Abstract
Drugs targeting aquaporins have broad potential clinical applications, including cancer, obesity, edema, glaucoma, skin diseases and others. The astrocyte water channel aquaporin-4 is a particularly compelling target because of its role of brain water movement, neuroexcitation and glia scarring, and because it is the target of pathogenic autoantibodies in the neuroinflammatory demyelinating disease neuromyelitis optica. There has been considerable interest in the identification of small molecule inhibitors of aquaporins, with various candidates emerging from testing of known ion transport inhibitors, as well as compound screening and computational chemistry. However, in general, the activity of reported aquaporin inhibitors has not been confirmed on retesting, which may be due to technical problems in water transport assays used in the original identification studies, and the challenges in modulating the activity of small, compact, pore-containing membrane proteins. We review here the state of the field of aquaporin-modulating small molecules and biologics, and the challenges and opportunities in moving forward.
Keywords: Water channel, AQP, Neuromyelitis optica, Brain edema, Drug discovery
16.1 Introduction and Potential Indications of Aquaporin Modulators
As reviewed in elsewhere in this book, more than a dozen mammalian aquaporins (AQPs) have been identified, many of which function as water channels, and some, the aquaglyceroporins, also transport various small, polar non-electrolytes including glycerol and hydrogen peroxide. Structurally, AQP monomers are small, membrane-spanning proteins of molecular size ~30 kDa, each containing one narrow aqueous pore. AQP monomers assemble in membranes as tetramers, with some AQPs such as AQP4 assembling further into supramolecular aggregates called orthogonal arrays of particles. There is high-resolution crystal structure data for some AQPs, as well as molecular dynamics simulations of how water and small polar molecules might traverse the aqueous pore. The AQPs have broad tissue expression, which includes absorptive and secretory epithelia, astrocytes, myocytes, adipocytes, epidermal cells, and others. A priori challenges in AQP drug discovery include: (i) the wide distribution and the many structurally similar AQP isoforms; (ii) the structural features of a narrow, drug-excluding pore and a compact tetramer; (iii) the lack of physiological regulation of intrinsic AQP function; and (iv) the unique ability of water, which is present at 55 molar concentration, to circumvent obstacles. Nevertheless, there is no single compelling reason to exclude the possibility of identifying useful inhibitors of AQP function, modulators of AQP expression, or blockers of AQP-targeted pathogenic antibodies in certain diseases. Indeed, mercury-containing and other heavy metal-containing sulfhydryl-reactive compounds inhibit the function of some AQPs by chemical modification of cysteine residues, though their marked toxicity and lack of selectivity preclude their development as drugs.
Notwithstanding the caveats listed above, there are compelling potential opportunities for AQP-targeted therapeutics in human disease. The physiological functions of AQPs, which have been elucidated largely from phenotype studies on knockout mice, are reviewed elsewhere [39]. Some interesting potential applications are mentioned briefly. Inhibitors of AQP1 water transport are predicted to act as unique diuretics, inhibit tumor angiogenesis and growth, reduce intraocular pressure in glaucoma, and potentially reduce nociception [7, 19, 31, 32, 47, 49]. Inhibitors of AQP4 water transport are predicted to reduce brain swelling in ischemic stroke [22]. Inhibitors of AQP3 glycerol and/or hydrogen peroxide transport are predicted to prevent or retard skin tumor growth and inflammatory skin diseases [9, 10]. Though it is unlikely that AQP ‘activators’ can be identified, as the AQPs are probably already maximally active, selective transcriptional upregulators of AQP7 may reduce adipocyte hypertrophy in obesity, of AQP3 may promote wound healing, of AQP4 may have anti-epileptic activity, and of AQP5 might increase glandular fluid secretion. There is evidence that AQP gene transfer may increase epithelial water permeability, promoting saliva secretion in salivary gland disorders [2] and bile secretion in liver diseases associated with cholestasis [23]. Finally, blockers of binding of pathogenic AQP4-targeted autoantibodies to astrocyte AQP4 might prevent or reduce neuropathology and neurological deficit in neuromyelitis optica [28].
Herein, we review approaches to assay for AQP-modulating compounds, the state of the field in the identification and validation of AQP modulators, and potential directions in moving forward.
16.2 Aquaporin Functional Measurements
Reliable assay of AQP function is central to the identification and validation of pharmacological AQP modulators. This section focuses on measurements of AQP water permeability, some of which are suitable for primary high-throughput screening, with discussion of assay limitations and potential artifacts. Alternative assays of transport functions of some AQPs, including glycerol, hydrogen peroxide and gas transport, merit consideration, but are not discussed further as available assays are not sufficiently robust for primary screening applications.
Measurement of water transport across an intact epithelium is accomplished by determination of net volume movement in response to a transepithelial osmotic gradient, which can be accomplished by a variety of methods such as displaced volume and dye dilution as measured by electrical of fluorescence methods (Fig. 16.1a). The epithelial cell layer can consist of cells cultured on a porous support or a native epithelium such as a kidney tubule or urinary bladder. Measurement of transepithelial water transport is robust and reliable, though not highly precise or suitable for high-throughput measurements. Additionally, because transcellular water transport involves two barriers in series (apical and basolateral membranes) the results probe mainly the rate-limiting barrier.
