Abstract
Introduction: Repeated administration of abused drugs, including Δ9-tetrahydrocannabinol (THC), induces the stable transcription factor ΔFosB in dopaminergic terminal field regions of the mesolimbic system. These studies investigated the effect of prior repeated THC treatment on THC-induced ΔFosB expression and regulation of downstream targets in the forebrain.
Methods: Mice received THC (10 mg/kg) or vehicle twice daily for 13 days, and then half of each group received a single injection of THC or vehicle 45 min before brain collection. ΔFosB messenger RNA (mRNA) and protein were measured by polymerase chain reaction and immunoblotting, respectively. Potential downstream targets of ΔFosB induction were measured by immunoblot.
Results: THC injection in mice with a history of repeated THC treatment enhanced ΔFosB expression as compared with vehicle in the prefrontal cortex (PFC), nucleus accumbens (NAc), and amygdala. This change occurred concomitantly with an increase in ΔFosB mRNA in the PFC and NAc. THC injection in mice with a history of repeated THC treatment increased expression of cyclin-dependent kinase 5 (Cdk5) and its regulatory protein p35 only in the PFC. This increase in Cdk5 and p35 expression in PFC was also found in mice that had only received repeated THC administration, suggesting that this effect might be due to induction of ΔFosB. Extracellular signal-regulated kinase (ERK) phosphorylation was increased in PFC after THC injection in repeated THC-treated mice. Phosphorylation of glycogen synthase kinase-3β (GSK3β), a Cdk5 target, was reduced in PFC after repeated THC treatment regardless of THC history, and phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) at the Cdk5-regulated threonine 75 site was unchanged.
Conclusion: These results suggest that a history of repeated THC administration primes THC-mediated induction of ΔFosB in the NAc and PFC, and that expression of both downstream targets of ΔFosB (e.g., Cdk5 and p35) and upstream activators (e.g., pERK) in the PFC is dependent on THC history, which might have functional implications in addiction and neuropsychiatric disease.
Keywords: : amygdala, cannabinoid, cyclin-dependent kinase 5, dopamine receptor, nucleus accumbens, prefrontal cortex
Introduction
Δ9-tetrahydrocannabinol (THC), cannabis's primary psychoactive constituent, modulates behavior by activating brain cannabinoid type 1 receptors (CB1Rs).1,2 Repeated cannabis use can produce dependence and is associated with cognitive impairment and psychosis.3,4 Repeated THC administration in rodents produces adaptation in CB1R signaling and/or expression5,6 and induces transcription factors, including ΔFosB,7 in brain. ΔFosB is a C-terminally truncated splice variant of FosB, which confers stability and allows accumulation with repeated drug administration.8 THC,9,10 other abused drugs, and natural rewards8,11 induce ΔFosB in forebrain dopaminergic fields. Transgenic overexpression of ΔFosB in the striatum enhanced the rewarding effects of cocaine and morphine, indicating that ΔFosB-mediated regulation of target genes alters drug effects after repeated administration.8,12 Moreover, ΔFosB induction by acute drug administration can be enhanced by prior repeated drug exposure. For example, cocaine administration to mice with a history of cocaine treatment showed enhanced ΔFosB induction compared with drug-naive mice receiving an acute cocaine injection, a finding linked to epigenetic priming mechanisms.13,14
ΔFosB dimerizes with Jun proteins to form activator protein-1 (AP-1) complexes that regulate transcription.8 Several ΔFosB target genes, including cyclin-dependent kinase 5 (Cdk5) and its coactivator p35, are increased in the nucleus accumbens (NAc) of transgenic ΔFosB overexpressing or repeated cocaine-treated mice.15 Cdk5 and p35 regulate synaptic plasticity, neurotransmitter release, and dopamine signaling,16,17 which could regulate processes implicated in drug abuse and neuropsychiatric disorders. Cdk5 also phosphorylates dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) at threonine 75 (T75), which inhibits dopamine type 1 receptor (D1R)-mediated protein kinase A (PKA) activity.18 THC-mediated regulation of Cdk5 has not been defined, but similarities in ΔFosB induction among abused drugs suggest that downstream targets might overlap. These studies showed that a history of repeated THC treatment produced an apparent priming of ΔFosB induction in the prefrontal cortex (PFC) and NAc, but this effect was associated with increased Cdk5 expression only in PFC. These findings suggest that repeated THC exposure could affect plasticity in the PFC and have implications for drug abuse and neuropsychiatric disorders.
Materials and Methods
Male C57Bl/6J mice (8 weeks; Jackson Laboratories, Indianapolis, IN) were housed four to six/cage (12-h light/dark cycle at 20–22°C) with food and water ad libitum. THC (10 mg/kg; NIDA Drug Supply Program, Rockville, MD) was dissolved in 1:1:18 ethanol, emulphor, and saline (vehicle). Mice were injected subcutaneously with vehicle (VEH) or THC at 07:00 h and 16:00 h for 13 days. On day 14, VEH- and THC-treated groups were divided into half and received a single injection of either VEH or THC (10 mg/kg) to produce four groups: VEH-VEH (VEH control), VEH-THC (acute THC), THC-VEH (repeated THC), and THC-THC (THC challenge after repeated THC). Brains were collected 45 min later to maximize observation of FosB/ΔFosB messenger RNA (mRNA) and DARPP-32 phosphorylation.13,19 PFC, NAc, caudate-putamen (CPu), and amygdala (Amyg) were dissected as published.9,20 Experiments were approved by VCU IACUC in accordance with the NIH Guide for Care and Use of Laboratory Animals.
