Abstract
Background and objectives
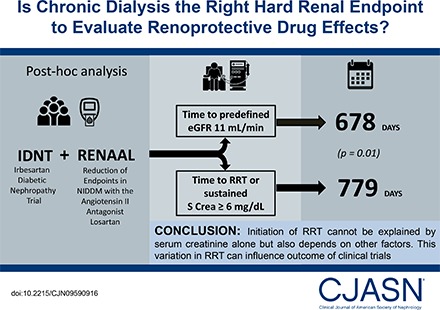
RRT and doubling of serum creatinine are considered the objective hard end points in nephrology intervention trials. Because both are assumed to reflect changes in the filtration capacity of the kidney, drug effects, if present, are attributed to kidney protection. However, decisions to start RRT are not only on the basis of filtration capacity of the kidney, but also on other factors. We therefore compared the time to RRT with the time to a fixed eGFR threshold and assessed the effect of the renoprotective drug irbesartan on both components.
Design, setting, participants, & measurements
Post hoc analysis of two clinical trials, the Irbesartan Diabetic Nephropathy Trial (IDNT) and Reduction of End points in Non-insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial, in patients with type 2 diabetes and nephropathy. The time to a predefined eGFR level of 11 ml/min per 1.73 m2 (eGFR11), calculated by within-patient linear regression, was compared with the time to RRT or sustained serum creatinine ≥6 mg/dl.
Results
A large difference was observed in the median time to RRT (779 days) compared with eGFR11 (678 days; P=0.01). We also observed a large variation in the difference between the time to RRT and eGFR11. In IDNT, the hazard ratio for the effect of irbesartan on the serum creatinine ≥6.0 mg/dl end point was 0.60 (95% confidence interval, 0.39 to 0.91; P=0.02), whereas it was smaller for the RRT end point (hazard ratio, 0.78; 95% confidence interval, 0.58 to 1.07; P=0.12).
Conclusions
This study shows a difference in the time to RRT and a fixed eGFR threshold, and shows that the effect of an angiotensin receptor blocker on a filtration-based end point versus RRT varies. This implies that evaluating renoprotective effects of drugs with a combined RRT and doubling of serum creatinine end point may result in evaluating other effects beyond renoprotection alone. Future trials should consider registering all parameters that lead to RRT decisions.
Keywords: Angiotensin II; Biphenyl Compounds; creatinine; Diabetes Mellitus, Type 2; Diabetic Nephropathies; glomerular filtration rate; Humans; kidney; Linear Models; Losartan; nephrology; renal dialysis; Renal Replacement Therapy; Tetrazoles; irbesartan
Introduction
Trials to test the efficacy of novel drugs to slow progression of CKD should use well defined end points. RRT (chronic dialysis or kidney transplantation) and doubling of serum creatinine (DSCr) are currently considered to be the best objective renal end points and are therefore the obvious clinically relevant end point in trials on slowing CKD progression (1,2).
Both RRT and DSCr are assumed to measure the filtration capacity of the kidney, and thus drugs tested in trials with this combined end point are assumed to be tested for an effect on kidney function/filtration. If there is a reduction in this combined end point with the experimental drug, it can be labeled as renoprotective. This is all on the basis of the assumption that both RRT and DSCr are indeed only reflecting changes in filtration capacity of the kidney. However, the decision for dialysis or transplantation is made not only on filtering capacity of the kidneys, but also on other parameters like judgment and decision of the physician, patient’s wellbeing and comorbidities, uremic symptoms, local habits and guidelines, and/or availability of RRT. These factors may influence the time to the RRT end point and could potentially lead to different treatment effects when considering a filtration-based end point (e.g., DSCr or fixed serum creatinine threshold) with the RRT end point. Because drugs are usually developed on the basis of an expectation that they will slow loss of the filtration capacity of the kidney (i.e., serum creatinine or eGFR), the result of trials that use a combined RRT/DSCr end point may result in unexpected outcomes through potential effects on parameters other than filtering capacity.
To test whether a change in the RRT end point actually reflects filtering capacity changes, we evaluated if initiation of RRT was on the basis of reaching a predefined eGFR level in the Irbesartan Diabetic Nephropathy Trial (IDNT) and Reduction of End points in Non-insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan (RENAAL) Trial. We also assessed and compared the treatment effect on the time to a fixed serum creatinine threshold versus RRT in the IDNT Trial.
Materials and Methods
Study Design
We performed post hoc analyses in the IDNT and RENAAL (Clinicaltrials.gov identifier 00308347) trials. Both trials demonstrated that an angiotensin receptor blocker (irbesartan in the IDNT Trial and losartan in the RENAAL Trial) delays the onset of a composite end point consisting of DSCr, RRT, or death of any cause in patients with type 2 diabetes and nephropathy. The rationale, study design, and primary outcomes of both trials have been described in detail elsewhere (3–6). Both trials were conducted from 1996 to 2000 and the average eGFR threshold at that time to start dialysis was 11 ml/min per 1.73 m2. Inclusion criteria for both trials were presence of type 2 diabetes, nephropathy, overt proteinuria, and age between 30 and 70 years. Individuals with insulin-dependent diabetes or renal disease not related to diabetes were excluded in both trials. All participants gave written informed consent. Both trials were approved by local medical ethics committees and conducted according to guidelines of the Declaration of Helsinki.
