Abstract
Objective
To assess the risk factors associated with recurrence, progression and survival in high-risk non-muscle-invasive bladder cancer (NMIBC) patients treated with bacillus Calmette–Guérin (BCG) and validate the European Organization for Research and Treatment of Cancer (EORTC) and Spanish Urological Club for Oncological Treatment (CUETO) scores.
Patients and methods
We retrospectively analyzed all BCG-treated NMIBC patients from 1998 to 2012. Multiple variables were tested as risk factors for recurrence-free survival and progression-free survival (PFS). Variables included age, sex, grade, stage, tumor size, number of tumors, carcinoma in situ (CIS), recurrence status, BCG strain used, smoking status, use of re-staging transurethral resection and use of single immediate postoperative instillation. We also tested the accuracy of EORTC and CUETO scores in predicting recurrence and progression.
Results
Overall, 123 patients were analyzed. Median (interquartile range) follow-up was 49 months. The 5-year overall survival, cancer-specific survival, recurrence-free survival and PFS were 75.0%, 89.3%, 59.4% and 79.2%, respectively. On univariate analysis, multiple tumors (≥3), concomitant CIS and smoking influenced recurrence. Regarding progression, multiple tumors, concomitant CIS and Connaught strain (vs Tice) negatively influenced PFS on univariate and multivariate analyses were independent prognostic factors. CUETO scores were accurate, with a slight overestimation, while EORTC score was not predictive of recurrence or progression.
Conclusion
In this study, CIS and tumor multiplicity were unfavorable predictors of recurrence and progression in patients with NMIBC receiving BCG. CUETO model was superior to EORTC risk tables in predicting recurrence and progression in our BCG-treated patient population. Nonetheless, both scores overestimated recurrence and progression rates. Prospective trials are needed to validate our findings.
Keywords: BCG, bladder cancer, progression, recurrence
Introduction
Bladder cancer is by far the most common malignancy of the urinary tract. In Europe, it represents the 4th most common neoplasia in men and the 14th in women. In the European Union, its incidence is estimated at 123,000 cases per year, with a yearly mortality rate of over 40,000 people.1 Beyond the medical burden, bladder cancer also represents an important economic issue, being the most expensive cancer per case from diagnosis to death.2 Two-thirds of new bladder cancers are non-muscle-invasive bladder carcinomas (NMIBCs).3
High-risk NMIBC, such as pT1G3 and carcinoma in situ (CIS), with unfavorable long-term outcome, represents a therapeutic dilemma with two main treatment options available: intravesical bacillus Calmette–Guérin (BCG) instillations for a conservative approach and radical cystectomy.4 The first option exhibits tolerability issues5,6 and might decrease the chances of healing if treatment fails.7,8 Furthermore, BCG production has recently shown its limits, with worldwide BCG shortage. Radical cystectomy, on the other hand, is a major surgery associated with high complication rates and could represent overtreatment for many NMIBC patients. Various prognostic factors have been described, and scoring models have been developed to predict the risk of recurrence and progression, in order to offer the best possible treatment to NMIBC patients.9,10
The aim of this study was to explore risk factors for NMIBC recurrence and progression and validate the European Organization for Research and Treatment of Cancer (EORTC)9 and Spanish Urological Club for Oncological Treatment (CUETO)10 scoring systems on an external cohort of high-risk patients who received BCG as the initial treatment and discern potential new risk factors of poor outcome.
Patients and methods
After obtaining approval from the local ethics committee, we retrospectively reviewed the clinical and pathologic features of 200 successive patients treated with BCG instillations in the Department of Urology, Cliniques Universitaires de Bruxelles, Hôpital Erasme-ULB, from January 1998 to December 2012. Patients gave informed consent to use their data for this study. The following data were included: age, sex, pathologic grade and stage, tumor size, number of tumors, presence of CIS, recurrence status, BCG instillations scheme used, BCG strain used, smoking status, use of restaging transurethral resection (re-TUR), use of single immediate postoperative instillation of chemotherapy (SIPIC), and EORTC9 and CUETO10 scores. Adverse events and exact cycle duration were recorded, but will be described in detail separately. The evaluated end-points were overall survival, cancer-specific survival (CSS), recurrence-free survival, progression-free survival and muscle-invasive free survival (MIFS).