Immunoblot
Immunoblotting was performed as published.20 Tissue was homogenized and loaded in 10% or 12% (Cdk5, p35, and p25) Tris-HCl gels and separated by electrophoresis. Gels were transferred onto nitrocellulose paper, blocked, and incubated in antibody in 0.1 M Tris-buffered saline (0.9%; pH 7.4; TBS) containing 0.1% Tween-20 (TBST) with 5% nonfat dry milk or 5% bovine serum albumin (for phosphorylated proteins). Each protein and respective control (total protein or α-tubulin) was observed on a separate membrane except for Cdk5, p35, and p25, which were observed on the same membrane. Antibodies are provided in Supplementary Table S1. Blots were washed in TBST and incubated with appropriate IRDye®-labeled secondary antibodies (LI-COR, Lincoln, NE) in TBST for 45 min. Fluorescent intensity was observed with the Odyssey LI-COR infrared scanner.20
Quantitative reverse transcriptase polymerase chain reaction
RNA was extracted from tissue in Trizol® and homogenized. RNA (5 μg) was converted into complementary DNA (cDNA) using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Inc., Foster City, CA). cDNA (10 ng) was added to master mix from QuantiFast® SYBR® Green polymerase chain reaction kit (Qiagen, Valencia, CA), with specific primers based on publications (FosB,21 ΔFosB,21 and β-actin22) at a final concentration of 0.4 μM and water in a 25 μl final volume. Samples without cDNA were no template controls. Samples were placed in a BioRad real-time thermocycler programed to a two-step cycling protocol, with a melt curve step at the end of the reaction. Cycle threshold (Ct) values were normalized to ΔCt values by subtracting sample Ct values from internal control (β-actin) Ct values. Data were further converted to ΔΔCt values relative to VEH-VEH control and final mRNA quantification was calculated: 2^(−ΔΔCt)×100=% mRNA expression.
Analysis
Data were analyzed with Prism® version 6.0 (GraphPad Software, San Diego, CA) using two-way analysis of variance with Dunnett's post hoc test. Data were normalized to the VEH-VEH group and presented as %VEH control±standard error of the mean. To determine differences in expression between acute THC (VEH-THC) and THC challenge after repeated THC (THC-THC) for ΔFosB mRNA and protein, data were expressed as acute THC [(VEH-THC) − (VEH-VEH)] and THC challenge [(THC-THC) − (THC-VEH)]. These data were analyzed by Student's t-test and are presented in Table 1. For all studies, significance was determined with p<0.05.
Table 1.
Net Change in FosB/ΔFosB Messenger RNA or Protein After Acute Δ9-Tetrahydrocannabinol Administration After Repeated Vehicle or Repeated Δ9-Tetrahydrocannabinol Treatment
| FosB mRNA | ΔFosB mRNA | ΔFosB protein | ||||
|---|---|---|---|---|---|---|
| Brain region | Acute THC | THC challenge | Acute THC | THC challenge | Acute THC | THC challenge |
| Prefrontal cortex | 95±36 | 12±26 | 1%±10 | 60±22a | 4±12 | 41±6a |
| Nucleus accumbens | −8±5 | 5±5 | 5%±3 | 43±9b | 11±8 | 17±13 |
| Caudate-putamen | −20±8 | −57±10a | −4%±8 | 15±22 | 4±7 | −15±11 |
| Amygdala | −29±22 | 38±10a | −39%±19 | 26±11a | 22±15 | 37±10 |
Data represent the net difference from respective repeated treatment for acute THC (VEH-THC − VEH-VEH) and THC challenge (THC-THC − THC-VEH), expressed as %VEH-VEH derived from Figures 1 and 2.
p<0.05, bp<0.01 different from acute THC by two-tailed Student's t-test (n=5–8 mice per treatment group).
mRNA, messenger RNA; THC, Δ9-tetrahydrocannabinol; VEH, vehicle.
Results
THC differentially induced ΔFosB expression in the forebrain depending on THC history
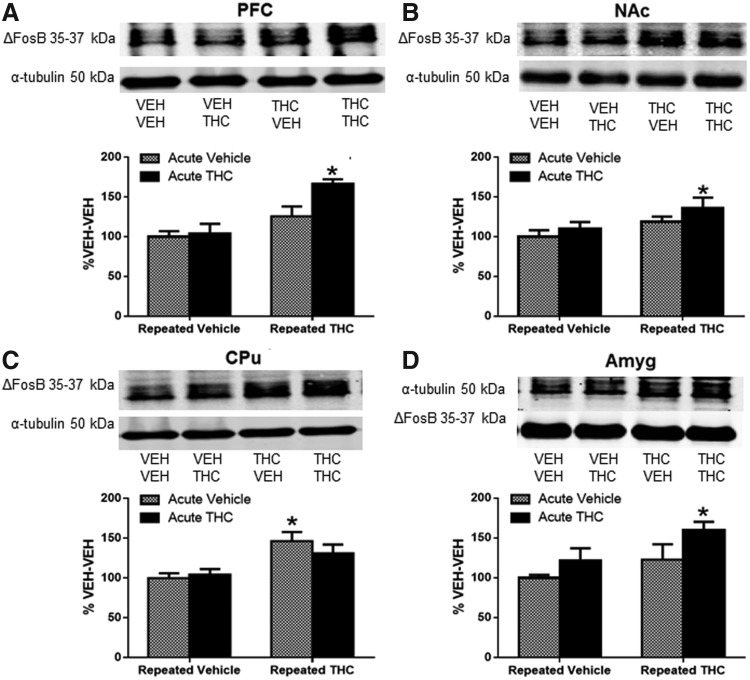
ΔFosB-ir was significantly increased in forebrain regions of all mice that received repeated THC, as indicated by a significant main effect of repeated THC treatment (statistical analyses presented in figure legends). There was also a main effect of THC challenge in both PFC and amygdala. Post hoc tests showed that THC challenge increased ΔFosB expression only in mice that had previously received repeated THC, which significantly differed compared with vehicle control mice in the PFC (66%, p<0.05, Fig. 1A), NAc (36%, p<0.05, Fig. 1B), and amygdala (60%, p<0.05, Fig. 1D). In CPu, post hoc analysis showed a significant increase in ΔFosB only in repeated THC-treated mice compared with VEH control mice (46%, p<0.05, Fig. 1C), although the THC-THC group approached significance (p=0.06).
