10.1021/np50109a007. [DOI] [PubMed] [Google Scholar]
- 28.Navarro V, Villarreal ML, Rojas G, Lozoya X. Antimicrobial evaluation of some plants used in Mexican traditional medicine for the treatment of infectious diseases. J Ethnopharmacol. 1996;53:143–7. doi: 10.1016/0378-8741(96)01429-8. [DOI] [PubMed] [Google Scholar]
- 29.Rios JL, Recio MC, Villar A. Screening methods for natural products with antimicrobial activity: A review of the literature. J Ethnopharmacol. 1988;23:127–49. doi: 10.1016/0378-8741(88)90001-3. [DOI] [PubMed] [Google Scholar]
- 30.Deans S, Simpson E, Noble R, MacPherson A, Penzes L. Natural antioxidants from Thymus vulgaris (thyme) volatile oil: The beneficial effects upon mammalian lipid metabolism. Acta Hortic. 1993;332:177–82. [Google Scholar]
- 31.Dorman HJ, Deans SG. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–16. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–4. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann CM, Markham JL. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol. 1998;84:538–44. doi: 10.1046/j.1365-2672.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 34.Skandamis PN, Nychas GJ. Effect of oregano essential oil on microbiological and physico-chemical attributes of minced meat stored in air and modified atmospheres. J Appl Microbiol. 2001;91:1011–22. doi: 10.1046/j.1365-2672.2001.01467.x. [DOI] [PubMed] [Google Scholar]
- 35.Burt SA, Reinders RD. Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett Appl Microbiol. 2003;36:162–7. doi: 10.1046/j.1472-765x.2003.01285.x. [DOI] [PubMed] [Google Scholar]
- 36.Shahzad M, Millhouse E, Culshaw S, Edwards CA, Ramage G, Combet E. Selected dietary (poly) phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2015;6:719–29. doi: 10.1039/c4fo01087f. [DOI] [PubMed] [Google Scholar]
- 37.Taguri T, Tanaka T, Kouno I. Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol Pharm Bull. 2006;29:2226–35. doi: 10.1248/bpb.29.2226. [DOI] [PubMed] [Google Scholar]
- 38.Geissman T. Flavonoid compounds, tannins, lignins and related compounds. In: Florkin M, Stotz E, editors. Pyrrole Pigments, Isoprenoid Compounds and Phenolic Plant Constituents. Vol. 9. New York: Elsevier; 1963. p. 265. [Google Scholar]
- 39.Mason T, Wasserman B. Inactivation of red beet betaglucan synthase by native and oxidized phenolic compounds. Phytochemistry. 1987;26:2197–202. [Google Scholar]
- 40.Lim A, Subhan N, Jazayeri JA, John G, Vanniasinkam T, Obied HK. Plant phenols as antibiotic boosters In vitro interaction of olive leaf phenols with ampicillin. Phytother Res. 2016;30:503–9. doi: 10.1002/ptr.5562. [DOI] [PubMed] [Google Scholar]
- 41.Aires A, Marques E, Carvalho R, Rosa EA, Saavedra MJ. Evaluation of biological value and appraisal of polyphenols and glucosinolates from organic baby-leaf salads as antioxidants and antimicrobials against important human pathogenic bacteria. Molecules. 2013;18:4651–68. doi: 10.3390/molecules18044651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cueva C, Mingo S, Muñoz-González I, Bustos I, Requena T, del Campo R, et al. Antibacterial activity of wine phenolic compounds and oenological extracts against potential respiratory pathogens. Lett Appl Microbiol. 2012;54:557–63. doi: 10.1111/j.1472-765X.2012.03248.x. [DOI] [PubMed] [Google Scholar]
- 43.Sarjit A, Wang Y, Dykes GA. Antimicrobial activity of gallic acid against thermophilic Campylobacter is strain specific and associated with a loss of calcium ions. Food Microbiol. 2015;46:227–33. doi: 10.1016/j.fm.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Borges A, Ferreira C, Saavedra MJ, Simões M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist. 2013;19:256–65. doi: 10.1089/mdr.2012.0244. [DOI] [PubMed] [Google Scholar]
