Abstract
Background and objectives
Marijuana use has become more widely accepted in the United States and has been legalized in many areas. Although it is biologically plausible that marijuana could affect kidney function, epidemiologic data are lacking.
Design, setting, participants, & measurements
We conducted a cohort study among young adults with preserved eGFR (i.e., eGFR≥60 ml/min per 1.73 m2) using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study. At scheduled examinations occurring every 5 years and starting at study year 10 (calendar years, 1995–1996), cystatin C was collected over a 10-year period, and urine albumin-to-creatinine ratio was collected over a 15-year period. We investigated the cross-sectional association between current and cumulative marijuana use (in marijuana-years; one marijuana-year equals 365 days of marijuana use) and eGFR by cystatin C (eGFRcys) at year 10. In longitudinal analyses, we investigated the association between cumulative marijuana use and eGFRcys change and rapid (≥3%/year) eGFRcys decline over two 5-year intervals and prevalent albuminuria (urine albumin-to-creatinine ratio ≥30 mg/g) over a 15-year period.
Results
Past or current marijuana use was reported by 83% (3131 of 3765) of the cohort, and the mean eGFRcys was 111 ml/min per 1.73 m2 at year 10. Over the following 10 years, 504 had rapid eGFRcys decline, and over the following 15 years, 426 had prevalent albuminuria. Compared with no use, daily current use and ≥5 marijuana-years of cumulative use were associated with lower eGFRcys at year 10: −4.5% (95% confidence interval, −8.1 to −0.7%; P=0.02) and −3.0% (95% confidence interval, −5.6 to −0.4%; P=0.03), respectively. Marijuana use was not significantly associated with eGFRcys change, rapid eGFRcys decline, or prevalent albuminuria.
Conclusions
Although we identified a modest cross-sectional association between higher marijuana exposure and lower eGFRcys among young adults with preserved eGFR, our findings were largely null and did not demonstrate a longitudinal association between marijuana use and eGFRcys change, rapid eGFRcys decline, or prevalent albuminuria.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2017_08_24_CJASNPodcast_17_10.mp3
Keywords: Marijuana; kidney disease; clinical epidemiology; Adult; Albumins; albuminuria; Cannabis; Cohort Studies; Coronary Vessels; creatinine; Cross-Sectional Studies; Cystatin C; glomerular filtration rate; Humans; Kidney Function Tests; Marijuana Abuse; Marijuana Smoking; Receptor, Epidermal Growth Factor; United States; Young Adult; EGFR protein, human
Introduction
In the United States, legalization of marijuana for medical and recreational purposes has become more common, and marijuana use has increased over the last decade according to some reports (1–6). Associations between marijuana use and adverse psychosocial, cognitive, and respiratory outcomes have been demonstrated (7–11), but the effect of marijuana use on kidney disease has been largely unexplored. CKD affects >20 million Americans (12), and the identification of potentially modifiable risk factors for adverse kidney outcomes is of public health importance.
Marijuana is derived from the hemp plant (Cannabis sativa) (13), which contains >60 cannabinoid molecules, including Δ9-tetrahydrocannabinol, which is primarily responsible for its psychoactive properties (14,15). The initial discovery of Δ9-tetrahydrocannabinol prompted the identification of the endogenous cannabinoid system, consisting of the cannabinoid receptors CB1 and CB2 and their endogenous ligands, and its recognition as an important physiologic regulator (14,16). In the kidney, the endogenous cannabinoid system plays a role in regulating kidney hemodynamics and sodium transport (14), and animal models suggest that CB1 activity may contribute to the pathogenesis of diabetic and obesity-related nephropathy and kidney fibrosis (17–21), whereas CB2 activity may be reno-protective (22–24).
Although it is biologically plausible that marijuana may play a role in the pathogenesis of kidney disease, data in humans are limited to case reports of AKI in the setting of synthetic cannabinoid use (25–27) and single-center studies of small sample size and relatively short duration (28–30). The Coronary Artery Risk Development in Young Adults (CARDIA) study provides an opportunity to investigate whether marijuana use has implications for kidney disease in a large cohort of young adults with preserved eGFR and marijuana exposure documented over the course of 25 years. Our primary aim was to investigate the association between marijuana exposure and eGFR. We hypothesized that marijuana exposure would be associated with lower eGFR.
Materials and Methods
Study Design and Population
We conducted a cohort study using data from the CARDIA study, which was designed to investigate the development and determinants of cardiovascular disease in young adults (31). Briefly, 5115 healthy black and white women and men aged 18–30 years were enrolled between 1985 and 1986 from four centers in the United States (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). Follow-up examinations were completed at study years 2, 5, 7, 10, 15, 20, and 25. Marijuana measures were collected at each study visit; cystatin C was collected at years 10, 15, and 20; and urine albumin-to-creatinine ratio was collected at years 10, 15, 20, and 25. All participants provided written informed consent, and the institutional review boards at each center approved the study protocol.
Our study cohort consisted of 3765 persons with a cystatin C measurement at year 10 and preserved eGFR (i.e., eGFR≥60 ml/min per 1.73 m2 on the basis of the available serum creatinine at year 0). We restricted the cohort to persons with preserved eGFR in order to have a homogeneous and generalizable study population. In a cross-sectional analysis, we investigated the association between marijuana use and eGFR by cystatin C (eGFRcys) at year 10. In longitudinal analyses, we investigated the association between marijuana use and change in eGFRcys and rapid (≥3%/year) eGFRcys decline over two 5-year intervals (i.e., years 10–15 and 15–20) among persons with cystatin C measures at two consecutive study visits (n=3118). In a longitudinal analysis, we also examined the association between marijuana use and prevalent albuminuria (urine albumin-to-creatinine ratio ≥30 mg/g) over a 15-year period (from years 10 to 25) among persons with a urine albumin-to-creatinine ratio measurement at year 10 (n=3732).
