Abstract
Background
Treatment/prevention of shoulder muscle strength imbalances are major therapeutic goals for children with obstetrical brachial plexus palsy. The study aims were to characterize muscle atrophy in children/adolescents with unilateral obstetrical brachial plexus palsy, to quantify the agonist-antagonist muscle volume balance and the association between muscle volume and strength.
Methods
Eight boys and four girls (age=12.1, standard deviation=3.3) participated in this case-control study. Three-dimensional magnetic resonance images of both shoulders were acquired. The unimpaired shoulder served as a reference. Volumes of deltoid, pectoralis major, supraspinatus, infraspinatus, teres major, subscapularis were calculated based on 3D models, derived through image segmentation. Maximal isometric torques were collected in six directions.
Findings
All the major muscles studied were significantly atrophied. The teres major demonstrated the biggest difference in atrophy between groups (51 percentage points), the pectoralis major was the least atrophied (23 percentage points). The muscle volume distribution was significantly different between shoulders. Muscle volume could predict maximal voluntary isometric torques, but the regression coefficients were weaker on the impaired side (72% to 91% of the strength could be predicted in the uninvolved side and 24% to 90% in the involved side and external rotation strength could not be predicted).
Interpretation
This study demonstrates muscle atrophy varied across all the main shoulder muscles of the glenohumeral joint, leading to significant muscle volume imbalances. The weaker coefficients of determination on the impaired side suggest that other variables may contribute to the loss of strength in addition to atrophy.
Keywords: Obstetrical brachial plexus palsy, Children, Muscle Volume, MRI, Strength, Shoulder
1. Introduction
Obstetrical brachial plexus palsy (OBPP) is one of the most common birth injuries, with an incidence around 1.5 per 1000 births1. While many injuries are transient, 18–34% of patients have long-term or permanent impairments/disability1–3. The upper (C5C6) and/or middle (C7) trunks of the brachial plexus and their distal elements are the most commonly injured elements3. These nerve injuries lead to varying degrees of muscle denervation in shoulder girdle muscles (prime movers and scapular stabilizers), inducing a complex profile of muscle atrophy and force imbalances between agonist and antagonist muscle pairs4. The combined effects of shoulder muscle force imbalances, weakness, and joint pathology can severely diminish a child’s functional use of their arm and/or hand for activities of daily living and other tasks.
Although the primary interventional goals when treating OBPP related impairments is to improve function by minimizing force imbalances across the shoulder muscles, there are little data defining the pattern or severity of muscle atrophy in children/adolescents with OBPP either individually or as a group. Studies evaluating atrophy in OBPP have typically used qualitative assessments5,6, substituted cross-sectional areas for volumes7,6,8,9, or defined the volume using only a portion of the muscle10,11. Additionally, these studies evaluated a limited subset of shoulder muscles, primarily the subscapularis and infraspinatus6,12,10,11,9. Therefore, the full extent and pattern of shoulder muscle atrophy in OBPP is not well established. The lack of information related to the pectoralis major (PM) and teres major (TM) is a major deficit when developing treatments for children with OBPP, as these muscles are often targeted for lengthening, transfer procedures13–17 or botulinum toxin injections18,19. Thus, a more complete understanding of the pattern and degree of muscle atrophy across shoulder muscles in patients with OBPP is needed to improve interventional outcomes.
An understanding of the volume-torque relationship in children/adolescents with OBPP will provide valuable patient-specific information, including the optimal target muscles for a given procedure. For example, Aydin and colleagues20 state that a preoperative examination should determine whether the muscle being transferred has sufficient power to justify the procedure. Yet, it is not possible to directly measure the power of an individual muscle. A recent study in typically developing children/adolescents established the relationship between shoulder muscle volume and torque in all three rotational degrees of freedom21. However, the in vivo relationship between muscle volume and torque has not been established in children with OBPP. A direct relationship between muscle volume and strength may not exist in these children, as other factors, such as glenohumeral deformity22 or fatty infiltration5, may alter the relationship.
The primary purpose of this study was to quantify the pattern and severity of muscle atrophy of the major shoulder muscles (anterior deltoid, posterior deltoid, PM, TM, combined infraspinatus and teres minor, subscapularis, and supraspinatus) in the involved, relative to the uninvolved, shoulder in children/adolescents with unilateral OBPP using a 3D magnetic resonance (MR) based methodology. The secondary purposes were to (1) quantify the agonist-antagonist muscle volume balance and (2) determine if the maximum voluntary isometric joint moment in all three degrees of freedom could be predicted by the muscle volumes using a multiple regression analysis. As this study set out to evaluate the muscle volume to torque relationship, it was known from the outset that an older population, inclusive of children with previous surgical interventions, would be targeted. Thus, a separate analysis addressed if muscle atrophy and volume distribution was different in individuals with and without previous reconstructive surgery. In support of these purposes, the inter-rater reliability of obtaining muscle volumes in children/adolescents with OBPP was evaluated.
2. Methods
Data from an ongoing IRB (National Institute of Child Health and Human Development, intramural, MD, USA) approved study4,22,21,23 formed the basis of this case-controlled study. Sixteen children/adolescents with unilateral OBPP were recruited as a sample of convenience. A legal guardian or subject over 18 years of age provided written consent. Written assent was obtained from subject’s under 18 years of age. The exclusion criteria were: 1) less than 30° active flexion and abduction, 2) other neurological problems (e.g., cerebral palsy, arthrogryposis), 3) contraindications for MR imaging, and 4) shoulder surgery and/or botulinum toxin injections within the 6 months prior to inclusion. A pediatric physiatrist performed a history and physical examination. Data were included only if a complete three-dimensional MR dataset was available for both shoulders. The final cohort consisted of eight boys and four girls [age=12.1 (SD 3.3) years, height = 155.9 (SD 20.8) cm, 52.6 (SD 17.9) kg] with five of these children having had past surgical interventions (supplementary material 1).
