Abstract
Background and objectives
Fibrosis is a major cause of kidney allograft injury. Currently, the only means of assessing allograft fibrosis is by biopsy, an invasive procedure that samples <1% of the kidney. We examined whether magnetic resonance elastography, an imaging-based measure of organ stiffness, could noninvasively estimate allograft fibrosis and predict progression of allograft dysfunction.
Design, setting, participants, & measurements
Kidney allograft recipients >1 year post-transplant undergoing an allograft biopsy first underwent free-breathing, flow-compensated magnetic resonance elastography on a 3.0-T magnetic resonance imaging scanner. Each patient had serial eGFR measurements after the elastography scan for a follow-up period of up to 1 year. The mean stiffness value of the kidney allograft was compared with both the histopathologic Banff fibrosis score and the rate of eGFR change during the follow-up period.
Results
Sixteen patients who underwent magnetic resonance elastography and biopsy were studied (mean age: 54±9 years old). Whole-kidney mean stiffness ranged between 3.5 and 7.3 kPa. Whole-kidney stiffness correlated with biopsy-derived Banff fibrosis score (Spearman rho =0.67; P<0.01). Stiffness was heterogeneously distributed within each kidney, providing a possible explanation for the lack of a stronger stiffness-fibrosis correlation. We also found negative correlations between whole-kidney stiffness and both baseline eGFR (Spearman rho =−0.65; P<0.01) and eGFR change over time (Spearman rho =−0.70; P<0.01). Irrespective of the baseline eGFR, increased kidney stiffness was associated with a greater eGFR decline (regression r2=0.48; P=0.03).
Conclusions
Given the limitations of allograft biopsy, our pilot study suggests the potential for magnetic resonance elastography as a novel noninvasive measure of whole-allograft fibrosis burden that may predict future changes in kidney function. Future studies exploring the utility and accuracy of magnetic resonance elastography are needed.
Introduction
Chronic allograft injury limits the lifespan of transplanted kidneys, leading to morbidity and/or graft loss (1). Fibrosis is a common, irreversible, and progressive form of chronic allograft injury (1), and it is an important predictor of kidney allograft outcomes (2,3). Despite the importance of fibrosis, no safe and effective antifibrotic therapies exist, meaning that scarring represents irreversible disease burden. Importantly, many other reversible causes of allograft injury, such as inflammation, can coexist with fibrosis. Because treatments for inflammation and other reversible causes of allograft injury are often toxic and expensive, the extent of irreversible allograft scarring is a critical factor influencing choice and timing of therapy. With minimal scarring, for example, clinicians are more likely to aggressively treat reversible causes of allograft dysfunction, such as rejection, and less likely to initiate chronic kidney replacement therapies, such as dialysis.
Currently, the most definitive means of assessing the cause of allograft dysfunction is a kidney biopsy. The invasiveness of a biopsy coupled with its small sampling size have limited its utility in quantifying and monitoring changes in allograft fibrotic burden, especially because fibrosis can be heterogeneously distributed. Accordingly, a noninvasive modality that assesses fibrosis on a kidney-wide scale would be of clinical utility.
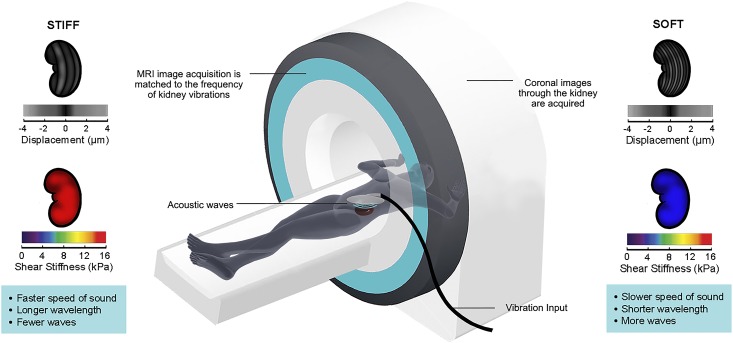
By replacing soft healthy tissue with stiff extracellular matrix, fibrosis stiffens organs (4), a phenomenon that has led multiple groups to explore whether tissue stiffness could be used as a surrogate measure for fibrosis. Magnetic resonance elastography (MRE) is a novel magnetic resonance imaging (MRI)–based technique that measures tissue stiffness. During MRE, the organ of interest is gently vibrated by applying acoustic waves through a pad placed on the overlying skin. The resulting microscopic vibrational waves passing through the organ generate shear waves that can be imaged with motion-synchronized MRI. The velocity of wave propagation is dependent on organ stiffness, with stiffer fibrotic tissue leading to more rapidly moving waves with longer wavelength (Figure 1). Capturing shear waves at multiple time points enables the reconstruction of a quantitative stiffness map (5).
Figure 1.