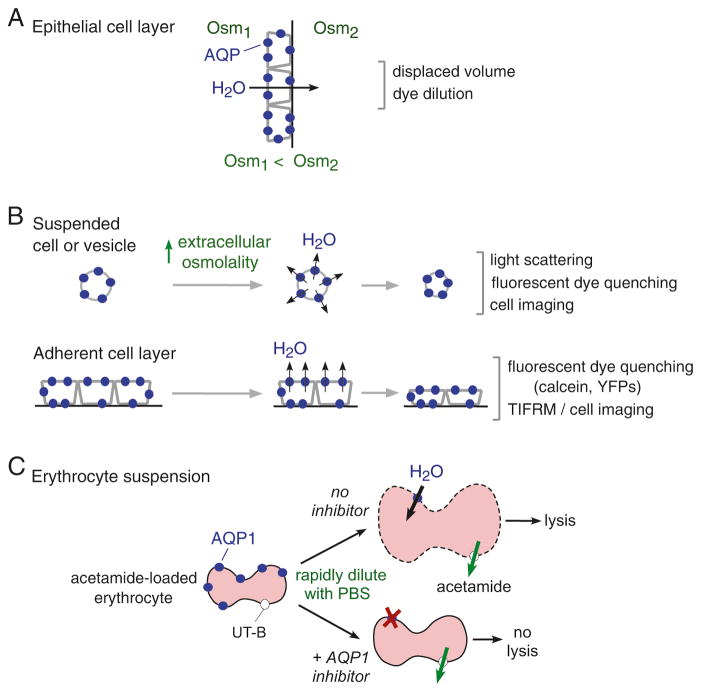
Fig. 16.1. Assays of osmotic water permeability.
(a) Water transport across an epithelial cell monolayer in response to a transepithelial osmotic gradient produces net volume flux. Osm, osmolality. (b) Water permeability across cells or vesicles in suspension (top) or across immobilized cells (bottom) in response to an osmotic gradient produces time-dependent volume change. Figure depicts water efflux in response to an inward osmotic gradient and cell shrinkage. (c) AQP1 water transport measured in erythrocytes, which express AQP1 and the urea/acetamide transporter UT-B. Dilution of acetamide-loaded erythrocytes into an acetamide-free solution drives water influx, cell swelling and lysis, which are reduced by AQP1 inhibition. See text for further explanations
Osmotic water transport in suspended or adherent cells is measured from the kinetics of cell volume in response to an osmotic gradient that is imposed over a time much less than the osmotic equilibration time (Fig. 16.1b). For suspended cells (or vesicles or liposomes) an osmotic gradient can be imposed using a stopped-flow instrument in which cell-containing and anisomolar solutions are mixed within milliseconds or less. For adherent cells such as AQP-expressing transfected or transduced cells, an osmotic gradient can be imposed using a perfusion chamber, or, for high-throughput applications, by rapid solution addition in a multi-well plate format. Cell volume readouts include light scattering, fluorescence of a volume-sensing dye, or direct imaging. Light scattering is used mainly to study small cells in suspension, such as erythrocytes, and is subject to various artifacts because the intensity of light scattered from cells depends not only on cell volume but on cell shape, the refractive index of intracellular and extracellular solutions, and membrane optical properties, each of which can be influenced by a putative AQP modulator. In addition, a ‘mixing artifact’ results from changes in light scattering as flow slows, which is unrelated to cell water permeability. Fluorescence methods are relatively insensitive to cell shape, membrane properties and mixing artifact, but can be affected directly by test compounds and confounded by dye leakage and binding to cell membranes, particularly in the calcein method in which calcein fluorescence is quenched by cytoplasmic proteins as cells shrink [35]. An alternative, genetically encoded chloride-sensing yellow fluorescent protein [3] does not suffer from leakage artifact, but is affected by changes in intracellular pH and anion concentrations, and has limited time resolution.
One particular cell type, the Xenopus oocyte, has been used in many studies to measure water permeability. Xenopus oocytes are injected with cRNA encoding an AQP, and the kinetics of oocyte swelling is measured in response to an osmotic gradient. Generally oocyte volume is inferred from its cross-sectional area (shadow) as measured with transmitted light microscopy using a low magnification lens. Though the oocyte swelling method was valuable in the original identification of AQP1 as a water channel, where large and unambiguous increases in the rate of swelling were seen, leading to oocyte bursting, oocytes have limited value for study of potential AQP modulators because of many potential artifacts. Changes in oocyte cross-sectional area depend not only on oocyte osmotic water permeability, but on oocyte geometry, membrane properties, solute transport, cytoplasmic and extracellular unstirred layers, and other factors. For example, preincubation of oocytes with an ion transport inhibitor can alter oocyte volume and cytoplasmic ionic concentrations, precluding meaningful measurement of osmotic water permeability. A further problem with many published measurements is that oocyte volume responses are measured over minutes, rather than seconds, a time scale where mechanical restrictions can affect swelling and solute transport can affect the osmotic gradient.
For measurement of erythrocyte AQP1 water permeability, we developed a simple method that relies on a single read-out of cell lysis (Fig. 16.1c). Erythrocytes, which natively express AQP1 and urea transporter UT-B, are preloaded with acetamide, a urea analog that is transported by UT-B and equilibrates across the erythrocyte membrane over a time course similar to that of osmotic water transport. Dilution of erythrocytes into an acetamide-free solution produces rapid, AQP1-dependent cell swelling and lysis, as assayed by solution absorbance in a platereader. Inhibition of AQP1 water permeability reduces cell lysis, as water influx is slower than dissipation of the osmotic gradient by acetamide efflux. A variation of this approach has been used to identify inhibitors of UT-B urea transport with low nanomolar potency [18], but a small-molecule screen for AQP1 inhibitors did not produce useful active compounds (unpublished data).