The MR data were acquired as a subset of a previous study23, but the muscle volume was not analyzed as part of that study. Each participant was placed supine on the scanning bed of a 3T MR scanner (Verio: Siemens, Germany). A flexible cardiac coil was placed posterior to the shoulder while its pair was wrapped around the subject’s shoulder and chest. The arm was in a neutral position with the hand pronated. A T1-gradient recalled echo sequence was independently acquired for each shoulder, enabling optimal shoulder position relative to the MR unit. Neither sedation nor anesthesia was used. The imaging parameters, based on recommended parameters for denervated muscle24,25, were held constant for each subject (416×312×192 pixels, slice thickness=1.2mm, TR=16.6msec, TE=5.1msec, imaging time=4min 22sec). The in-plane resolution varied from 0.55mmX0.55mm to 0.63mmX0.63mm across subjects, allowing for finer resolution in smaller subjects. The minimum scan volume ranged from the inferior scapular angle to 5mm proximal of the acromion superior edge and from the lateral edge of the arm to the mid-spine. Throughout data processing the research team was blinded to the subjects’ identity and side of impairment.
The 3D volume calculation was carried out as previously done21. The muscles of interest were manually segmented using MIPAV (Medical Image Processing Analysis and Visualization, Bethesda, MD, USA). The first and last five images containing a specific muscle were always used in segmentation. In the belly of the muscle every other, every fourth, or every sixth slice was used, based on the length of the muscle. Segmentation was guided by tracking minor area changes through the “skipped” images and using a tri-planar view to identify the muscular fascial planes, origins, and insertions. In cases of severe atrophy, segmentations were allowed to deviate from the uniform slice separation to more precisely capture the volume. The infraspinatus and teres minor were segmented as a single muscle (infraspinatus-teres minor (I-tm)). Similarly, the distal border of the TM was assumed to be at the level of the most distal scapular point, resulting in a small portion of the latissimus dorsi being included within the TM volume.
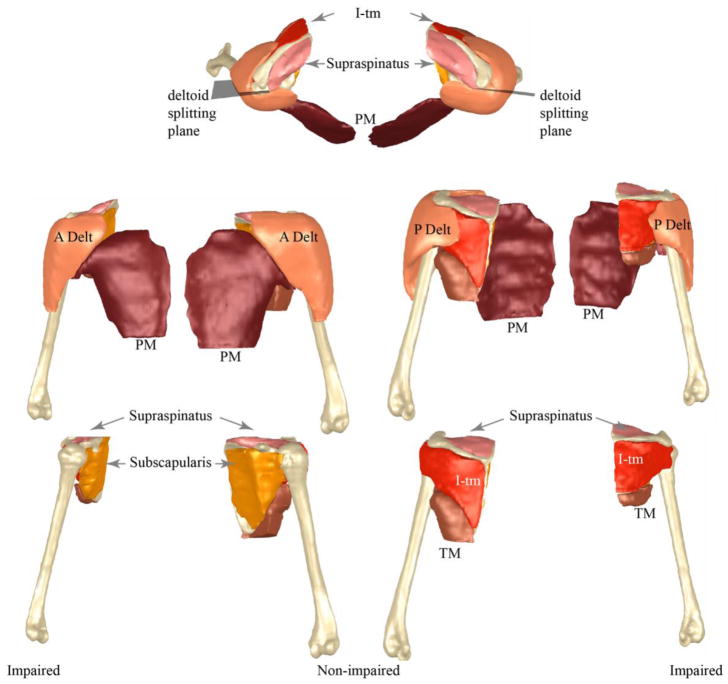
From the segmentation, a three-dimensional model (Fig. 1) of each muscle was created (Geomagic, Research Triangle Park, NC, Morrisville, USA). Smoothing filters were not used and minor modeling errors (e.g., overlapping surfaces, holes) were manually corrected. Muscle volume was computed from the 3D model. The deltoid was split into functional segments, based on previous work in typically developing children21, and the volume of its functional sub-sections were quantified.
Fig. 1.
Comparison of muscle volumes between sides for a single subject. The musculoskeletal models are both from a single male subject (subject 2). This subject has a Narakas score of 3 and a Mallet of 15. Top row is a superior to inferior view (involved arm on the right of the image), the left column provides an anterior to posterior view (involved side on the left of the image), and the right column provides a posterior view (involved arm on the right side of the image). For the bottom four images the identical scale is used. The identical scale is used in the top two images. Thus, the smaller muscle and bone volumes seem for the involved side are a result of atrophy/hypotrophy and are not a scaling artefact. The 3D MR images were acquired independently for both arms, as per the protocol, but used the identical resolution. Thus, the pose of each arm relative to each the other was based on a visual approximation and not a true anatomical position.
Each subject’s contralateral shoulder was used as a control for the involved side. The atrophy was defined as the ratio of muscle volume from the involved side relative to that of the uninvolved side for each muscle. The proportional volumes of each muscle were obtained by dividing the corresponding volumes by the total muscle volume of the six muscles volumes quantified. These proportional volumes defined the muscle volume distribution. The ratio of agonist to antagonist muscle volume was computed for both shoulders in each of the three movement planes. The external rotation muscle group was the I-tm, the internal rotation group was the subscapularis and PM. The extensor group was the posterior deltoid and TM. The flexor group was the PM, supraspinatus, and the anterior deltoid. The abduction group was the entire deltoid, I-tm, and supraspinatus. The adductor group was the PM and subscapularis (Table 1).
Table 1. Adjusted coefficient of determination (R2) for muscle Volume-Torque relationship.