Exposure Measures
Marijuana use was assessed at each study visit (years 0, 2, 5, 7, 10, 15, 20, and 25) with a self-administered questionnaire. Current use was reported as the number of days of marijuana use within the 30 days preceding each study visit, and lifetime use was reported as the number of times marijuana had been used in one’s lifetime. Until year 10, the quantity of marijuana used on a typical day (in joints or filled pipe bowls smoked) was assessed.
Using data on lifetime marijuana use reported at year 0, the current frequency of marijuana use, and the quantity of marijuana smoked per day, we calculated cumulative marijuana exposure in marijuana-years (one marijuana-year is equivalent to 365 days of marijuana use) and joint-years (one joint-year is equivalent to 365 joints or filled pipe bowls smoked) at each study visit as previously described (Supplemental Appendix 1) (10,32).
The primary exposures were cumulative and current marijuana use. We considered marijuana-years as the primary means of assessing cumulative exposure because these data were available for the entire duration of follow-up. We also performed an exploratory analysis with cumulative use expressed in joint-years, with use beyond year 10 estimated as detailed in Supplemental Appendix 1, in order to capture associations with quantity of use. As previously defined by our research group (10,32), marijuana use was categorized as follows: cumulative use in marijuana-years (never, >0–<0.5, 0.5–<2, 2–<5, and ≥5), cumulative use in joint-years (never, >0–5, >5–10, and >10), and current use (none, 1–10, 11–29, and 30 days).
Outcome Measures
Using stored sera collected at years 10, 15, and 20 as part of an ancillary study, cystatin C measurements were performed simultaneously at the University of Minnesota by nephelometry with the N Latex cystatin C kit (Dade Behring, now Siemens, Munich, Germany) and calibrated for drift as previously described (33). Kidney function was estimated using the 2012 CKD Epidemiology Collaboration cystatin C equation and expressed as eGFRcys (ml/min per 1.73 m2) (34). As in prior CARDIA analyses, we estimated kidney function using cystatin C rather than creatinine because cystatin C–based methods have been demonstrated to have superior accuracy among those with preserved kidney function (35,36) and allow for earlier detection of decrements in kidney function (37). Additionally, simultaneous measurements of cystatin C performed in a single laboratory optimized precision and our ability to interpret changes in kidney function. Albumin-to-creatinine ratio was measured from untimed (spot) urine samples collected at years 10, 15, 20, and 25 and expressed in milligrams of albumin per gram of creatinine. Urine albumin was measured using nephelometry, and urine creatinine was assessed using the Jaffé method (38).
Our primary outcomes were: (1) log-transformed eGFRcys at year 10; (2) annualized changes in eGFRcys between years 10–15 and 15–20, as percentages of the value at the beginning of the interval; and (3) rapid eGFRcys decline (≥3%/year) between years 10–15 and 15–20 (n=518 events). Prevalent albuminuria (urine albumin-to-creatinine ratio ≥30 mg/g) at year 10, 15, 20, or 25 (n=640 events) was a secondary outcome. Cystatin C was available in 3765, 3118, and 2995 participants in years 10, 15, and 20, respectively, but was not available at year 25.
Covariates
Age, race, sex, income, employment, education, and substance use were obtained with a self-administered questionnaire. Substance use was expressed in cumulative years of tobacco smoking; heavy and binge alcohol use; and cocaine, amphetamine, and heroin use as previously described (10). At each study visit, three seated systolic and diastolic BPs were obtained with a sphygmomanometer in years 0–15 and automated oscillometric BP monitor in years 20 and 25, and the mean of the second and third readings was calculated. Diabetes was defined as a fasting blood glucose ≥126 mg/dl or use of insulin and/or oral hypoglycemic medications. Hyperlipidemia was defined as an LDL cholesterol >130 mg/dl or use of lipid-lowering medications. Body mass index was calculated from weight measured with light clothing and height without shoes (39). Physical activity score was obtained by a validated interviewer-administered questionnaire and log-transformed (10,39).
Statistical Analyses
We compared baseline characteristics at year 10 by category of marijuana use using chi-squared and Kruskal–Wallis testing as appropriate. At year 10, we used linear regression to estimate the associations of cumulative and current marijuana use, modeled as separate exposures, with log-transformed eGFRcys. The back-transformed estimates for each category of marijuana use can be interpreted as the percent difference in eGFRcys compared with the referent category. We chose year 10 as the baseline timepoint because this was the first visit at which cystatin C was available.
Models for the longitudinal outcomes focused only on cumulative marijuana use because this was thought to be the relevant exposure for outcomes that may reflect cumulative toxicity rather than acute effects. We used linear mixed models to estimate the association of cumulative marijuana use, treated as a time-dependent exposure, with repeated annualized changes in eGFRcys over the year 10–15 and 15–20 intervals, as percentages of the year 10 and 15 values, respectively. We modeled eGFRcys changes in 5-year intervals in order to account for nonlinearity in rates of decline. Within-participant correlation was modeled using an unstructured correlation matrix for the residuals. We then used generalized estimating equation Poisson models with robust SEMs to evaluate the association between cumulative marijuana use, treated as a time-dependent exposure, and the following repeated binary outcomes: (1) rapid decline in eGFRcys in the year 10–15 and 15–20 intervals and (2) prevalent albuminuria at year 10, 15, 20, or 25. In the model for prevalent albuminuria, participants contributed to the analysis at all years in which they experienced the outcome.
For each outcome, we performed an unadjusted and multivariable-adjusted analysis controlling for age, race, sex, study site, income, education, employment, systolic and diastolic BP, diabetes, hyperlipidemia, body mass index, physical activity, and cumulative years of tobacco smoking, heavy and binge alcohol use, and cocaine, amphetamine, and heroin use. Adjustment variables were evaluated at year 10 in the analysis for eGFRcys and treated as time-varying (if subject to change) in the analyses for the longitudinal outcomes. We performed tests for trend across categories of marijuana use and tested for interaction by race, sex, and tobacco smoking status categorized as never, former, or current. In order to account for the possibility of informative censoring due to loss to follow-up, we performed sensitivity analyses with inverse probability of censoring weighting. The tests for statistical significance were two-tailed, and P<0.05 was considered significant. Analyses were performed with STATA version 14 (StataCorp LP, College Station, TX).
