Hepatitis C virus (HCV) is a small RNA flavivirus that was discovered in 1989 as the major cause of non-A, non-B hepatitis. Untreated, acute HCV infection may persist in up to 90% of patients, often with minimal symptoms over years, only to later manifest as chronic active hepatitis, cirrhosis, or hepatocellular carcinoma. Chronic activation of the immune system by HCV can also result in extrahepatic manifestations, such as GN, arthralgia, purpura, and lymphoproliferative diseases, frequently accompanied with essential mixed (types 2 and 3) cryoglobulinemia. The most common form of glomerular disease associated with HCV is membranoproliferative GN. In laboratory examinations, hypocomplementemia, cryoglobulinemia, HCV-RNA, and rheumatoid factor are frequently detected as well as proteinuria, hematuria, and kidney dysfunction (1). HCV is not uncommon in the patients with CKD, and its presence is associated with increased risk for progression to ESRD as well as increased morbidity and mortality (2,3). Proteinuria due to overt or underdiagnosed cryoglobulinemia or arteriosclerosis induced by chronic inflammation could contribute to the increased frequency of CKD. Early treatment of HCV primarily consisted of IFN-based regimens (IFN-α or pegylated IFN), often combined with ribavirin; although remission could be obtained in the majority of patients, side effects were often major (depression and malaise from IFN and hemolytic anemia from ribavirin), and relapse was common, especially in the subject with CKD.
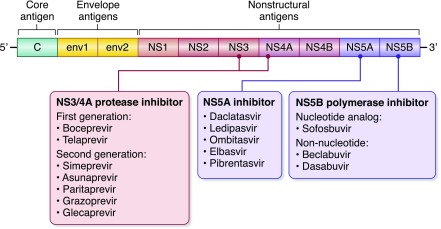
The major breakthrough occurred with the introduction of direct-acting antiviral agents that act on specific molecules driving replication pathways of the virus. The earliest agents were first generation protease inhibitors, such as boceprevir and telaprevir, but later, antivirals were introduced aimed at second generation protease inhibitors, NS5A inhibitors, and NS5B polymerase inhibitors in the nonstructural regions (Figure 1). One agent, sofosbuvir (NS5B polymerase inhibitor; Gilead Sciences), has been found to be quite effective in treating HCV genotype 1 in patients without CKD, especially when combined with NS3/4A protease inhibitors or NS5A inhibitors. Thus, the Cosmos Study reported that the combination of sofosbuvir and simeprevir (NS3/4A protease inhibitor; Olysio; Janssen Therapeutics) resulted in 92%–94% of patients having sustained virologic response (SVR) in patients without CKD with HCV genotype 1 (4). Likewise, the combination of sofosbuvir with ledipasvir (NS5A inhibitor), also known as Harvoni (Gilead Sciences), was reported to be highly effective, with an SVR rate of 93%–99% in patients without CKD with HCV genotype 1 (5).
Figure 1.
Schematic of hepatitis C virus and classification of direct-acting antiviral agents.
A problem with sofosbuvir-based regimens is that sofosbuvir is excreted primarily by the kidneys and accumulates when eGFR is <30 ml/min per 1.73 m2. Nevertheless, there have been a few reports of successful treatment of HCV genotype 1 in the advanced CKD, even in patients on dialysis with sofosbuvir-based regimens, but the numbers of patients were very small (6). However, importantly, there have also been reports of AKI with sofosbuvir-based regimens, especially in patients with cirrhosis. Although AKI may be due to concomitant use of agents, such as nonsteroidal anti-inflammatory agents, one study reported that, of 426 patients receiving direct-acting antiviral agent with HCV infection without CKD, the risk for AKI was 11% in the group receiving sofosbuvir-based regimens and 18% in the group receiving the older telaprevir- or boceprevir-based regimens (7). It is not known how much of this kidney injury is hemodynamic, toxin based, or immune based, but at least one report of steroid-responsive acute interstitial nephritis has been reported in a subject receiving sofosbuvir (8).