Recurrence was defined as follows: reappearance of tumor (any grade and any stage) during follow-up. Progression was defined as progression to muscle-invasive disease, development of lymph node (N+) disease or distant metastasis (M1).11 Exclusion criteria were the following: history of muscle-invasive urothelial cancer, of pelvic irradiation and previous BCG instillations. re-TUR was proposed to patients in whom the primary resection was macroscopically incomplete or no muscle tissue was found on pathologic examination of the transurethral resection (TUR) specimen.
Instillation scheme
Induction scheme of six-weekly instillations started 2–6 weeks after the initial TUR or re-TUR. Full dose of BCG was instilled with a Connaught strain (Immucyst-Sanofi-Pasteur® 81 mg) from 1998 to 2006, followed by Tice strain (Oncotice-MSD® 60 mg) from 2006 to 2012. Cystoscopy and cytology, eventually associated with bladder biopsies, were performed after the induction cycle. If no residual tumor was found, maintenance instillations could be offered in a scheme of three-weekly instillations every 3 months for a maximum duration of 3 years. NMIBC found after induction instillations was not considered as recurrence, but as persistence, and six new BCG induction instillations were generally offered. Time zero was then reset at the day of the second TUR, and the first induction instillations were not counted in the instillation scheme.
Follow-up
During the first 2 years, patients were offered cystoscopy and cytology every 3 months and during the following 3 years, cystoscopy and cytology were offered every 6 months and yearly thereafter.
Statistical analysis
Kaplan–Meier survival method and a stepwise forward Cox logistic regression model were used to compare risk factors for overall survival, CSS, recurrence-free survival, progression-free survival and MIFS on univariate analysis (log-rank test). Variables analyzed included age (<60, 60–70, >70), sex, pathologic grade (G1, G2, G3) and stage (pTa vs pT1), tumor size (<3 vs >3 cm), number of tumors (<3 vs ≥3), presence of CIS, recurrence status (primary vs recurrent <1/year vs recurrent ≥1/year), BCG strain used (Tice vs Connaught), smoking status (never, stopped vs active), use of re-TUR and use of SIPIC. The actual recurrence and progression rates across EORTC and CUETO categories were compared to the expected rates based on such nomograms. We considered p values <0.05 as statistically significant, and statistically significant risk factors of interest were evaluated in multivariate analysis. All statistical analyses were carried out with Medcalc® version 13.2.2 for Windows® (Medcalc Software, Oostende, Belgium).
Ethics
This study was approved by the Local Ethics Review Board at the Cliniques Universitaires de Bruxelles, Hôpital Erasme-ULB under approval number 021/406.
Results
Overall, we identified 200 patients treated with BCG. Seventy-seven were excluded from the analysis, as 35 had history of former BCG instillations, 11 of muscle-invasive urothelial neoplasia, 5 of pelvic irradiation, 1 had BCG instillations on the upper urinary tract and 2 due to missing oncologic data. Twenty-three patients had CIS only and were excluded as many evaluated factors did not apply (number, size, stage, EORTC and CUETO scores). One hundred and twenty-three patients were finally retained for the analysis. Characteristics of the population are shown in Table 1. Mean (interquartile range) follow-up was 49 (28–81) months and the median was 56 months (range 2–181). Survival statistics of the global cohort are shown in Table 2. In this study, 42/123 (32%) had a recurrence, 19/123 (17%) had a progression and 9/123 (7%) died during the follow-up available. Globally, 95.9% of patients were treated with at least a six-weekly induction course; 69.9% had some sort of maintenance therapy and 28.9% had a maintenance therapy of over 1 year.
Table 1.