Results
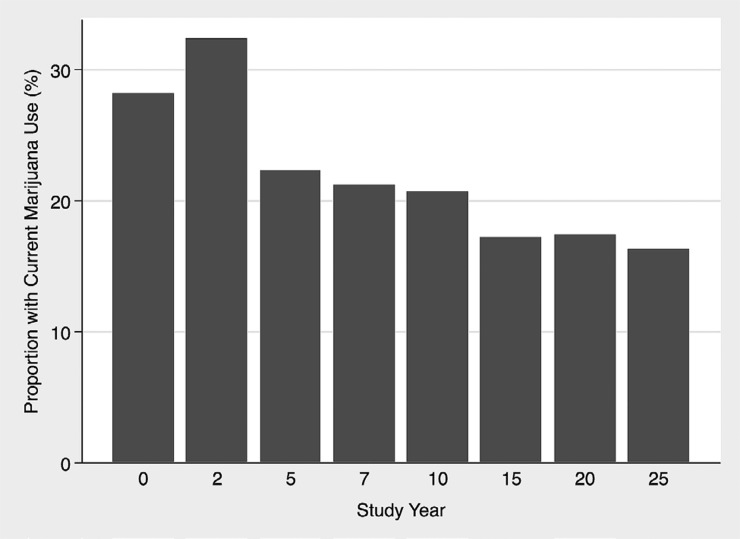
At year 10, the majority of the study cohort (3131 out of 3765 [83%]) reported past or current marijuana use; the mean age was 35 years, and the mean eGFRcys was 111 ml/min per 1.73 m2. The proportion of current marijuana use was highest at years 0 and 2 but remained persistently >15% throughout the entire follow-up period (Figure 1). Characteristics associated with cumulative marijuana use included age, sex, race, study site, income, employment, education, BP, body mass index, and physical activity (Table 1). Current marijuana use and use of other substances were also higher among those with higher cumulative marijuana use.
Figure 1.
Proportion of cohort with current marijuana use from CARDIA (Coronary Artery Risk Development in Young Adults) study year 0 (calendar years, 1985–1986) through study year 25 (calendar years, 2010–2011). The proportion of current marijuana use was highest at years 0 and 2 but remained greater than 15% throughout the entire follow-up period. Study year 10 (calendar years, 1995–1996) was used as the baseline timepoint for this study. The mean age of the cohort at study year 0 was 35 years (range, 26–45 years).
Table 1.
Characteristics of 3765 participants by cumulative marijuana use category at Coronary Artery Risk Development in Young Adults Study year 10 (calendar years, 1995–1996)
| Characteristic | Cumulative Marijuana Use at Yr 10 (Marijuana-Yr) | ||||
|---|---|---|---|---|---|
| Never Used (n=634) | >0–<0.5 (n=1744) | 0.5–<2 (n=930) | 2–<5 (n=330) | ≥5 (n=127) | |
| Mean age (SD), yr | 34 (4) | 35 (4) | 35 (3) | 35 (4) | 35 (3) |
| Race and sex, n (%) | |||||
| Black women | 237 (37) | 560 (32) | 165 (18) | 74 (22) | 15 (12) |
| Black men | 113 (18) | 253 (15) | 251 (27) | 120 (36) | 34 (27) |
| White women | 153 (24) | 572 (33) | 241 (26) | 41 (12) | 16 (13) |
| White men | 131 (21) | 359 (21) | 273 (29) | 95 (29) | 62 (49) |
| Study site, n (%) | |||||
| Birmingham, AL | 271 (43) | 402 (23) | 144 (15) | 55 (17) | 21 (17) |
| Chicago, IL | 157 (25) | 379 (22) | 213 (23) | 52 (16) | 17 (13) |
| Minneapolis, MN | 129 (20) | 428 (25) | 310 (33) | 125 (38) | 42 (33) |
| Oakland, CA | 77 (12) | 535 (31) | 263 (28) | 98 (30) | 47 (37) |
| Annual income, n (%) | |||||
| <$25,000 | 132 (21) | 294 (17) | 212 (23) | 114 (35) | 31 (24) |
| $25,000–<$50,000 | 248 (39) | 596 (34) | 333 (36) | 114 (35) | 49 (39) |
| ≥$50,000 | 254 (40) | 850 (49) | 385 (41) | 102 (31) | 47 (37) |
| Employment, n (%) | |||||
| Working | 536 (86) | 1474 (86) | 781 (85) | 266 (82) | 108 (87) |
| Unemployed | 61 (10) | 148 (9) | 110 (12) | 52 (16) | 14 (11) |
| Homemaker | 29 (4) | 89 (5) | 28 (3) | 7 (2) | 2 (2) |
| Highest level of education, n (%) | |||||
| Less than high school | 21 (3) | 53 (3) | 67 (7) | 27 (8) | 11 (9) |
| High school completed | 124 (20) | 279 (16) | 224 (24) | 108 (33) | 36 (28) |
| College or more | 489 (77) | 1408 (81) | 639 (69) | 195 (59) | 80 (63) |
| Mean systolic BP (SD), mmHg | 110 (12) | 109 (13) | 111 (13) | 113 (13) | 113 (11) |
| Mean diastolic BP (SD), mmHg | 73 (10) | 72 (10) | 72 (11) | 74 (10) | 72 (10) |
| Diabetes, n (%) | 50 (8) | 119 (7) | 55 (6) | 17 (5) | 5 (4) |
| Hyperlipidemia, n (%) | 151 (24) | 390 (22) | 222 (24) | 67 (20) | 34 (27) |
| Mean body mass index (SD), kg/m2 | 28.4 (6.7) | 27.4 (6.9) | 27.2 (5.8) | 27.9 (6.6) | 26.4 (4.4) |
| Mean physical activity score (SD) | 239 (229) | 280 (245) | 310 (259) | 335 (282) | 342 (272) |
| Current marijuana use, days per month, n (%) | |||||
| None | 634 (100) | 1703 (98) | 748 (80) | 119 (36) | 17 (13) |
| 1–10 d | 0 (0) | 41 (2) | 179 (19) | 144 (44) | 24 (19) |
| 11–29 d | 0 (0) | 0 (0) | 3 (0.3) | 55 (17) | 51 (40) |
| 30 d (daily) | 0 (0) | 0 (0) | 0 (0) | 12 (4) | 35 (28) |
| Tobacco smoking, n (%) | |||||
| Never | 550 (87) | 1055 (60) | 264 (28) | 86 (26) | 32 (25) |
| Former | 45 (7) | 361 (21) | 285 (31) | 87 (26) | 32 (25) |
| Current | 39 (6) | 328 (19) | 381 (41) | 157 (48) | 63 (50) |
| Cumulative yr of tobacco use until yr 10 among ever tobacco smokers, mean (SD) | 1.4 (4.5) | 4.5 (7.3) | 9.2 (8.4) | 9.9 (8.8) | 10.2 (8.7) |