Muscles were identified as torque contributors to a given direction based on published moment arm data26–28. R2 values are presented when p< 0.05.
| Torque direction | Muscle | Moment arm (mm) | R2 value | |
|---|---|---|---|---|
| Involved | Uninvolved | |||
| Flexion | Anterior Deltoid | 15.926 | 0.60 | 0.87 |
| Supraspinatus | 727 | -- | 0.81 | |
| PM | 1027 | 0.90 | 0.78 | |
|
| ||||
| Extension | Posterior deltoid | 45.4 26 | 0.37 | 0.89 |
| TM | 3827 | 0.86 | 0.86 | |
|
| ||||
| Abduction | Supraspinatus | 1927 | 0.24 | 0.83 |
| Total deltoid | 19.526 | 0.45 | 0.86 | |
| I-tm | 1927 | 0.58 | 0.81 | |
|
| ||||
| Adduction | PM | 2127 | 0.80 | 0.83 |
| Subscapularis | 827 | 0.44 | 0.72 | |
|
| ||||
| Internal rotation | Subscapularis | 22.528 | 0.46 | 0.76 |
| PM | 2028 | 0.88 | 0.82 | |
|
| ||||
| External rotation | I-tm | 2428 | -- | 0.91 |
Abbreviations: I-tm= Infraspinatus-teres minor, PM= pectoralis major, TM=Teres Major
Functional groups (Table 1) were determined based on moment arms from in vitro adult studies that used identical testing positions26–28. Muscles with a moment arm greater than 6 mm in a specific torque direction were considered as contributors to that torque. This qualitative cut-off was slightly higher than our previous study21 due to the potential alteration in moment arms secondary to the bone deformities and humeral head migration in OBPP22. This resulted in the entire deltoid being considered an abductor, its anterior segment a flexor, and its posterior segment an extensor26.
Two investigators independently assessed inter-rater reliability of calculating the muscle volumes from MR images in fifteen randomly selected shoulders (five affected and 10 healthy shoulders) for three of the six muscles evaluated (deltoid, supraspinatus and I-tm). In total the volumes of 45 muscles were segmented twice. The selected muscles represented a diversity of shapes, sizes, depths, and functions. The reliability of calculating the anterior and posterior deltoid volume was quantified.
As part of a previous study4, maximal voluntary isometric joint torques were acquired in all three degrees of freedom using a hand held dynamometer (JTech Commander PowerTrack II). The position of the child, dynamometer, and observer; the order of the torque production (flexion, abduction, external rotation, internal rotation, adduction, and extension); and verbal encouragement cues were standardized. Children rested at least 10 sec between trials and at least 1min between directions. The highest maximal voluntary isometric torque value produced in three trials was used for the multiple regression analysis.
An a priori power analysis, based on a Wilcoxon paired test, determined that at least 10 subjects were required (α=0.05 and β=0.9) assuming 50% atrophy in the subscapularis volume, as previously reported11. A signed rank Wilcoxon paired-test was used for between-side comparisons and a signed Mann-Whitney test was used for sub-group comparisons. Using a linear regression analysis, the adjusted coefficient of determination between groups (R2) was calculated between each muscle and the strength in the related direction. All statistical analyses were run in SPSS (IBM, version 22). To quantify inter-rater reliability, the intra-class correlation coefficients (ICCs), using a two-way mixed effects model, were computed. A P-value <0.05 was considered significant.
3. Results
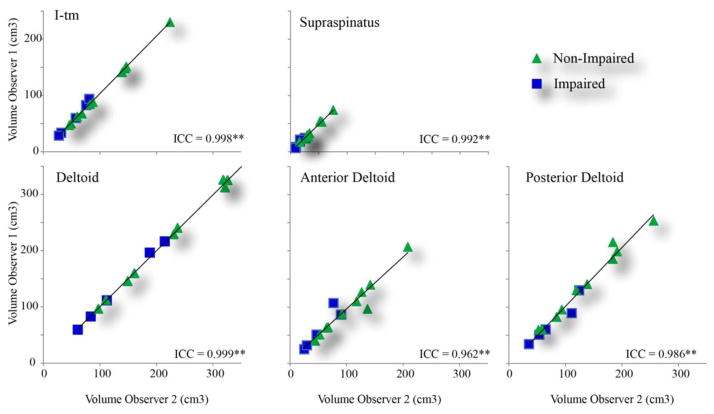
The inter-rater reliability for quantifying muscle volumes was excellent (ICC= 0.962–0.999, Fig. 2). Removing the data from the involved shoulder created minimal changes in the reliability. The percent error between raters ranged from −2.0% to 3.5%. The larger percent errors were due to the small volumes of the muscle.
Fig. 2. Interobserver reliability of muscle volume measurement.
