Abstract
Introduction:
The combination of traumatic brain injury (TBI) and long-bone fractures has previously been reported to lead to exuberant callus formation. The aim of this experimental study was to radiographically and biomechanically study the effect of TBI on bone healing in a mouse model.
Materials and methods:
138 female C57/Black6N mice were assigned to four groups (fracture (Fx) / TBI / combined trauma (Fx/TBI) / controls). Femoral osteotomy and TBI served as variables: osteotomies were stabilized with external fixators, TBI was induced with controlled cortical impact injury. During an observation period of four weeks, in vivo micro-CT scans of femora were performed on a weekly basis. Biomechanical testing of femora was performed ex vivo.
Results:
The combined-trauma group showed increased bone volume, higher mineral density, and a higher rate of gap bridging compared to the fracture group. The combined-trauma group showed increased torsional strength at four weeks.
Discussion:
TBI results in an increased formation of callus and mineral density compared to normal bone healing in mice. This fact combined with a tendency towards accelerated gap bridging leads to increased torsional strength. The present study underscores the empirical clinical evidence that TBI stimulates bone healing. Identification of underlying pathways could lead to new strategies for bone-stimulating approaches in fracture care.
Keywords: Traumatic Brain Injury, Fracture Healing, Polytrauma, microCT
Introduction
The effect of traumatic brain injury (TBI) in patients with concomitant long-bone fractures has long been discussed. In the 1960s, Calandriello was the first to describe a radiographically exuberant bone formation in patients with severe TBI and concomitant fractures[1]. Several clinical studies with equivocal results followed, coming to similar[2-5] or even contradictory[6] conclusions. However, none of these investigations provided definitive evidence whether this interaction leads to quicker bone healing, increased callus formation, or a certain type of heterotopic ossification. Up to now it remains unclear whether the above described phenomenon represents physiological bone healing in terms of “physiological restoration of bone tissue, structure and function after injury”[7,8] or is a side effect of other central or peripheral signaling cascades known to involve bone morphogenetic pathways.
The inherent difficulties in the evaluation of bone healing using conventional x-rays, the variety of fracture types and treatment protocols used in the clinical practice, as well as the ethical concerns associated with the repeated use of CT-scans in patients additionally complicate a quantitative analysis[9]. In an effort to overcome those restrictions, a series of experimental animal studies have been elaborated. Despite the progress that has been made in the understanding of the biological mechanisms underlying the interaction between brain and bone[10-16], the majority of the existing literature consists of animal studies that have focused on the radiographic evaluation of bone healing using conventional x-rays. In studies using more sophisticated diagnostic tools, however, the fractures were fixed by intramedullary nailing that adversely affects a detailed evaluation of fracture healing over time due to an impairment of endosteal callus formation, increased metal artifacts, and iatrogenic structural damage to the medullary cavity during implant removal.
To overcome these restrictions, we have developed a standardized mouse model combining TBI and externally fixated femoral osteotomy[17], allowing for consecutive and serial micro-CT measurements without intramedullar compromise of endosteal callus formation and the problem of metal artifacts during quantitative CT analysis. Using this new model, the aim of this study was to investigate in vivo the effect of TBI on the radiographic and biomechanical outcome of femoral bone healing in a murine trauma model.
Materials and methods
Animal care
All experiments were carried out with ethical permission according to the policies and principles established by the German Animal Welfare Act (Federal Law Gazette I, p.1094) and the National Institutes of Health Guide for Care and Use of Laboratory Animals18 and were approved by the local legal representative animal rights protection authorities (Landesamt für Gesundheit und Soziales, Berlin, G 0009/12). Female C57/Black6N mice (Charles River, Sulzfeld, Germany, n=138, age: 12-15 weeks, body weight: 20-25 g) were housed under conditions of controlled temperature (20°C±2°C) in standardized cages with a 12h light-darkness cycle and access to food and water ad libitum. Prior to study inclusion, the animals were kept in the laboratory premises for at least one week in order to minimize stress. All surgical procedures were performed on a heating pad (37°C) in spontaneously breathing animals, anesthetized with isoflurane 1.6 vol % (FORENE, Abbot, Wiesbaden, Germany) in a 2:1 mixture of N2O/O2 (0.5 and 0.3 l/min). Analgesia was ensured by subcutaneous (s.c.) application of buprenorphin 0.1 ml/kg BW (TEMGESIC, Reckitt Benckiser, Mannheim, Germany). Clindamycin (0.02 ml) was administered subcutaneously for antibiotic treatment. Postoperatively, tramadol 100 mg/ml (TRAMAL, Grünenthal, Aachen, Germany) was added to the drinking water (8 drops / 250 ml of water) for three days, and the s.c. application of buprenorphine was repeated. The weekly micro-CT examinations were performed after intra-peritoneal (i.p.) application of medetomidine 0.3 ml/kg BW (DORMITOR, Orion Pharma, Bad Homburg, Germany) and ketamine 0.6 ml/kg BW (KETAMIN, Actavis, Munich, Germany).
