Abstract
Thyroid nodules are prevalent in upto 68% of randomly selected individuals in whom high resolution ultrasound is performed. The majority of nodules are benign. The use of ultrasound coupled with FNAC has dramatically reduced the number of patients who undergo surgery for nodules. The six tier Bethesda scoring system has reduced variability and increased the ability to clinicians to guide patients with thyroid nodules. There is good correlation between cytology and histopathologic outcomes. A significant proportion of patients will however fall into an indeterminate category. The availability of molecular markers enhanced with next generation sequencing technology and the expression classifier are added diagnostic aids that can help in management. However these are not available in many countries and in resource limited settings. A pragmatic approach to the diagnosis of indeterminate nodules includes utilising pre and post test probability, clinical acumen, correlation of ultrasound findings and expert opinion in some settings. Using this approach high risk patients can be appropriately chosen for surgery while relegating patients with lower risk to watchful followup.
Keywords: Atypia of undetermined significance follicular lesion of undetermined, Bethesda scoring, follicular neoplasm/suspicious for follicular neoplasm, indeterminate nodule, suspicious for malignancy, Thyroid Imaging Reporting and Data System
INTRODUCTION
A thyroid nodule may be defined as a discrete lesion within the thyroid gland that is radiologically distinct from the surrounding gland.[1] Palpable thyroid nodules are present in approximately 5% of women and 1% of men in iodine sufficient areas. If high-resolution ultrasound (USG) was used, up to 68% of randomly selected individuals may have nodules – more in women and increasing with age in both sexes.[2] There is a linear increase in the prevalence from almost none at age 15%–50% by the age of 65 years.[3]
Thyroid nodules are the clinical manifestation of a myriad of pathologic processes. Nonneoplastic nodules are the result of glandular hyperplasia arising spontaneously or following partial thyroidectomy.[4] Hashimoto's thyroiditis may present with a nodular feel but do not represent an example of true nodule formation. Adenomas are characterized by orderly architecture and few mitosis with no lymphatic or vascular invasion. Necrosis is common in nodules resulting in cyst formation. Nodules are monoclonal and grow slowly reflecting the long time taken by thyroid cells to divide. Most nodules are detected incidentally. Symptoms of growth and invasion such as dysphagia dystonia and stridor are rare. Bleeding into the nodule occurs rarely and presents with increase in size pain and tenderness or even transient thyrotoxicosis.
Thyroid cancers are uncommon and account for <0.5% of cancer deaths.[5] However, the incidence of thyroid cancer is increasing rapidly and currently appears to be the most rapidly increasing malignancy among men and women in the general population.[6] This appears to be a worldwide phenomenon.[7] The mortality rates appear to be unchanged largely reflecting the fact that the increase is primarily in early stage papillary carcinoma. In part, this increase may be attributed to greater use of imaging since the early 1990s.
The clinician's approach to the thyroid nodule over the years is to primarily distinguish the small number of nodules that harbor a malignancy from the majority that do not. At autopsy, up to 30% of thyroid glands will harbor malignant nodules which are under 1 cm (microcarcinomas); many but not all of them these will have an indolent course.
SCORING SYSTEMS AND THEIR IMPACT ON DECISION-MAKING IN SOLITARY NODULES
Conventionally, the approach to the thyroid nodule has been muddled by the lack of standardization of both imaging and cytologic techniques. The availability of high-frequency USG and the development of risk scores that can quantify the risk of thyroid malignancy is a significant advance that has demystified decision-making in thyroid nodules. Several USG features have been identified in multivariate analysis as associated with malignancy, specifically papillary cancer of the thyroid (PTC). These include the presence of microcalcifications and nodule hypoechogenicity when compared with strap muscles, irregular margins (infiltrative microlobulated and spiculated), shape taller than wide on transverse view, central vascularity, and twinkling on B flows imaging.[8] Follicular thyroid cancer (FTC) has somewhat different features. They are more often iso- or hyper-echoic, noncalcified, round with grater AP dimensions, and regular smooth margins. Follicular variant of PTC has similar dimensions.[9]
Some features on USG are associated with a low risk of differentiated thyroid cancer. A spongiform appearance defined as the aggregation of multiple microcystic components in more than 50% of then nodule is strongly suggestive of a benign nodule.[10] Other USG features include hyperechogenicity, large coarse calcification, peripheral calcifications, puff pastry appearance, and comet tail shadowing.