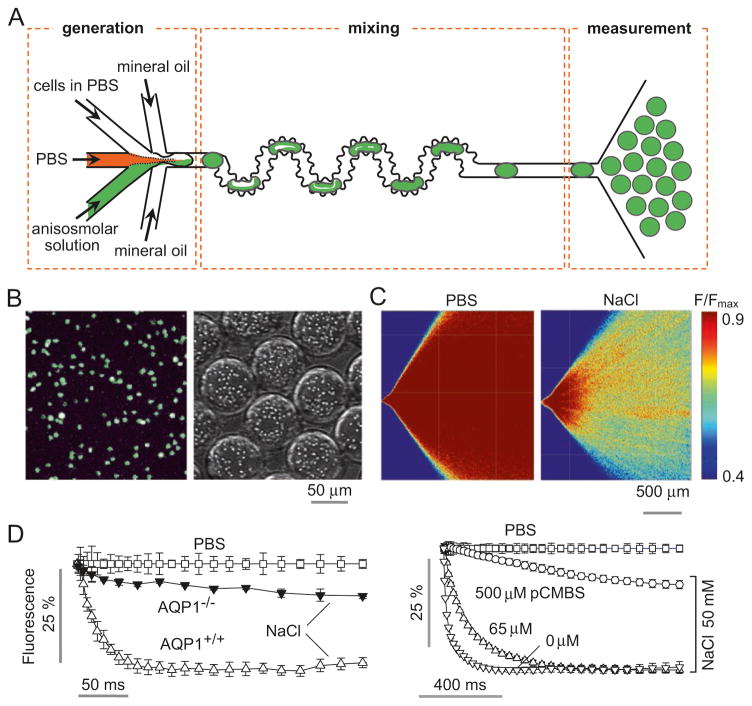
We have been developing microfluidics methods to measure water permeability, as microfluidics can provide a technically robust platform for rapid assays using very small samples. In one study, a perfusion channel was developed to measure volume changes of epithelial organoids in which the organoids are entrapped by pillars and volume measured by dye exclusion [16]. In another study [15], a microfluidic channel was designed to mimic rapid stopped-flow mixing methodology, in which cells are subjected to an osmotic gradient in milliseconds by solution mixing inside a ~ 0.1 nL droplet surrounded by oil (Fig. 16.2a). Rapid mixing of cells with an anisomolar solution is accomplished in a mixing channel, which then deposits the cell-containing droplets in an observation area in which time after mixing is determined by spatial position. Osmotic water permeability is then determined from a single, time-integrated fluorescence image of the observation area. As an example, water permeability was measured in calcein-labeled erythrocytes (Fig. 16.2b). Fluorescence of the observation area showed reduced fluorescence in the presence of an osmotic gradient (Fig. 16.2c), from which the kinetics of water transport can be deduced. Figure 16.2d shows the deduced kinetics data from wildtype and AQP1-null mouse erythrocytes (left) and from control and pCMBS (a mercurial)-treated human erythrocytes (right), which agree with results using the conventional stopped-flow light scattering method. Compared with costly stopped-flow instrumentation, this microfluidics platform utilizes sub-microliter blood sample volume, does not suffer from mixing artifact, and replaces kinetic measurements by a single image capture using a standard laboratory fluorescence microscope. However, microfluidics methods are in general not yet suited for automated high-throughput screening and do not obviate potential measurement artifacts in conventional suspended cell measurements.
Fig. 16.2. Microfluidic ‘stopped-flow’ approach to measure water permeability.
(a) Microfluidic channel design, in which mixture of cells with an anisomolar solution in droplets drives osmotic water transport and cell volume change, as measured by fluorescence in a measurement area. (b) Fluorescence micrograph of calcein-loaded erythrocytes on a coverglass (left) and in aqueous droplets in the microfluidic channel (right). (c) The microfluidic channel was perfused with an erythrocyte suspension in PBS, PBS (in central channel), and PBS containing 500 mm NaCl (bottom channel), to give a 200-mM NaCl gradient. Fluorescence micrographs of the measurement region for zero gradient (left) or a 200-mM NaCl gradient (right). (d) Deduced time course of erythrocyte calcein fluorescence in erythrocytes from wild-type and AQP1-knockout mice for a 200-mM NaCl gradient, and in the absence of an osmotic gradient (PBS) (left). Measurements on human erythrocytes preincubated with indicated concentration of the mercurial AQP1 inhibitor pCMBS (right) (Adapted from Ref. [15])
16.3 Aquaporin Inhibitors – State of the Field
16.3.1 Older Literature on Aquaporin Inhibitors
It has long been known that mercurial sulfhydryl-reactive compounds, including mercuric chloride and p-chloromercuribenzene sulfonate (pCMBS), inhibit water transport in erythrocytes and various epithelia [20]. After the discovery of AQPs the cysteine(s) involved in mercurial water transport inhibition were identified, such as Cys-187 in AQP1 [48]. More recently, gold-containing compounds were reported to inhibit AQP3, with auphen being the most potent [24]. Various non-metal-containing small molecules were reported to inhibit water permeability in some AQPs, including the K+ channel blocker tetraethylammonium (TEA+), the carbonic anhydrase inhibitor acetazolamide, several anti-epileptic drugs and dimethylsulfoxide (DMSO) [5, 12, 34]. Subsequent testing, however, did not confirm AQP inhibition by these small molecules [34, 43–45], suggesting measurement artifact in the oocyte swelling studies used to identify the compounds, which, as discussed above, are prone to artifact, especially for compounds that inhibit ion transport processes.