FIG. 1.
ΔFosB protein increased in all regions examined after repeated THC treatment. Graphs show the effect of challenge with acute vehicle or THC on ΔFosB protein in repeated vehicle- or THC-treated mice in (A) PFC, (B) NAc, (C) CPu, and (D) Amyg. Data are expressed as percentage protein in repeated vehicle-treated mice that received vehicle challenge (%VEH-VEH). (A) Significant main effect of acute treatment [F(1,27)=4.64, p<0.05] and significant main effect of repeated treatment [F(1,27)=17.84, p<0.001] in PFC; (B) significant main effect of repeated treatment [F(1,27)=5.92, p<0.05] in NAc; (C) significant main effect of repeated treatment [F(1,28)=15.26, p<0.001] in CPu; and (D) significant main effect of acute treatment [F(1,19)=4.89, p<0.05] and repeated treatment [F(1,19)=5.09, p<0.05] in Amyg. Significance was determined with two-way ANOVA and Dunnett's post hoc test. *p<0.05 compared with VEH-VEH-treated mice. Data are presented as mean±SEM from seven to eight mice per treatment group. Amyg, amygdala; ANOVA, analysis of variance; CPu, caudate-putamen; NAc, nucleus accumbens; PFC, prefrontal cortex; SEM, standard error of the mean; THC, Δ9-tetrahydrocannabinol; VEH, vehicle.
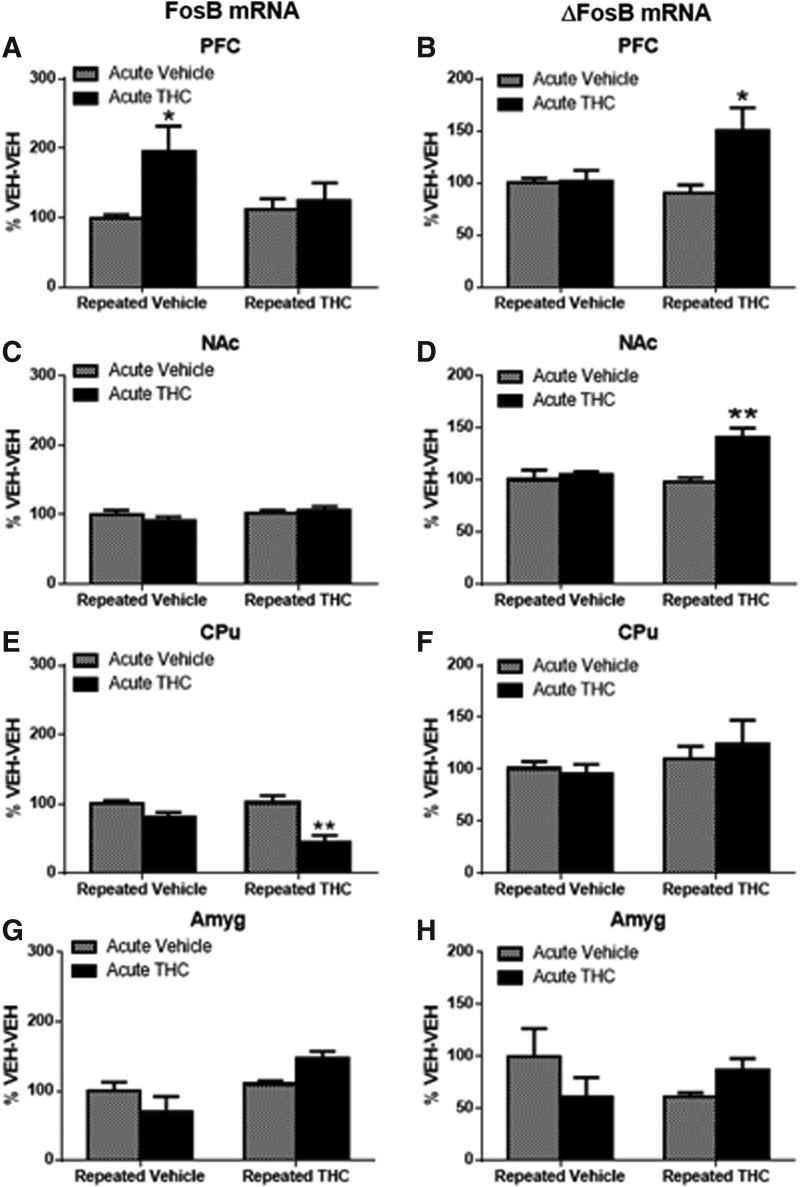
ΔFosB is produced by alternative splicing of FosB mRNA. Therefore, FosB and ΔFosB mRNA were examined in the same experimental groups as ΔFosB protein. FosB mRNA was significantly increased in PFC after acute THC (96% in VEH-THC group, p<0.05, Fig. 2A) and decreased in CPu after THC challenge in repeated THC-treated mice (−56% in THC-THC group, p<0.01, Fig. 2E). No other significant differences were found in FosB mRNA. THC challenge after repeated THC treatment increased ΔFosB mRNA by 51% in PFC (p<0.05, Fig. 2B) and 41% in NAc (p<0.05, Fig. 2D). These results indicate that THC challenge induced FosB in PFC of repeated VEH-treated mice, but not mice with a history of THC. In contrast, ΔFosB mRNA was significantly induced in PFC and NAc only after THC challenge in mice with a history of repeated THC treatment.