All Intraclass Correlation Coefficients (ICC) all had p-values less than 0.001, as indicated by the double star. Abbreviations: I-tm= Infraspinatus-teres minor
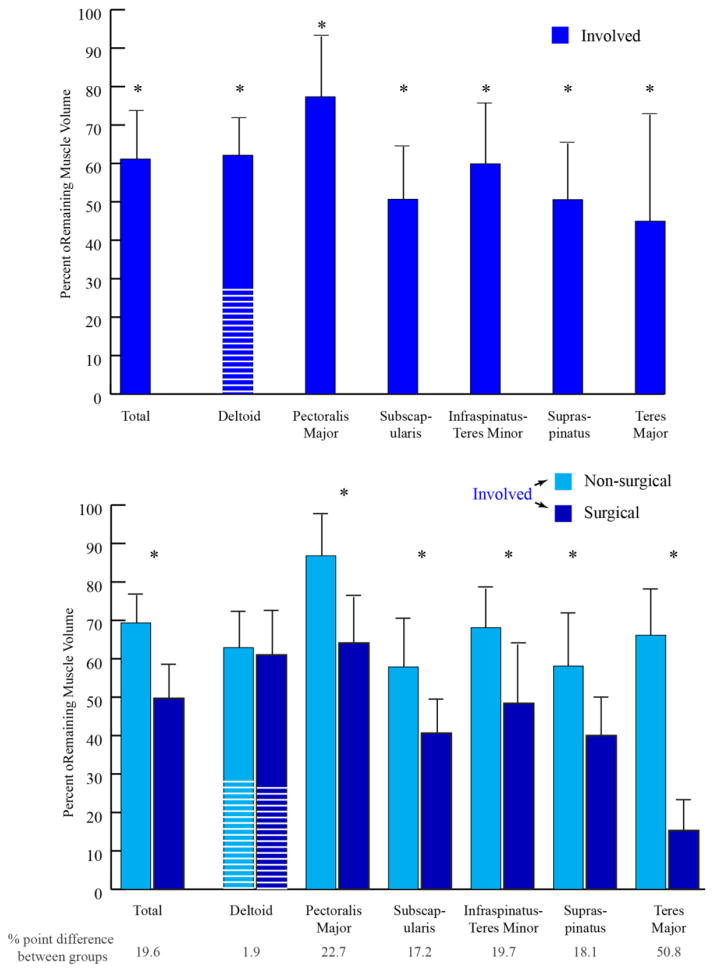
For all subjects every muscle on the involved side was smaller relative to the uninvolved side (Fig. 3, top graph), with one exception for one muscle for one subject (3.8% hypertrophy in the PM). The TM, supraspinatus, and subscapularis muscle volumes were the most affected. In contrast, the PM and posterior deltoid volumes were the least affected. The children with previous surgeries tended to have more severe functional limitations (a trend towards lower Mallet scores) and significantly greater atrophy in all muscles, excluding the deltoid (Fig. 3, bottom graph). The loss of muscle volume was approximately 20 percentage points higher in the post-surgical group, except for the TM, which was 51 percentage points higher and the deltoid that demonstrated little difference between groups.
Fig. 3. Percentage of Remaining Muscle.
One standard deviation is represented by the black bar and line. The white lines on the bar representing the deltoid demonstrate the percentage of the entire deltoid assigned to the anterior deltoid. “Total” represents the total remaining muscle volume of all muscles evaluated.
Top: percent remaining muscle for the entire cohort (n=12). Bottom: percent remaining muscle for the separated into the non-surgical group (light blue, n= 7) and the surgical group (dark blue, n=5). The numbers at the bottom of the graph represent the differences between the two groups. Significant differences between sides (top) and between surgical/non-surgical group (bottom) are represented by: * = p<0.05.
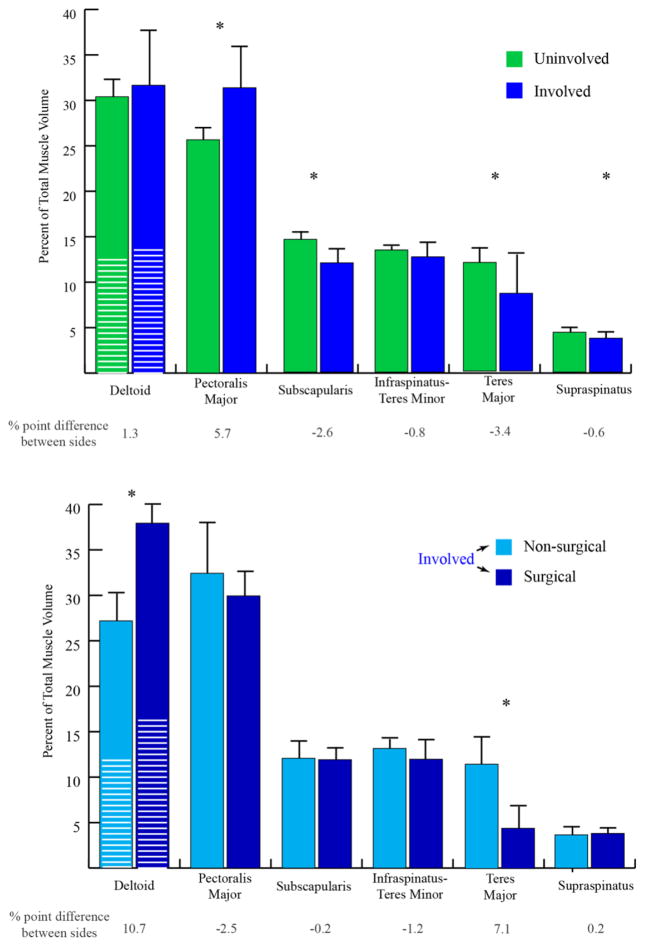
The muscle volume distribution was significantly different between shoulders (Fig. 4, top graph). For the involved side, the PM had a larger proportional volume (P=0.002), relative to the uninvolved shoulder. In contrast, the TM, subscapularis, and supraspinatus made up a significantly lower proportional volume (P=0.024, 0.003 and 0.010), as compared with the uninvolved shoulder. The muscle volume distribution on the involved side was not significantly different between the surgical and non-surgical groups for all muscles, with the exception of the deltoid and I-tm (Fig. 4, bottom graph).
Fig. 4. Muscle Volume Distribution Changes.
Uninvolved (left columns) and involved (right columns) muscle volume distributions and standard deviations (error bars) are displayed. The distribution was calculated as a percentage that an individual muscle’s volume contributes to the total volume of all six muscles. The white lines in the deltoid column represents the anterior deltoid. The between side differences expressed as a percentage of the control is provided below the graph. A significant difference is denoted with a * if p<0.05. The anterior and posterior deltoid between side volume distributions were not significantly different.
