Abstract
Aims and Objectives:
The aim of this study is to determine the prevalence of hypogonadism in human immunodeficiency virus (HIV)-infected males and to study its relation to age, CD4 count, body mass index (BMI), duration of highly active antiretroviral therapy (HAART), and metabolic status.
Methodology:
Eighty-one HIV positive cases and 82 healthy controls were included in this case–control study. Each case underwent a complete physical examination and serum fasting plasma glucose, A1c, lipid profile, total testosterone (TT), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) levels were estimated. Serum TT level <300 ng/dl, or TT >300 ng/dl with high LH and FSH (compensatory hypogonadism) were taken as markers for hypogonadism, and it was correlated with age, CD4 count, duration of HAART, and metabolic status of the patient.
Results:
Out of 81 cases, 21 (25.9%) were found to have hypogonadism as compared to 4 (4.9%) out of 82 controls. Of these 21, 14 cases had secondary hypogonadism, five had primary, and the remaining two had compensatory hypogonadism. The mean serum TT value among cases (371.7 ± 102.9 ng/dl) was significantly lower than that among controls (419.7 ± 71.5 ng/dl) (P = 0.007). Hypogonadism was found to be significantly associated with the age of the patient (P = 0.007), CD4 count (P = 0.002), and duration of HAART (P = 0.04) and was independent of the BMI (P = 0.9) and the waist circumference (P = 0.8). Dyslipidemia and dysglycemia were significantly more common among cases as compared to controls (P < 0.05) but were not associated with hypogonadism.
Conclusion:
The prevalence of hypogonadism is higher among HIV-infected males as compared to healthy individuals. Hypogonadism was significantly associated with age, CD4 count, and duration of HAART and was independent of BMI, glycemic status, and dyslipidemia.
Keywords: Human immunodeficiency virus, hypogonadism, metabolic status, serum testosterone
INTRODUCTION
Human immunodeficiency virus (HIV) infection is a global pandemic. According to the World Health Organization (WHO), there were 36.7 million people living with HIV worldwide in 2015.[1] The advent of antiretroviral therapy (ART) for the treatment of HIV has lead to a marked decline in mortality among HIV patients. Nevertheless, whereas mortality due to HIV-related events is sharply declining, non-AIDS conditions are emerging as significant causes of morbidity and mortality in this population.[2] ART and improvement in mortality have also lead to a dramatic increase in the prevalence of endocrine and metabolic abnormalities among HIV patients. Endocrine changes in the form of thyroid, adrenal, gonadal, bone, and metabolic dysfunction have all been reported in both early and late stages of HIV infection.[3] Hypogonadism is relatively common among HIV patients. Reduced testosterone concentrations have been associated with lower CD4 cell count, advanced stage of illness, use of certain drugs (e.g., megestrol acetate, glucocorticoids, or ketoconazole), and weight loss.[4] HIV patients have also been found to have a higher prevalence of metabolic derangements as compared to the normal population. In turn, hypogonadism has also been found to be associated with metabolic derangements. This study was conducted to determine the prevalence of hypogonadism among HIV-infected male patients and to assess its association with various factors, including CD4 count, age and duration of highly active antiretroviral therapy (HAART). The correlation of hypogonadism with the metabolic status of the HIV-infected males was also assessed.
METHODOLOGY
This case–control study was conducted in the Medicine Department and ART Centre of a Tertiary Care Center of North India and was approved by the Institutional Ethics Committee. The study was conducted over a period of 15 months from May 2015 to August 2016.