FIG. 2.
FosB mRNA increased after acute THC in PFC, whereas ΔFosB mRNA increased in both PFC and NAc after THC challenge in repeated THC-treated mice. Graphs show the effect of challenge with acute vehicle or THC on FosB mRNA (A, C, E, G) and ΔFosB mRNA (B, D, F, H) in repeated vehicle- or THC-treated mice expressed as percentage of the VEH-VEH-treated group. (A) Significant main effect of acute treatment in PFC [F(1,19)=5.766, p<0.05]; (B) significant interaction [F(1,18)=6.07, p<0.05] in PFC; (D) significant interaction [F(1,20)=7.360, p<0.05] in NAc; and (E) significant main effect of repeated treatment [F(1,12)=19.77), p<0.001] in CPu. (A–H) Significance was determined with two-way ANOVA and Dunnett's post hoc test. *p<0.05, **p<0.01 compared with VEH-VEH-treated mice. mRNA, messenger RNA.
These data suggest that THC-induced regulation of FosB/ΔFosB mRNA and ΔFosB protein differs depending on THC history. The effect of THC history was compared by calculating net differences in FosB mRNA, ΔFosB mRNA, and ΔFosB protein between acute THC or THC challenge after repeated THC and the respective vehicle control in each region (Table 1). In CPu, THC challenge after repeated THC significantly decreased FosB mRNA compared with acute THC (p<0.05). In amygdala, both FosB and ΔFosB mRNA were decreased by acute THC, but increased after THC challenge in repeated THC-treated mice (p<0.05). In PFC (p<0.05) and NAc (p<0.01), increases in ΔFosB mRNA were significantly greater after THC challenge after repeated THC when compared with an acute THC injection, but this translated into a significant further increase in ΔFosB protein only in PFC (p<0.05).
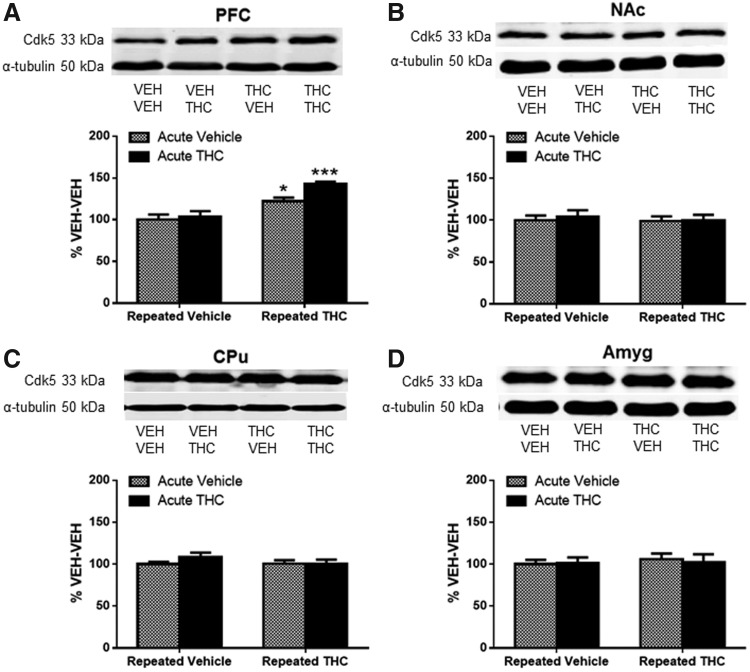
Repeated THC increased Cdk5 and p35 in PFC
Cdk5-ir was measured in the same regions of the four treatment groups as ΔFosB. Results in PFC showed a significant main effect of repeated THC treatment. Cdk5-ir was significantly increased by 22% in mice that received repeated THC (p<0.05, Fig. 3A) and by 43% after THC challenge in repeated THC-treated mice (p<0.001, Fig. 3A) as compared with vehicle control mice using post hoc tests. In contrast, there were no significant differences in Cdk5-ir between any treatment groups in NAc (Fig. 3B), CPu (Fig. 3C), or amygdala (Fig. 3D).
FIG. 3.
Cdk5 expression increased in the PFC after repeated THC administration. Graphs show Cdk5 protein after acute vehicle or THC challenge in repeated vehicle- or THC-treated mice expressed as percentage Cdk5 expression in VEH-VEH mice in (A) PFC, (B) NAc, (C) CPu, and (D) Amyg. (A) Significant main effect of repeated treatment [F(1,25)=29.79, p<0.001] and main effect of acute treatment [F(1,25)=4.67, p<0.05] in PFC. Significance was determined with two-way ANOVA and Dunnett's post hoc test. *p<0.05, ***p<0.001 compared with VEH-VEH-treated mice. Data are presented as mean±SEM from N=5–8 mice per treatment group. Cdk5, cyclin-dependent kinase 5.