Experimental design
The animals were randomly assigned to four groups (fracture (Fx) group: n=30 / TBI group: n=30 / combined trauma (Fx/TBI) group: n=30 / control group: n=30) with bone injury and TBI serving as variables. Bone injury was induced with a standardized femoral osteotomy that was stabilized with an external fixator[19,20]. For the mid-diaphyseal approach to the femur, a lateral longitudinal skin incision (2 cm length) along an imaginary line from the knee to the hip joint was performed. The femoral bone was exposed by dissection of the fascia lata and by blunt preparation of the Musc. vastus lateralis and the Musc. biceps femoris, carefully sparing the sciatic nerve. The first pin hole was drilled with a fine hand-drill (diameter: 0.45 mm) just proximal to the distal metaphysis of the femur, perpendicular to the longitudinal femoral axis and cortical surface. Thereafter, serial drilling for pin placement through the connectors of the external fixator (MouseExFix, RIsystem, Davos, Switzerland) was performed, resulting in a fixation of the external fixator construct strictly parallel to the femur. Following rigid fixation, a 0.70 mm osteotomy was performed between both middle pins using a Gigli wire saw (RISystem, Davos, Switzerland). Wounds were closed with Ethilon 5-0 suture (Ethicon, Johnson&Johnson, Norderstedt, Germany).
The TBI was induced with the standardized model of “Controlled Cortical Impact Injury” (CCII)[21,22]. After animal fixation on a stereotactic device (Stoelting, Wood Dale, Illinois/USA), skin mobilization and preparation of the temporal muscle using a bipolar coagulation device were performed, followed by a craniotomy of the parietotemporal region with a micro drill and lifting of a 7x7 mm bone window. Extra care was taken to leave the dura mater intact. A standardized TBI was induced using a pneumatically-driven impactor (penetration depth 0.25 mm, impact velocity 3.5 m/s, and contact duration 150 ms). Finally, the preserved piece of cranial bone was repositioned and fixed with dental cement (Hoffmann, Berlin, Germany) in order to simulate closed brain injury. Both injuries were sequentially combined in the combined-trauma group with initial induction of CCI followed by subsequent femoral osteotomy. The control group underwent no surgical procedures.
In vivo micro-computed tomography (micro-CT) analysis
The animals in the Fx group and in the combined trauma (Fx/TBI) group underwent in vivo micro-CT examination on a weekly basis (Scanco vivaCT 40, Scanco Medical, Brüttisellen, Switzerland). At the level of the osteotomy, 212 slices (thickness 15 µm) were scanned at a resolution of 1024x1024 pixels. A volume of interest (VOI) of 1,05 mm (equivalent to 70 slices) was set, beginning with the last slice showing bone cortex not yet affected and ending with the first slice showing bone cortex not affected anymore by the osteotomy. The boundaries of the pre-existing cortices and the newly-formed callus areas where defined, using the software (Scanco software IPL version 5.15, Scanco Medical, Brüttisellen, Switzerland) to quantify the volume of the newly formed callus (mm3) and the mean mineral density (hydroxyapatite mg (HA)/cm3). We defined a threshold of 244 equaling 576.3 mg HA/cm3 in order to separate callus tissue from background noise, using established data from our institute. A semi-quantitative analysis of bone healing was performed by two independent reviewers according to the modified scoring system by Mehta et al.[23] at week three and week four postoperatively, using the sagittal and coronal reconstructions of the micro-CT scans. Each osteotomy was classified in one of the four following groups: A=complete bridging (four cortices bridged by callus), B=incomplete bridging (two to three cortices bridged by callus), C=no bridging (callus present, but no bridging visible), and D=delayed healing (rounded cortices, minimal presence of callus)[23].