Population characteristics (N=123)
| Age (years) | Tumor size, mm | |||
|---|---|---|---|---|
| Mean–median (extremes) | 68–69 (37–97) | >30 | 37 (30%) | |
| Sex | ≤30 | 86 (70%) | ||
| Men | 109 (89%) | |||
| Women | 14 (11%) | Recurrence | ||
| Histologic stage | Primary | 78 (63%) | ||
| Ta | 14 (11%) | >1 recurrence/year | 29 (24%) | |
| Ta+CIS | 7 (6%) | ≤1 recurrence/year | 16 (13%) | |
| T1 | 61 (50%) | Smoking status | ||
| T1+CIS | 41 (33%) | Active | 61 (49%) | |
| Stopped | 32 (26%) | |||
| Histologic grade (WHO 1973)24 | Never smoked | 29 (24%) | ||
| G1 | 8 (7%) | Unknown | 1 (1%) | |
| G2 | 19 (15%) | |||
| G3 | 96 (78%) | SIPIC | ||
| Histologic grade (WHO 2004)25 | 24 (19%) | |||
| Low | 15 (12%) | re-TUR | ||
| High | 108 (88%) | 27 (22%) | ||
| Number of tumors | BCG strain, mg | |||
| Single | 70 (57%) | Tice 60 | 102 (83%) | |
| Multiple | 53 (43%) | Connaught 81 | 21 (17%) | |
Abbreviations: BCG, bacillus Calmette–Guérin; CIS, carcinoma in situ; re-TUR, restaging transurethral resection; SIPIC, single immediate postoperative instillation of chemotherapy; WHO, World Health Organization.
Table 2.
Overall survival statistics
| 1 year | 2 years | 3 years | 4 years | 5 years | 6 years | 7 years | 8 years | |
|---|---|---|---|---|---|---|---|---|
| OS | 95.7% | 91.9% | 87.4% | 80.00% | 74.9% | 70.0% | 68.0% | 62.4% |
| CSS | 99.3% | 97.6% | 95.7% | 92.3% | 89.2% | 87.3% | 84.8% | 84.8% |
| RFS | 82.7% | 73.9% | 65.7% | 60.0% | 58.6% | 56.9% | 54.7% | 54.7% |
| PFS | 92.1% | 89.0% | 85.4% | 83.3% | 83.3% | 81.6% | 81.6% | 81.6% |
| BIS | 92.8% | 90.4% | 86.6% | 84.4% | 81.5% | 79.9% | 79.9% | 79.9% |
| Nb at risk | 130 | 118 | 92 | 72 | 54 | 40 | 31 | 21 |
Abbreviations: BIS, bladder in situ; CSS, cancer-specific survival; Nb, number; OS, overall survival; PFS, progression-free survival; re-TUR, restaging transurethral resection; RFS, recurrence-free survival.
Results from univariate and multivariate analyses are shown in Tables 3 and 4, respectively. Concerning recurrence, univariate analysis showed significant correlation with the number (≥3), CIS and smoking (even stopped). In multivariate analyses, concomitant CIS and tumor multiplicity showed significant correlation (Table 4). When exploring progression to muscle-invasive disease, a significant correlation with number of tumors (≥3), concomitant CIS and type of strain (Connaught) was found on univariate analysis (all p<0.05). In multivariate analyses, concomitant CIS and tumor multiplicity showed significant correlation to the risk of progression (Table 5).
Table 3.