| Cumulative yr of heavy and binge drinking until yr 10 among ever drinkers, mean (SD) | 3.2 (4.3) | 4.0 (4.8) | 6.2 (5.3) | 7.5 (5.8) | 9.4 (5.9) |
| Cumulative yr of cocaine, amphetamine, and heroin use until yr 10 among ever users, mean (SD) | 0.1 (0.2) | 0.2 (0.4) | 0.4 (0.7) | 0.7 (1.0) | 0.7 (0.9) |
We did not observe a dose-response relationship between cumulative marijuana use and eGFRcys at year 10. However, there was an association with lower eGFRcys at year 10 among those with at least 5 marijuana-years of use (Table 2). There was a statistically significant interaction with race (P value for interaction=0.03) (Table 2), but the findings appeared similar for men and women (P value for interaction=0.56). There was a statistically significant interaction by tobacco smoking status (P<0.001). Among those with at least 5 marijuana-years of use, the point estimates for the marijuana associations with eGFRcys were in the direction of harm in multivariable-adjusted models for never (percent difference eGFRcys, −1.4%; 95% CI, −5.9 to 3.4%; P=0.56) and former (percent difference eGFRcys, −6.4%; 95% CI, −11.8 to −0.7%; P=0.03), but not current (percent difference eGFRcys, 6.7%; 95% CI, 1.2 to 12.6%; P=0.02), tobacco smokers. Higher levels of current marijuana use were associated with lower eGFRcys at year 10 in multivariable-adjusted models, and the interaction with race was statistically significant (P value for interaction=0.04) (Supplemental Table 1). Findings appeared similar for men and women (P value for interaction=0.82).
Table 2.
Percent difference in eGFRcys at Coronary Artery Risk Development in Young Adults Study year 10 (calendar years, 1995–1996) among participants with cumulative marijuana use (in marijuana-years) compared with never-users
| Cumulative Marijuana Use at Yr 10 (Marijuana-Yr) | Participants (n) | eGFRcys, mean (SD) | Unadjusted | Multivariable-Adjusted | ||
|---|---|---|---|---|---|---|
| % Difference eGFRcys (95% CI) | P Valuea | % Difference eGFRcys (95% CI) | P Valuea | |||
| Overall cohort | ||||||
| Never used | 634 | 111 (13) | Referent | Referent | ||
| >0–<0.5 | 1744 | 111 (13) | 0.0 (−1.2 to 1.3) | 0.97 | 0.2 (−1.1 to 1.4) | 0.79 |
| 0.5–<2 | 930 | 111 (13) | −0.2 (−1.6 to 1.2) | 0.74 | 1.0 (−0.5 to 2.5) | 0.18 |
| 2–<5 | 330 | 110 (14) | −0.8 (−2.6 to 1.1) | 0.42 | 0.3 (−1.6 to 2.2) | 0.77 |
| ≥5 | 127 | 108 (15) | −3.2 (−5.7 to −0.6) | 0.02b | −3.0 (−5.6 to −0.4) | 0.03b |
| P value trendc | <0.01b | 0.04b | ||||
| Raced | ||||||
| White | ||||||
| Never used | 284 | 110 (12) | Referent | Referent | ||
| >0–<0.5 | 931 | 110 (12) | −0.5 (−2.3 to 1.3) | 0.56 | −0.9 (−2.7 to 0.8) | 0.30 |
| 0.5–<2 | 514 | 109 (12) | −1.3 (−3.2 to 0.7) | 0.20 | −0.5 (−2.5 to 1.5) | 0.61 |
| 2–<5 | 136 | 108 (13) | −2.5 (−5.2 to 0.3) | 0.08 | −1.9 (−4.5 to 0.9) | 0.19 |
| ≥5 | 78 | 104 (15) | −6.1 (−9.3 to −2.8) | <0.001b | −6.0 (−9.2 to −2.7) | <0.001b |
| P value trendc | <0.001b | <0.001b | ||||
| Black | ||||||
| Never used | 350 | 112 (14) | Referent | Referent | ||
| >0–<0.5 | 813 | 113 (14) | 0.9 (−0.8 to 2.7) | 0.30 | 1.0 (−0.6 to 2.7) | 0.22 |
| 0.5–<2 | 416 | 113 (14) | 1.2 (−0.8 to 3.2) | 0.25 | 2.5 (0.5 to 4.6) | 0.02b |
| 2–<5 | 194 | 112 (15) | 0.2 (−2.2 to 2.7) | 0.85 | 2.1 (−0.4 to 4.6) | 0.10 |
| ≥5 | 49 | 113 (12) | 1.5 (−2.6 to 5.8) | 0.48 | 1.0 (−3.1 to 5.2) | 0.64 |
| P value trendc | 0.60 | 0.51 | ||||
Multivariable model adjusted for age, race, sex, study site, income, education, employment, systolic BP, diastolic BP, diabetes, hyperlipidemia, body mass index, physical activity, and cumulative years of tobacco smoking, heavy and binge alcohol use, and cocaine, amphetamine, and heroin use. The results can be interpreted as the percent difference in eGFRcys at year 10 compared with the referent category. For example, in the multivariable-adjusted model, compared with participants who never used marijuana, those with ≥5 marijuana-years of use had a 3.0% lower eGFRcys, and this difference was statistically significant (P=0.03). eGFRcys, eGFR as calculated with the Chronic Kidney Disease Epidemiology Collaboration cystatin C equation (ml/min per 1.73 m2); 95% CI, 95% confidence interval.