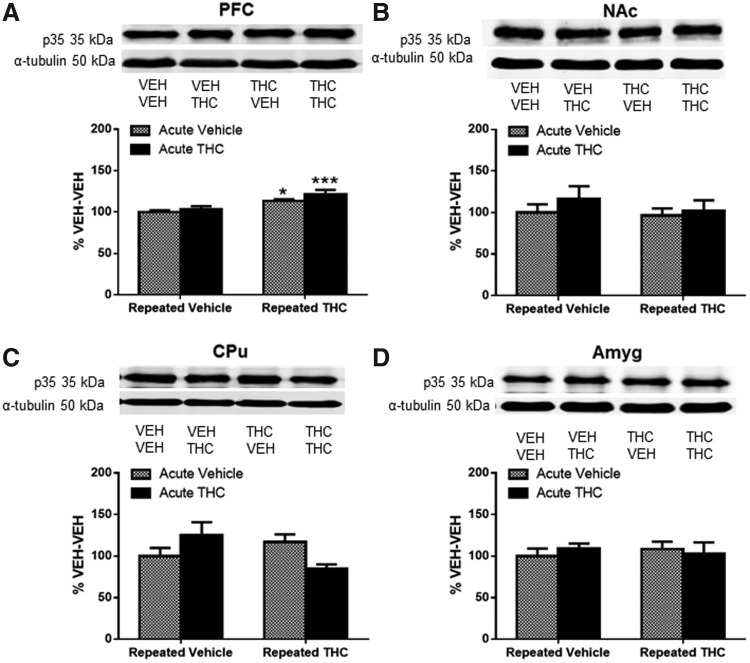
Cdk5 activation requires association with the coactivator p35 or its truncated form p25.21 Analysis in PFC revealed a significant main effect of repeated THC treatment on p35-ir. Expression of p35-ir was significantly increased by repeated THC (14% increase, p<0.05, Fig. 4A) and after THC challenge in repeated THC-treated mice (21% increase, p<0.001, Fig. 4A) compared with vehicle control as shown by post hoc analysis. There were no significant differences in p35-ir between treatment groups in NAc (Fig. 4B), CPu (Fig. 4C), or amygdala (Fig. 4D). The levels of p25-ir did not significantly differ between groups in any region (Supplementary Fig. S1).
FIG. 4.
Expression of p35 increased in the PFC after repeated THC administration. Graphs show p35 protein after acute vehicle or THC challenge in repeated vehicle- or THC-treated mice expressed as percentage of p35 expression in VEH-VEH-treated mice in (A) PFC, (B) NAc, (C) CPu, and (D) Amyg. (A) Significant main effect of repeated treatment [F(1, 24)=18.07, p<0.001] in PFC. Significance was determined with two-way ANOVA and Dunnett's post hoc test. *p<0.05, ***p<0.001 compared with VEH-VEH-treated mice. Data are presented as mean±SEM from N=5–8 mice per group.
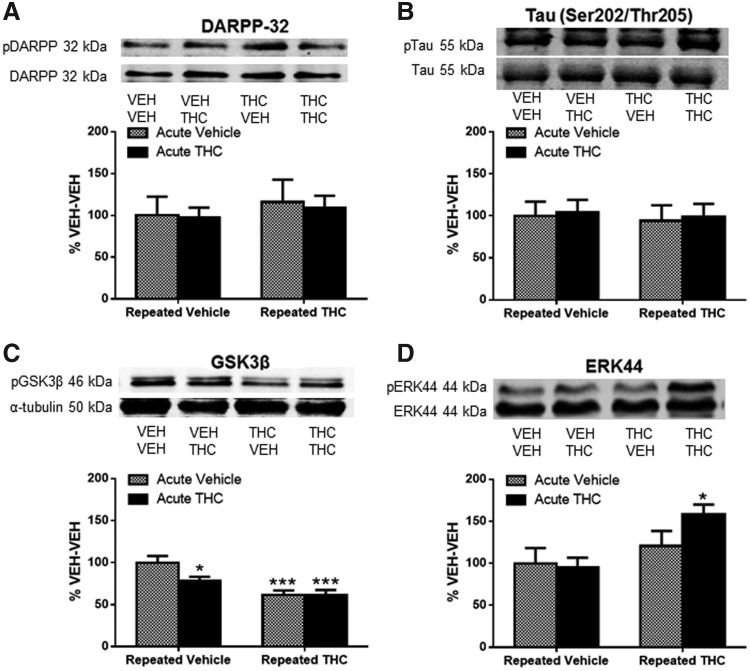
Repeated THC decreased pGSK3β and increased pERK1 in PFC
Phosphorylation of Ser9 of glycogen synthase kinase-3β (GSK3β) was significantly decreased after acute THC (22% decrease, p<0.05, Fig. 5C), repeated THC (38% decrease, p<0.001), and in mice that received THC challenge after repeated THC (38% decrease, p<0.001). Extracellular signal-regulated kinase (ERK) phosphorylation at Thr202/Tyr204 was significantly increased by 58% in mice challenged with THC after repeated THC (p<0.05, Fig. 5D), but not after acute THC. Neither DARPP-32 nor tau phosphorylation was affected by THC in the PFC (Fig. 5A, B).
FIG. 5.
Phosphorylation of GSK3β and ERK was altered, whereas phosphorylation of DARPP-32 and tau was unchanged in the PFC of THC-treated mice. Graphs show expression of (A) pThr75 DARPP-32, (B) pSer202/The205 Tau, (C) pSer9 GSK3β, and (D) pERK in the PFC after acute vehicle or THC challenge in repeated vehicle- or THC-treated mice expressed as %VEH-VEH controls. (C) Significant main effect of repeated treatment [F(1, 28)=20.41, p<0.001] on pGSK3β, and (D) significant main effect of repeated treatment [F(1,28)=7.56, p<0.05] on pERK. Significance was determined with two-way ANOVA and Dunnett's post hoc test. *p<0.05 and ***p<0.001 compared with VEH-VEH-treated mice. Data are presented as mean±SEM from N=5–8 mice per treatment group. DARPP-32, dopamine- and cAMP-regulated phosphoprotein of 32 kDa; ERK, extracellular signal-regulated kinase; GSK3β, glycogen synthase kinase-3β.