Univariate analysis of risk factors
| Factor | Recurrence
|
Progression
|
||||||
|---|---|---|---|---|---|---|---|---|
| Nb (%) | OR | 95% CI | p | OR | 95% CI | p | ||
| Sex | Male | 109 (89) | 1.33 | 0.53–3.35 | NS | 1.15 | 0.29–4.60 | |
| Female | 14 (11) | 0.75 | 0.30–1.90 | 0.87 | 0.22–3.49 | NS | ||
| Age (years) | <60 | 26 (21.1) | Ref | Ref | Ref | Ref | Ref | Ref |
| 60–70 | 37 (30.1) | 1.77 | 0.77–4.07 | 1.87 | 0.55–6.39 | |||
| >70 | 60 (48.8) | 1.59 | 0.75–3.39 | NS | 3.08 | 0.98–9.69 | NS | |
| Grade | 1 | 10 (8.1) | Ref | Ref | Ref | Ref | Ref | Ref |
| 2 | 27 (22.0) | 0.44 | 0.14–1.37 | 0.57 | 0.19–1.72 | |||
| 3 | 86 (69.9) | 0.98 | 0.35–2.73 | NS | 1.75 | 0.58–5.26 | NS | |
| Number | <3 | 97 (78.9) | 0.37 | 0.17–0.81 | 0.38 | 0.13–1.14 | ||
| ≥3 | 26 (21.1) | 2.70 | 1.24–5.86 | <0.01 | 2.61 | 0.88–7.75 | <0.05 | |
| Size (mm) | <30 | 86 (69.9) | 0.98 | 0.49–1.95 | 1.12 | 0.41–3.02 | ||
| >30 | 37 (30.1) | 1.02 | 0.51–2.03 | NS | 0.90 | 0.33–2.42 | NS | |
| CIS | No | 75 (61.0) | 0.48 | 0.26–0.91 | 0.33 | 0.13–0.84 | ||
| Yes | 48 (39.0) | 2.07 | 1.09–3.92 | <0.05 | 3.02 | 1.18–7.69 | <0.05 | |
| Stage | Ta | 22 (18) | 0.57 | 0.28–1.19 | ||||
| T1 | 101 (82) | 1.74 | 0.84–3.61 | NS | – | – | <0.05 | |
| Recurrence | Primary | 78 (63.4) | 0.76 | 0.40–1.43 | 0.83 | 0.33–2.09 | ||
| Recurrent | 45 (36.6) | 1.31 | 0.70–2.47 | NS | 1.21 | 0.48–3.06 | NS | |
| <1/year | 29 (23.6) | 0.95 | 0.46–1.94 | 0.91 | 0.31–2.65 | |||
| >1/year | 16 (13.0) | 2.10 | 0.79–5.59 | NS | 1.77 | 0.45–7.05 | NS | |
| Strain | Tice | 87 (70.7) | 1.18 | 0.61–2.30 | 0.41 | 0.15–1.14 | ||
| Connaught | 36 (29.3) | 0.85 | 0.44–1.65 | NS | 2.43 | 0.88–6.68 | <0.05 | |
| SIPIC | No | 100 (81.3) | 1.21 | 0.54–2.72 | 1.87 | 0.58–6.02 | ||
| Yes | 23 (18.7) | 0.83 | 0.37–1.86 | NS | 0.53 | 0.17–1.72 | NS | |
| Smoking | Never | 30 (24.4) | Ref | Ref | Ref | Ref | Ref | Ref |
| Stopped | 32 (26.0) | 2.88 | 1.18–7.03 | 2.09 | 0.59–7.35 | |||
| Active | 61 (49.6) | 1.07 | 0.52–2.20 | <0.01 | 0.93 | 0.31–2.77 | NS | |
| re-TUR | No | 96 (78.0) | 0.73 | 0.34–1.54 | 0.36 | 0.12–1.10 | ||
| Yes | 27 (22.0) | 1.38 | 0.65–2.92 | NS | 2.74 | 0.91–8.29 | <0.05 | |
| 0–4 | 24 (19.5) | Ref | Ref | Ref | Ref | Ref | Ref | |
| CUETO | 5–6 | 40 (32.5) | 4.21 | 1.84–9.64 | – | – | ||
| score | 7–9 | 40 (32.5) | 6.22 | 2.67–14.52 | 0.44 | 0.16–1.23 | ||
| ≥10 | 19 (15.5) | 9.24 | 3.27–26.14 | <0.01 | 2.27 | 0.81–6.34 | <0.05 | |
| EORTC | 0–4 | 26 (21.1) | Ref | Ref | Ref | Ref | Ref | Ref |
| score | 5–9 | 85 (69.1) | 1.57 | 0.76–3.24 | 0.37 | 0.14–0.97 | ||
| ≥10 | 12 (9.8) | 1.78 | 0.57–5.63 | NS | 2.73 | 1.03–7.27 | <0.05 | |
Abbreviations: CIS, carcinoma in situ; CUETO, Spanish Urological Club for Oncological Treatment; EORTC, European Organization for Research and Treatment of Cancer; Nb, number; NS, not significant; OR, odds ratio; Ref, reference; re-TUR, restaging transurethral resection; SIPIC, single immediate postoperative instillation of chemotherapy.
Table 4.