Magnetic resonance elastography can be used to image transplant kidney stiffness. In magnetic resonance elastography, a gentle vibrational wave is applied on the skin overlying the kidney using an acoustic generator. The magnetic resonance imaging (MRI) scanner then images the small displacements produced by the resulting shear waves generated in the kidney. Because the waves are longer and travel more rapidly through stiff tissue, kidney stiffness can be calculated on the basis of the measured displacements and the amount of vibrational force applied. Postimaging quantitative reconstruction enables the generation of stiffness maps (elastograms) of the imaged kidney. Displacement images are shown to the left and right of the scanner. Below each displacement image is the corresponding pseudocolorized elastogram (stiffness map); blue depicts softer tissue, and red depicts stiffer tissue.
MRE is already a well established, gold standard technique for liver fibrosis imaging, having been shown to accurately reflect biopsy-derived liver fibrosis measurements (6–10). In contrast, MRE for nonhepatic applications is still early in its development, and its technique has not yet been standardized. Small pilot studies of MRE have been performed in native kidneys (11–13) and kidney allografts (14,15), attempting to correlate kidney stiffness with either fibrosis burden or kidney function. Although MRE in porcine kidneys showed a correlation between stiffness and fibrosis in the medulla (12), the results of small pilot human studies thus far have been conflicting, and no studies as yet have assessed whether stiffness measured by MRE predicts progression of kidney dysfunction.
In this pilot study, we examined the association of two-dimensional MRE-derived stiffness with (1) fibrosis scores from histologic analysis of allograft biopsies and (2) the rate of eGFR change post-MRE.
Materials and Methods
Experimental Design
We performed a prospective cohort study of kidney allograft recipients at St. Michael’s Hospital. Our institutional review board approved the study protocol (10–303), which adhered to the Declaration of Helsinki. All patients provided written informed consent. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Study Population
Patients were recruited from the Kidney Transplant Clinic of our institution, being considered eligible if they had a kidney transplanted >1 year ago and were undergoing an allograft biopsy. A biopsy was either clinically indicated for allograft dysfunction or performed as a protocol biopsy as part of an independent, parallel clinical trial in which our center was participating, which was assessing the effects of high- or low-dose tacrolimus with or without renin-angiotensin system blockade on allograft structure and function (clinicaltrials.gov; no. NCT00933231). Exclusion criteria for our study were as follows: (1) declining consent, (2) claustrophobia or other standard MRI contraindications, and (3) recent allograft instrumentation.
Baseline Clinical Data
Patient age, sex, initial cause of ESRD, transplant type, transplant vintage, serum creatinine, and urinary albumin-to-creatinine ratio were recorded within 1 month of MRI scanning. eGFR was calculated from serum creatinine values using the Modification of Diet in Renal Disease Equation (16).
MRE
Imaging was performed on a Siemens Skyra 3.0-T scanner (software version VD13A). Patients were positioned head first and supine, and they were imaged with an 18-channel torso phased array coil centered over the allograft. The mechanical vibrations required for MRE were supplied by an active pneumatic driver system (9,14). MRE acquisitions were performed using 60.1-Hz vibrations and a free-breathing, flow-compensated two-dimensional gradient echo coronal MRE pulse sequence to derive magnitude images and a corresponding stiffness map. The imaging orientation was standardized to the coronal plane, with the frequency encoding direction superior to inferior. Other imaging parameters were as follows: field of view, 32–40 cm; acquisition matrix, 128×128 reconstructed to 1.48×1.48-mm in-plane resolution; no parallel imaging; five two-dimensional slices through the allograft, with each slice being 5-mm thick with a 1-mm interslice gap; repetition time, 50 ms; and echo time, 20.79 ms. Four time points of the motion and three mutually perpendicular directions of the vector motion were sampled. The acquisition time per scan was 4.5 minutes.
MRI Analyses
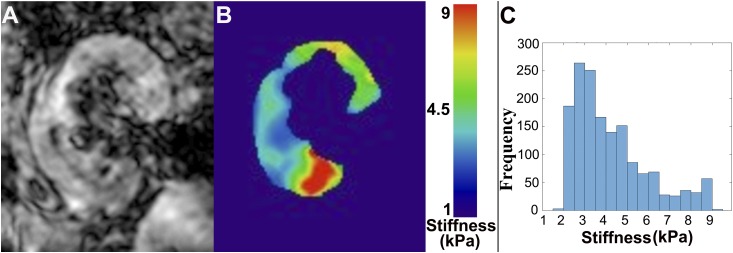
Stiffness maps were generated by averaging all three directions and all four motion time points for each slice using the vendor-supplied built-in software. A 95% confidence interval stiffness map was generated for each slice by automatic rejection of the regions where stiffness calculation was not optimal. A minimum of three stiffness map coronal slices was generated for each patient for stiffness measurements. The allograft parenchyma was manually traced as a region of interest on each of the imaged slices using the magnitude MRE images. These regions of interest were copied onto the corresponding 95% confidence interval stiffness map slices. The regions of interest were edited on the stiffness map, if necessary, to avoid regions outside the 95% confidence interval. The mean stiffness from all regions of interest on all coronal slices was recorded as the mean stiffness of the whole organ. The MRE stiffness map reader was blinded to the patient’s blood work, clinical, and histopathologic results. For one representative subject, a histogram analysis of the voxelwise stiffness in one slice was also performed to examine stiffness heterogeneity (Figure 2). ImageJ (NIH, Bethesda, MD) and Matlab (version 2015b; Mathworks, MA) were used to draw the regions of interest and compute image statistics.