P values for percent difference in eGFRcys compared with the referent (never use).
P values <0.05.
P values for test for trend across categories of marijuana use.
P value for test for interaction by race=0.03.
There were no statistically significant associations of cumulative marijuana use with changes per year in eGFRcys between years 10–15 and 15–20 (Table 3). Among the study cohort, 504 had experienced rapid decline in eGFRcys in at least one interval between visits and 426 had prevalent albuminuria. There were no statistically significant associations between the higher categories of cumulative marijuana use and rapid eGFRcys decline or prevalent albuminuria in multivariable-adjusted models (Table 4).
Table 3.
Percent difference in eGFRcys change per year during Coronary Artery Risk Development in Young Adults Study years 10–15 (calendar years, 1995–1996 to 2000–2001) and 15–20 (calendar years, 2000–2001 to 2005–2006) among participants with cumulative marijuana use (in marijuana-years) compared with never-users
| Cumulative Marijuana Use (Marijuana-Yr)a | 5-Yr Person-Intervals (n) | eGFRcys Change per Yr, Mean (SD) | Unadjusted | Multivariable-Adjusted | ||
|---|---|---|---|---|---|---|
| % Difference eGFRcys (95% CI) | P Valueb | % Difference eGFRcys (95% CI) | P Valueb | |||
| Never used | 995 | −0.7 (4.3) | Referent | Referent | ||
| >0–<0.5 | 2709 | −0.6 (6.8) | −0.1 (−0.4 to 0.1) | 0.33 | −0.2 (−0.5 to 0.1) | 0.16 |
| 0.5–<2 | 1411 | −0.8 (2.1) | −0.1 (−0.4 to 0.2) | 0.57 | −0.2 (−0.5 to 0.1) | 0.25 |
| 2–<5 | 462 | −0.6 (2.3) | −0.1 (−0.4 to 0.3) | 0.79 | −0.1 (−0.6 to 0.3) | 0.58 |
| ≥5 | 238 | −0.9 (2.2) | −0.2 (−0.7 to 0.4) | 0.52 | −0.1 (−0.7 to 0.5) | 0.72 |
| P value trendc | 0.63 | 0.82 | ||||
Multivariable model adjusted for age, race, sex, study site, income, education, employment, systolic BP, diastolic BP, diabetes, hyperlipidemia, body mass index, physical activity, and cumulative years of tobacco smoking, heavy and binge alcohol use, and cocaine, amphetamine, and heroin use. The results can be interpreted as the percent difference in change in eGFRcys per year during years 10–15 and 15–20 compared with the referent category. For example, in the multivariable-adjusted model, compared with participants who never used marijuana, the annualized change in eGFRcys relative to the value at the beginning of each 5-yr interval was smaller by 0.1% per year among those with ≥5 marijuana-years of use, but this difference was not statistically significant (P=0.72). CARDIA, Coronary Artery Risk Development in Young Adults; eGFRcys, estimated glomerular filtration rate as calculated with the Chronic Kidney Disease Epidemiology Collaboration cystatin C equation (ml/min per 1.73 m2); 95% CI, 95% confidence interval.
Cumulative marijuana use was evaluated at CARDIA years 10 (calendar years, 1995–1996) and 15 (calendar years, 2000–2001).
P values for percent difference in eGFRcys change per year compared with the referent (never use).
P values for test for trend across categories of marijuana use.
Table 4.
Kidney outcomes measured during Coronary Artery Risk Development in Young Adults Study year 10 (calendar years, 1995–1996) through year 20 (calendar years, 2005–2006) or year 25 (calendar years, 2010–2011) among participants with cumulative marijuana use (in marijuana-years) compared with never-users
| Cumulative Marijuana Use (Marijuana-Yr)a | Events (n) | 5-Yr Person-Intervals or Visits (n)b | Urine ACR, median (IQR) | Unadjusted | Multivariable-Adjusted | ||
|---|---|---|---|---|---|---|---|
| PRR (95% CI) | P Valuec | PRR (95% CI) | P Valuec | ||||
| Rapid eGFRcys declined | |||||||
| Never used | 95 | 995 | 1.00 (Referent) | 1.00 (Referent) | |||
| >0–<0.5 | 233 | 2709 | 0.90 (0.72 to 1.13) | 0.35 | 1.02 (0.80 to 1.30) | 0.87 | |
| 0.5–<2 | 121 | 1411 | 0.90 (0.70 to 1.15) | 0.40 | 0.98 (0.73 to 1.30) | 0.87 | |
| 2–<5 | 39 | 462 | 0.90 (0.64 to 1.27) | 0.55 | 0.98 (0.66 to 1.45) | 0.92 | |
| ≥5 | 30 | 238 | 1.18 (0.81 to 1.74) | 0.39 | 1.14 (0.73 to 1.78) | 0.56 | |
| P value trende | 0.42 | 0.66 | |||||
| Prevalent albuminuriaf | |||||||
| Never used | 134 | 1995 | 4 (3–7) | 1.00 (Referent) | 1.00 (Referent) | ||
| >0–<0.5 | 269 | 5604 | 4 (3–6) | 0.71 (0.54 to 0.93) | 0.01g | 0.80 (0.61 to 1.05) | 0.11 |
| 0.5–<2 | 140 | 2938 | 4 (3–6) | 0.76 (0.57 to 1.02) | 0.07 | 0.73 (0.54 to 1.00) | 0.05 |
| 2–<5 | 55 | 980 | 4 (3–7) | 0.86 (0.59 to 1.25) | 0.42 | 0.80 (0.55 to 1.17) | 0.26 |
| ≥5 | 42 | 740 | 4 (3–6) | 0.82 (0.53 to 1.27) | 0.38 | 0.79 (0.47 to 1.32) | 0.37 |
| P value trende | 0.68 | 0.41 | |||||
Definitions: rapid eGFRcys decline =≥3%/year; albuminuria = ACR≥30 mg/g. Multivariable model adjusted for age, race, sex, study site, income, education, employment, systolic BP, diastolic BP, diabetes, hyperlipidemia, body mass index, physical activity, and cumulative years of tobacco smoking, heavy and binge alcohol use, and cocaine, amphetamine, and heroin use. CARDIA, Coronary Artery Risk Development in Young Adults; ACR, urine albumin-to-creatinine ratio (mg/g); IQR, interquartile range; PRR, prevalence rate ratio; 95% CI, 95% confidence interval; eGFRcys, eGFR as calculated with the Chronic Kidney Disease Epidemiology Collaboration cystatin C equation.