Biomechanical testing
The biomechanical testing of the femora was conducted ex vivo. The femora were harvested, the external fixators were carefully removed, and the surrounding soft tissues dissected leaving a layer of soft tissue around the osteotomy site to avoid any damage to the callus. The bone was affixed through Technovit, a quick hardening 2-component plastic on a methyl meth acrylate base (MMA). It was then mounted on a biomechanical testing device (Bose ElectroForce Systems, Friedrichsdorf, Germany), and a torsional test was performed. The rate of twist was 0,6°/s while the axial preload remained constant. Digital control and set-up was performed via WinTest software (WinTest 7 Controls, ElectroForce System Group, Minnesota/USA), enabling real-time measurement of torque (torsional moment, Nmm) and angular degree (°). These parameters were used for the calculation of stiffness (Nmm/°) and torsional strength (maximum torsional moment, Nmm).
Statistical analysis
Continuous variables were expressed as means ± standard deviation (SD), whereas categorical variables were expressed as percentages (%). The Kolmogorov-Smirnov test was used in order to assess distribution normality. For parametric variables the Student t-test was used for the comparison of two groups; for non-parametric variables the Mann-Whitney or the Wilcoxon-rank test were implemented. Differences for categorical variables were assessed with the χ2-test or Fisher’s exact test. Differences were considered statistically significant if the null hypothesis could be rejected with >95% confidence (p<0.05).
Results
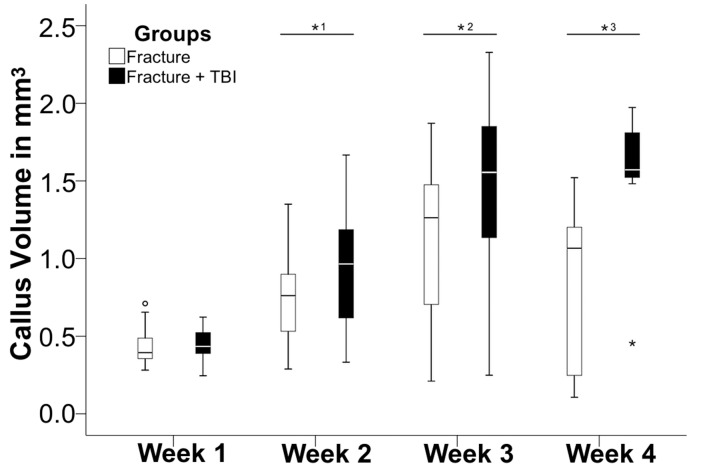
The combined trauma and the fracture group both showed an increase in callus volume over the first three weeks postoperatively, followed by a moderate decrease in week four (Figure 1). The combined trauma group already showed a significantly higher bone volume in the second week with 1,2- 1,5-fold higher values in week 3 and 4, respectively, compared to the fracture group (p<0.05, Mann-Whitney-U-test), (Figure 1).
Figure 1.
Temporal profile of evolving callus volume (in mm3) over 4 weeks, comparing fracture group (open bar) and combined trauma group (solid bar). Note the significantly (*p<0.05) increased callus volume in the combined trauma group already at two weeks, remaining increased throughout the entire study period.
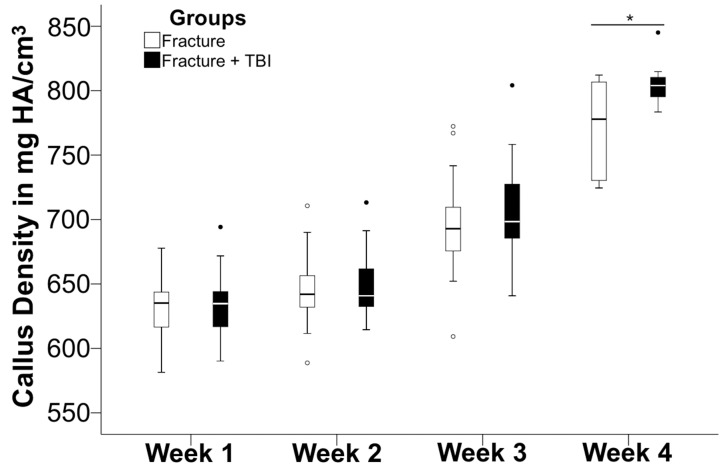
Regarding the mineral density (mg HA/cm3) of the newly formed callus, there was no significant difference as both groups showed a steady increase of mineral density, reaching the highest value after four weeks. In week four, the combined-trauma group showed a tendency towards higher values (804 mg HA/cm3) than the fracture group (778 mg HA/cm3) (p=0.053, Mann-Whitney-U test) (Figure 2).