All HIV positive (diagnosed as per the WHO guidelines 2015) male patients aged 18–60 years attending the outpatient medicine department and ART Centre were included in the study as cases. Patients with chronic liver insufficiency, chronic kidney disease, chronic systemic illnesses such as tuberculosis and those who had undergone prior treatment with drugs that could affect testosterone levels such as androgens, sex steroids, dehydroepiandrosterone, antiandrogens, anabolic agents, GnRH agonists, and psycholeptic agents were excluded from the study. Controls were age-matched healthy HIV negative male individuals in the age group of 18–60 years. All individuals were assessed clinically by detailed history taking and general physical examination including anthropometric measurements that included waist circumference and body mass index (BMI). The study population underwent a venous sampling in the morning after undergoing an overnight fast of 8 h for serum total testosterone (TT), follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and also for fasting plasma glucose (FPG) and fasting lipid profile. The hormonal tests were done using chemiluminescence assays. CD4 count was done for all cases using flow cytometry using a BD FACSCount system kits supplied by the National AIDS Control Organization of India to ART Centre. Hypogonadism was defined as a serum TT level of < 300ng/dl or a serum TT level of ≥300 ng/dl with high FSH (>12 mIU/L) or LH (>12 mIU/L) level.[5] Eugonadism was defined as normal TT and normal FSH and LH levels. Compensatory hypogonadism was defined as normal TT but high FSH or LH levels. Primary hypogonadism was defined as low TT levels with high LH or FSH while secondary hypogonadism was defined as low TT with low or normal FSH or LH.
Assessment of the metabolic status of the cases and controls was done by estimating the waist circumference, BMI on clinical evaluation, and investigating the patient for FPG levels, A1c, and fasting plasma lipid profile.
A total of 81 HIV positive cases and 82 healthy controls were included in the study. The GraphPad software version 6.0 (San Diego, California, USA) was used for analysis of data. The numerical data were compared using t-test for independent variables and Chi-square tests for nonparametric variables. The level of significance was considered 0.05.
RESULTS
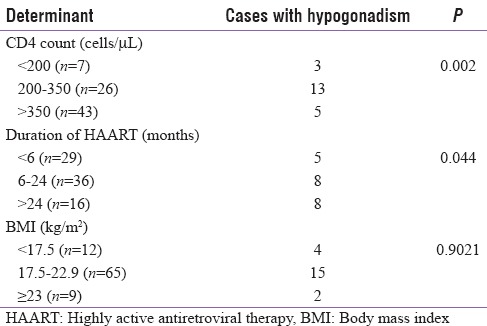
A total of 81 cases and 82 controls were enrolled in the study. Of the 81 cases, a total of 21, that is 25.9%, had hypogonadism while only three out of the 82 controls (3.6%) had hypogonadism (P = 0.001). Primary hypogonadism was seen in 5/82 cases (6.2%), secondary in 14/82 cases (17.3%), and compensatory in 2/82 (2.5%) cases. The mean serum TT level among cases were 360.6 ± 111.5 ng/dl (mean ± 2 standard deviation [SD]) and among controls were 422.0 ± 74.4 ng/dl (mean ± 2SD) (P = 0.007). The mean serum TT level was also significantly lower among cases as compared to controls when compared for different age groups [Table 1]. The mean serum TT level also decreased with decreasing CD4 count among cases [Figure 1]. The prevalence of hypogonadism increased significantly among cases with decrease in CD4 count (P = 0.002), increase in duration of HAART (P = 0.044) but was not significantly associated with BMI (P = 0.9021) [Table 2].
Table 1.
Mean serum total testosterone levels at different age groups
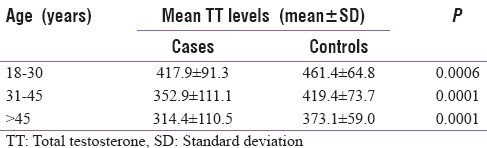
Figure 1.
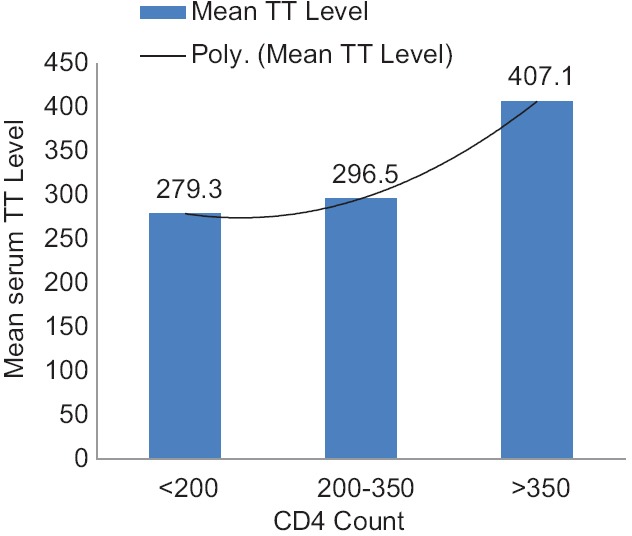
Mean total testosterone level at different CD4 counts
Table 2.