Figure 2.
Representative magnetic resonance elastography images demonstrate the heterogeneous distribution of stiffness in the kidney. (A) A representative magnetic resonance elastography magnitude image of a kidney allograft and (B) the corresponding stiffness map for the same kidney, where blue represents softer tissue and red represents stiffer tissue (the color bar is in kilopascals). (C) A histogram of magnetic resonance elastography stiffness value frequency derived from the stiffness map in B, showing stiffness heterogeneity across the entire slice. As can be seen on this histogram, measured stiffness values (per pixel) exhibit a wide range (from 1.9 to 10.7 kPa) for this representative slice.
Pathology
All patients underwent ultrasound-guided kidney allograft biopsy after their MRE. All biopsies were reported according to Banff criteria (1,17) by a blinded pathologist. Interstitial fibrosis scores (ci scores) were reported as follows: ci0 (<5% of cortex occupied by fibrosis), ci1 (5%–25% cortex fibrotic), ci2 (26%–50% cortex fibrotic), and ci3 (>50% cortex fibrotic).
Follow-Up Assessment of eGFR
Study participants were monitored with routine clinic blood work (e.g., serum creatinine) scheduled at a minimum of every 3 months for up to 1 year after the MRE. eGFR was calculated for each follow-up visit.
Statistical Analyses
We used Spearman correlation analysis to assess for relationships between (1) Banff fibrosis score and MRE-derived stiffness, (2) baseline eGFR and MRE-derived stiffness, (3) a history of current or past rejection and MRE-derived stiffness, and (4) eGFR change over time and (4a) MRE-derived stiffness, (4b) baseline eGFR, and (4c) baseline urine albumin-to-creatinine ratio. To capture the trend of eGFR change over the follow-up period, eGFR measurements taken in the first month post-MRE (and biopsy) were excluded. This exclusion ensured that any acute cause of kidney dysfunction that could lead to transient eGFR changes in patients with a clinically indicated biopsy would not affect the overall eGFR trend. To determine whether MRE-derived stiffness was independently associated with eGFR change over time, a linear mixed model with random intercepts (to account for patients’ baseline eGFR variability) and slopes (to account for variability between patients’ rates of change) was fit to the data. Stiffness, baseline eGFR, and their interaction were included in the model as predictor variables. All analyses were performed using Matlab (version 2015b). Statistical significance was defined if P<0.05.
Results
Study Population and Clinical and Histologic Data
Seventeen patients were recruited post-transplant between April of 2014 and April of 2016. One patient was excluded because of the severe and sudden onset of decompensated liver failure unrelated to the kidney transplant shortly after allograft biopsy, leading to death within 3 months of the MRI. Demographic and transplant-related clinical data for the 16 patients included in the study, including initial cause of kidney disease and biopsy indication, are summarized in Table 1. A biopsy was clinically indicated for allograft dysfunction in ten of 16 patients, whereas six of 16 patients underwent a research protocol biopsy as part of the clinical trial testing the effects of low- and high-dose tacrolimus. Seven patients had evidence of acute or chronic rejection, two had interstitial fibrosis and tubular atrophy without evidence of any specific etiology, two had IgA nephropathy, two had lupus nephritis, one had membranous nephropathy, and two had no significant pathologic findings. Banff ci scores revealed severe fibrosis (ci3) in two, intermediate fibrosis (ci2) in four, and mild fibrosis (ci1) in eight patients, whereas two patients had no or minimal fibrosis (ci0). Tables 2 and 3 list baseline eGFR and urine albumin-to-creatinine ratio at the time of study entry, MRE-derived stiffness, and Banff histology scores.
Table 1.