Discussion
This study revealed two novel major findings. First, a history of repeated THC treatment enhanced acute THC-mediated increases in ΔFosB mRNA in PFC and NAc. We previously reported that repeated THC treatment induced ΔFosB protein in PFC, NAc, CPu, and amygdala20,23 as replicated here, and prior studies showed that repeated treatment with psychostimulants24 or morphine25 induced ΔFosB in these same regions. Second, induction of ΔFosB by repeated THC treatment was associated with increased expression of Cdk5 and p35 only in PFC. Prior studies showed that Cdk5 was a target of ΔFosB in the NAc after psychostimulant treatment,15 whereas THC did not induce Cdk5 in NAc. These findings demonstrate regional differences in the induction of downstream targets of ΔFosB between these two drug classes.
ΔFosB was not induced by acute THC in any region, similar to findings after acute morphine25 or cocaine24 administration. However, the finding of enhanced induction of ΔFosB mRNA and ΔFosB protein after repeated THC in PFC and NAc suggests that repeated THC treatment might prime FosB gene induction. A previous study showed that although acute cocaine injection induced both FosB and ΔFosB mRNA in the NAc, only induction of ΔFosB mRNA was significantly enhanced in the NAc of cocaine-experienced mice challenged with cocaine.13 Previous cocaine exposure appeared to prime ΔFosB induction by chromatin modifications at the FosB/ΔFosB promoter.13 The mechanism by which repeated THC enhanced ΔFosB induction is not known. Increases in both ΔFosB and FosB mRNA would be predicted if THC induced chromatin modifications of the FosB gene, as seen after cocaine treatment. However, FosB mRNA was increased in PFC after THC injection in drug-naive, but not in THC-experienced, mice. Therefore, it is possible that this apparent priming effect in THC-experienced mice occurs at the post-transcriptional level, possibly by differential degradation of FosB versus ΔFosB mRNA.21,26
FosB/ΔFosB gene induction by THC in CPu and amygdala differed from PFC and NAc. In CPu, ΔFosB was induced by repeated THC, but there was no enhancement with THC challenge, nor was ΔFosB mRNA induced under any THC treatment condition. FosB or ΔFosB mRNA induction was not expected in mice treated repeatedly with THC and challenged with vehicle because FosB and ΔFosB mRNA degrade to control levels by 12 h after drug injection,21 and tissue was collected 24 h after THC injection. However, FosB mRNA was significantly decreased in CPu after THC injection in THC-experienced mice. Partial desensitization of FosB induction was previously shown in the striatum with repeated amphetamine treatment,21 but here we observed repression of FosB by THC challenge in drug-experienced mice. In amygdala, ΔFosB was elevated by THC challenge in THC-experienced mice, but ΔFosB mRNA was not significantly different from mice treated with vehicle only. However, analysis of net FosB and ΔFosB induction by THC challenge in THC-experienced mice showed significant induction of both transcripts relative to the levels in drug-naive mice receiving THC acutely. This effect was probably because THC injection had small but opposite effects on FosB/ΔFosB mRNA in THC-naive (decreased) versus THC-experienced (increased) mice. These findings indicate bidirectional regulation of FosB/ΔFosB transcription by THC in CPu and amygdala, dependent on drug history.
ΔFosB transcriptionally regulates the expression of Cdk5 and p3527–30; therefore, these proteins were also examined in brains from THC-treated mice. Acute THC injection did not alter Cdk5 in drug-naive mice, whereas repeated THC increased Cdk5 expression in PFC, which was further augmented by THC challenge. Acute THC also enhanced ΔFosB mRNA in the PFC of THC-experienced, but not drug-naive, mice, which suggests that THC-induced enhancement of Cdk5 expression might occur through ΔFosB-regulated transcription, as previously shown in the striatum of cocaine-treated animals.15,30 The reason that Cdk5 is induced in different regions by THC versus cocaine is not known. ΔFosB induction by both THC and psychostimulants requires D1R activation,20,24 suggesting a common upstream site of action. Both drug classes induce ΔFosB in D1R/dynorphin-expressing medium spiny neurons (MSNs) in the NAc.11,20 Thus, differential induction of the Cdk5 pathway cannot be explained by target cell type alone, and likely depends on the specific signaling pathways modulated in these cells by each drug class.
ERK could be linked to the particular responsiveness of ΔFosB induction in the PFC to prior THC experience, and potentially coupling to the Cdk5 pathway. Phosphorylation of ERK was significantly enhanced in PFC after THC challenge in THC-experienced mice, similar to ΔFosB induction in this region. ERK activation is necessary for ΔFosB induction,31 which could explain the enhanced induction of ΔFosB in PFC of THC-experienced mice. A previous study did not find enhanced ERK phosphorylation in PFC after repeated THC administration for 6.5 days,32 but it is possible that a longer duration of repeated THC treatment is required, as in this study. We found that acute THC did not activate ERK in PFC, in agreement with prior studies,32,33 although one study showed acute THC activated ERK in this region.34 However, that response was seen only at lower THC doses (<1 mg/kg).