Several risk scoring systems have been developed which aim to reduce interobserver variability and allow clinicians to make decisions regarding further workup and follow-up. The most useful of these is the Thyroid Imaging Reporting and Data System (TIRADS) classification [Table 1]. Similar to the Breast Imaging-Reporting and Data System for breast lesion, the TIRADS system allows the user understand and explain to the patient the risk of malignancy in a nodule and the need for further workup including aspiration.[8] In the author's experience, the TIRADS system correlates exceptionally well with the Bethesda system for cytology.[11] The American Thyroid Association uses a different system based on an estimated risk of malignancy from centers that deal with a high volume of patients with thyroid nodules and malignancy [Table 2].[1] There is a significant correlation between both systems. However, some nodules that do not meet the criteria for malignancy in the American Thyroid Association guidelines appeared have increased risk of malignancy (18.2%).[12]
Table 1.
Thyroid Imaging Reporting and Data System scoring system[8]

Table 2.
The American Thyroid Association risk scoring system[1]

Similarly, USG-guided cytology and the standardization of interpretation of thyroid cytology has reduced ambiguity. The diagnostic groups reported under the six-tiered Bethesda system for reporting thyroid cytopathology have gained widespread acceptance [Table 3].[13] An adequate specimen is defined as composing of at least six groups of cells each having 10–15 cells. When this is not present, the fine-needle aspiration cytology (FNAC) is deemed inadequate or nondiagnostic. Approximately 5% of all aspirations in experienced hands will fall into this category. Several factors contribute to nondiagnostic specimens including nodule component and FNAC technique.
Table 3.
Bethesda scoring system[13]

Adequate specimens are categorized as benign, malignant, or indeterminate with the latter being divided into three specific categories each correlating with a different malignancy risk. These include atypia of undetermined significance (AUS), follicular or Hurthle cell neoplasms, and suspicious for malignancy [Table 3]. 2%–3% of benign nodules as determined by FNAC will subsequently prove to be malignant. Conversely, the same amount of malignant nodules on FNAC will prove to be benign.[14] Large studies showed a high degree of concordance between the system and pathology, especially in the definitively benign and the definitively malignant categories with variability in the intermediate categories.
Decision-making in the “indeterminate” category possesses the greatest challenge for the clinical endocrinologist and will be the focus of this review.
SETTING THE CONTEXT
For the purpose of this review, the indeterminate thyroid nodule will be defined as those nodules that have after an initial evaluation (history, physical examination, ultrasound, and FNAC) have received Bethesda classification of either III, IV, or V (BIII, BIV, and BV). This indeterminate category falls into a malignancy risk between 5% and 75% and represents up to 40% of all FNACs. At the lower end of the spectrum which is AUS follicular lesion of undetermined (FLUS), we will need to rule out disease – we need an approach that has high negative predictive value (NPV). An ideal rule out will have the NPV of Bethesda II (BII) cytology (96.3%). At the higher end of the spectrum, we will need an approach that has high positive predictive value. An ideal “rule in” will have the positive predictive value of Bethesda VI (BVI) cytology (98.6%). Conceptually, our approach to AUS/FLUS and follicular neoplasm (FN)/suspicious for follicular neoplasm should be to rule out malignancy, so we need an approach with tools that have high sensitivity and high NPV; similarly, our approach to suspicious for malignancy should be to rule in malignancy and our approach should have tools that have high specificity and positive predictive value [Figure 1].
Figure 1.

Clinical decision-making in indeterminate nodules based on probability of malignancy
AVAILABLE MALIGNANCY MARKERS AND THEIR UTILITY IN INDETERMINATE THYROID NODULES
The association of gene mutations and translocation fusions with thyroid cancer has been described extensively.[15] Over the years, several markers of malignancy have been evaluated. Many of the early markers were suboptimal for clinical use. Panels of markers have been developed to improve efficiency and accuracy and commercialized. These panels and their ability to rule out and rule in indeterminate nodules are briefly summarized.