The varying degrees of muscle atrophy led to significant changes in antagonist/agonist volume ratios (Fig. 5). This was true for extension/flexion (sagittal plane imbalance, P=0.002), external/internal rotation (transverse plane imbalance, P=0.016), and ab/adduction (frontal plane imbalance, p=0.025).
Fig. 5. Shoulder muscle group ratio (balance).
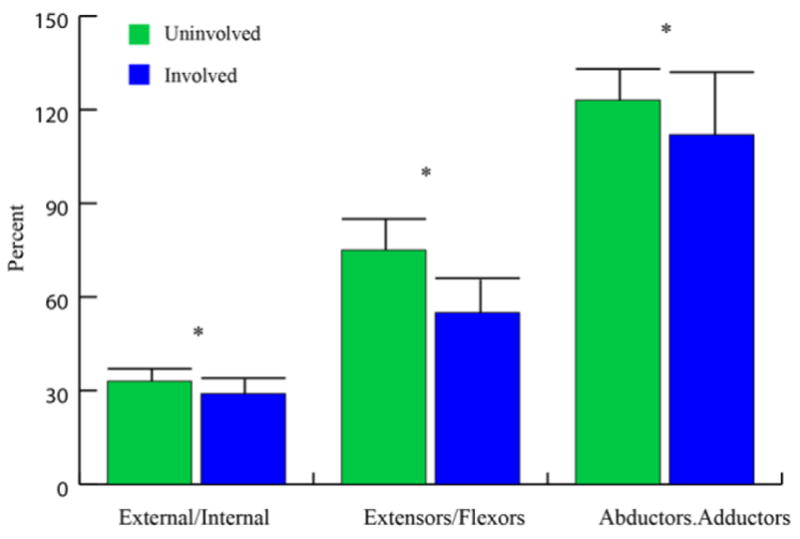
The volume for each group was calculated based on the categorization provided in Table 1. For example, extensors/flexors ratio= (volume of anterior deltoid + volume of supraspinatus + volume of pectoralis major)/(volume of posterior deltoid + volume of teres major). Mean and standard deviations (error bars) are displayed. A significant between side difference is denoted with a * if p<0.05
Abbreviations: external = external rotators, and internal = internal rotators.
For the uninvolved shoulder, 72% to 91% of the maximal voluntary isometric torques could be predicted using the muscle volumes (Table 1). For the involved side, 24% to 90% of the strength could be predicted, but external rotation strength could not be predicted (Table 1).
4. Discussion
This study advances our understanding of the complexity of shoulder muscle atrophy in OBPP by providing the first comprehensive evaluation of muscle volume loss across all the main shoulder muscles of the glenohumeral joint in children/adolescents with unilateral OBPP. In the impaired shoulder, all the muscles are atrophied, the TM, supraspinatus, and subscapularis muscle volumes are the most affected and the PM the least affected, inducing different distribution of shoulder muscle volume and three-dimensional imbalance around the palsied shoulder. Quantifying this variable pattern and severity of muscle atrophy fosters understanding of the impairments and functional limitations associated with OBPP, as well as, promotes improved subject-specific treatment planning. Substantiating the volume-strength relationship allows for deeper insights into how atrophy across various muscles relates to weakness at the shoulder. Identifying differential atrophy in the post-operative subgroup provides novel data which enhances our understanding of the long term musculoskeletal sequela associated with OBPP.
The inclusion of all “main” muscles of the shoulder provides a more detailed picture of the variability of atrophy across the shoulder in patients with OBPP. The greater atrophy in the current participants, relative to past studies6,12,10,11,9, is likely due to the older age of the current participants. Specifically, Talbert and colleagues12 demonstrated a correlation between age and I-tm atrophy in a group of patients with OBPP (age 1.1 to 13.2 years). Of note is the 23% loss of volume in the PM, which contrasts past reports9 that assumed the PM was “unaffected”. One possible explanation of this difference might be atrophy induced by a lack of use of the arm. The 3D muscle models take into account the entire volume and geometrical complexity of the shoulder muscles, which previous techniques did not, with the exception of one study evaluating the I-tm12. This volumetric information is particularly important in atrophic muscle where the relationship between the entire muscle volume and the area within a single slice may be quite different than in uninvolved muscles (Fig. 1). The length of the affected muscles may also be altered. These factors combined with the knowledge that muscle volume has been found to correlate more with age than cross sectional area29,30 support the concept that a 3D, volume-based, method is needed for accurately measuring muscle atrophy in individuals with OBPP.
The shifts in the muscle volume distribution between affected and unaffected shoulders likely relate to muscle innervation and the site and severity of the injury, as well as the extent of re-innervation. The lower percent contribution to the total muscle volume for the subscapularis, supraspinatus, and TM muscles highlights their potential key role in OBPP. Similarly, the PM’s increase in percent muscle volume is likely due to the relative preservation of this muscle, which has dual innervation from multiple trunks and both the medial and lateral cords. The alterations in muscle volume balance in all three anatomical planes, with a greater volume imbalance in the sagittal plane, clearly demonstrates that treatment focused solely on the axial plane is too restrictive. The ratio of muscle volumes producing external rotation (I-tm) to muscle volume producing internal rotation (subscapularis and PM) matched previous results9. If the PM is removed from the internal rotation group, then the previously reported decreased ratio is seen9. These comparisons highlight the fact that subscapularis atrophy was compensated for by the fairly well preserved PM for both internal rotation and adduction, leading to the transverse and coronal plane imbalances. This makes the PM the major player in generating muscle volume imbalance. Since imbalance, more than muscle atrophy, is likely the cause of the glenohumeral deformities31–34, evaluating and targeting the imbalances in all three planes may improve global shoulder muscle balance, posture, motion, and joint integrity.