Figure 2.
Comparison of mineral density (in mg HA/cm3) between week 1, 2, 3, and 4 following isolated fracture (Fx group; open bar) and combined trauma (Fx/TBI-group; solid bar). In week 4, the combined trauma group showed a tendency (*p=0.053) towards higher values than fracture group.
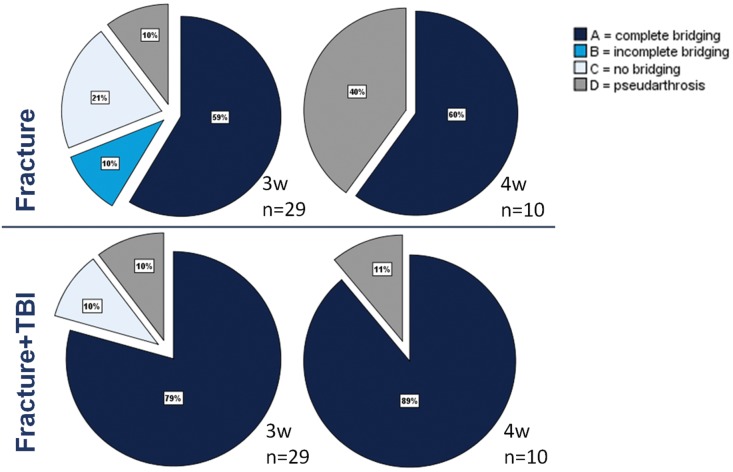
The combined trauma group compared to the fracture group showed a higher rate of osteotomy gap bridging at week three (79% vs. 59%) that further increased at week four (89% vs. 60%) (Figure 3). However, this difference did not reach statistical significance (p>0.05).
Figure 3.
Semi-quantitative evaluation of fracture gap bridging in week 3 and 4 after isolated fracture or combined trauma. A=four cortices bridged by callus, B=two to three cortices bridged by callus, C=callus present, but no bridging visible, D=rounded cortices, minimal presence of callus.
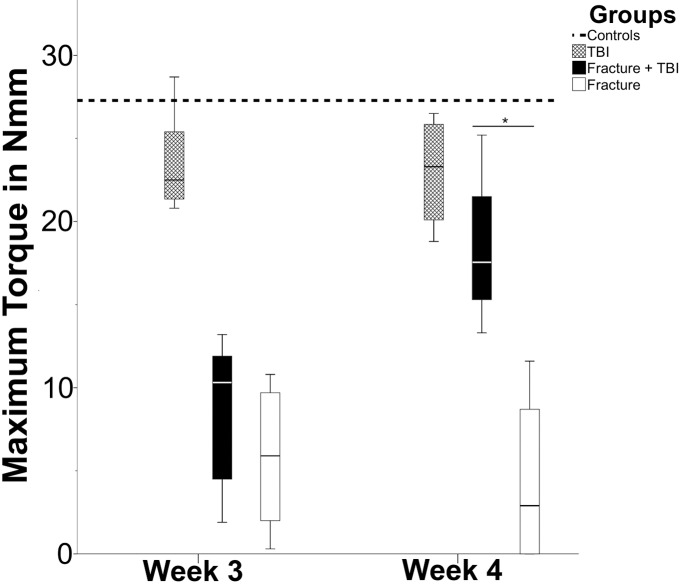
The biomechanical analysis showed increasing values for torsional strength and stiffness at week three and four in the fracture and the combined trauma group (Figures 4 and 5). In week four, torsional strength in the combined trauma group was significantly increased as opposed to the fracture group (p=0.029) while the stiffness displayed only a tendency towards increased values in the combined trauma group when compared to the fracture group (p=0.057). Interestingly, there was also a significant decrease in torsional strength (p=0.021) and stiffness (p=0.04) at week three following TBI group relative to the non-injured control group. This difference could not be detected anymore after 4 weeks.
Figure 4.