The Afirma Gene Expression Classifier (GEC) (Veracyte, Inc., South San Francisco, California, USA) uses microarray technology to analyze mRNA expression of 167 different genes, 142 of which are commonly, and 25 which are uncommonly seen with thyroid cancer. Only BIII and BIV are accepted for analysis and generate two possible results, benign and suspicious. In the BIII and BIV setting, the GEC has an NPV of 95% and 94%, respectively. It must be noted that in the BV category, the NPV was only 85%.[16] The PPV in BIV and BV are low at 38% and 37%, respectively, reaffirming the role of this test as a rule out (benign) than a rule in test. The usefulness of this test is largely determined by the institutional prevalence of malignancy in nodules.[17] and appears to be most useful in a practice setting with the prevalence of malignancy in indeterminate lesions of 15%–21%. The value of a “suspicious” result is less well categorized because of the low PPV. The GEC also reports a number of Hurthle cell-rich but benign lesions as suspicious limiting its use in this category. The value of the GEC is testified by the dramatic reduction in resection of cytologically indeterminate nodules from 74% to 7.6% in a multi-cohort study.[18] The Afirma Malignancy Classifier (AMC) is an extension of the GEC. This is performed only on BV or BVI lesion or a suspicious GEC and tests through an mRNA profile for medullary thyroid carcinoma (MTC) (five genes) and DTC through the BRAF V600E mutation. The test is yet to be validated extensively. As of the time of this writing, the GEC is not widely used in India. The cost is comparable to or lower than the cost of thyroidectomy in an average surgical center in India.
BRAF is most commonly seen in up to 45% while rat sarcoma viral oncogene homolog (RAS) and (Ret proto-oncogene) RET/PTC gene mutations are identified in 10%–20% of PTCs. Approximately 70% of PTCs harbor one of the BRAF/RAS/RET PTC or TRK rearrangements. Mutually exclusive RAS (40%–50%) or PAX8/PPARγ (30%–35%) is seen in up to 75% of FTC. Mutations in TP53 and CTNNB1 genes occur more commonly in anaplastic cancer while RET gene point mutations are seen in sporadic and familiar MTC.
The ThyGenX Test uses a next-generation sequencing (NGS) platform to identify over 100 mutations in eight genes and is an improvement on an earlier panel available. The test requires one pass of FNAC of 50 ng of cellular material. It only accepts BIII and BIV cytology for testing. The ThyraMir is based on the analysis of 10 available miRNAs involved in the cell-cycle progression, differentiation, and proliferation in thyroid pathology and is meant to be used when ThyGenX is negative. Combined these tests offer an NPV of 94% in BIII and BIV which is comparable to the GEC. The PPV at 74% is higher than the GEC. Importantly, when both tests are negative, the residual risk of cancer is low at 6%.
ThyroSeq v2 is an NGS-based test which again is an enhanced version of a previous platform that now tests for 14 genes with over 1000 mutations, 42 RNA alterations. The panel reported a sensitivity and specificity of 90% and 92% translating into an NPV, PPV, and accuracy of 96%, 83%, and 92%, respectively.[19] When applied to a population in whom the risk of malignancy in BIII BIV lesions is between 5% and 15%, the panel is expected to have an NPV of 98%–99% and a PPV of 40%–69% as based on Bayesian modeling making it an effective “rule out test” but not a good “rule in” test. Further, data are required to confirm the effectiveness of this panel. To the best of the author's knowledge, there is no significant experience of the use of this panel in India.
BETHESDA III (ATYPIA OF UNDETERMINED SIGNIFICANCE/FOLLICULAR LESION OF UNDETERMINED)
The BIII describes a group of FNAC specimens that contain cells with architectural or nuclear atypia that would not qualify it for BII but does not contain enough suspicious features that would warrant a higher class assignment. This category was intended for limited use and expected to have a frequency of about 7%. Usage of this category by cytologists has been variable with studies reporting usage up to 27%. When patients in this category underwent surgery, malignancy was seen up to 14.5%.[20]
Using USG features to estimate malignancy, risk in BIII lesions has been examined. The reported cancer risk in BIII lesions and high suspicion sonographic features was between 90% and 100%. The prevalence of at least one suspicious feature on USG in BIII lesions ranged from 18% to 50% and increased the risk of malignancy to 60%–90%.[21] It must be noted that the overall malignancy rate in these studies was 40%–45%.
Fludeoxyglucose-positron emission tomography (FDG-PET) has been reported to have a high NPV when applied to the diagnosis of cytologically indeterminate thyroid nodules. In a systematic review and meta-analysis of six studies, FDG-PET had a low PPV (39%) and a high NPV (96%) when performed in thyroid nodules with BIII or BIV cytology.[22] This approach is however not recommended.