16.3.2 Screening to Identify Aquaporin Inhibitors
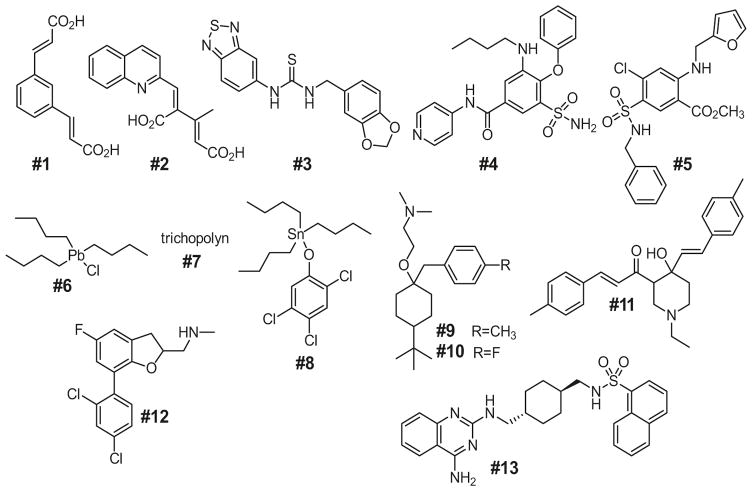
Additional putative small molecule AQP inhibitors have emerged from experimental and computational screens, with structures of 12 proposed AQP1 inhibitors, and one AQP1 activator, shown in Fig. 16.3. Compounds #1, #2, and #3 were identified by virtual (computational) screening involving docking to the extracellular surface of human AQP1, and testing 14 compounds for inhibition of osmotic swelling in AQP1-expressing Xenopus oocytes [33]. These compounds reduced osmotic swelling of oocytes by ~80% with IC50 of 8–20 μM, but were reported not to inhibit AQP1 in erythrocytes. Compound #4 (AqB013), an analog of the NKCC1 inhibitor bumetanide, came from a small synthesis study, and was claimed to inhibit AQP1 and AQP4 with IC50 ~20 μM [25], though it did not show the predicted in vivo beneficial effect when tested in a brain injury model [27]. The same group also reported that an analog of the loop diuretic furosemide, compound #5 (AqF026), activated AQP1 by ~20% in the oocyte assay [46], which is probably well below the limited reliability of such assays. Compounds #6, #7, #8 and #9, identified in small screen using the calcein fluorescence assay, were reported to inhibit AQP1 with IC50 values of 25–50 μM [26]; however the organolead and organotin structures are not drug-like and likely toxic, and compound #7 (trichopolyn I) is a 10-residue fungal, pore-forming lipopeptide. More recently, compounds #10 and #11 emerged from a small screen using a yeast freeze-thaw assay, of unclear rationale, done in E. coli expressing AQP1 [36]. Compounds #12 and #13 emerged from a small screen [29], though their reported activities were quite variable in oocyte, erythrocyte ghost and AQP1 proteoliposome assays. As described below, we have retested each of these compounds using several sensitive assays of AQP1 water permeability [6].
Fig. 16.3.
Chemical structures of putative small-molecule AQP1 inhibitors and an AQP1 activator (Compounds shown are reported in Refs. [25, 26, 29, 33, 36, 46]. See text for further explanations)
16.3.3 Screening by Computational Chemistry
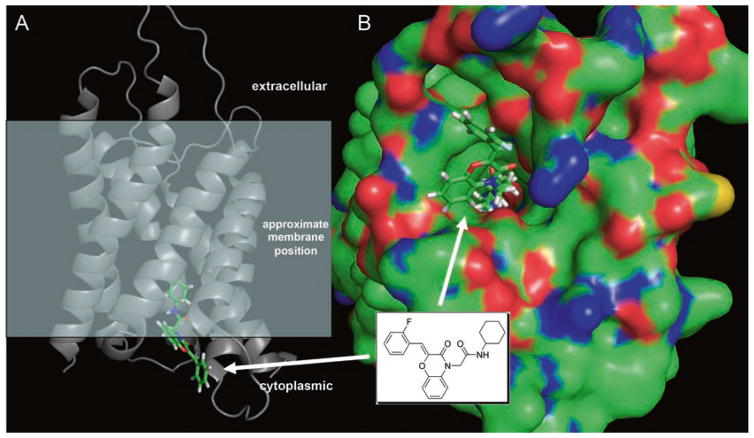
Several reports utilize computational methods (virtual screening, some with molecular dynamics (MD) simulations) to identify putative inhibitors of various AQPs. Surprisingly, multiple chemically unrelated antiepileptic drugs, which were selected from docking computation using an electron diffraction structure of rat AQP4, were reported to inhibit oocyte swelling [12]. The same investigators reported non-antiepileptic drugs as AQP4 inhibitors with IC50 of 2–11 μM, including 2-(nicotinamido)-1,3,4-thiadiazole, sumatriptan, and rizatriptan [13]. However, retesting of the compounds in Refs. [12, 13] did not confirm activity [45]. As mentioned above, several compounds emerged from a docking screen of ~106 compounds from the UCSF-ZINC library against an MD-refined structure of human AQP1 at a site near the ar/R selectivity filter [33]; docked conformations of two of the more promising structures were subjected to several hundred-ns MD simulations to confirm the stability of the docked poses. In a recent study, docking and MD simulations were done using homology models of mouse AQP9 [41], which identified a small set of inhibitors with IC50 <50 μM from a shrinking assay in AQP9-expressing CHO cells, though compound activities have not been independently tested to date. In our lab, we carried out large-scale docking studies against high-resolution structures of AQP1 and AQP4, with testing of the best-scoring ~2000 compounds, which, disappointingly, showed <20% inhibition at 50 μM (unpublished data). An example of a well-scored compound of the ben-zoxazin-3-one class is shown in Fig. 16.4a bound to the cytoplasmic pore region of mouse AQP1. A surface depiction of the complex (Fig. 16.4b) shows a complementary fit, with the nonpolar cyclohexyl substituent projecting deep into the channel, positioned to interact with residues Ile-60, Leu-149, and Val-79.
Fig. 16.4. Computational approach to identify aquaporin-interacting small molecules.