Association of hypogonadism with CD4 count, body mass index, and duration of highly active antiretroviral therapy
The mean FPG for cases was 102.7 ± 34.9 mg/dl and that for controls was 93.0 ± 21.2 mg/dl (P = 0.047). 10/81 cases had impaired fasting glucose (IFG) levels, and 9/81 cases had diabetes mellitus (DM). Thus, 19/81 cases (23.4%) had dysglycemia. 10/82 controls (12.2%) had dysglycemia, (6/82 controls had IFG, and 4/82 cases had DM). Thus, dysglycemia was significantly more common among cases as compared to controls (P = 0.0451). 13/62 cases (20.6%) with euglycemia had hypogonadism while 8/19 cases (42.1%) with dysglycemia had hypogonadism (P = 0.066).
A comparison of the mean lipid levels among cases with and without hypogonadism was also done. Besides, serum HDL level (P = 0.011), the difference between the lipid levels of the two groups was not found to be significant.
DISCUSSION
Hypogonadism was found to be significantly more common among HIV-infected male patients (25.9%) as compared to the normal population (3.6%). Secondary hypogonadism was the most common form of hypogonadism accounting for 66.7% of hypogonadal cases. In a cross-sectional study conducted by Mandal et al.[6] on HIV-infected male patients from Eastern India, the prevalence of male hypogonadism was 33% among the HIV-infected male population. Among male hypogonadism, 30.8% were primary, and 69.2% were secondary.
When mean serum TT levels of the HIV-infected males and normal population was compared, mean serum TT levels were found be lower among the HIV patients for all age groups. Rochira et al.[5] also found mean serum TT levels to be lower among HIV-infected patients as compared to the normal population. However, in a study conducted by Rietschel et al.,[7] the mean serum TT levels were found to be higher in HIV-infected male patients as compared to healthy HIV uninfected controls. The mean serum free testosterone levels were, however, lower for the HIV-infected patients as compared to the normal population. This was attributed to increase in SHBG levels among HIV-infected patients as compared to normal healthy population.
Hypogonadism was found to be significantly associated with age of HIV patients, their CD4 count and also with the duration of HAART in this study; however, the association with BMI was not significant. Studies by Laudat et al.[8] and Meena et al.[9] also found a direct correlation between serum testosterone levels and lower CD4 counts among HIV patients. Rietschel et al.[7] found hypogonadism to be more common among patients with AIDS wasting, but no correlation was found with CD4 count and ART status.
Dysglycemia was found to be significantly more common among HIV patients (31.4%) as compared to the normal population (12.2%) in this study, but the association with hypogonadism was not found to be significant. In a study conducted by Gazzaruso et al.[10] on 553 HIV positive patients, 133 (24.0%) showed high fasting glucose or antidiabetes medication use. In a Multi-ethnic Study of Atherosclerosis conducted by Colangelo et al.,[11] it was found that despite adjustment for BMI and waist circumference, there were a significant inverse associations of TT and SHBG with diabetes and IFG.
Serum triglycerides (TGs), low-density lipoprotein, and total cholesterol levels were observed to be significantly higher among HIV population as compared to the normal population in this study. However, there was no significant difference in the lipid levels of hypogonadal and eugonadal HIV patients on comparison. A study by Almeida et al.[12] showed that the concentrations of total cholesterol, TGs and glucose were significantly higher among patients taking HAART. In a prospective case–control study conducted in Nigeria,[13] a strong association was seen between low testosterone levels and hypertriglyceridemia.