Since there is significant interobserver variability in this category,[23] one recommended approach is to obtain a second opinion from a high volume cytopathologist. Central cytopathologists from institutions with high volume make fewer indeterminate diagnosis (55% vs. 42%) than community-based cytopathologists[23] In one study, a second opinion for a nodule originally read as indeterminate and subsequently reclassified as benign had an NPV 95%. The second opinion improves diagnostic accuracy from 60% to 74% and avoids diagnostic surgery in 25% of patients.
A repeat FNA may reclassify the lesion into a more definitive diagnosis. A repeat diagnosis recategorizes an AUS lesion into a benign category the majority of the time with an indeterminate diagnosis persisting only a third of the time.[24] Malignancy rates are similar with single BIII and two successive BIII diagnoses. This approach has been recently questioned.
When available, the GEC appears to be an ideal rule out test in BIII with a reported NPV of 95% in patients with BIII cytology.[16] The Afirma GEC is most useful in excluding malignancy in settings where the overall incidence of malignancy is between 12% and 25%. Recent retrospective studies confirm this high NPV though this may be lower in community-based hospital settings.[25] The PPV of the GEC is 38% in a BIII lesion. The value of a suspicious lesion by GEC is thus limited. The AMC extends the PPV of the GEC and may be useful in suspicious lessons. As observed above, there is very limited experience for this modality in India. An enhanced version of the Thyroseq 2 with PPV of 83%, an NPV of 96%, and accuracy of 92% makes it a good rule in a rule out test.
A composite of clinical ultrasound and cytology and patient preference may be used to decide if surgery is required when molecular testing is not available. A pragmatic approach to the diagnosis of AUS/FLUS (BIII)[26] lesions is summarized in Figure 2.
Figure 2.

Clinical decision-making in Bethesda III lessons
BETHESDA IV (SECONDARY NEOPLASM/SUSPICIOUS FOR FOLLICULAR NEOPLASM)
This diagnostic category consists of either (a) an arrangement of follicular cells with cell crowding and microfollicle formation and lacking nuclear features of PTC or (b) almost exclusively of Hurthle (oncocytic cells).[27] The majority of tumors are benign follicular adenomas driven by the oncogenic RAS mutation with uncertain malignant potential. The risk for malignancy is intermediate (15%–30%). The application of this category has provided a mean prevalence of 10% (1%–25%) and mean cancer risk of 26% (14%–33%). Traditionally, diagnostic excision has been used in this category. Molecular markers have added considerably to the diagnostic assessment in this category.
The GEC was reported to have a 94% NPV and 37% PPV in BIV. Subsequent studies have confirmed both the not so high NPV and the low PPV. In a prospective registry, the PPV is 15% and NPV 75% in BIV lesions. There is a tendency for the GEC to report Hurthle cell-rich lesions as suspicious leading to a significant decrease in PPV to 15%.[28] Thus, the GEC may not be ideal to be used to avoid diagnostic lobectomy in the majority of patients when classified as GEC suspicious.
An initial seven-gene panel including BRAF, RAS, RET PTC, and PPARY has an NPV of up to 86%.[29] However, this does not avoid diagnostic lobectomies since only 70% of cancers harbor one of the mutations. Expanding this with additional mutations and gene rearrangements and using to NGS extended the NPV to 96% (95% CI 92%–100%) and PPV to 83% (95% CI 72%–95%). The Thyroseq v2 assay has a positive predictive value of 83% (95% CI, 72%–95%), an NPV of 96% (95% CI, 92%–100%), and 92% accuracy (95% CI, 88%–97%) in BIV lesions. Patients with BIV cytology and negative NGS-based testing may be followed without surgery. Exceptions include populations with unusual prevalence of malignancy or high pretest probability of disease including family history high-risk sonographic features or prior irradiation. In the presence of these features, the pretest probability will often exceed 50% reducing the NPV to <90%; this would be considered too low to avoid diagnostic thyroidectomy.[30] The clinical approach to the patient with the BIV lesion is summarized in Figure 3.
Figure 3.

Clinical decision-making in Bethesda IV lesions
BETHESDA V (SUSPICIOUS FOR MALIGNANCY)
Aspirates with cytologic features that raise a strong suspicion of malignancy but insufficient for conclusive diagnosis are assigned BV. Approximately 1%–6% of patients are assigned to this category and at an average 75% (53%–87%) of patients have malignancy diagnosed at surgery. The pretest probability of disease is high necessitating surgery in patients with this category. Mutational testing has high specificity with low sensitivity. GEC has a PPV that is similar to cytology alone and an NPV of 85% and is not indicated. The seven-panel gene of mutations is associated with a PPV of 80%–95% and an NPV of 72%–75%.[29] Conceivably, a positive test may help plan the extent of surgery; a negative test does not obviate the need for one. The AMC may be useful in this setting though not extensively validated.