In this paper by Sise et al. (9), a sofosbuvir-based regimen with either simeprevir or ledipasvir with or without ribavirin was administered to 98 patients with HCV infection and CKD stages 1–3. SVR was obtained in 81%; it was higher in those with genotype 1b and lower in those with genotypes 3 and 4, and it also seemed to be more effective in patients with more advanced CKD (which the authors posited may be due to higher blood levels of sofosbuvir). In the patients with CKD stage 3, there was a 9-ml/min per 1.73 m2 increase in eGFR at 6 months, suggesting reversibility in many patients with HCV and CKD and an overall benefit. However, there was evidence of some AKI, with a transient rise of serum creatinine of 0.3 mg/dl or more in one quarter of subjects, with seven patients having a rise in serum creatinine of 1.5 times greater than baseline. Unfortunately, there was no control group to determine if this was related to the treatments.
Although this study suggests that sofosbuvir may be useful in the patient with CKD if monitored carefully, there are other regimens that have come to market that are effective in the subject with CKD. A combination treatment consisting of elbasvir (NS5A inhibitor) and grazoprevir (NS3/4A protease inhibitor), known commonly as Zepatier (Merck), has been found to be effective for HCV genotypes 1 and 4 in the C-Surfer Trial, which enrolled 224 patients with HCV genotype 1 infection and CKD stages 4 and 5 or on dialysis (10). These agents are not excreted by the kidney, and they were exceptionally effective (99.1% SVR at 12 weeks), with minimal side effects. Indeed, Zepatier was Food and Drug Administration (FDA) approved for HCV genotypes 1 and 4 in patients with CKD stage 4 or 5 or on dialysis in 2016.
Another combination agent that has been used in patients with CKD is Viekira (Abbvie), which is a combination of ombitasvir (NS5A inhibitor), paritaprevir (NS3/4A serine protease inhibitor), ritonavir (CYP3A4 inhibitor, a booster of protease inhibitor), and dasabuvir (NS5A inhibitor). In the Ruby-1 Trial, 20 patients with CKD stage 4 or 5 or on dialysis with HCV genotype 1 were treated for 12 weeks, with the patients with HCV genotype 1a infection also receiving ribavirin (200 mg daily) (11). The vast majority (18 of 20) achieved SVR with no serious side effects, although anemia was common in the ribavirin-treated group. Viekira is FDA approved in the patient on dialysis with HCV genotype 1 infection and needs no dose adjustment.
However, the most promising combination therapy to date is combination glecaprevir (NS3/4A protease inhibitor) and pibrentasvir (NS5A inhibitor). This regimen was given in the Expedition Trial to 104 patients with CKD stage 4 or 5 or on dialysis and included all HCV genotypes. In November of 2016, the results were announced, and a 12-week course of therapy was associated with 98% SVR, with minimal side effects. The formal publication of this trial is being eagerly awaited.
Antiviral treatment should also be a part of treatment of HCV-associated GN and essential mixed cryoglobulinemia, and a case report of successful remission in a Japanese woman with cryoglobulinemic membranoproliferative GN with yet another regimen (daclatasvir and asunaprevir) has recently been reported (12). However, whether direct antiviral agents should be used solely in the patient with HCV-associated vasculitis or GN is questionable, because immunosuppression is important to arrest the acute inflammatory components of the disease. Indeed, rituximab-based regimens seem to be the most beneficial (13,14) as summarized in a recent review by Cacoub and Comarmond (15). It is suggested that optimized antiviral treatment alone be used in mild to moderate disease. In addition to the optimized antiviral treatment, rituximab is recommended in severe conditions, including progressive kidney disease, or patients with skin ulcers. In life-threatening conditions, such as rapidly progressive GN, or with vasculitis involving the central nervous system or lungs, the combination of corticosteroids, plasma exchanges, and rituximab could be considered.
In summary, HCV is now a curable disease. All patients with HCV infection should be evaluated for potential treatment. Sofosbuvir-based therapy may be useful in the management of patients with CKD and HCV genotypes 1 and 4. However, we would like to see more studies investigating the potential nephrotoxicity of sofosbuvir. Furthermore, this latter regimen is not FDA approved in the United States in the patient with CKD at this time. Alternative therapies are becoming available that may be safer and more effective. We predict that HCV, like hepatitis B virus and HIV, will slowly disappear as a major medical problem for patients with kidney disease.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “Effect of Sofosbuvir-Based Hepatitis C Virus Therapy on Kidney Function in Patients with CKD,” on pages 1615–1623.