Biomechanical testing of maximum torque (in Nmm) comparing TBI group (cross-hatched bar), combined trauma group (solid bar), and Fx group (open bar). The dotted line shows the control group. In week 3, control group and TBI group show a significant difference (p<0.05), as Fx group and combined trauma group do in week 4 (*, p<0.05).
Figure 5.
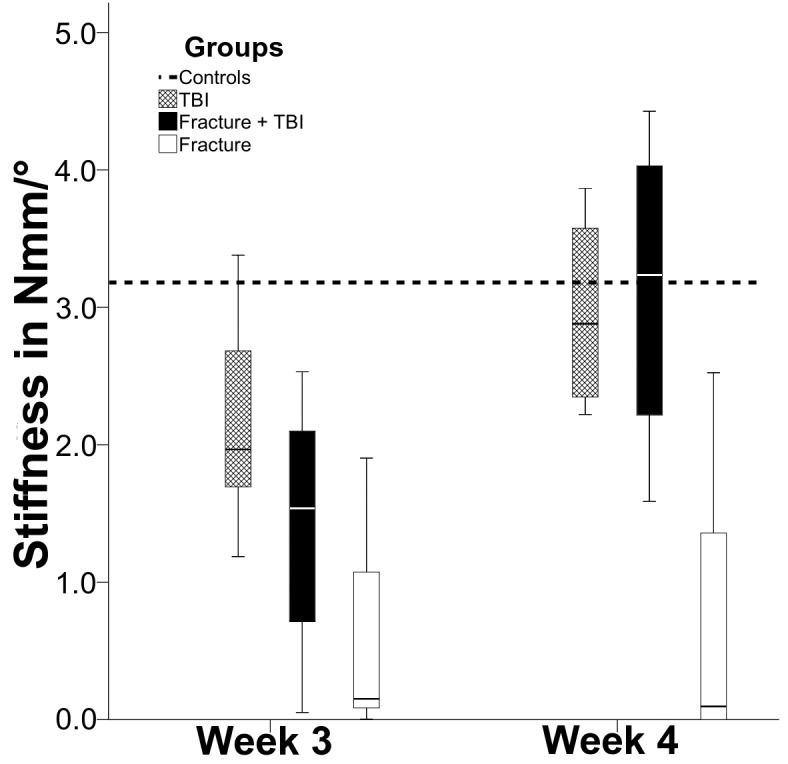
Biomechanical testing of stiffness (in Nmm/°) comparing TBI group (crosshatched bar), combined trauma group (solid bar), and Fx group (open bar). The dotted line shows the control group. Interestingly, in week 3, the control group showed a significantly higher stiffness (p=0.04) than TBI group.
Discussion
Our study provides further evidence for the long stated hypothesis that the combination of TBI and fracture results in exacerbated callus formation. In comparison to the fracture group, a significantly increased callus volume and biomechanical stability in terms of torsional strength with enhanced mineral density and higher rates of bony gap bridging was observed following fracture and concomitant TBI (combined trauma group).
In the combined trauma group of our study, the markedly increased callus volume was already observed two weeks following trauma and continued to increase further throughout the entire study period. Previous experimental and clinical studies are consistent with our findings[1-5,24-27]. Using a rat model, Boes et al. observed that three weeks after combined trauma (Fx and TBI) the callus volume of a closed femoral fracture stabilized with an intramedullary Kirschner-wire – as assessed through ex vivo measurement of the mediolateral and anteroposterior diameter of callus[26] – is markedly increased compared with the fracture-only group. In the studies of Wei and Wang the callus diameter of intramedullary fixated femoral fractures in rats was assessed in conventional x-rays with the use of the Perkins volume formula. The authors report an increase in callus volume in the combined trauma group up to week 8[24,25]. Finally, Maegele et al. used a new rat neurotrauma model and performed an ex vivo histomorphometrical evaluation of newly-formed callus in non-fixated tibia fractures after implant removal Similar to our findings, the authors demonstrated markedly increased callus mass in the combined trauma group two weeks after trauma[27].
The advantage of our study is the fact that the osteotomy gap was left untouched and was not crossed by any type of extra- or intramedullar implant, thus enabling a very precise and almost artefact-free micro-CT evaluation of bone healing over time. Repeating the in vivo micro-CT examination on a weekly basis, we were able to monitor the progress and dynamics of the temporal profile of bone healing, while additionally addressing the mineral density of the newly formed bone.