Demographic and transplant-related clinical data
| Patient No. (Age, yr/Sex) | Initial Cause of Kidney Failure | Transplant Donor Type | Transplant Vintage, mo | Prior Rejection History | Indication for Study Biopsy | Clinical Diagnosis at Time of Biopsy (Treatment) |
|---|---|---|---|---|---|---|
| 1 (51/M) | PCKD | Living | 25 | No | Research protocol | No significant pathology |
| 2 (60/M) | PCKD | Deceased | 24 | No | Research protocol | No significant pathology |
| 3 (68/M) | Diabetes type 2 | Deceased | 55 | No | Allograft dysfunction | Transplant glomerulopathy (rituximab) |
| 4 (57/M) | Pyelo/interstitial nephritis | Living | 17 | No | Research protocol | Mild tubulitis and interstitial infiltrate (no treatment) |
| 5 (64/W) | Lupus nephritis | Living | 148 | Acute cellular rejection (6.2 yr prestudy) | Allograft dysfunction | Recurrent mesangial lupus nephritis (prednisone pulse) |
| 6 (55/M) | IgA nephropathy | Living | 105 | No | Allograft dysfunction | Interstitial fibrosis and tubular atrophy with significant interstitial inflammation (MMF dose increased, angiotensin receptor blocker initiated) |
| 7 (51/M) | PCKD | Deceased | 24 | No | Research protocol | Borderline acute cellular rejection (prednisone pulse) |
| 8 (37/M) | Obstructive uropathy | Living | 24 | Borderline acute cellular rejection (1.7 yr prestudy) | Research protocol | IgA nephropathy (prednisone pulse) |
| 9 (53/M) | IgA nephropathy | Living | 120 | No | Allograft dysfunction | IgA nephropathy (no treatment) |
| 10 (43/M) | Henoch–Schonlein purpura | Living | 39 | Borderline acute cellular rejection (3.2 yr prestudy) | Allograft dysfunction | Borderline acute cellular rejection superimposed on transplant glomerulopathy (prednisone pulse, rituximab infusions) |
| 11 (48/M) | Membranous nephropathy | Deceased | 24 | No | Research protocol | Membranous nephropathy (angiotensin receptor blocker) |
| 12 (67/W) | Diabetes type 2 | Deceased | 43 | No | Allograft dysfunction | Transplant glomerulopathy (increase angiotensin receptor blocker dose) |
| 13 (46/W) | Lupus nephritis | Living | 37 | No | Allograft dysfunction | Banff grade 1B acute cellular rejection (solumedrol pulse with tapering prednisone) |
| 14 (51/M) | IgA nephropathy | Living | 108 | No | Allograft dysfunction | Interstitial fibrosis and tubular atrophy (no treatment) |
| 15 (66/M) | IgA nephropathy | Deceased | 70 | No | Allograft dysfunction | Acute antibody-mediated rejection (solumedrol pulse with tapering prednisone, intravenous Ig) |
| 16 (50/W) | Lupus nephritis | Living | 45 | No | Allograft dysfunction | Class 3 lupus nephritis (dolumedrol pulse and increase MMF dose) |
M, man; PCKD, polycystic kidney disease; W, woman; MMF, mycophenolate mofetil.
Table 2.
Baseline kidney function and stiffness
| Patient No. | Baseline eGFR, ml/min per 1.73 m2 | Baseline Urine Albumin-to-Creatinine Ratio, mg/g | Stiffness ± SD, kPa |
|---|---|---|---|
| 1 | 54 | 11 | 3.7±0.2 |
| 2 | 48 | 14 | 4.0±1.2 |
| 3 | 31 | 3649 | 4.9±0.9 |
| 4 | 74 | 13 | 3.8±0.4 |
| 5 | 37 | 10 | 3.7±0.2 |
| 6 | 41 | 1305 | 3.5±0.6 |
| 7 | 42 | 22 | 4.4±0.6 |
| 8 | 38 | 368 | 4.7±1.0 |
| 9 | 24 | 320 | 4.7±0.7 |
| 10 | 26 | 2488 | 5.1±1.2 |
| 11 | 69 | 62 | 4.1±0.4 |
| 12 | 32 | 1245 | 7.3±1.3 |
| 13 | 28 | 61 | 4.3±0.9 |
| 14 | 19 | 1684 | 4.8±0.5 |
| 15 | 44 | 3109 | 4.6±0.8 |
| 16 | 28 | 1491 | 5.8±1.4 |
Baseline eGFR and urine albumin-to-creatinine ratio refer to the values measured at the start of the study (within 1 month of magnetic resonance elastography).
Table 3.
Banff histopathologic scores
| Patient No. | i | t | ptc | g | v | cg | mm | cv | ah | ct | ci |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 1 |
| 3 | 0 | 0 | 2 | 3 | 0 | 2 | 2 | 2 | 3 | 3 | 3 |
| 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 1 | 3 | 0 |
| 7 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 8 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 2 |
| 10 | 1 | 1 | 2 | 2 | 0 | 1 | 1 | 1 | 1 | 2 | 2 |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 12 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 2 | 1 | 1 | 1 |
| 13 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| 14 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 3 | 3 |
| 15 | 2 | x | 1 | x | x | x | x | x | x | 1 | 2 |
| 16 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 0 | 2 | 2 |
For patient 15, insufficient biopsy tissue was available for full Banff classification. i, Mononuclear cell interstitial inflammation score; t, tubulitis score; ptc, peritubular capillaritis score; g, glomerulitis score; v, intimal arteritis score; cg, chronic glomerulopathy score; mm, mesangial matrix score; cv, chronic vascular injury score; ah, arteriolar hyaline thickening score; ct, tubular atrophy score; ci, interstitial fibrosis score.