CONCLUSION
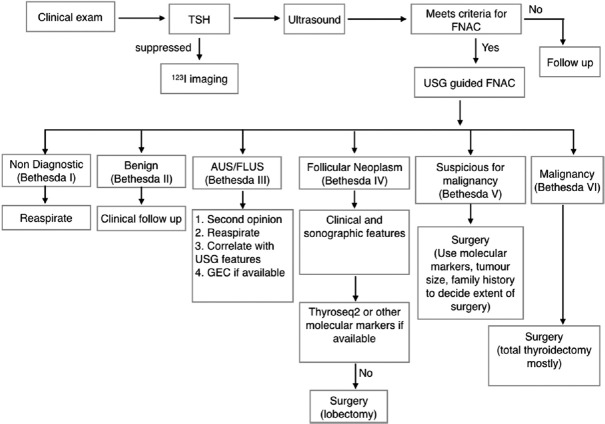
The author's current approach to thyroid nodules is summarized in Figure 4. Decision-making in thyroid nodules has significantly improved because of processes available[31] including collaborative work between the endocrinologist, sonologist, cytologist, and surgeon, high-resolution ultrasound, USG-guided FNAC, and on the spot testing for adequacy. The consistent use of ultrasound- and cytology-based scoring systems has greatly reduced uncertainty. The use of clinical data that assesses the risk of malignancy coupled with adequate knowledge of the prevalence malignancy in the population, use of sonographic features in conjunction with the Bethesda scoring system allows for informed decision-making in the “indeterminate nodule.” When available molecular markers supplement clinical judgment; A Bayesian-based approach will in the event of their nonavailability allow the endocrinologist to reduce surgery as a sure fire choice in low-risk lesions.
Figure 4.
A pragmatic approach to the management of solitary thyroid nodules
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guth S, Theune U, Aberle J, Galach A, Bamberger CM. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699–706. doi: 10.1111/j.1365-2362.2009.02162.x. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–9. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 4.DeGroot LJ, Pacini F. Thyroid Nodules. [Last accessed on 2017 Jan 04]. Available from: http://www.thyroidmanager.org/chapter/thyroid-nodules/
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; 2015; 2015. [Last accessed on 2017 Jan 04]. Based on November, 2014 SEER Data Submission, Posted to the SEER web site, April, 2015. Available from: http://www.seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 7.Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, et al. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20:525–31. doi: 10.1007/s10552-008-9260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology. 2011;260:892–9. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 9.Jeh SK, Jung SL, Kim BS, Lee YS. Evaluating the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid tumor. Korean J Radiol. 2007;8:192–7. doi: 10.3348/kjr.2007.8.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation – Multicenter retrospective study. Radiology. 2008;247:762–70. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 11.Seshadri KG, Gokulakrishnan PR. Personal communication [Google Scholar]
- 12.Yoon JH, Lee HS, Kim EK, Moon HJ, Kwak JY. Malignancy risk stratification of thyroid nodules: Comparison between the thyroid imaging reporting and data system and the 2014 American Thyroid Association Management Guidelines. Radiology. 2016;278:917–24. doi: 10.1148/radiol.2015150056. [DOI] [PubMed] [Google Scholar]
- 13.Baloch ZW, LiVolsi VA, Asa SL, Rosai J, Merino MJ, Randolph G, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425–37. doi: 10.1002/dc.20830. [DOI] [PubMed] [Google Scholar]
- 14.Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration. Cancer Cytopathol. 2009;117:195–202. doi: 10.1002/cncy.20029. [DOI] [PubMed] [Google Scholar]
- 15.de Biase D, Visani M, Pession A, Tallini G. Molecular diagnosis of carcinomas of the thyroid gland. Front Biosci (Elite Ed) 2014;6:1–14. doi: 10.2741/e685. [DOI] [PubMed] [Google Scholar]
- 16.Alexander EK, Kennedy GC, Baloch ZW, Cibas ES, Chudova D, Diggans J, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–15. doi: 10.1056/NEJMoa1203208. [DOI] [PubMed] [Google Scholar]