The Cdk5 coactivator p35 was increased in the PFC after repeated THC administration, suggesting that downstream targets of Cdk5 might also be regulated. DARPP-32 is phosphorylated at T75 by Cdk5, but was not affected by THC. This agrees with studies showing no effect of CP55,940 on phosphorylation of T75 DARPP-32.35 THC-induced increases in Cdk5 and p35 occurred concomitantly with attenuated phosphorylation of GSK3β in the PFC, but GSK3β phosphorylation was also significantly decreased after acute THC administration. Cdk5 inhibits activity of GSK3β by increasing its phosphorylation, possibly by inhibition of protein phosphatase 1,36,37 providing a possible link between these observations. However, while THC increased Cdk5 expression it decreased inhibitory phosphorylation of GSK3β, so it is likely that THC affects GSK3β phosphorylation in the PFC through an alternative mechanism. Dysregulation of GSK3β-related signaling has been implicated in schizophrenia,38 which is interesting given the potential role of cannabinoids in cognitive impairment and psychosis. Although Cdk5 and GSK3β phosphorylate tau, phosphorylation of tau at Ser202/Thr205 was unaffected by THC. This result agrees with our finding that p25, which is more closely associated with tau phosphorylation, was not altered in PFC.
This repeated THC paradigm also desensitized CB1R-mediated G-protein activation in the PFC and amygdala and produced tolerance to hypolocomotion, antinociception, and hypothermia, but did not produce desensitization in CPu or NAc or tolerance to catalepsy.9,20 We have also reported that ΔFosB induction correlated inversely with CB1R desensitization as a function of brain region after repeated THC treatment.9 Moreover, inducible transgenic expression of ΔFosB in D1R-expressing striatal MSNs inhibited CB1R desensitization, whereas similar expression of a dominant negative inhibitor of AP-1-mediated transcription (ΔcJun) in striatum enhanced CB1R desensitization, which was associated with enhanced tolerance to the locomotor suppressive action of THC.23 Thus, THC-induced changes in the ΔFosB pathway in the PFC could modulate CB1R adaptation. Alternatively, changes in signaling proteins in the PFC could induce neuroadaptations independent of CB1R regulation. For example, Cdk5 can induce dendritic spine outgrowth in striatum39 and cannabinoids have been shown to modulate dendritic branching.40–42
PFC dysregulation has been associated with addiction, cognitive impairment, and schizophrenia.43,44 Cannabinoids can produce psychotic-like symptoms and cognitive impairment acutely, in part through regulation of PFC function.3,44 Some studies have suggested an association between cannabis use and schizophrenia,3,4 although underlying mechanisms are not well defined. Our finding that repeated THC treatment induced ΔFosB and Cdk5, and that THC challenge in repeated THC-treated mice further enhanced expression, provides one possible candidate mechanism. In fact, virally mediated overexpression of ΔFosB in the medial PFC of rodents impaired prepulse inhibition and inhibitory avoidance, which have been used to model negative behavioral outcomes of schizophrenia.45
Conclusion
Repeated THC administration increased ΔFosB in all regions examined, but increased Cdk5/p35 expression only in the PFC. THC treatment history is an important factor, because ΔFosB mRNA was significantly induced in PFC and NAc by THC challenge only in mice previously treated with repeated THC. These data are consistent with functional priming of ΔFosB induction by prior THC experience. ΔFosB induction in the PFC occurred in conjunction with induction of the Cdk5 pathway, which is a downstream target of ΔFosB. In this regard, THC differed from psychostimulants, which induced the Cdk5 pathway only in NAc. THC-mediated induction of ΔFosB and the Cdk5 pathway in PFC provide a potential mechanism by which cannabinoids might alter PFC functions associated with addiction and other neuropsychiatric disorders.
Supplementary Material
Abbreviations Used
- AP-1
activator protein-1
- CB1R
cannabinoid type 1 receptor
- Cdk5
cyclin-dependent kinase 5
- cDNA
complementary DNA
- CPu
caudate-putamen
- Ct
cycle threshold
- D1R
dopamine type 1 receptor
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of 32 kDa
- ERK
extracellular signal-regulated kinase
- GSK3β
glycogen synthase kinase-3β
- mRNA
messenger RNA
- MSN
medium spiny neuron
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- PKA
protein kinase A
- T75
threonine 75
- THC
Δ9-tetrahydrocannabinol
- VEH
vehicle
Acknowledgment
This study was supported by U.S. Public Health Service Grants DA030404, DA014277, and F31 DA030227.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202 [DOI] [PubMed] [Google Scholar]
- 2.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180 [DOI] [PubMed] [Google Scholar]
- 3.Radhakrishnan R, Wilkinson ST, D'Souza DC. Gone to pot—a review of the association between cannabis and psychosis. Front Psychiatry. 2014;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370:2219–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin BR, Sim-Selley LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci. 2004;25:325–330 [DOI] [PubMed] [Google Scholar]
- 6.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15:91–119 [DOI] [PubMed] [Google Scholar]
- 7.Lazenka MF, Selley DE, Sim-Selley LJ. Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci. 2013;92:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClung CA, Ulery PG, Perrotti LI, et al. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–154 [DOI] [PubMed] [Google Scholar]
- 9.Lazenka MF, Selley DE, Sim-Selley LJ. DeltaFosB induction correlates inversely with CB(1) receptor desensitization in a brain region-dependent manner following repeated Delta(9)-THC administration. Neuropharmacology. 2014;77:224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perrotti LI, Weaver RR, Robison B, et al. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobo MK, Zaman S, Damez-Werno DM, et al. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zachariou V, Bolanos CA, Selley DE, et al. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211 [DOI] [PubMed] [Google Scholar]