Cumulative marijuana use was evaluated at CARDIA years 10 (calendar years, 1995–1996) and 15 (calendar years, 2000–2001) for rapid eGFRcys decline and years 10 (calendar years, 1995–1996), 15 (calendar years, 2000–2001), 20 (calendar years, 2005–2006), and 25 (calendar years, 2010–2011) for prevalent albuminuria.
5-yr person-intervals for rapid eGFRcys decline and visits for prevalent albuminuria.
P values for prevalence rate of outcome compared with the referent (never use).
Measured between CARDIA years 10–15 and 15–20.
P values for test for trend across categories of marijuana use.
Measured at CARDIA years 10, 15, 20, and 25.
P values <0.05.
The results were similar in exploratory analyses evaluating cumulative marijuana use in joint-years. Higher exposure in joint-years was associated with lower eGFRcys at year 10, and the interaction with race was statistically significant (P value for interaction <0.01) (Supplemental Table 2). Findings appeared similar for men and women (P value for interaction=0.76). There was a statistically significant interaction by tobacco smoking status (P value <0.001), and among those with at least 10 joint-years of use, we observed similar results for the analysis stratified by tobacco smoking status as we did for marijuana-years. There were no statistically significant associations between the higher categories of joint-years and change in eGFRcys, rapid eGFRcys decline, or prevalent albuminuria in the multivariable-adjusted models (Supplemental Tables 3 and 4). In a sensitivity analysis using inverse probability of censoring weighting, the associations of both marijuana-years and joint-years with log-transformed eGFRcys at year 10 were similar in magnitude to those of the original analyses (Supplemental Tables 5 and 6).
Discussion
In a large cohort of young adults with preserved eGFR followed for up to 15 years, we found that greater marijuana exposure was associated with worse eGFRcys at year 10, but we did not detect an association between marijuana use and subsequent change in eGFRcys, rapid eGFRcys decline, or prevalent albuminuria. The magnitude of the association with eGFRcys at year 10 was more pronounced among whites than blacks and among never or former smokers relative to current smokers.
To our knowledge, our study is the first to examine the association between marijuana use and kidney function in the general population. Previous literature is limited to case reports of AKI associated with synthetic cannabinoid use (25–27) and studies of small sample size and relatively shorter duration. In a single-center study investigating the effect of illicit drug use and kidney function among hypertensive men (n=647), marijuana users did not have a significantly higher risk of kidney function decline (defined as an increase in serum creatinine of ≥0.6 mg/dl) over a median follow-up of 7 years compared with nonusers (28). In a prospective cohort study designed to assess the safety of marijuana use for chronic noncancer pain, cannabis use for 1 year did not significantly change serum creatinine among 78 users (29). Finally, among kidney transplant recipients (n=1225), marijuana use was not associated with death, graft failure, or worse graft function at 1 year post-transplant (30). Differences in study populations and methodology preclude direct comparison of our results with those of previous studies.
The mechanisms underlying marijuana’s potential influence on kidney function are uncertain, but it is possible that marijuana use could either acutely lower kidney function or result in cumulative toxicity. The cannabinoid receptor CB1 is expressed in various regions and cell types within the kidneys of animals and humans; data regarding the expression of CB2 receptors in the kidney are less consistent (14). In normal rat kidneys, the endogenous ligand anandamide, acting via CB1 receptors expressed in afferent and efferent arterioles, decreased eGFR by preferential vasodilation of the efferent arteriole, an effect that was blocked by CB1 antagonists (40). Animal models suggest that CB1 activity may be pathogenic, whereas CB2 activity may be reno-protective. In mouse and rat models of diabetic and obesity-related nephropathy and kidney fibrosis, CB1 antagonism and CB2 activation were associated with improved kidney parameters (e.g., reduced albuminuria, proteinuria, and kidney fibrosis, and increased creatinine clearance) (17–23), and CB2 antagonism was associated with worsened kidney parameters (e.g., increased albuminuria, reduced creatinine clearance) (23,24).
Our study has several limitations. We only observed an association cross-sectionally but not longitudinally, which may reflect greater susceptibility of cross-sectional analyses to confounding due to unmeasured interindividual differences. Although we adjusted for a comprehensive list of variables, it is possible that the associations we observed at year 10 could be explained by confounding, such as by smoking; tobacco use is common among marijuana users (41) and has been associated with worse kidney function in several reports (42–45). Additionally, our cohort consisted of young adults with preserved eGFR, so rapid decline and albuminuria were rare. Thus, our power to detect longitudinal associations was limited, and we cannot exclude benefit or harm for these outcomes.