Docking computation using a homology model of mouse AQP1. (a) Side view of an AQP1-ligand complex with the approximate membrane position indicated. (b) Surface view of the same complex, showing the cyclohexyl group of the ligand projecting deep into the channel, interacting with a hydrophobic surface
16.3.4 Reevaluation of Proposed AQP1 Inhibitors
In a recent study [6] we reevaluated the 13 compounds shown in Fig. 16.3 for AQP1-modulating activity. The compounds were tested at 50 μM, a concentration predicted from published data to strongly inhibit (or weakly activate) AQP1 water permeability. One approach was stopped-flow light scattering in freshly obtained human erythrocytes. Representative light scattering curves are shown in Fig. 16.5 (left), with averaged data summarized in the right panel. Whereas HgCl2 strongly inhibited osmotic water permeability in erythrocytes, no significant effect was seen for 12 of the 13 test compounds, with the small apparent effect of compound #13 related to cell toxicity. In addition, to rule out the possibility that the lack of inhibition might be due to hemoglobin, which might bind compounds, similar studies done in sealed, hemoglobin-free ghost membranes also showed no inhibition (or activation). Several of the compounds (#6, #9, #10, #12 and #13) showed toxicity as evidenced by erythrocyte crenation and aggregation. Multiple additional assays supported the conclusion that compounds #1 to #13 do not inhibit (or activate) AQP1 water permeability, including erythrocyte swelling assays, erythrocyte water transport assays using calcein fluorescence, and water transport assays in plasma membrane vesicles from AQP1-transfected CHO cells.
Fig. 16.5. Testing of putative AQP1 modulators in human erythrocytes.
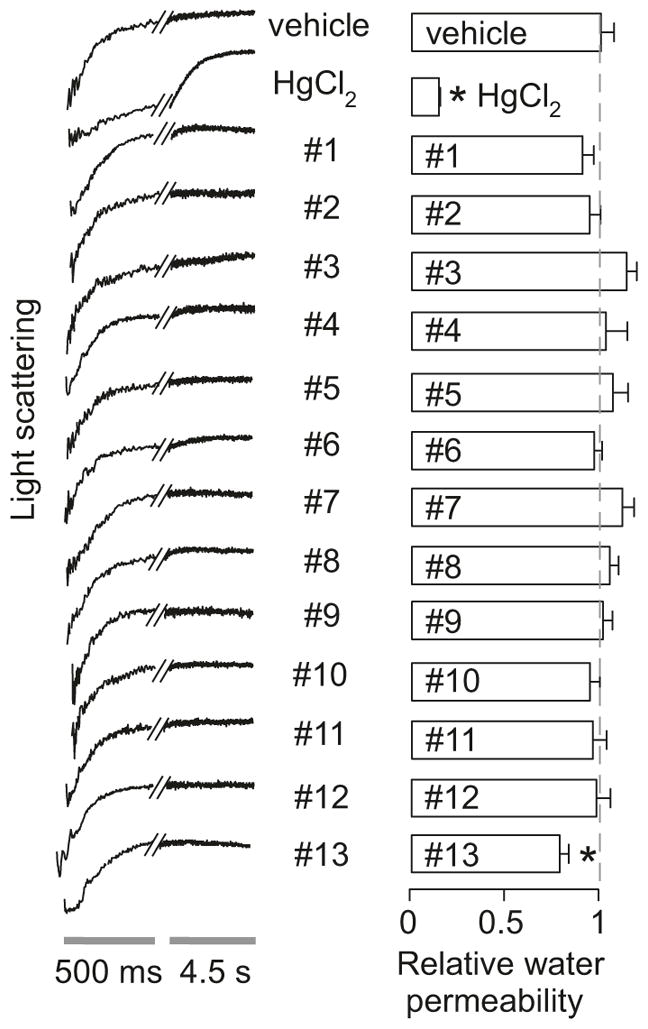
Osmotic water permeability was measured in human erythrocytes from the time course of scattered light intensity at 530 nm in response to a 250-mM inwardly directed sucrose gradient. Representative original light scattering data shown on the left for negative control (DMSO vehicle alone) and positive control (HgCl2), and indicated compounds at 50 μM. Summary of relative osmotic water permeability shown on the right (S.E., n = 4, *P < 0.05 compared to control) (Adapted from Ref. [6])
It is uncertain why activity could not be confirmed of the many putative AQP modulators reported in the literature. As discussed above the oocyte swelling or calcein fluorescence assays used in most of the studies are subject to considerable artifact in which apparent inhibition of osmotic cell swelling could result from changes in cell size or shape, cell volume regulation, activities of non-AQP ion or solute transporters, etc. Inhibitors of known cell membrane transporter processes, such as bumetanide, acetazolamide and tetraethylammonium, may affect resting cell volume and volume regulation. Because of the very low probability of identifying AQP inhibitors, as suggested from screening work, it is unlikely that testing of common drugs, such as loop diuretics, carbonic anhydrase inhibitors, and antiepileptics, without large-scale screening, would yield bona fide AQP inhibitors.
16.3.5 Antibodies as AQP Therapeutics
Given the challenges and limited progress in small molecule AQP-targeted therapeutics, consideration of biologic therapeutics is warranted. Of particular interest are IgG1 anti-AQP4 autoantibodies (“AQP4-IgG”) in neuromyelitis optica (NMO), an inflammatory demyelinating disease of the central nervous system that can cause paralysis and blindness. It is thought that AQP4-IgG produces neuropathology by binding to AQP4 on astrocytes (Fig. 16.6a) to cause complement- and cell-mediated astrocyte cytotoxicity, which produces inflammation, blood-brain barrier disruption, oligodendrocyte injury, demyelination and neurological deficit [28]. The AQP4-IgG autoantibodies are directed against various 3-dimensional epitopes on the AQP4 extracellular surface. Though it was initially proposed from oocyte swelling studies that inhibition of AQP4 water permeability plays a causal role in NMO [11], subsequent studies showed that AQP4-IgGs, even at saturating concentrations, do not inhibit AQP4 water permeability [30]. Interestingly, autoantibodies against AQP2 [17] and AQP5 [1] have been found recently in interstitial nephritis and Sjogren’s syndrome, respectively, though their involvement in disease pathogenesis is not known.