Allograft Stiffness
Whole-kidney MRE-derived stiffness ranged between 3.5 and 7.3 kPa. Figure 2 shows a sample magnitude MRE image (Figure 2A) and the corresponding stiffness map (Figure 2B) for one slice in a representative subject. The MRE stiffness maps showed significant intrakidney heterogeneity in all subjects as indicated by histogram analysis (Figure 2C) as well as the large SD for each individual’s stiffness measurement (Table 2).
Relationship between MRE-Derived Stiffness, Rejection, and Banff Fibrosis Score
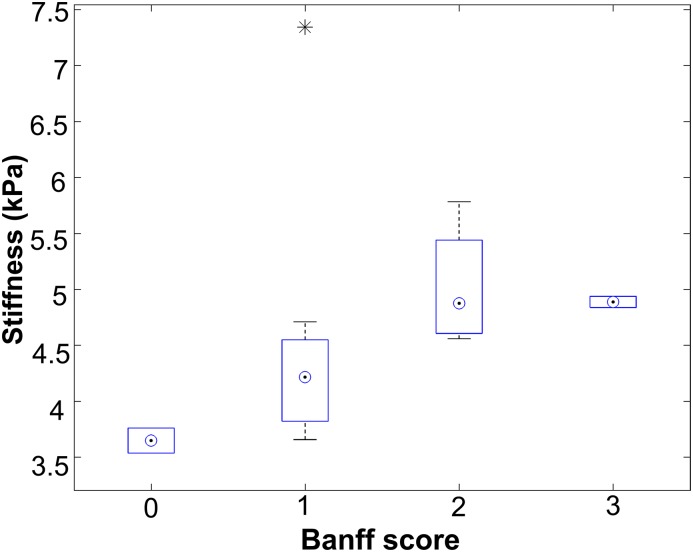
A history of prior or current rejection did not correlate with MRE-derived stiffness (Spearman rho =0.39; P=0.13) (Table 4). Whole-kidney stiffness showed a positive correlation with biopsy-derived fibrosis score (Spearman rho =0.67; P<0.01) (Figure 3, Table 4), suggesting that stiffer kidneys were more fibrotic than their softer counterparts.
Table 4.
Spearman correlation analyses
| Groups of Measures | Spearman rho or r2 | P Value |
|---|---|---|
| Whole-kidney MRE stiffness versus Banff fibrosis score | Rho =0.67 | <0.01 |
| Whole-kidney MRE stiffness versus baseline eGFR | Rho =−0.65 | <0.01 |
| Whole-kidney MRE stiffness versus history of prior or current rejection | Rho =0.39 | 0.13 |
| Whole-kidney MRE stiffness versus slope of eGFR change over the 12-mo follow-up period | Rho =−0.70 | 0.004 |
| Baseline eGFR versus slope of eGFR change over the 12-mo follow-up period | Rho =0.63 | 0.01 |
| Baseline urine albumin-to-creatinine ratio versus slope of eGFR change over the 12-mo follow-up period | Rho =−0.65 | 0.01 |
| Whole-kidney MRE stiffness as a predictor of the slope of eGFR change over the 12-mo follow-up period (regression analysis) | r2=0.48 | 0.03 |
Rho represents the strength of Spearman correlation, whereas r2 represents the strength of regression. MRE, magnetic resonance elastography.
Figure 3.
Magnetic resonance elastography–derived stiffness scores increase with increasing Banff fibrosis score. Patients were categorized according to their biopsy-derived Banff interstitial fibrosis score (ci), and mean magnetic resonance elastography–derived whole-kidney stiffness scores for each group are plotted. The numbers of patients in each group were as follows: ci0 (n=2), ci1 (n=8), ci2 (n=4), and ci3 (n=2). *Outlier measurement.
Relationship between MRE-Derived Stiffness and eGFR
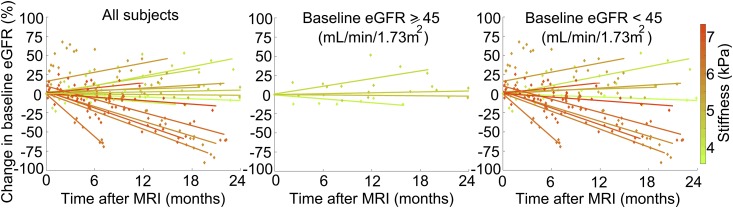
Although stiffness heterogeneity might have impaired the ability of whole-kidney mean stiffness measurements to reflect local fibrotic changes within a small biopsy sample, we reasoned that, if stiffness does reflect fibrotic burden, whole-kidney stiffness should also be related to baseline eGFR and more importantly, change in eGFR over time. As expected, we found a significant inverse correlation between MRE-derived kidney stiffness and baseline eGFR in our cohort (Spearman rho =−0.65; P<0.01) (Table 4). Interestingly, MRE-derived kidney stiffness was also inversely correlated with eGFR change for the 12 months after the MRE scan (Spearman rho =−0.70, P<0.01) (Table 4), suggesting that a higher kidney stiffness was associated with a greater eGFR decline. Moreover, regression analysis indicated that, when controlling for baseline eGFR, kidney stiffness independently predicted eGFR change over the follow-up period (r2=0.48; P=0.03) (Table 4). To illustrate this pictorially, change in eGFR over time was plotted for each patient in Figure 4, with each line representing an individual patient. The lines are color coded to reflect each patient’s MRE-derived kidney stiffness. Because eGFR is an important predictor of future changes in allograft function, we next focused on patients with preexisting allograft dysfunction using an eGFR cutoff of 45 ml/min per 1.73 m2 (17) to determine whether MRE-derived stiffness still predicted eGFR change in patients with reduced function. As shown in Figure 4, although patients with baseline eGFR <45 ml/min per 1.73 m2 generally had stiffer kidneys compared with patients with baseline eGFR ≥45 ml/min per 1.73 m2, a higher stiffness value still tended to predict a greater eGFR decline in patients with reduced function.