Marijuana exposure was determined on the basis of self-report, although misclassification should have been mitigated by use of a self-administered questionnaire and is unlikely to be differential according to outcome status. We lacked the information to account for changes in marijuana exposure (e.g., quality, composition) and self-reporting that may have changed over time and may vary by geographic location. Active marijuana use was more common during the earlier years of follow-up before kidney measures were available, so we were unable to evaluate the influence of marijuana when its use was maximal. We are also unable to discern whether current or cumulative use was responsible for the association we observed at year 10, and we cannot distinguish whether this represents a transient or enduring effect. The spectrum of marijuana use may also be biased toward the moderate side because heavy users in the general population may be less likely to participate in the ongoing evaluations required of CARDIA participants. Our results are not generalizable to older adults among whom marijuana use has been increasing (1) and who are at higher risk for adverse kidney outcomes (46). Finally, we were unable to determine whether the larger associations of marijuana with lower eGFRcys among whites than blacks reflect a true biologic difference or a chance finding. However, investigation of whether marijuana has different associations with eGFRcys across racial groups merits further study.
Strengths of our study are the large sample size, long duration of follow-up, and repeated assessments of marijuana use and kidney outcomes. We have reason to be confident in the quality of our exposure measures given that previous CARDIA papers have demonstrated robust associations with other health outcomes (10,32) using the same methods of assessing marijuana use.
Because marijuana use is becoming increasingly accepted in the United States (1), there is a critical need for epidemiologic data to assess the risk-to-benefit ratio of a substance that may be poised for more widespread use. Although our findings were largely negative, we observed that higher marijuana use was associated with modestly lower eGFRcys among adults with preserved eGFR. This result may not translate into a clinically meaningful difference and may be insufficient to inform decision-making concerning marijuana use. However, it is possible that the association could be stronger among patients with established kidney disease, and additional research to define the effect of marijuana use on kidney outcomes in other study populations is warranted.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to acknowledge Feng Lin (Master of Science, Department of Epidemiology and Biostatistics, University of California, San Francisco) for statistical programming support.
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants 5K23DK103963 (J.H.I), 5K24DK085153 (K.L.J.), and 5K24DK103992 (K.B.D.). The Coronary Artery Risk Development in Young Adults (CARDIA) Study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300026C), Northwestern University (HHSN268201300027C), the University of Minnesota (HHSN268201300028C), the Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005).
This manuscript has been reviewed by CARDIA for scientific content. The NHLBI had input into the overall design and conduct of the CARDIA study. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the NHLBI, the National Institutes of Health, or the US Department of Health and Human Services.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01530217/-/DCSupplemental.
References
- 1.Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF: Prevalence of marijuana use disorders in the united states between 2001-2002 and 2012-2013. JAMA Psychiatry 72: 1235–1242, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of National Drug Control Policy: Marijuana resource center: State laws related to marijuana, 2016. Available at https://www.whitehouse.gov/ondcp/state-laws-related-to-marijuana. Accessed November 21, 2016
- 3.Bonn-Miller MO, Harris AH, Trafton JA: Prevalence of cannabis use disorder diagnoses among veterans in 2002, 2008, and 2009. Psychol Serv 9: 404–416, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Pacek LR, Mauro PM, Martins SS: Perceived risk of regular cannabis use in the United States from 2002 to 2012: Differences by sex, age, and race/ethnicity. Drug Alcohol Depend 149: 232–244, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azofeifa A, Mattson ME, Grant A: Monitoring marijuana use in the united states: Challenges in an evolving environment. JAMA 316: 1765–1766, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Compton WM, Han B, Jones CM, Blanco C, Hughes A: Marijuana use and use disorders in adults in the USA, 2002-14: Analysis of annual cross-sectional surveys. Lancet Psychiatry 3: 954–964, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Volkow ND, Baler RD, Compton WM, Weiss SR: Adverse health effects of marijuana use. N Engl J Med 370: 2219–2227, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall W: The adverse health effects of cannabis use: What are they, and what are their implications for policy? Int J Drug Policy 20: 458–466, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Hall W, Degenhardt L: Adverse health effects of non-medical cannabis use. Lancet 374: 1383–1391, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Auer R, Vittinghoff E, Yaffe K, Künzi A, Kertesz SG, Levine DA, Albanese E, Whitmer RA, Jacobs DR Jr, Sidney S, Glymour MM, Pletcher MJ: Association between lifetime marijuana use and cognitive function in middle age: The coronary artery risk development in young adults (CARDIA) study. JAMA Intern Med 176: 352–361, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tashkin DP: Effects of marijuana smoking on the lung. Ann Am Thorac Soc 10: 239–247, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 13.National Institute on Drug Abuse: DrugFacts: Marijuana. 2016. Available at https://www.drugabuse.gov/publications/drugfacts/marijuana. Accessed November 21, 2016