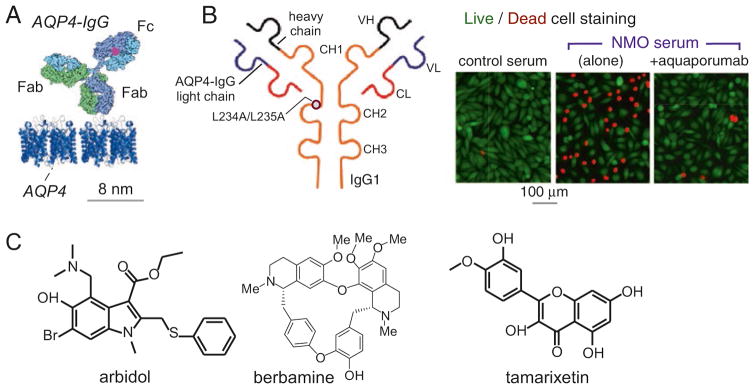
Fig. 16.6. Antibody and small molecule blockers of AQP4 binding of anti-AQP4 autoantibodies causing neuromyelitis optica.
(a) To-scale diagram of IgG antibody binding to AQP4 on membranes. (b) Anti-AQP4 IgG1 antibody with L234A/L235A mutations that eliminate effector functions (left). Live/dead (green/red) cell staining of AQP4-expressing cells exposed to control or human NMO serum, showing protection by aquaporumab. (c) Structures of small molecule blockers of autoantibody binding to AQP4 (Adapted from Refs. [37, 38])
Though the identification of a neutralizing anti-AQP antibody is unlikely because of its large molecular size and binding to extracellular loop regions far from the narrow AQP pore, AQP-binding antibodies have other therapeutic applications. In one application, we generated a high-affinity anti-AQP4 antibody (“aquaporumab”) in which the antibody Fc portion was mutated to eliminate effectors functions involved in complement- and cell-mediated cytotoxicity (Fig. 16.6b, left) [37]. The antibody prevented cytotoxicity from NMO patient sera in cell cultures (Fig. 16.6b, right) and prevented pathology and demyelination in animal models of NMO, suggesting its application for primary therapy of NMO. Screening and computational analysis of small molecule blockers of AQP4-IgG binding to AQP4 produced candidate molecules (Fig. 16.6c) [21, 38]; however, their affinities are too low for development as NMO therapeutics, which is not unexpected given the recognized challenges in identifying potent small molecule blockers of protein-protein interactions.
16.4 Perspective and Future Directions
Though there is much speculation about the utility of AQP-targeted therapeutics [4, 8, 14, 40, 42], as well as compelling animal data to support AQP drug development, progress in the field has been disappointing. Reports of AQP inhibition by common ion transport inhibitors, such as loop diuretics and antiepileptics, have confused the literature, as have reports of small molecule AQP inhibitors that could not be confirmed on retesting. The potential pitfalls in assays of AQP function merit appreciation, as does the importance of showing robust, cell context-independent compound action. Well-conceived, large-scale functional screens of random, drug-like small molecules may yield useful, bona fide AQP inhibitors, as might smaller screens of compounds collections biased by computation chemistry. A relatively unexplored subject is antibody- and peptide-based AQP therapeutics, and small molecule transcriptional regulators of AQP expression. The interest in commercializing AQP-targeted therapeutics, and the need for useful research tools to overcome the limitations of transgenic animal models, will likely drive further developments in the field.
Acknowledgments
This work was supported by grants DK101373, DK35124, DK72517, EB00415, EY13574 and DK99803 from the National Institutes of Health, and grants from the Guthy-Jackson Charitable Foundation and the Cystic Fibrosis Foundation.
Contributor Information
Lukmanee Tradtrantip, Departments of Medicine and Physiology, University of California, San Francisco, CA 94143-0521, USA.
Bjung-Ju Jin, Departments of Medicine and Physiology, University of California, San Francisco, CA 94143-0521, USA.
Xiaoming Yao, Departments of Medicine and Physiology, University of California, San Francisco, CA 94143-0521, USA.