Multivariate analysis of risk factors for NMIBC recurrence
| Factor | HR | 95% CI | p-value |
|---|---|---|---|
| Number of tumors ≥3 | 2.46 | 1.31–4.65 | <0.01 |
| CIS | 1.85 | 1.00–3.44 | 0.05 |
Abbreviations: CIS, carcinoma in situ; HR, hazard ratio; NMIBC, non-muscle-invasive bladder cancer.
Table 5.
Multivariate analysis of risk factors for NMIBC progression
| Factor | HR | 95% CI | p-value |
|---|---|---|---|
| Number of tumors ≥3 | 2.49 | 1.00–6.17 | 0.05 |
| CIS | 2.9 | 1.15–7.38 | <0.05 |
Abbreviations: CIS, carcinoma in situ; HR hazard ratio; NMIBC, non-muscle-invasive bladder cancer.
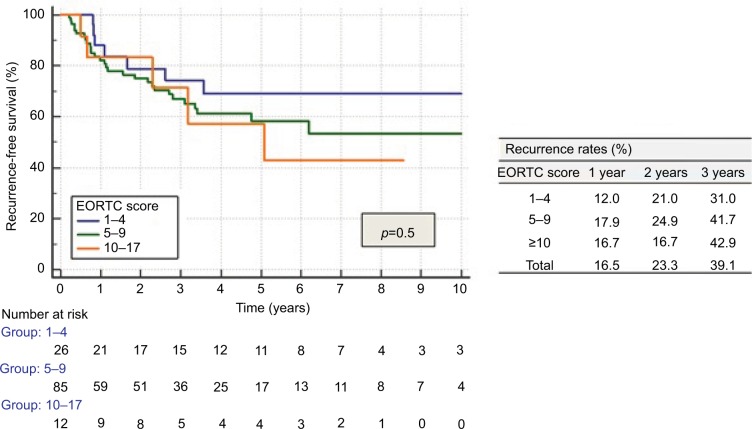
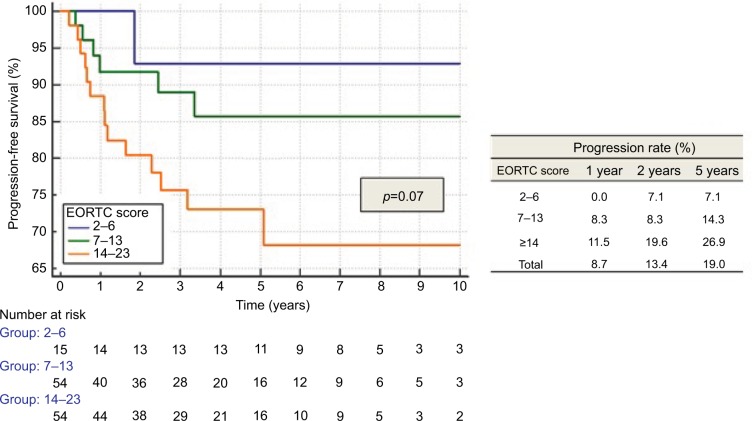
EORTC risk tables did not distinctly separate patients for recurrence (Figure 1) and progression (Figure 2), but only for MIFS (p<0.05). Recurrence rates for all categories, as well as progression rates for the highest risk category (EORTC progression score ≥14) were significantly lower than those described by EORTC.9
Figure 1.
Recurrence according to EORTC score.
Abbreviation: EORTC, European Organization for Research and Treatment of Cancer.
Figure 2.
Progression according to EORTC score.
Abbreviation: EORTC, European Organization for Research and Treatment of Cancer.