- 17.Marti JL, Avadhani V, Donatelli LA, Niyogi S, Wang B, Wong RJ, et al. Wide inter-institutional variation in performance of a molecular classifier for indeterminate thyroid nodules. Ann Surg Oncol. 2015;22:3996–4001. doi: 10.1245/s10434-015-4486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duick DS, Klopper JP, Diggans JC, Friedman L, Kennedy GC, Lanman RB, et al. The impact of benign gene expression classifier test results on the endocrinologist-patient decision to operate on patients with thyroid nodules with indeterminate fine-needle aspiration cytopathology. Thyroid. 2012;22:996–1001. doi: 10.1089/thy.2012.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikiforov YE, Carty SE, Chiosea SI, Coyne C, Duvvuri U, Ferris RL, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;120:3627–34. doi: 10.1002/cncr.29038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bongiovanni M, Crippa S, Baloch Z, Piana S, Spitale A, Pagni F, et al. Comparison of 5-tiered and 6-tiered diagnostic systems for the reporting of thyroid cytopathology: A multi-institutional study. Cancer Cytopathol. 2012;120:117–25. doi: 10.1002/cncy.20195. [DOI] [PubMed] [Google Scholar]
- 21.Kim DW, Lee EJ, Jung SJ, Ryu JH, Kim YM. Role of sonographic diagnosis in managing Bethesda class III nodules. AJNR Am J Neuroradiol. 2011;32:2136–41. doi: 10.3174/ajnr.A2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vriens D, de Wilt JH, van der Wilt GJ, Netea-Maier RT, Oyen WJ, de Geus-Oei LF. The role of [18F]-2-fluoro-2-deoxy-d-glucose-positron emission tomography in thyroid nodules with indeterminate fine-needle aspiration biopsy: Systematic review and meta-analysis of the literature. Cancer. 2011;117:4582–94. doi: 10.1002/cncr.26085. [DOI] [PubMed] [Google Scholar]
- 23.Cibas ES, Baloch ZW, Fellegara G, LiVolsi VA, Raab SS, Rosai J, et al. A prospective assessment defining the limitations of thyroid nodule pathologic evaluation. Ann Intern Med. 2013;159:325–32. doi: 10.7326/0003-4819-159-5-201309030-00006. [DOI] [PubMed] [Google Scholar]
- 24.VanderLaan PA, Marqusee E, Krane JF. Clinical outcome for atypia of undetermined significance in thyroid fine-needle aspirations: Should repeated FNA be the preferred initial approach? Am J Clin Pathol. 2011;135:770–5. doi: 10.1309/AJCP4P2GCCDNHFMY. [DOI] [PubMed] [Google Scholar]
- 25.Harrell RM, Bimston DN. Surgical utility of Afirma: Effects of high cancer prevalence and oncocytic cell types in patients with indeterminate thyroid cytology. Endocr Pract. 2014;20:364–9. doi: 10.4158/EP13330.OR. [DOI] [PubMed] [Google Scholar]
- 26.Gupta N, Dasyam AK, Carty SE, Nikiforova MN, Ohori NP, Armstrong M, et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98:E914–22. doi: 10.1210/jc.2012-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faquin WC, Michael CW, Renshaw AA. Follicular neoplasm, Hurthle cell type/suspicious for a follicular neoplasm, Hürthle cell type. In: Ali SZ, Cibas ES, editors. The Bethesda System for Reporting Thyroid Cytopathology. New York: Springer; 2010. pp. 59–7. [Google Scholar]
- 28.Lastra RR, Pramick MR, Crammer CJ, LiVolsi VA, Baloch ZW. Implications of a suspicious Afirma test result in thyroid fine-needle aspiration cytology: An institutional experience. Cancer Cytopathol. 2014;122:737–44. doi: 10.1002/cncy.21455. [DOI] [PubMed] [Google Scholar]
- 29.Nikiforov YE, Steward DL, Robinson-Smith TM, Haugen BR, Klopper JP, Zhu Z, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–8. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 30.Kargi AY, Bustamante MP, Gulec S. Genomic profiling of thyroid nodules: Current role for thyroseq next-generation sequencing on clinical decision-making. Mol Imaging Radionucl Ther. 2016;26(Suppl 1):24–35. doi: 10.4274/2017.26.suppl.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seshadri KG. Improving decision-making in thyroid nodules. Thyroid Res Pract. 2016;13:99–100. [Google Scholar]