Figure 4.
Magnetic resonance elastography–derived stiffness scores may predict future changes in kidney allograft function. Shown are eGFR trajectories over the follow-up period, normalized to baseline eGFR, for (A) all patients (n=16), (B) patients with baseline eGFR ≥45 ml/min (n=4), and (C) patients with baseline eGFR <45 ml/min (n=12). Data points and best fit lines for eGFR trajectories are color coded to the mean stiffness of each allograft (the color bar on the right shows stiffness values in kilopascals). MRI, magnetic resonance imaging.
We next investigated whether baseline eGFR and urine albumin-to-creatinine ratio, two known predictors of allograft injury progression, correlated with eGFR change in our cohort and how the strengths of these correlations compared with those for stiffness and eGFR change. As expected, both baseline eGFR and urine albumin-to-creatinine ratio correlated with eGFR change (Spearman rho =0.63; P=0.01 and Spearman rho =−0.65; P=0.01, respectively) (Table 4). However, the strength of correlation was marginally stronger for stiffness and eGFR change (Spearman rho =−0.70; P<0.01).
Discussion
Our study shows the feasibility of performing MRE of human transplant kidneys. Furthermore, we are the first to report a significant relationship between MRE-derived stiffness and both allograft fibrosis on biopsy and change in eGFR over a 12-month follow-up period.
Biopsy, the only current means of assessing kidney fibrosis, is limited by its invasiveness and small sampling size (18–20). Because kidney fibrosis can be heterogeneously distributed, this reference standard is flawed, because the amount of fibrosis in the biopsy may not reflect fibrotic burden in the remainder of the kidney. Because we could not exactly localize the biopsy site on the MRI scan, we compared whole-kidney stiffness with Banff fibrosis scores from these small biopsy samples. Despite the limitations of this analysis, we were still able to show a correlation between whole-kidney MRE-derived stiffness and biopsy-derived fibrosis scores.
Unlike in the kidney, MRE-derived liver stiffness correlates very tightly with liver fibrosis. The weaker correlation between MRE-derived stiffness and biopsy-derived fibrosis in the transplant kidney may reflect differences between these two organs. In contrast to the liver, the kidney is equipped with a microvascular network carrying approximately 20% of cardiac output that undergoes dramatic perfusion changes in response to multiple hemodynamic and neurohormonal stimuli (21–24). Post-transplantation, kidney perfusion may also be uneven, leading to heterogeneous blood flow patterns and thus, a patchy distribution of fibrosis. The kidney also contains a separate network of tubules that carry urine (25). Because stiffness is determined by not only fibrosis but also hydrostatic pressure (10,12,13,15), such differences between the liver and kidney may partially explain the difference in correlation between stiffness and fibrosis in these two organs. Although we excluded gross hydronephrosis in our subjects, we did not directly measure tubular pressures or kidney perfusion. Future studies combining MRE-derived stiffness mapping with other imaging methods capable of quantifying kidney microvascular and urinary flow will be required to dissect out the individual contributions of fibrotic matrix, perfusion, and urinary flow to kidney stiffness measurements.
If truly a surrogate measure of allograft fibrosis, we reasoned that whole-kidney stiffness should correlate with baseline eGFR and predict changes in kidney function over time. We found that stiffness was indeed associated with baseline eGFR (Table 4). This relationship was relatively modest, possibly because other factors active at the time of biopsy might have affected eGFR but did not affect allograft stiffness (e.g., rejection). More importantly, stiffness also predicted future changes in kidney function, with stiffer allografts experiencing a more rapid eGFR decline compared with softer allografts, even when adjusting for baseline eGFR. Indeed, in the 12 patients with low baseline eGFR (<45 ml/min per 1.73 m2), those who exhibited a more rapid decline over the 12-month follow-up period had stiffer kidneys. Future studies of larger cohorts are required to test whether the predictive value of MRE-derived kidney stiffness is retained when controlling for other predictors of eGFR change, including proteinuria and degree of inflammation.