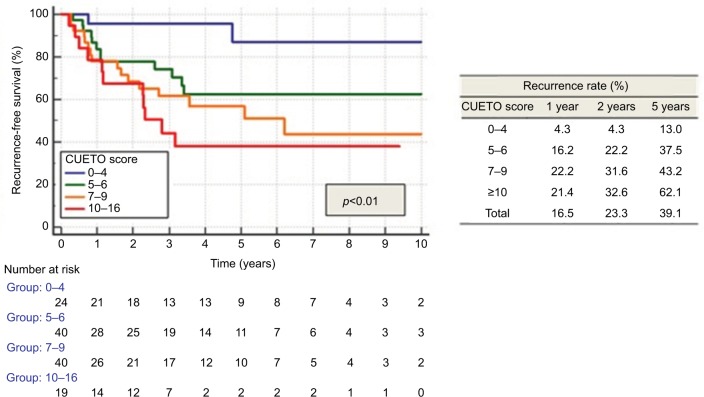
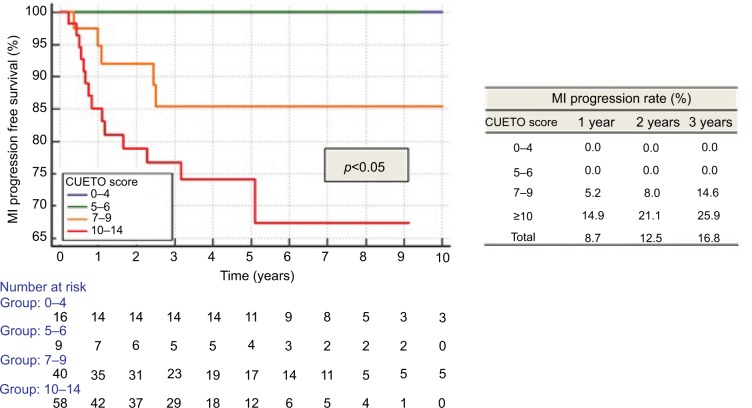
CUETO scoring model significantly separated patients into distinctive groups for recurrence (p<0.01; Figure 3), progression (p<0.05) and MIFS (p<0.05; Figure 4). Recurrence rates matched the lower limits described by CUETO, except for the lowest risk group (CUETO recurrence score <5), where we found even lower rates. MIFS was 100% for patients at the lowest risk of progression. Progression rates for patients at higher risk corresponded to the lower limit described by CUETO.10
Figure 3.
Recurrence according to CUETO score.
Abbreviation: CUETO, Spanish Urological Club for Oncological Treatment.
Figure 4.
Progression according to CUETO score.
Abbreviations: MI, muscle invasive; CUETO, Spanish Urological Club for Oncological Treatment..
Discussion
The two most widely used NMIBC scoring systems, EORTC and CUETO, were applied to this retrospective, BCG-treated cohort to determine which model could be preferred as a prognostic model in patients with NMIBC.
EORTC risk tables are mainly based on non-BCG-treated patients (<200 patients were treated by BCG and no maintenance regimen was administered).9 They were not adequate in our population due to overestimation and poor discrimination of recurrence and progression rates. This overestimation is consistent with the conclusions reported by large multi-institutional studies.12–14
CUETO scoring model, especially designed for BCG-treated patients, was more accurate than EORTC model in our BCG-treated cohort to discriminate patients at risk of recurrence and progression. CUETO score still overestimated recurrence and progression rates, but to a lesser extent than EORTC score. Our recurrence and progression rates are comparable to the lower limits described by the CUETO group, which is consistent with the results reported by Xylinas et al.13 CUETO score was able to discriminate our BCG-treated patients in distinct risk groups. It could be particularly helpful for patients at very low risk of recurrence and progression in individualizing patient treatment and follow-up. Not a single CUETO low-risk patient progressed to muscle-invasive disease. In this study, patients at highest risk of progression had a 5-year progression rate of around 25% to muscle-invasive disease. This value cannot justify aggressive (radical cystectomy) primary treatment in all highest risk patients and most of them can safely be treated with intravesical BCG instillations.
Although CUETO scoring model has been found to be superior to EORTC in defining the risk of recurrence and progression in this study, there are several publications comparing both scores and reflecting not only some identical interpretations, but also different results with controversial discussions in predicting recurrence or progression in NMIBC patients.13–16 The discriminative power of both scoring models, in terms of recurrence and progression, is quiet poor when applied to external cohorts.13–16 The question is challenging due to the basic heterogeneous characteristics of patients in such studies. Cambier et al confirmed heterogeneous prognosis in NMIBC high-risk patients treated with 1–3 years of maintenance BCG.17 Unfortunately, as CIS patients were excluded from that study, we could not apply their nomogram to our patient population. There is, therefore, an urgent need to find more accurate ways to define very high-risk patients who might be candidates for alternative treatment or early cystectomy.