- 14.Tam J: The emerging role of the endocannabinoid system in the pathogenesis and treatment of kidney diseases. J Basic Clin Physiol Pharmacol 27: 267–276, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Ameri A: The effects of cannabinoids on the brain. Prog Neurobiol 58: 315–348, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Pertwee RG: Cannabinoid pharmacology: The first 66 years. Br J Pharmacol 147[Suppl 1]: S163–S171, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barutta F, Corbelli A, Mastrocola R, Gambino R, Di Marzo V, Pinach S, Rastaldi MP, Perin PC, Gruden G: Cannabinoid receptor 1 blockade ameliorates albuminuria in experimental diabetic nephropathy. Diabetes 59: 1046–1054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nam DH, Lee MH, Kim JE, Song HK, Kang YS, Lee JE, Kim HW, Cha JJ, Hyun YY, Kim SH, Han SY, Han KH, Han JY, Cha DR: Blockade of cannabinoid receptor 1 improves insulin resistance, lipid metabolism, and diabetic nephropathy in db/db mice. Endocrinology 153: 1387–1396, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Janiak P, Poirier B, Bidouard JP, Cadrouvele C, Pierre F, Gouraud L, Barbosa I, Dedio J, Maffrand JP, Le Fur G, O’Connor S, Herbert JM: Blockade of cannabinoid CB1 receptors improves renal function, metabolic profile, and increased survival of obese Zucker rats. Kidney Int 72: 1345–1357, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Jenkin KA, O’Keefe L, Simcocks AC, Grinfeld E, Mathai ML, McAinch AJ, Hryciw DH: Chronic administration of AM251 improves albuminuria and renal tubular structure in obese rats. J Endocrinol 225: 113–124, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Lecru L, Desterke C, Grassin-Delyle S, Chatziantoniou C, Vandermeersch S, Devocelle A, Vernochet A, Ivanovski N, Ledent C, Ferlicot S, Dalia M, Saïd M, Beaudreuil S, Charpentier B, Vazquez A, Giron-Michel J, Azzarone B, Durrbach A, François H: Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int 88: 72–84, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Barutta F, Piscitelli F, Pinach S, Bruno G, Gambino R, Rastaldi MP, Salvidio G, Di Marzo V, Cavallo Perin P, Gruden G: Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 60: 2386–2396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkin KA, O’Keefe L, Simcocks AC, Briffa JF, Mathai ML, McAinch AJ, Hryciw DH: Renal effects of chronic pharmacological manipulation of CB2 receptors in rats with diet-induced obesity. Br J Pharmacol 173: 1128–1142, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barutta F, Grimaldi S, Franco I, Bellini S, Gambino R, Pinach S, Corbelli A, Bruno G, Rastaldi MP, Aveta T, Hirsch E, Di Marzo V, Gruden G: Deficiency of cannabinoid receptor of type 2 worsens renal functional and structural abnormalities in streptozotocin-induced diabetic mice. Kidney Int 86: 979–990, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Bhanushali GK, Jain G, Fatima H, Leisch LJ, Thornley-Brown D: AKI associated with synthetic cannabinoids: A case series. Clin J Am Soc Nephrol 8: 523–526, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazory A, Aiyer R: Synthetic marijuana and acute kidney injury: An unforeseen association. Clin Kidney J 6: 330–333, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pendergraft WF 3rd , Herlitz LC, Thornley-Brown D, Rosner M, Niles JL: Nephrotoxic effects of common and emerging drugs of abuse. Clin J Am Soc Nephrol 9: 1996–2005, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vupputuri S, Batuman V, Muntner P, Bazzano LA, Lefante JJ, Whelton PK, He J: The risk for mild kidney function decline associated with illicit drug use among hypertensive men. Am J Kidney Dis 43: 629–635, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ware MA, Wang T, Shapiro S, Collet JP; COMPASS study team : Cannabis for the management of pain: Assessment of safety study (COMPASS). J Pain 16: 1233–1242, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Greenan G, Ahmad SB, Anders MG, Leeser A, Bromberg JS, Niederhaus SV: Recreational marijuana use is not associated with worse outcomes after renal transplantation. Clin Transplant 30: 1340–1346, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ: CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41: 1105–1116, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, Lin F, Kertesz S: Association between marijuana exposure and pulmonary function over 20 years. JAMA 307: 173–181, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peralta CA, Vittinghoff E, Bansal N, Jacobs D Jr, Muntner P, Kestenbaum B, Lewis C, Siscovick D, Kramer H, Shlipak M, Bibbins-Domingo K: Trajectories of kidney function decline in young black and white adults with preserved GFR: Results from the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Kidney Dis 62: 261–266, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hojs R, Bevc S, Ekart R, Gorenjak M, Puklavec L: Serum cystatin C as an endogenous marker of renal function in patients with mild to moderate impairment of kidney function. Nephrol Dial Transplant 21: 1855–1862, 2006 [DOI] [PubMed] [Google Scholar]
- 36.White C, Akbari A, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA: Estimating glomerular filtration rate in kidney transplantation: a comparison between serum creatinine and cystatin C-based methods. J Am Soc Nephrol 16: 3763–3770, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Herget-Rosenthal S, Pietruck F, Volbracht L, Philipp T, Kribben A: Serum cystatin C--a superior marker of rapidly reduced glomerular filtration after uninephrectomy in kidney donors compared to creatinine. Clin Nephrol 64: 41–46, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Murtaugh MA, Jacobs DR Jr, Yu X, Gross MD, Steffes M; Coronary Artery Risk Development in Young Adults Study : Correlates of urinary albumin excretion in young adult blacks and whites: The Coronary Artery Risk Development in Young Adults study. Am J Epidemiol 158: 676–686, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Grubbs V, Lin F, Vittinghoff E, Shlipak MG, Peralta CA, Bansal N, Jacobs DR, Siscovick DS, Lewis CE, Bibbins-Domingo K: Body mass index and early kidney function decline in young adults: A longitudinal analysis of the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Kidney Dis 63: 590–597, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koura Y, Ichihara A, Tada Y, Kaneshiro Y, Okada H, Temm CJ, Hayashi M, Saruta T: Anandamide decreases glomerular filtration rate through predominant vasodilation of efferent arterioles in rat kidneys. J Am Soc Nephrol 15: 1488–1494, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Richter KP, Kaur H, Resnicow K, Nazir N, Mosier MC, Ahluwalia JS: Cigarette smoking among marijuana users in the United States. Subst Abus 25: 35–43, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Briganti EM, Branley P, Chadban SJ, Shaw JE, McNeil JJ, Welborn TA, Atkins RC: Smoking is associated with renal impairment and proteinuria in the normal population: The AusDiab kidney study. Australian Diabetes, Obesity and Lifestyle Study. Am J Kidney Dis 40: 704–712, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Goetz FC, Jacobs DR Jr, Chavers B, Roel J, Yelle M, Sprafka JM: Risk factors for kidney damage in the adult population of Wadena, Minnesota. A prospective study. Am J Epidemiol 145: 91–102, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Hall ME, Wang W, Okhomina V, Agarwal M, Hall JE, Dreisbach AW, Juncos LA, Winniford MD, Payne TJ, Robertson RM, Bhatnagar A, Young BA: Cigarette smoking and chronic kidney disease in african americans in the jackson heart study. J Am Heart Assoc 5: e003280, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regalado M, Yang S, Wesson DE: Cigarette smoking is associated with augmented progression of renal insufficiency in severe essential hypertension. Am J Kidney Dis 35: 687–694, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Taal MW, Brenner BM: Predicting initiation and progression of chronic kidney disease: Developing renal risk scores. Kidney Int 70: 1694–1705, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.