The markedly greater atrophy seen in the surgical, relative to the non-surgical, subgroup is noted in one previous study6. Unique to the current study, the shoulder muscles do not show a consistent difference between groups. The deltoid shows no difference in atrophy across groups and the difference in TM atrophy between groups (66% and 15% remaining for the non-surgical and surgical groups) is more than double all other muscle evaluated. As all children in this subgroup had a TM transfer, from a functional perspective, these results call into question the efficacy of the TM transfer and its potential long term benefits in the surgery subgroup. This is true regardless of whether the TM atrophy occurred preoperatively or post operatively. Longitudinal and controlled studies involving more children undergoing reconstructive/transfer surgeries are thus needed to evaluate accurately the biomechanical impacts of these interventions.
Accurately assessing muscle strength is of critical importance when considering reconstructive surgery in patients with OBPP20. It has long been recognized that the multiple muscles contributing to a given movement inhibit the direct assessment of an individual muscle’s contribution to an overall joint torque. Further, overall joint torque cannot be assessed in individuals who cannot follow directions. Thus, establishing that muscle volume does predict strength in this population with unilateral OBPP provides the most direct insights into the remaining strength for individual muscles and will help advance the decision making process for reconstructive procedures37. However, the weaker R2 values on the involved side coupled with a non-significant relationship between muscle volume and external rotation torque suggests that additional variables such as rheological changes in non-contractile muscle elements, fatty infiltration5, changes in muscle architecture and/or osseous deformation35,34,36 may also contribute to the loss of strength.
A limitation of this study is the use of the contralateral side as a control, as the uninvolved side may be altered relative to that of a typically developing child. Yet, an evaluation of nine typically developing children21, age and sex matched to nine children in the current study, demonstrated no significant differences in the dominant arm muscle volumes (deltoid, pectoralis major, supraspinatus, infraspinatus, teres major, subscapularis) or in the average height and weight in typically developing children relative to the children with unilateral OBPP. Using the dominant arm as a control provides the best anthropometric match, which is a main methodological issue in pediatric research. In order to avoid the use of anesthesia in this research study and to accurately assess strength, the subjects were older than those in previous studies5,6,12,10,11,9. Further, the current segmentation and reconstruction methods are, at present, time-consuming. This limits their use in routine clinical practice, but for decisions involving invasive procedures, the time could easily be justified. Future work on developing/validating a more efficient, reliable technique is warranted38,39.
This study clearly demonstrates muscle atrophy across all the main shoulder muscles of the glenohumeral joint. This atrophy varied across the muscle groups, leading to significant 3D muscle volume imbalances. As the PM is relatively preserved, compared to other muscles, this muscle is a key factor in generating 3D volumetric and strength imbalances. Based on the regression analysis, this study also emphasizes that other variables in addition to atrophy (e.g., joint deformity and/or muscle inefficiency) may contribute to the loss of strength, especially in external rotation. An individualized, comprehensive, 3D musculoskeletal evaluation, including a muscle volume specific evaluation should be considered a prerequisite for interventions in OBPP children with complex clinical presentation.
Supplementary Material
Highlights.
Muscle atrophy is present across all the main muscles of the glenohumeral joint in children/adolescents with obstetrical brachial plexus palsy (OBPP).
Because of the varied atrophy, muscle volume imbalances occur in children/adolescents with OBPP.
Pectoralis major is a key factor in generating 3D volumetric and strength imbalances in in children/adolescents with OBPP.
Other variables in addition to atrophy may contribute to the loss of strength in children/adolescents with OBPP.
Acknowledgments
We thank Diane Damiano, PhD, Christopher Hollingsworth, Lindsey Curatalo, Christopher Stanley, and Lori Ohlrich for their help and support in the work. In addition, we would like to thank the Radiology Department, headed by Dr. David Bluemke, at the Clinical Center of the National Institutes of Health for their support of this work.
Funding sources
This work was funded by grants awarded to Dr Brochard from the University Hospital of Brest, the French Society of Physical Medicine and Rehabilitation (SOFMER), the French Society of Research in Children with Disabilities (SFERHE) and by the Intramural Research Program of the National Institutes of Health Clinical Center, Bethesda, MD, USA.