- 45.Nowacka N, Nowak R, Drozd M, Olech M, Los R, Malm A. Antibacterial, antiradical potential and phenolic compounds of thirty-one polish mushrooms. PLoS One. 2015;10:e0140355. doi: 10.1371/journal.pone.0140355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi JG, Mun SH, Chahar HS, Bharaj P, Kang OH, Kim SG, et al. Methyl gallate from Galla rhois successfully controls clinical isolates of Salmonella infection in both in vitro and in vivo systems. PLoS One. 2014;9:e102697. doi: 10.1371/journal.pone.0102697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madikizela B, Aderogba MA, Van Staden J. Isolation and characterization of antimicrobial constituents of Searsia chirindensis L. (Anacardiaceae) leaf extracts. J Ethnopharmacol. 2013;150:609–13. doi: 10.1016/j.jep.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 48.Wang SS, Wang DM, Pu WJ, Li DW. Phytochemical profiles, antioxidant and antimicrobial activities of three Potentilla species. BMC Complement Altern Med. 2013;13:321. doi: 10.1186/1472-6882-13-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomás-Menor L, Barrajón-Catalán E, Segura-Carretero A, Martí N, Saura D, Menéndez JA, et al. The promiscuous and synergic molecular interaction of polyphenols in bactericidal activity: An opportunity to improve the performance of antibiotics? Phytother Res. 2015;29:466–73. doi: 10.1002/ptr.5296. [DOI] [PubMed] [Google Scholar]
- 50.Soberón JR, Sgariglia MA, Dip Maderuelo MR, Andina ML, Sampietro DA, Vattuone MA. Antibacterial activities of Ligaria cuneifolia and Jodina rhombifolia leaf extracts against phytopathogenic and clinical bacteria. J Biosci Bioeng. 2014;118:599–605. doi: 10.1016/j.jbiosc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 51.Jiamboonsri P, Pithayanukul P, Bavovada R, Chomnawang MT. The inhibitory potential of Thai mango seed kernel extract against methicillin-resistant Staphylococcus aureus. Molecules. 2011;16:6255–70. doi: 10.3390/molecules16086255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rúa J, Fernández-Álvarez L, de Castro C, Del Valle P, de Arriaga D, García-Armesto MR. Antibacterial activity against foodborne Staphylococcus aureus and antioxidant capacity of various pure phenolic compounds. Foodborne Pathog Dis. 2011;8:149–57. doi: 10.1089/fpd.2010.0659. [DOI] [PubMed] [Google Scholar]
- 53.Cushnie TP, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 54.Rukayadi Y, Lee K, Han S, Yong D, Hwang JK. In vitro activities of panduratin A against clinical Staphylococcus strains. Antimicrob Agents Chemother. 2009;53:4529–32. doi: 10.1128/AAC.00624-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shiu WK, Rahman MM, Curry J, Stapleton P, Zloh M, Malkinson JP, et al. Antibacterial acylphloroglucinols from Hypericum olympicum. J Nat Prod. 2012;75:336–43. doi: 10.1021/np2003319. [DOI] [PubMed] [Google Scholar]
- 56.Taglialatela-Scafati O, Pollastro F, Chianese G, Minassi A, Gibbons S, Arunotayanun W, et al. Antimicrobial phenolics and unusual glycerides from Helichrysum italicum subsp. microphyllum. J Nat Prod. 2013;76:346–53. doi: 10.1021/np3007149. [DOI] [PubMed] [Google Scholar]
- 57.Shiu WK, Gibbons S. Anti-staphylococcal acylphloroglucinols from Hypericum beanii. Phytochemistry. 2006;67:2568–72. doi: 10.1016/j.phytochem.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 58.Yam TS, Hamilton-Miller JM, Shah S. The effect of a component of tea (Camellia sinensis) on methicillin resistance, PBP2’synthesis, and beta-lactamase production in Staphylococcus aureus. J Antimicrob Chemother. 1998;42:211–6. doi: 10.1093/jac/42.2.211. [DOI] [PubMed] [Google Scholar]
- 59.Hu ZQ, Zhao WH, Asano N, Yoda Y, Hara Y, Shimamura T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:558–60. doi: 10.1128/AAC.46.2.558-560.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sudano Roccaro A, Blanco AR, Giuliano F, Rusciano D, Enea V. Epigallocatechin-gallate enhances the activity of tetracycline in staphylococci by inhibiting its efflux from bacterial cells. Antimicrob Agents Chemother. 2004;48:1968–73. doi: 10.1128/AAC.48.6.1968-1973.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gibbons S, Moser E, Kaatz GW. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus. Planta Med. 2004;70:1240–2. doi: 10.1055/s-2004-835860. [DOI] [PubMed] [Google Scholar]
- 62.Shibata H, Kondo K, Katsuyama R, Kawazoe K, Sato Y, Murakami K, et al. Alkyl gallates, intensifiers of beta-lactam susceptibility in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2005;49:549–55. doi: 10.1128/AAC.49.2.549-555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jayaraman P, Sakharkar MK, Lim CS, Tang TH, Sakharkar KR. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa, in vitro. Int J Biol Sci. 2010;6:556–68. doi: 10.7150/ijbs.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saavedra MJ, Borges A, Dias C, Aires A, Bennett RN, Rosa ES, et al. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med Chem. 2010;6:174–83. doi: 10.2174/1573406411006030174. [DOI] [PubMed] [Google Scholar]