References
- 1.Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Corey L, Wener MH, Alpers CE, Willson R: Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 328: 465–470, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Molnar MZ, Alhourani HM, Wall BM, Lu JL, Streja E, Kalantar-Zadeh K, Kovesdy CP: Association of hepatitis C viral infection with incidence and progression of chronic kidney disease in a large cohort of US veterans. Hepatology 61: 1495–1502, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabrizi F, Verdesca S, Messa P, Martin P: Hepatitis C virus infection increases the risk of developing chronic kidney disease: A systematic review and meta-analysis. Dig Dis Sci 60: 3801–3813, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Lawitz E, Sulkowsk, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, DeJesus E, Pearlman B, Rabinovitz M, Gitlin N, Lim JK, Pockros PJ, Scott JD, Fevery B, Lambrecht T, Ouwerkerk-Mahadevan S, Callewaert K, Symonds WT, Picchio G, Lindsay KL, Beumont M, Jacobson IM: Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: The COSMOS randomised study. Lancet 384: 1756–1765, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Jensen CM, Holle LM: Ledipasvir-Sofosbuvir: A once-daily oral treatment option for chronic hepatitis C virus genotype 1 infection. Pharmacotherapy 36: 562–574, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Hundemer GL, Sise ME, Wisocky J, Ufere N, Friedman LS, Corey KE, Chung RT: Use of sofosbuvir-based direct-acting antiviral therapy for hepatitis C viral infection in patients with severe renal insufficiency. Infect Dis (Lond) 47: 924–929, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maan R, Al Marzooqi SH, Klair JS, Karkada J, Cerocchi O, Kowgier M, Harrell SM, Rhodes KD, Janssen HLA, Feld JJ, Duarte-Rojo A: The frequency of acute kidney injury in patients with chronic hepatitis C virus infection treated with sofosbuvir-based regimens. Aliment Pharmacol Ther 46: 46–55, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Ashraf T, Majoni W: Acute interstitial nephritis associated with sofosbuvir and daclatasvir. ACG Case Rep J 4: e84, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sise ME, Blackman E, Ortiz G, Hundemer GL, Ufere N, Chute DF, Brancate J, Xu D, Wisocky J, Lin MV, Kim AY, Thadhani R, Chung RT: Effect of sofosbuvir-based hepatitis C virus therapy on kidney function in patients with chronic kidney disease. Clin J Am Soc Nephrol 12: 1615–1623, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour Jr. H, Martin P, Pol S, Londoño MC, Hassanein T, Zamor PJ, Zuckerman E, Wan S, Jackson B, Nguyen BY, Robertson M, Barr E, Wahl J, Greaves W: Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4-5 chronic kidney disease (the C-SURFER study): A combination phase 3 study. Lancet 386: 1537–1545, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Pockros PJ, Reddy KR, Mantry PS, Cohen E, Bennett M, Sulkowski MS, Bernstein DE, Cohen DE, Shulman NS, Wang D, Khatri A, Abunimeh M, Podsadecki T, Lawitz E: Efficacy of direct-acting antiviral combination for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology 150: 1590–1598, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Shimada M, Nakamura N, Endo T, Yamabe H, Nakamura M, Murakami R, Narita I, Tomita H: Daclatasvir/asunaprevir based direct-acting antiviral therapy ameliorate hepatitis C virus-associated cryoglobulinemic membranoproliferative glomerulonephritis: A case report. BMC Nephrol 18: 109, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vita S, Quartuccio L, Isola M, Mazzaro C, Scaini P, Lenzi M, Campanini M, Naclerio C, Tavoni A, Pietrogrande M, Ferri C, Mascia MT, Masolini P, Zabotti A, Maset M, Roccatello D, Zignego AL, Pioltelli P, Gabrielli A, Filippini D, Perrella O, Migliaresi S, Galli M, Bombardieri S, Monti G: A randomized controlled trial of rituximab for the treatment of severe cryoglobulinemic vasculitis. Arthritis Rheum 64: 843–853, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Saadoun D, Resche Rigon M, Sene D, Terrier B, Karras A, Perard L, Schoindre Y, Coppere B, Blanc F, Musset L, Piette JC, Rosenzwajg M, Cacoub P: Rituximab plus Peg-interferon-alpha/ribavirin compared with Peg-interferon-alpha/ribavirin in hepatitis C-related mixed cryoglobulinemia. Blood 116: 326–334, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Cacoub P, Comarmond C: New insights into HCV-related rheumatologic disorders: A review. J Adv Res 8: 89–97, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]