Footnotes
Conflicts of interests
none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chauhan SP, Blackwell SB, Ananth CV. Neonatal brachial plexus palsy: Incidence, prevalence, and temporal trends. Semin Perinatol. 2014;38:210–218. doi: 10.1053/j.semperi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Hoeksma AF, Steeg T, Marie A, Nelissen RG, Van Ouwerkerk WJ, Lankhorst GJ, et al. Neurological recovery in obstetric brachial plexus injuries: an historical cohort study. Dev Med Child Neurol. 2004;46(2):76–83. doi: 10.1017/s0012162204000179. [DOI] [PubMed] [Google Scholar]
- 3.Lagerkvist AL, Johansson U, Johansson A, Bager B, Uvebrant P. Obstetric brachial plexus palsy: a prospective, population-based study of incidence, recovery, and residual impairment at 18 months of age. Dev Med Child Neurol. 2010;52(6):529–534. doi: 10.1111/j.1469-8749.2009.03479.x. [DOI] [PubMed] [Google Scholar]
- 4.Brochard S, Alter K, Damiano D. Shoulder strength profiles in children with and without brachial plexus palsy. Muscle Nerve. 2014;50(1):60–66. doi: 10.1002/mus.24099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hogendoorn S, van Overvest KLJ, Watt I, Duijsens AHB, Nelissen RGHH. Structural changes in muscle and glenohumeral joint deformity in neonatal brachial plexus palsy. J Bone Joint Surg Am. 2010;92(4):935–942. doi: 10.2106/JBJS.I.00193. [DOI] [PubMed] [Google Scholar]
- 6.Pöyhiä TH, Nietosvaara YA, Remes VM, Kirjavainen MO, Peltonen JI, Lamminen AE. MRI of rotator cuff muscle atrophy in relation to glenohumeral joint incongruence in brachial plexus birth injury. Pediatr Radiol. 2005;35(4):402–409. doi: 10.1007/s00247-004-1377-3. [DOI] [PubMed] [Google Scholar]
- 7.Eismann EA, Little KJ, Laor T, Cornwall R. Glenohumeral Abduction Contracture in Children with Unresolved Neonatal Brachial Plexus Palsy. J Bone Joint Surg Am. 2015;97(2):112–118. doi: 10.2106/JBJS.N.00203. [DOI] [PubMed] [Google Scholar]
- 8.Ruoff JM, van der Sluijs JA, Van Ouwerkerk WJ, Jaspers RT. Musculoskeletal growth in the upper arm in infants after obstetric brachial plexus lesions and its relation with residual muscle function. Dev Med Child Neurol. 2012;54(11):1050–1056. doi: 10.1111/j.1469-8749.2012.04383.x. [DOI] [PubMed] [Google Scholar]
- 9.Waters PM, Monica JT, Earp BE, Zurakowski D, Bae DS. Correlation of radiographic muscle cross-sectional area with glenohumeral deformity in children with brachial plexus birth palsy. J Bone Joint Surg Am. 2009;91(10):2367–2375. doi: 10.2106/JBJS.H.00417. [DOI] [PubMed] [Google Scholar]
- 10.Van Gelein Vitringa VM, Jaspers R, Mullender M, Ouwerkerk WJ, van der Sluijs JA. Early effects of muscle atrophy on shoulder joint development in infants with unilateral birth brachial plexus injury. Dev Med Child Neurol. 2011;53(2):173–178. doi: 10.1111/j.1469-8749.2010.03783.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Gelein Vitringa VM, van Kooten EO, Mullender MG, van Doorn-Loogman MH, van der Sluijs JA. An MRI study on the relations between muscle atrophy, shoulder function and glenohumeral deformity in shoulders of children with obstetric brachial plexus injury. Journal of brachial plexus and peripheral nerve injury. 2009;4(1):5. doi: 10.1186/1749-7221-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talbert RJ, Michaud LJ, Mehlman CT, Kinnett DG, Laor T, Foad SL, et al. EMG and MRI are Independently Related to Shoulder External Rotation Function in Neonatal Brachial Plexus Palsy. J Pediatric Orthop. 2011;31(2):194–204. doi: 10.1097/BPO.0b013e3182092892. [DOI] [PubMed] [Google Scholar]
- 13.Kirkos J, Kyrkos M, Kapetanos G, Haritidis J. Brachial plexus palsy secondary to birth injuries long-term results of anterior release and tendon transfers around the shoulder. J Bone Joint Surg Br. 2005;87(2):231–235. doi: 10.1302/0301-620x.87b2.14739. [DOI] [PubMed] [Google Scholar]
- 14.Kozin SH, Boardman MJ, Chafetz RS, Williams GR, Hanlon A. Arthroscopic treatment of internal rotation contracture and glenohumeral dysplasia in children with brachial plexus birth palsy. J Shoulder Elbow Surg. 2010;19(1):102–110. doi: 10.1016/j.jse.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Odeh R, Odeh M. A modified Sever-L’Episcopo procedure for restoration of shoulder joint function in Erb’s palsy. Int Orthop. 2015;39(2):309–317. doi: 10.1007/s00264-014-2592-7. [DOI] [PubMed] [Google Scholar]
- 16.Ozben H, Atalar AC, Bilsel K, Demirhan M. Transfer of latissmus dorsi and teres major tendons without subscapularis release for the treatment of obstetrical brachial plexus palsy sequela. J Shoulder Elbow Surg. 2011;20(8):1265–1274. doi: 10.1016/j.jse.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Ozturk K, Bulbul M, Demir BB, Buyukkurt CD, Ayanoglu S, Esenyel CZ. Reconstruction of shoulder abduction and external rotation with latissimus dorsi and teres major transfer in obstetric brachial plexus palsy. Acta orthopaedica et traumatologica turcica. 2010;44(3):186–193. doi: 10.3944/AOTT.2010.2332. [DOI] [PubMed] [Google Scholar]
- 18.Ezaki M, Malungpaishrope K, Harrison RJ, Mills JK, Oishi SN, Delgado M, et al. OnabotulinumtoxinA injection as an adjunct in the treatment of posterior shoulder subluxation in neonatal brachial plexus palsy. J Bone Joint Surg Am. 2010;92(12):2171–2177. doi: 10.2106/JBJS.I.00499. [DOI] [PubMed] [Google Scholar]