Ours is only the third published study to evaluate MRE in human kidney allografts. Lee et al. (14) performed MRE on stable transplant recipients, comparing MRE-derived stiffness with biopsy fibrosis scores in nine patients. Although they found no significant correlation, they showed a trend similar to our findings, with stiffer allografts in the two patients with ci2 fibrosis compared with the seven patients with ci0 or ci1 fibrosis. More recently, Marticorena Garcia et al. (15) assessed 27 kidney allografts in 22 patients with MRE and found, unlike in our study, a positive association between MRE-derived stiffness and a 4-month averaged eGFR before the MRE. The authors attributed their findings to kidney perfusion pressure being the major determinant of kidney stiffness, with dysfunctioning transplants assumed to be perfused at lower pressures. Although hydrostatic pressure can affect stiffness (12,13), it is difficult to compare the results of this study with our results. First, the authors did not directly measure kidney perfusion pressure, and they did not compare MRE-derived stiffness with biopsy-derived fibrosis scores. As a result, the relative contributions of fibrosis and perfusion to stiffness could not be evaluated. Second, patients with allograft dysfunction in this study had all undergone at least one session of dialysis. This dialysis requirement may partially explain the discrepant findings, because allograft perfusion would likely be compromised in this setting. In such patients, impaired perfusion might play a more dominant role in regulating kidney stiffness, potentially explaining why MRE-derived stiffness declined with falling eGFR. In contrast, none of our patients had progressed to dialysis (lowest baseline eGFR =19 ml/min per 1.73 m2), and thus, perfusion pressure may not have played as significant a role in determining kidney stiffness in our cohort. Finally, no clinical follow-up was reported in the study by Marticorena Garcia et al. (15), and thus, the predictive value of MRE was not assessed.
An important limitation of our study was that our MRE technique was not optimal for kidney stiffness assessment in several ways. First, we used a two-dimensional instead of a three-dimensional inversion algorithm to construct stiffness maps. This was chosen, because this was the only technique available from our MRI vendor. Assessment of kidney stiffness may be more accurate with three-dimensional MRE, because the complex wave patterns generated by the anisotropy of the kidney would theoretically be more fully captured. Second, because of vendor limitation, we used gradient echo–based MRE for this study instead of spin echo–based MRE, which can cause susceptibility artifact. Fortunately, the effect of this artifact was minimized by the superficial location of the allograft, leading to less shear wave attenuation, and thus, adequate quality images with high reproducibility could be obtained (10). Third, we used a single low driver frequency of 60.1 Hz. MRE-derived stiffness is dependent on driver frequency, and multifrequency approaches may add improved accuracy (26). These limitations reflect the fact that kidney MRE is still early in its development, and more studies are needed to develop standardized techniques across multiple vendor platforms (10,27). Despite these limitations, our results suggest the potential for MRE as a noninvasive measure of whole-kidney fibrotic burden, which we hope will motivate larger confirmatory studies with a more accurate three-dimensional wave-field MRE technique.
Despite our study’s limitations, we were able to show, for the first time, that MRE-derived stiffness correlates positively with fibrosis in transplanted kidneys. Although biopsy is currently the gold standard for fibrosis assessment, it is associated with bleeding risks, difficult to perform serially, and subject to potential sampling bias. These limitations have prevented more extensive use of biopsy as a means to assess allograft health and have been a major barrier for clinical trials of promising new antifibrotic agents. Because MRE is a rapid, noninvasive technique that may estimate whole-kidney fibrotic burden, our results suggest that MRE may offer an opportunity to overcome these limitations by serving as an adjunctive test for fibrosis quantification. Interestingly, our data also suggest that MRE-derived stiffness may predict the rate of eGFR change over a 12-month follow-up period, with stiffer allografts experiencing a more rapid eGFR decline. Together, these initial results suggest that MRE warrants further evaluation as a noninvasive measure of allograft fibrosis. Future work will be required to confirm our results, with (1) larger prospective studies that test the ability of MRE to predict eGFR change when controlling for other variables that influence injury progression, such as degree of inflammation and proteinuria, and (2) mechanistic studies that tease out the various pathophysiologic contributors to allograft stiffness.
Disclosures
None.
Acknowledgments
The authors thank technologists Anthony Sheen and Cindy Hamid for performing the research magnetic resonance imaging scans and research staff Lindita Rapi, Camalene Chrysostoum, Weiqiu Yuan, Michelle Nash, and Shalini Anthwal for assistance in ethics board approvals, patient recruitment, data collection, and study coordination. The authors also thank Drs. Ramesh Prasad and Jeffrey Zaltzman for facilitating patient recruitment from their busy transplant clinics and Drs. Andrew Common, Danny Marcuzzi, and Vikram Prabhudesai for performing all allograft biopsies.