Marc O. Anderson, Department of Chemistry and Biochemistry, San Francisco State University, San Francisco, CA 94132-4136, USA
Alan S. Verkman, Departments of Medicine and Physiology, University of California, San Francisco, CA 94143-0521, USA
References
- 1.Alam J, Koh JH, Kim N, Kwok SK, Park SH, Song YW, Park K, Choi Y. Detection of autoantibodies against aquaporin-5 in the sera of patients with primary Sjögren’s syndrome. Immunol Res. 2016;64:849–856. doi: 10.1007/s12026-016-8786-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, Nottingham LK, Rudy SF, Van Waes C, Whatley MA, Brahim JS, Chiorini JA, Danielides S, Turner RJ, Patronas NJ, Chen CC, Nikolov NP, Illei GG. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci U S A. 2012;109:19403–19407. doi: 10.1073/pnas.1210662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgart F, Rossi A, Verkman AS. Light inactivation of water transport and protein-protein interactions of aquaporin-Killer Red chimera. J Gen Physiol. 2012;139:83–91. doi: 10.1085/jgp.201110712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beitz E, Golldack A, Rothert M, von Bülow J. Challenges and achievements in the therapeutic modulation of aquaporin functionality. Pharmacol Ther. 2015;155:22–35. doi: 10.1016/j.pharmthera.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Brooks HL, Regan JW, Yool AJ. Inhibition of aquaporin-1 water permeability by tetraethylammonium: involvement of the loop E pore region. Mol Pharmacol. 2000;57:1021–1026. [PubMed] [Google Scholar]
- 6.Esteva-Font C, Jin BJ, Lee S, Phuan PW, Anderson MO, Verkman AS. Experimental evaluation of proposed small-molecule inhibitors of water channel aquaporin-1. Mol Pharmacol. 2016;89:686–693. doi: 10.1124/mol.116.103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteva-Font C, Jin BJ, Verkman AS. Aquaporin-1 gene deletion reduces breast tumor growth and lung metastasis in tumor-producing MMTV-PyVT mice. FASEB J. 2014;28:1446–1453. doi: 10.1096/fj.13-245621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frigeri A, Nicchia GP, Svelto M. Aquaporins as targets for drug discovery. Curr Pharm Des. 2007;13:2421–2427. doi: 10.2174/138161207781368738. [DOI] [PubMed] [Google Scholar]
- 9.Hara-Chikuma M, Satooka M, Watanabe S, Honda T, Miycahi Y, Watanabe T, Verkman AS. Aquaporin-3-mediated hydrogen peroxide transport required for NF-κB signaling in keratinocytes and development of psoriasis. Nat Commun. 2015;6:7454. doi: 10.1038/ncomms8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara-Chikuma M, Verkman AS. Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol. 2008;28:328–332. doi: 10.1128/MCB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hinson SR, Romero MF, Popescu BF, Lucchinetti CF, Fryer JP, Wolburg H, Fallier-Becker P, Noell S, Lennon VA. Molecular outcomes of neuromyelitis optica (NMO)-IgG binding to aquaporin-4 in astrocytes. Proc Natl Acad Sci U S A. 2012;109:1245–1250. doi: 10.1073/pnas.1109980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber VJ, Tsujita M, Kwee IL, Nakada T. Inhibition of aquaporin 4 by antiepileptic drugs. Bioorg Med Chem. 2009;17:418–424. doi: 10.1016/j.bmc.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Huber VJ, Tsujita M, Nakada T. Identification of Aquaporin 4 inhibitors using in vitro and in silico methods. Bioorg Med Chem. 2009;17:411–417. doi: 10.1016/j.bmc.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 14.Jeyaseelan K, Sepramaniam S, Armugam A, Wintour EM. Aquaporins: a promising target for drug development. Expert Opin Ther Targets. 2006;10:889–909. doi: 10.1517/14728222.10.6.889. [DOI] [PubMed] [Google Scholar]
- 15.Jin BJ, Esteva-Font C, Verkman AS. Droplet-based microfluidic platform for measurement of rapid erythrocyte water transport. Lab Chip. 2015;15:3380–3390. doi: 10.1039/c5lc00688k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin BJ, Battula S, Zachos N, Kovbasnjuk O, Fawlke-Abel J, In J, Donowitz M, Verkman AS. Microfluidics platform for measurement of volume changes in immobilized intestinal enteroids. Biomicrofluidics. 2014;8:024106. doi: 10.1063/1.4870400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landegren N, Pourmousa Lindberg M, Skov J, Hallgren Å, Eriksson D, Lisberg Toft-Bertelsen T, MacAulay N, Hagforsen E, Räisänen-Sokolowski A, Saha H, Nilsson T, Nordmark G, Ohlsson S, Gustafsson J, Husebye ES, Larsson E, Anderson MS, Perheentupa J, Rorsman F, Fenton RA, Kämpe O. Autoantibodies targeting a collecting duct-specific water channel in tubulointerstitial nephritis. J Am Soc Nephrol. 2016;27:3220–3228. doi: 10.1681/ASN.2015101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levin MH, de la Fuente R, Verkman AS. Urearetics: a small molecule screen yields nanomolar potency inhibitors of urea transporter UT-B. FASEB J. 2007;21:551–563. doi: 10.1096/fj.06-6979com. [DOI] [PubMed] [Google Scholar]
- 19.Ma T, Song Y, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Nephrogenic diabetes insipidus in mice lacking aquaporin-3 water channels. Proc Natl Acad Sci U S A. 2000;97:4386–4391. doi: 10.1073/pnas.080499597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macey RI, Farmer RE. Inhibition of water and solute permeability in human red cells. Biochim Biophys Acta. 1970;211:104–106. doi: 10.1016/0005-2736(70)90130-6. [DOI] [PubMed] [Google Scholar]
- 21.Mangiatordi GF, Alberga D, Sirgusa L, Goracci L, Lattandi G, Nicolotti O. Challenging AQP4 drug-gability for NMO-IgG antibody binding using molecular dynamics and molecular interaction field. 2015;1848:1462–1471. doi: 10.1016/j.bbamem.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen A, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 23.Marrone J, Soria LR, Danielli M, Lehmann GL, Larocca MC, Marinelli RA. Hepatic gene transfer of human aquaporin-1 improves bile salt secretory failure in rats with estrogen-induced cholestasis. Hepatology. 2016;64:534–548. doi: 10.1002/hep.28564. in press. [DOI] [PubMed] [Google Scholar]