The fact that the mineral density of the callus in the combined group was equal to that of the fracture group with a tendency towards increased values at week four compared with the fracture group demonstrates that the newly formed bone is of sufficient quality. The combination of increased callus formation at a stable quality leads to increased biomechanical parameters of the combined trauma group over the fracture group.
The combined trauma group showed a tendency for higher stiffness in comparison with the fracture group after four weeks. These results are to some extent consistent with those of Boes et al., who reported significantly higher stiffness values in femora of rats having sustained combined trauma[26] at a time point of three weeks. Regarding their findings in radiographic analyses, the authors concluded fracture healing in combined trauma to be accelerated regarding load-transfer capability (e.g., stiffness), but not regarding load-carrying capability (strength). Similarly to the results of Boes et al, in the present study a difference in torsional strength after three weeks was not observed. Yet one week later, after four weeks, the combined trauma group showed significantly higher torsional strength than the fracture group, indicating continuing dynamics in bone healing (or bone remodeling) several weeks after trauma.
The qualitative analysis showed the combined trauma group to have a higher rate of fracture gap bridging both after three and four weeks compared with the fracture group. However, this difference did not reach statistical significance. Therefore, using the current model with a study period of four weeks, the question whether TBI leads to a quicker bone healing cannot be answered definitively, despite the fact that the present results are suggestive of such a conclusion.
The non-fractured femora of the TBI group, in comparison with controls, showed inferior biomechanical behavior (decreased torsional stiffness and strength three weeks after trauma), assimilating to “normal” values in the fourth week. This finding contradicts the results of Boes et al.[26], who found increased stiffness in the TBI only group, but no differences in torsional strength in rats. Whether this finding could be attributed to an actual interaction between brain and bone, or results from a temporary trauma-induced decreased mobilization of the animals with TBI, thus leading to a decline in mineral density, remains to be addressed in future studies. Nevertheless, this observation suggests that not a solitary TBI per se causes bone to develop improved biomechanical properties. Quite on the contrary: an action-counter-reaction only occurring when TBI and bone injury coincide might be the key to the above described phenomenon.
In most of the previous studies, rats have been used for the examination of the interaction between TBI and bone injury. It is now becoming increasingly clear that a mouse model offers relevant advantages over rat models due to the genetic similarity of mice to humans and the possibility to create and use knock-out species. In this context, knock-out strains have attracted major interest as they are essential in unraveling pathways known to be causatively involved in the interaction between central nervous system and skeletal repair[28].
The present study is the first to collect precise radiographic and biomechanical data from serial in vivo micro-CT measurements and biomechanical testing in the same mice for the evaluation of the stimulatory effect of TBI on bone healing over time. Our results therefore provide strong evidence for the hypothesis that TBI has a positive effect on bone healing. The confirmation of the stimulating effect of TBI on callus formation in mice raises new questions on the biochemistry underlying this phenomenon as well as on its comprehension and therapeutic use in humans on the grounds of translational research. From a clinical perspective, elucidation of the underlying biochemical and hormonal cascade leading to distinctive stimulation of bone formation could prove important for the development of future therapies, for example of delayed fracture healing and non-unions.