- 19.Michaud LJ, Louden EJ, Lippert WC, Allgier AJ, Foad SL, Mehlman CT. Use of botulinum toxin type A in the management of neonatal brachial plexus palsy. PM R. 2014;6(12):1107–1119. doi: 10.1016/j.pmrj.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Aydın A, Bićer A, özkan T, Mersa B, Özkan S, Yıldırım Z. Does primary brachial plexus surgery alter palliative tendon transfer surgery outcomes in children with obstetric paralysis? BMC musculoskelet Disord. 2011;12(1):1. doi: 10.1186/1471-2474-12-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Im HS, Alter KE, Brochard S, Pons C, Sheehan FT. In vivo pediatric shoulder muscle volumes and their relationship to 3D strength. J Biomech. 2014;47(11):2730–2737. doi: 10.1016/j.jbiomech.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brochard S, Mozingo JD, Alter KE, Sheehan FT. Three dimensionality of glenohumeral deformities in obstetrical brachial plexus palsy. J Orthop Res. 2016;34(4):675–82. doi: 10.1002/jor.23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheehan FT, Brochard S, Behnam AJ, Alter KE. Three-dimensional humeral morphologic alterations and atrophy associated with obstetrical brachial plexus palsy. J Shoulder Elbow Surg. 2014;23(5):708–719. doi: 10.1016/j.jse.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamath S, Venkatanarasimha N, Walsh M, Hughes P. MRI appearance of muscle denervation. Skeletal Radiol. 2008;37(5):397–404. doi: 10.1007/s00256-007-0409-0. [DOI] [PubMed] [Google Scholar]
- 25.Menashe SJ, Tse R, Nixon JN, Ishak GE, Thapa MM, McBroom JA, Iyer RS. Brachial Plexus Birth Palsy: Multimodality Imaging of Spine and Shoulder Abnormalities in Children. AJR Am J Roentgenol. 2015;204(2):W199–W206. doi: 10.2214/AJR.14.12862. [DOI] [PubMed] [Google Scholar]
- 26.Brown J, Wickham J, McAndrew D, Huang X-F. Muscles within muscles: coordination of 19 muscle segments within three shoulder muscles during isometric motor tasks. J Electromyogr and Kinesiol. 2007;17(1):57–73. doi: 10.1016/j.jelekin.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Kuechle DK, Newman SR, Itoi E, Morrey BF, An K-N. Shoulder muscle moment arms during horizontal flexion and elevation. J Shoulder Elbow Surg. 1997;6(5):429–439. doi: 10.1016/s1058-2746(97)70049-1. [DOI] [PubMed] [Google Scholar]
- 28.Kuechle DK, Newman SR, Itoi E, Niebur GL, Morrey BF, An K-N. The relevance of the moment arm of shoulder muscles with respect to axial rotation of the glenohumeral joint in four positions. Clin Biomech. 2000;15(5):322–329. doi: 10.1016/s0268-0033(99)00081-9. [DOI] [PubMed] [Google Scholar]
- 29.Pitcher CA, Elliott CM, Williams SA, Licari MK, Kuenzel A, Shipman PJ, Valentine JP, Reid SL. Childhood muscle morphology and strength: alterations over six months of growth. Muscle Nerve. 2012;46(3):360–366. doi: 10.1002/mus.23326. [DOI] [PubMed] [Google Scholar]
- 30.Vidt ME, Daly M, Miller ME, Davis CC, Marsh AP, Saul KR. Characterizing upper limb muscle volume and strength in older adults: A comparison with young adults. J Biomech. 2012;45(2):334–341. doi: 10.1016/j.jbiomech.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahm J, Wein B, Alhares G, Dogan C, Radermacher K, Schuind F. Assessment and treatment of glenohumeral joint deformities in children suffering from upper obstetric brachial plexus palsy. J Pediatr Orthop B. 2007;16(4):243–251. doi: 10.1097/BPB.0b013e3280925681. [DOI] [PubMed] [Google Scholar]
- 32.Gharbaoui IS, Gogola GR, Aaron DH, Kozin SH. Perspectives on glenohumeral joint contractures and shoulder dysfunction in children with perinatal brachial plexus palsy. J Hand Ther. 2015;28(2):176–184. doi: 10.1016/j.jht.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Sibinski M, WoŸniakowski B, Drobniewski M, Synder M. Secondary gleno-humeral joint dysplasia in children with persistent obstetric brachial plexus palsy. Int Orthop. 2010;34(6):863–867. doi: 10.1007/s00264-010-0965-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Der Sluijs J, Van Ouwerkerk W, De Gast A, Wuisman P, Nollet F, Manoliu R. Retroversion of the humeral head in children with an obstetric brachial plexus lesion. J Bone Joint Surg Br. 2002;84(4):583–587. doi: 10.1302/0301-620x.84b4.12243. [DOI] [PubMed] [Google Scholar]
- 35.Eismann EA, Laor T, Cornwall R. Three-Dimensional Magnetic Resonance Imaging of Glenohumeral Dysplasia in Neonatal Brachial Plexus Palsy. J Bone Joint Surg Am. 2016;98(2):142–151. doi: 10.2106/JBJS.O.00435. [DOI] [PubMed] [Google Scholar]
- 36.Voigt C, Kreienborg S, Megatli O, Schulz A-P, Lill H, Hurschler C. How does a varus deformity of the humeral head affect elevation forces and shoulder function? A biomechanical study with human shoulder specimens. J Orthop Trauma. 2011;25(7):399–405. doi: 10.1097/BOT.0b013e31820beb80. [DOI] [PubMed] [Google Scholar]
- 37.Jeng CL, Thawait GK, Kwon JY, Machado A, Boyle JW, Campbell J, Carrino JA. Relative strengths of the calf muscles based on MRI volume measurements. Foot Ankle Int. 2012;33(5):394–399. doi: 10.3113/FAI.2012.0394. [DOI] [PubMed] [Google Scholar]
- 38.Blazevich AJ, Coleman DR, Horne S, Cannavan D. Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur J Appl Physiol. 2009;105(6):869–878. doi: 10.1007/s00421-008-0972-7. [DOI] [PubMed] [Google Scholar]
- 39.Lund H, Christensen L, Savnik A, Boesen J, Danneskiold-Samsøe B, Bliddal H. Volume estimation of extensor muscles of the lower leg based on MR imaging. Eur Radiol. 2002;12(12):2982–2987. doi: 10.1007/s00330-002-1334-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.