Recently, Gontero et al, in a large retrospective multicenter study, reported that a subgroup of pT1G3 patients with age >70 years, tumor size >3 cm and concomitant CIS had a higher risk of progression.18 In our retrospective population, age (>70 years), concomitant CIS and number of tumors (≥3) were the risk factors associated with progression in multivariate analysis (tumor size had no significant impact on outcome). If all three risk factors are present, more aggressive treatment options should be considered.
Trying to improve outcome prediction, we further analyzed other variables not included in the scoring systems. For example, smoking status was significantly associated with recurrence on univariate analysis. Patients who had stopped smoking had surprisingly higher recurrence rates than active and never smokers. As a well-known risk factor for developing bladder cancer,4 smoking status should, whenever possible, be reported in NMIBC patients. In univariate analysis, Connaught strain was adversely associated with MIFS and CSS. In multivariate analysis, Connaught strain stayed the only factor significantly associated with worse CSS. These results could, at least partially, be explained by the fact that Connaught-treated patients represent an older cohort (1998–2006) than Tice-treated patients (2006–2012). Indeed, Connaught-treated patients received significantly less SIPIC, although SIPIC did not significantly influence patient outcome in our population. Nonetheless, we must be cautious with any misinterpretation of this result, as contrasting results were recently reported in a prospective randomized trial where Connaught strain was superior to Tice.19 The effect of the BCG strain used needs to be further evaluated and should be explicitly specified in BCG-related studies.
In the future, especially in times of BCG shortage, further risk factors that were not evaluated in this study should be studied to help stratifying NMIBC patients and guide clinical decision making more accurately. Potential candidates are more precise details in pathologic diagnosis, such as lymphovascular involvement20 and extent of lamina propria invasion,21 evaluation of circulating tumor cells22 and next-generation sequencing with determination of genetic markers such as FGFR-3 (favorable) and Tp53 (unfavorable).23
The limits of this study are notably its retrospective design and the small sample size. All patients were not managed with a standardized treatment regimen following current standards, as in a real-life setting. It should be noted that the EORTC tables were designed for all stages of NMIBC, and not only for those receiving BCG, making the comparison of our results to those of the EORTC highly biased. Pathologic slides, although initially examined by a senior pathologist, were not reviewed for the purpose of this study. All patients, however, received BCG instillations after initial TUR. Also, 95.9% of patients received at least a six-weekly induction course and 69.9% received some sort of maintenance therapy. Nonetheless, only 28.9% received instillations for an optimal duration over 1 year. Nevertheless, our recurrence and progression rates correspond to the lower limits described by the CUETO scoring model. The actual recurrence and progression rates of patients treated with current standards of care are likely to be even lower, making the overestimation13–18 of EORTC and CUETO scores even more important.
Conclusion
High-risk NMIBC patients remain a urologic challenge. Current scoring systems, CUETO and EORTC, show low discrimination ability for very high-risk patient selection. CUETO scoring system overestimates rates of recurrence and progression in adequately treated high-risk NMIBC patients, but to a lesser extent than the EORTC scoring system. In this study, the patients at highest risk of progression were older (>70 years), had multiple tumors (≥3) and concomitant CIS, and might be candidates for early cystectomy. New accurate prognostic markers to define very high-risk NMIBC patients and prospective trials are mandatory and should contribute to optimize existing predictive models.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 2.Sievert KD, Amend B, Nagele U, et al. Economic aspects of bladder cancer: what are the benefits and costs? World J Urol. 2009;27(3):295–300. doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM. The global burden of urinary bladder cancer. Scand J UrolNephrol. 2008;218:12–20. doi: 10.1080/03008880802285032. [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Burger M, Zigeuner R, et al. European Association of Urology EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64(4):639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Lamm DL, Blumenstein BA, Crissman JD, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163(4):1124–1129. [PubMed] [Google Scholar]