Footnotes
Edited by: F. Rauch
References
- 1.Calandriello B. Callus formation in severe brain injuries. Bull Hosp Joint Dis. 1964;25:170–5. [PubMed] [Google Scholar]
- 2.Spencer RF. The effect of head injury on fracture healing. A quantitative assessment. J Bone Joint Surg Br. 1987;69:525–8. doi: 10.1302/0301-620X.69B4.3611151. [DOI] [PubMed] [Google Scholar]
- 3.Perkins R, Skirving AP. Callus formation and the rate of healing of femoral fractures in patients with head injuries. J Bone Joint Surg Br. 1987;69:521–4. doi: 10.1302/0301-620X.69B4.3611150. [DOI] [PubMed] [Google Scholar]
- 4.Newman RJ, Stone MH, Mukherjee SK. Accelerated fracture union in association with severe head injury. Injury. 1987;18:241–6. doi: 10.1016/0020-1383(87)90006-4. [DOI] [PubMed] [Google Scholar]
- 5.Giannoudis PV, Mushtaq S, Harwood P, Kambhampati S, Dimoutsos M, Stavrou Z, et al. Accelerated bone healing and excessive callus formation in patients with femoral fracture and head injury. Injury. 2006;37(Suppl 3):S18–24. doi: 10.1016/j.injury.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Garland DE, Rothi B, Waters RL. Femoral fractures in head-injuries adults. Clin Orthop Relat Res. 1982:219–25. [PubMed] [Google Scholar]
- 7.Morley J, Marsh S, Drakoulakis E, Pape HC, Giannoudis PV. Does traumatic brain injury result in accelerated fracture healing? Injury. 2005;36:363–8. doi: 10.1016/j.injury.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Einhorn TA. The science of fracture healing. J Orthop Trauma. 2005;19:S4–6. doi: 10.1097/00005131-200511101-00002. [DOI] [PubMed] [Google Scholar]
- 9.Kooistra BW, Dijkman BG, Busse JW, Sprague S, Schemitsch EH, Bhandari M. The radiographic union scale in tibial fractures: reliability and validity. J Orthop Trauma. 2010;24(Suppl 1):S81–6. doi: 10.1097/BOT.0b013e3181ca3fd1. [DOI] [PubMed] [Google Scholar]
- 10.Katsnelson A. Physiology: The bones of contention. Nature. 2010;466:914–5. doi: 10.1038/466914a. [DOI] [PubMed] [Google Scholar]
- 11.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142:309–19. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 15.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–17. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 16.Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–89. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsitsilonis S, Seemann R, Misch M, Wichlas F, Haas NP, Schmidt-Bleek K, et al. The effect of traumatic brain injury on bone healing: an experimental study in a novel in vivo animal model. Injury. 2015;46:661–5. doi: 10.1016/j.injury.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 18.Council NR. Guide for the Care and Use of Laboratory Animals: Eighth Edition. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 19.Cheung KM, Kaluarachi K, Andrew G, Lu W, Chan D, Cheah KS. An externally fixed femoral fracture model for mice. J Orthop Res. 2003;21:685–90. doi: 10.1016/S0736-0266(03)00026-3. [DOI] [PubMed] [Google Scholar]
- 20.Histing T, Garcia P, Holstein JH, Klein M, Matthys R, Nuetzi R, et al. Small animal bone healing models: standards, tips, and pitfalls results of a consensus meeting. Bone. 2011;49:591–9. doi: 10.1016/j.bone.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, et al. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–78. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 22.Thomale UW, Kroppenstedt SN, Beyer TF, Schaser KD, Unterberg AW, Stover JF. Temporal profile of cortical perfusion and microcirculation after controlled cortical impact injury in rats. J Neurotrauma. 2002;19:403–13. doi: 10.1089/08977150252932361. [DOI] [PubMed] [Google Scholar]
- 23.Mehta M, Strube P, Peters A, Perka C, Hutmacher D, Fratzl P, et al. Influences of age and mechanical stability on volume, microstructure, and mineralization of the fracture callus during bone healing: is osteoclast activity the key to age-related impaired healing? Bone. 2010;47:219–28. doi: 10.1016/j.bone.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y, Wang L, Clark JC, Dass CR, Choong PF. Elevated leptin expression in a rat model of fracture and traumatic brain injury. J Pharm Pharmacol. 2008;60:1667–72. doi: 10.1211/jpp/60.12.0013. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Yuan JS, Zhang HX, Ding H, Tang XG, Wei YZ. Effect of leptin on bone metabolism in rat model of traumatic brain injury and femoral fracture. Chin J Traumatol. 2011;14:7–13. [PubMed] [Google Scholar]
- 26.Boes M, Kain M, Kakar S, Nicholls F, Cullinane D, Gerstenfeld L, et al. Osteogenic effects of traumatic brain injury on experimental fracture-healing. J Bone Joint Surg Am. 2006;88:738–43. doi: 10.2106/JBJS.D.02648. [DOI] [PubMed] [Google Scholar]
- 27.Maegele M, Riess P, Sauerland S, Bouillon B, Hess S, McIntosh TK, et al. Characterization of a new rat model of experimental combined neurotrauma. Shock. 2005;23:476–81. doi: 10.1097/01.shk.0000159929.87737.5c. [DOI] [PubMed] [Google Scholar]
- 28.Karsenty G, Oury F. Biology without walls: the novel endocrinology of bone. Annu Rev Physiol. 2012;74:87–105. doi: 10.1146/annurev-physiol-020911-153233. [DOI] [PMC free article] [PubMed] [Google Scholar]