CONCLUSIONS
Hypogonadism is a common endocrine abnormality among HIV-infected male patients with prevalence being 25.9% among HIV males in this study. Primary hypogonadism was more common than secondary hypogonadism among the HIV population. Serum TT levels were also lower among HIV males as compared to normal population for all age groups. There was a significant association of hypogonadism with age, CD4 count, and duration of HAART, but the association with BMI was not found to be significant. Dysglycemia and dyslipidemia were also more prevalent among HIV males when compared to normal population but their association with hypogonadism was not found to be significant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.UN Joint Programme on HIV/AIDS (UNAIDS), Global AIDS Update. 2016. [Last accessed on 2017 Apr 28]. Available from: http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf .
- 2.Blanco F, San Román J, Vispo E, López M, Salto A, Abad V, et al. Management of metabolic complications and cardiovascular risk in HIV-infected patients. AIDS Rev. 2010;12:231–41. [PubMed] [Google Scholar]
- 3.Sinha U, Sengupta N, Mukhopadhyay P, Roy KS. Human immunodeficiency virus endocrinopathy. Indian J Endocrinol Metab. 2011;15:251–60. doi: 10.4103/2230-8210.85574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner G, Rabkin JG, Rabkin R. Illness stage, concurrent medications, and other correlates of low testosterone in men with HIV illness. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8:204–7. [PubMed] [Google Scholar]
- 5.Rochira V, Zirilli L, Orlando G, Santi D, Brigante G, Diazzi C, et al. Premature decline of serum total testosterone in HIV-infected men in the HAART-era. PLoS One. 2011;6:e28512. doi: 10.1371/journal.pone.0028512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mandal SK, Paul R, Bandyopadhyay D, Basu AK, Mandal L. Study on endocrinological profile of HIV infected male patients from Eastern India. Int Res J Pharm. 2013;4:220–3. [Google Scholar]
- 7.Rietschel P, Corcoran C, Stanley T, Basgoz N, Klibanski A, Grinspoon S. Prevalence of hypogonadism among men with weight loss related to human immunodeficiency virus infection who were receiving highly active antiretroviral therapy. Clin Infect Dis. 2000;31:1240–4. doi: 10.1086/317457. [DOI] [PubMed] [Google Scholar]
- 8.Laudat A, Blum L, Guéchot J, Picard O, Cabane J, Imbert JC, et al. Changes in systemic gonadal and adrenal steroids in asymptomatic human immunodeficiency virus-infected men: Relationship with the CD4 cell counts. Eur J Endocrinol. 1995;133:418–24. doi: 10.1530/eje.0.1330418. [DOI] [PubMed] [Google Scholar]
- 9.Meena LP, Rai M, Singh SK, Chakravarty J, Singh A, Goel R, et al. Endocrine changes in male HIV patients. J Assoc Physicians India. 2011;59:365–6, 371. [PubMed] [Google Scholar]
- 10.Gazzaruso C, Sacchi P, Garzaniti A, Fratino P, Bruno R, Filice G. Prevalence of metabolic syndrome among HIV patients. Diabetes Care. 2002;25:1253–4. doi: 10.2337/diacare.25.7.1253. [DOI] [PubMed] [Google Scholar]
- 11.Colangelo LA, Ouyang P, Liu K, Kopp P, Golden SH, Dobs AS, et al. Association of endogenous sex hormones with diabetes and impaired fasting glucose in men: Multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32:1049–51. doi: 10.2337/dc08-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almeida SE, Borges M, Fiegenbaum M, Nunes CC, Rossetti ML. Metabolic changes associated with antiretroviral therapy in HIV-positive patients. Rev Saude Publica. 2009;43:283–90. doi: 10.1590/s0034-89102009005000005. [DOI] [PubMed] [Google Scholar]
- 13.Akinloye O, Blessing Popoola B, Bolanle Ajadi M, Gregory Uchechukwu J, Pius Oparinde D. Hypogonadism and metabolic syndrome in Nigerian male patients with both type 2 diabetes and hypertension. Int J Endocrinol Metab. 2014;12:e10749. doi: 10.5812/ijem.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]