- 6.Brausi M, Oddens J, Sylvester R, et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomized phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65(1):69–76. doi: 10.1016/j.eururo.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Raj GV, Herr H, Serio AM. Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J Urol. 2007;177(4):1283–1286. doi: 10.1016/j.juro.2006.11.090. [DOI] [PubMed] [Google Scholar]
- 8.Denzinger S, Fritsche HM, Otto W, Blana A, Wieland WF, Burger M. Early vs deferred cystectomy for initial high-risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder-sparing approach? Eur Urol. 2008;53(1):146–152. doi: 10.1016/j.eururo.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 9.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–477. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Gomez J, Madero R, Solsona E, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182(5):2195–2203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Lamm D, Persad R, Brausi M, et al. Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. J Urol. 2014;191(1):20–27. doi: 10.1016/j.juro.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Gomez J, Madero R, Solsona E, et al. The EORTC Tables Overestimate the Risk of Recurrence and Progression in Patients with Non–Muscle-Invasive Bladder Cancer Treated with Bacillus Calmette-Guérin: External Validation of the EORTC Risk Tables. Eur Urol. 2011;60(3):423–430. doi: 10.1016/j.eururo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 13.Xylinas E, Kent M, Kluth L, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer. 2013;109(6):1460–1466. doi: 10.1038/bjc.2013.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi SY, Ryu JH, Chang IH, et al. Predicting Recurrence and Progression of Non–Muscle-Invasive Bladder Cancer in Korean Patients: A Comparison of the EORTC and CUETO Models. Korean J Urol. 2014;55:643–649. doi: 10.4111/kju.2014.55.10.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu T, Zhu Z, Zhang X, et al. Predicting recurrence and progression in Chinese patients with non- muscle-invasive bladder cancer using EORTC and CUETO scoring models. Urology. 2013;82:387–393. doi: 10.1016/j.urology.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Vedder MM, Márquez M, de Bekker-Grob EW, et al. Risk prediction scores for recurrence and progression of non-muscle invasive bladder cancer: an international validation in primary tumors. PLoS One. 2014 Jun 6;9(6):e96849. doi: 10.1371/journal.pone.0096849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cambier S, Sylvester RJ, Collette, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guérin. Eur Urol. 2016;69:60–69. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 18.Gontero p, Sylvester R, Pisano F, et al. Prognostic factors and risk groups in T1G3 non-muscle-invasive bladder cancer patients initially treated with Bacillus Calmette-Guérin: Results of a retrospective multicenter study of 2451 patients. Eur Urol. 2015;67(1):74–82. doi: 10.1016/j.eururo.2014.06.040. [DOI] [PubMed] [Google Scholar]
- 19.Rentsch CA, Birkhäuser FD, Biot C, et al. Bacillus Calmette-Guérin Strain Differences Have an Impact on Clinical Outcome in Bladder Cancer Immunotherapy. Eur Urol. 2014;66(4):677–688. doi: 10.1016/j.eururo.2014.02.061. [DOI] [PubMed] [Google Scholar]
- 20.Cho KS, Seo HK, Joung JY, et al. Lymphovascular invasion in trans-urethral resection specimens as predictor of progression and metastasis in patients with newly diagnosed T1 bladder urothelial cancer. J Urol. 2009;182(6):2625–2630. doi: 10.1016/j.juro.2009.08.083. [DOI] [PubMed] [Google Scholar]
- 21.van Rhijn BW, van der Kwast TH, Alkhateeb SS, et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol. 2012;61(2):378–384. doi: 10.1016/j.eururo.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Gazzaniga p, de Berardinis E, Raimondi C, et al. Circulating tumor cells detection has independent prognostic impact in high-risk non-muscle invasive bladder cancer. Int J Cancer. 2014;135(8):1978–1982. doi: 10.1002/ijc.28830. [DOI] [PubMed] [Google Scholar]
- 23.Netto GJ. Molecular genetics and genomics progress in urothelial bladder cancer. Semin Diagn Pathol. 2013;30(4):313–320. doi: 10.1053/j.semdp.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Mostofi FK, Sobin LH, Torloni H, World Health Organization Histological typing of urinary bladder tumours. [Accessed September 13, 2017]. Available from http://apps.who.int/iris/handle/10665/41533.
- 25.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. World Health Organization classification of tumours. pathology and genetics of tumours of the urinary system and male genital organs. IARC Press; Lyon: 2004. [Google Scholar]