- 13.Damez-Werno D, LaPlant Q, Sun H, et al. Drug experience epigenetically primes Fosb gene inducibility in rat nucleus accumbens. J Neurosci. 2012;32:10267–10272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bibb JA, Chen J, Taylor JR, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380 [DOI] [PubMed] [Google Scholar]
- 16.Benavides DR, Bibb JA. Role of Cdk5 in drug abuse and plasticity. Ann N Y Acad Sci. 2004;1025:335–344 [DOI] [PubMed] [Google Scholar]
- 17.Su SC, Tsai LH. Cyclin-dependent kinases in brain development and disease. Annu Rev Cell Dev Biol. 2011;27:465–491 [DOI] [PubMed] [Google Scholar]
- 18.Nairn AC, Svenningsson P, Nishi A, et al. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47 Suppl 1:14–23 [DOI] [PubMed] [Google Scholar]
- 19.Borgkvist A, Marcellino D, Fuxe K, et al. Regulation of DARPP-32 phosphorylation by Delta9-tetrahydrocannabinol. Neuropharmacology. 2008;54:31–35 [DOI] [PubMed] [Google Scholar]
- 20.Lazenka MF, Tomarchio AJ, Lichtman AH, et al. Role of dopamine type 1 receptors and dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa in delta9-tetrahydrocannabinol-mediated induction of DeltaFosB in the mouse forebrain. J Pharmacol Exp Ther. 2015;354:316–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alibhai IN, Green TA, Potashkin JA, et al. Regulation of fosB and DeltafosB mRNA expression: in vivo and in vitro studies. Brain Res. 2007;1143:22–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimaldi C, Capasso A. Role of lipid rafts/caveolae in the anticancer effect of endocannabinoids. Mini Rev Med Chem. 2012;12:1119–1126 [DOI] [PubMed] [Google Scholar]
- 23.Lazenka MF, David BG, Lichtman AH, et al. Delta FosB and AP-1-mediated transcription modulate cannabinoid CB receptor signaling and desensitization in striatal and limbic brain regions. Biochem Pharmacol. 2014;91:380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nye HE, Hope BT, Kelz MB, et al. Pharmacological studies of the regulation of chronic FOS-related antigen induction by cocaine in the striatum and nucleus accumbens. J Pharmacol Exp Ther. 1995;275:1671–1680 [PubMed] [Google Scholar]
- 25.Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645 [PubMed] [Google Scholar]
- 26.Marinescu V, Loomis PA, Ehmann S, et al. Regulation of retention of FosB intron 4 by PTB. PLoS One. 2007;2:e828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Zhang Y, Kelz MB, et al. Induction of cyclin-dependent kinase 5 in the hippocampus by chronic electroconvulsive seizures: role of [Delta]FosB. J Neurosci. 2000;20:8965–8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bibb JA, Snyder GL, Nishi A, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671 [DOI] [PubMed] [Google Scholar]
- 29.Peakman MC, Colby C, Perrotti LI, et al. Inducible, brain region-specific expression of a dominant negative mutant of c-Jun in transgenic mice decreases sensitivity to cocaine. Brain Res. 2003;970:73–86 [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Choi KH, Renthal W, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314 [DOI] [PubMed] [Google Scholar]
- 31.Fasano S, D'Antoni A, Orban PC, et al. Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol Psychiatry. 2009;66:758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubino T, Forlani G, Vigano D, et al. Modulation of extracellular signal-regulated kinases cascade by chronic delta 9-tetrahydrocannabinol treatment. Mol Cell Neurosci. 2004;25:355–362 [DOI] [PubMed] [Google Scholar]
- 33.Valjent E, Pages C, Herve D, et al. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836 [DOI] [PubMed] [Google Scholar]
- 34.Rubino T, Sala M, Vigano D, et al. Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Delta9-tetrahydrocannabinol in rats. Neuropsychopharmacology. 2007;32:2036–2045 [DOI] [PubMed] [Google Scholar]
- 35.Andersson M, Usiello A, Borgkvist A, et al. Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J Neurosci. 2005;25:8432–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morfini G, Szebenyi G, Brown H, et al. A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 2004;23:2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plattner F, Angelo M, and Giese KP. The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J Biol Chem. 2006;281:25457–25465 [DOI] [PubMed] [Google Scholar]
- 38.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norrholm SD, Bibb JA, Nestler EJ, et al. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolb B, Gorny G, Limebeer CL, et al. Chronic treatment with Delta-9-tetrahydrocannabinol alters the structure of neurons in the nucleus accumbens shell and medial prefrontal cortex of rats. Synapse. 2006;60:429–436 [DOI] [PubMed] [Google Scholar]
- 41.Carvalho AF, Reyes BA, Ramalhosa F, et al. Repeated administration of a synthetic cannabinoid receptor agonist differentially affects cortical and accumbal neuronal morphology in adolescent and adult rats. Brain Struct Funct. 2016;221:407–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubino T, Prini P, Piscitelli F, et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis. 2015;73:60–69 [DOI] [PubMed] [Google Scholar]
- 43.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Egerton A, Allison C, Brett RR, et al. Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev. 2006;30:680–695 [DOI] [PubMed] [Google Scholar]
- 45.Dietz DM, Kennedy PJ, Sun H, et al. DeltaFosB induction in prefrontal cortex by antipsychotic drugs is associated with negative behavioral outcomes. Neuropsychopharmacology. 2014;39:538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
Cite this article as: Lazenka MF, Kang M, De DD, Selley DE, Sim-Selley LJ (2017) Δ9-Tetrahydrocannabinol experience influences ΔFosB and downstream gene expression in prefrontal cortex, Cannabis and Cannabinoid Research 2:1, 224–234, DOI: 10.1089/can.2017.0022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.