- 24.Martins AP, Ciancetta A, de Almeida A, Marrone A, Re N, Soveral G, Casini A. Aquaporin inhibition by gold(III) compounds: new insights. Chem Med Chem. 2013;8:1086–1092. doi: 10.1002/cmdc.201300107. [DOI] [PubMed] [Google Scholar]
- 25.Migliati E, Meurice N, DuBois P, Fang JS, Somasekharan S, Beckett E, Flynn G, Yool A. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol Pharmacol. 2009;76:105–112. doi: 10.1124/mol.108.053744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mola MG, Nicchia GP, Svelto M, Spray DC, Frigeri A. Automated cell-based assay for screening of aquaporin inhibitors. Anal Chem. 2009;81:8219–8229. doi: 10.1021/ac901526k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliva AA, Kang Y, Truettner JS, Sanchez-Molano J, Furones C, Yool AJ, Atkins CM. Fluid-percussion brain injury induces changes in aquaporin channel expression. Neurobiologia. 2011;180:272–279. doi: 10.1016/j.neuroscience.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 28.Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurosci. 2014;10:493–506. doi: 10.1038/nrneurol.2014.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patil RV, Xu S, van Hoek AN, Rusinko A, Feng Z, May J, Hellberg M, Sharif NA, Wax MB, Irigoyen M, Carr G, Brittain T, Brown P, Colbert D, Kumari S, Varadaraj K, Mitra AK. Rapid identification of novel inhibitors of the human aquaporin-1 water channel. Chem Biol Drug Des. 2016;87:794–805. doi: 10.1111/cbdd.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi A, Ratelade J, Papadopoulos MC, Bennett JL, Verkman AS. Neuromyelitis optica IgG does not alter aquaporin-4 water permeability, plasma membrane M1/M23 isoform content, or supramolecular assembly. Glia. 2012;60:2027–2039. doi: 10.1002/glia.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- 32.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci U S A. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seeliger D, Zapater C, Krenc D, Haddoub R, Flitsch S, Beitz E, Cerda J, de Groot BL. Discovery of novel human aquaporin-1 blockers. ACS Chem Biol. 2013;8:249–256. doi: 10.1021/cb300153z. [DOI] [PubMed] [Google Scholar]
- 34.Søgaard R, Zeuthen T. Test of blockers of AQP1 water permeability by a high-resolution method: no effects of tetraethylammonium ions or acetazolamide. Pflugers Arch. 2008;456:285–292. doi: 10.1007/s00424-007-0392-2. [DOI] [PubMed] [Google Scholar]
- 35.Solenov E, Watanabe H, Manley GT, Verkman AS. Sevenfold-reduced osmotic water permeability in primary astrocyte cultures from AQP-4-deficient mice, measured by a fluorescence quenching method. Am J Phys. 2004;286:C426–C432. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- 36.To J, Yeo CY, Soon CH, Torres J. A generic high-throughput assay to detect aquaporin functional mutants: Potential application to discovery of aquaporin inhibitors. Biochim Biophys Acta. 1850;2015:1869–1876. doi: 10.1016/j.bbagen.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 37.Tradtrantip L, Zhang H, Saadoun S, Phuan PW, Lam C, Papadopoulos MC, Bennett JL, Verkman AS. Ann Neurol. 2012;71:314–322. doi: 10.1002/ana.22657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tradtrantip L, Zhang H, Anderson MO, Saadoun S, Phuan PW, Papadopoulos MC, Bennett JL, Verkman AS. Small molecule inhibitors of NMO-IgG binding to aquaporin-4 reduce astrocyte cytotoxicity in neuromyelitis optica. FASEB J. 2012;26:2197–2208. doi: 10.1096/fj.11-201608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012;63:303–316. doi: 10.1146/annurev-med-043010-193843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13:259–277. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wacker SJ, Aponte-Santamaria C, Kjellbom P, Nielsen S, De Groot BL, Rutzler M. The identification of novel, high affinity AQP9 inhibitors in an intracellular binding site. Mol Membr Biol. 2013;30:246–260. doi: 10.3109/09687688.2013.773095. [DOI] [PubMed] [Google Scholar]
- 42.Wang F, Feng XC, Li YM, Yang H, Ma TH. Aquaporins as potential drug targets. Acta Pharmacol Sin. 2006;27:395–401. doi: 10.1111/j.1745-7254.2006.00318.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi T, Iwata Y, Miura S, Kawada K. Reinvestigation of drugs and chemicals as aquaporin-1 inhibitors using pressure-induced hemolysis in human erythrocytes. Biol Pharm Bull. 2012;35:2088–2091. doi: 10.1248/bpb.b12-00581. [DOI] [PubMed] [Google Scholar]
- 44.Yang B, Kim JK, Verkman AS. Comparative efficacy of HgCl2 with candidate aquaporin-1 inhibitors DMSO, gold, TEA+ and acetazolamide. FEBS Lett. 2006;580:6679–6684. doi: 10.1016/j.febslet.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang B, Zhang H, Verkman AS. Lack of aquaporin-4 water transport inhibition by antiepileptics and arylsulfonamides. Bioorg Med Chem. 2008;16:7489–7493. doi: 10.1016/j.bmc.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yool AJ, Morelle J, Cnops Y, Verbavatz JM, Campbell EM, Beckett EA, Booker GW, Flynn G, Devuyst O. AqF026 is a pharmacologic agonist of the water channel aquaporin-1. J Am Soc Nephrol. 2013;24:1045–1052. doi: 10.1681/ASN.2012080869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang D, Vetrivel L, Verkman AS. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J Gen Physiol. 2002;119:561–569. doi: 10.1085/jgp.20028597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang R, van Hoek AN, Biwersi J, Verkman AS. A point mutation at cysteine 189 blocks the water permeability of rat kidney water channel CHIP28k. Biochemist. 1993;32:2938–2941. doi: 10.1021/bi00063a002. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Verkman AS. Aquaporin-1 tunes pain perception by interaction with Nav1.8 Na+ channels in dorsal root ganglion neurons. J Biol Chem. 2010;285:5896–5906. doi: 10.1074/jbc.M109.090233. [DOI] [PMC free article] [PubMed] [Google Scholar]