This work was supported by a Collaborative Health Research Project Grant from the Canadian Institutes of Health Research (CIHR) and the National Sciences and Engineering Research Council of Canada as well as grants from the Physicians’ Services Incorporated Foundation, Astellas Pharma Canada (SG-185), and the St. Michael’s Hospital Foundation. D.A.Y. is supported by a Kidney Research Scientist Core Education and National Training Program New Investigator and Canadian Diabetes Association Clinician Scientist salary support award and a recipient of a CIHR New Investigator Award.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ’05 meeting report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Djamali A, Samaniego M: Fibrogenesis in kidney transplantation: Potential targets for prevention and therapy. Transplantation 88: 1149–1156, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naesens M, Kuypers DR, De Vusser K, Evenepoel P, Claes K, Bammens B, Meijers B, Sprangers B, Pirenne J, Monbaliu D, Jochmans I, Lerut E: The histology of kidney transplant failure: A long-term follow-up study. Transplantation 98: 427–435, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Wells RG: Tissue mechanics and fibrosis. Biochim Biophys Acta 1832: 884–890, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muthupillai R, Lomas DJ, Rossman PJ, Greenleaf JF, Manduca A, Ehman RL: Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science 269: 1854–1857, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R, Horsmans Y, Van Beers BE: Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 135: 32–40, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL: Advanced fibrosis in nonalcoholic fatty liver disease: Noninvasive assessment with MR elastography. Radiology 268: 411–419, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkatesh SK, Wang G, Lim SG, Wee A: Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol 24: 70–78, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL: Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol 5: 1207–1213.e2, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venkatesh SK, Ehman RL: Magnetic resonance elastography of abdomen. Abdom Imaging 40: 745–759, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rouvière O, Souchon R, Pagnoux G, Ménager JM, Chapelon JY: Magnetic resonance elastography of the kidneys: Feasibility and reproducibility in young healthy adults. J Magn Reson Imaging 34: 880–886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korsmo MJ, Ebrahimi B, Eirin A, Woollard JR, Krier JD, Crane JA, Warner L, Glaser K, Grimm R, Ehman RL, Lerman LO: Magnetic resonance elastography noninvasively detects in vivo renal medullary fibrosis secondary to swine renal artery stenosis. Invest Radiol 48: 61–68, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warner L, Yin M, Glaser KJ, Woollard JA, Carrascal CA, Korsmo MJ, Crane JA, Ehman RL, Lerman LO: Noninvasive in vivo assessment of renal tissue elasticity during graded renal ischemia using MR elastography. Invest Radiol 46: 509–514, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CU, Glockner JF, Glaser KJ, Yin M, Chen J, Kawashima A, Kim B, Kremers WK, Ehman RL, Gloor JM: MR elastography in renal transplant patients and correlation with renal allograft biopsy: A feasibility study. Acad Radiol 19: 834–841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marticorena Garcia SR, Fischer T, Dürr M, Gültekin E, Braun J, Sack I, Guo J: Multifrequency magnetic resonance elastography for the assessment of renal allograft function. Invest Radiol 51: 591–595, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Kasiske BL, Israni AK, Snyder JJ, Skeans MA; Patient Outcomes in Renal Transplantation (PORT) Investigators : The relationship between kidney function and long-term graft survival after kidney transplant. Am J Kidney Dis 57: 466–475, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Naesens M, Kuypers DR, De Vusser K, Vanrenterghem Y, Evenepoel P, Claes K, Bammens B, Meijers B, Lerut E: Chronic histological damage in early indication biopsies is an independent risk factor for late renal allograft failure. Am J Transplant 13: 86–99, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Boor P, Ostendorf T, Floege J: Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Corapi KM, Chen JL, Balk EM, Gordon CE: Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am J Kidney Dis 60: 62–73, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Schneider MP, Janka R, Ziegler T, Raff U, Ritt M, Ott C, Veelken R, Uder M, Schmieder RE: Reversibility of the effects of aliskiren in the renal versus systemic circulation. Clin J Am Soc Nephrol 7: 258–264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Zhang Y, Yang X, Wang X, Zhang J, Fang J, Jiang X: Hemodynamic effects of furosemide on renal perfusion as evaluated by ASL-MRI. Acad Radiol 19: 1194–1200, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Schnermann J, Persson AE, Agerup B: Tubuloglomerular feedback. Nonlinear relation between glomerular hydrostatic pressure and loop of henle perfusion rate. J Clin Invest 52: 862–869, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichikawa I: Direct analysis of the effector mechanism of the tubuloglomerular feedback system. Am J Physiol 243: F447–F455, 1982 [DOI] [PubMed] [Google Scholar]
- 25.Sohn B, Kim MJ, Han SW, Im YJ, Lee MJ: Shear wave velocity measurements using acoustic radiation force impulse in young children with normal kidneys versus hydronephrotic kidneys. Ultrasonography 33: 116–121, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papazoglou S, Hirsch S, Braun J, Sack I: Multifrequency inversion in magnetic resonance elastography. Phys Med Biol 57: 2329–2346, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Serai SD, Yin M, Wang H, Ehman RL, Podberesky DJ: Cross-vendor validation of liver magnetic resonance elastography. Abdom Imaging 40: 789–794, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]