RRT and eGFR-Based End Points
RRT was defined as the decision for initiation of chronic dialysis (>4 weeks) or kidney transplantation. In the IDNT Trial, an additional RRT criterion for the primary analysis required a confirmed serum creatinine level ≥6.0 mg/dl (SCr6). For the purpose of this analysis, the SCr6 component was excluded from the RRT definition. The effect of irbesartan on RRT and SCr6 was assessed in the IDNT Trial because both components were adjudicated and prespecified only in the IDNT Trial; SCr6 end points were not recorded in the RENAAL Trial. In both trials, all RRT events were adjudicated by an independent adjudication committee, using rigorous definitions and guidelines.
Serum creatinine was measured in both trials regularly at 3-month intervals by a central laboratory. Once a patient reached RRT, study medication was discontinued and subsequent serum creatinine measurements were not recorded. eGFR was calculated using the Modification of Diet for Renal Disease equation on the basis of serum creatinine, age, race, and sex (7).
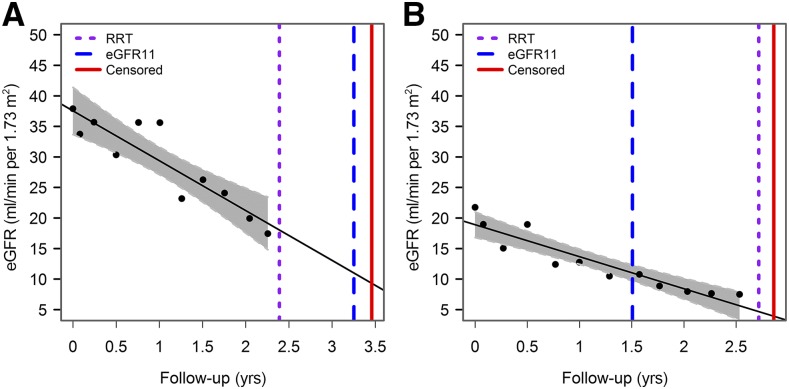
The eGFR threshold to define kidney failure was 11 ml/min per 1.73 m2. On the basis of the eGFR slope of each individual, calculated by within-patient linear regression, we interpolated or extrapolated the time until the individual reached 11 ml/min per 1.73 m2 (eGFR11). For illustration purposes, Figure 1 displays the eGFR trajectories and time to eGFR11 and RRT of two patients. The time to reach eGFR11 was subsequently compared with the time to the initiation of RRT. The threshold of 11 ml/min per 1.73 m2 was chosen because it is the average threshold that was used in clinical practice to initiate dialysis at the time the RENAAL and IDNT trials were conducted (8). In a sensitivity analysis, the eGFR-based threshold for kidney failure was 15 ml/min per 1.73 m2 because the current Kidney Disease Improving Global Outcomes guidelines define stage 5 CKD as eGFR<15 ml/min per 1.73 m2 (9).
Figure 1.
Examples of two patients who initiated RRT approximately 1 year before reaching eGFR11 (A) or initiated RRT 1 year after reaching eGFR11 (B). The purple dotted line indicates the initiation of dialysis, the vertical dotted blue line indicates the time point when eGFR11 was reached, and the vertical straight red line indicates the censor date of the individual. eGFR11, time to a predefined eGFR level of 11 ml/min per 1.73 m2.
The interpolation of the individual eGFR trajectory to calculate the time to RRT assumes linear eGFR trajectories in all patients. However, previous studies have shown that in a proportion of patients eGFR decline is not linear over time (10). To account for potential nonlinear eGFR trajectories, we conducted an additional analysis in which the time to the first eGFR measurement ≤11 ml/min per 1.73 m2 (confirmed by the subsequent measurement) was compared with the actual time to RRT. This analysis only includes patients in whom eGFR was recorded before RRT initiation because serum creatinine was not recorded after RRT initiation. To assess internal validity, we compared the time to the first confirmed eGFR11 with the time to eGFR11 calculated from the eGFR slope. To reduce the uncertainty of calculating the time to eGFR11, we analyzed a subgroup of patients in whom eGFR decline over time was optimally fitted by selecting all patients below the median residual sum of squares of the individual regression line. We subsequently compared the time to eGFR11 with the time to RRT in this subgroup of patients.
Statistical Analyses
The time to eGFR11 or SCr6 and RRT was compared by Mann–Whitney U test. The median difference between the time to eGFR11 and RRT was subsequently calculated and represented the bias. Because patients could reach eGFR11 before or after RRT, the time difference was calculated separately for patients who reached eGFR11 either before or after RRT. The time difference was zero if the time to eGFR11 was similar to the time to RRT. Accuracy was calculated and defined as the percentage of patients reaching eGFR11 within 90 days of the actual time to RRT.
A joint model of longitudinal and survival data were used to assess the relationship between time-varying eGFR and time to RRT, and time to reaching eGFR11 and SCr6. The joint model links two processes by unobserved random effects through the use of a shared parameter in order to model both survival and longitudinal data simultaneously. To model the longitudinal eGFR data we used a linear mixed effects model with a random intercept and random slope. The model included an interaction term between follow-up time and treatment. To model survival data we used a Cox proportional hazard model. The model included a term for randomized treatment assignment. The joint model included both RRT and SCr6 (or eGFR11) as competing events. Patients who did not reach an event were censored at their date of death, or for those still alive at the end of the trial, the date of their last clinic visit before the termination of the study. Estimation of the joint model was on the basis of the maximum likelihood approach. In the joint model, we assumed that the risk for SCr6 (or eGFR11) end point at a specific time depends on features of the longitudinal trajectory at the same time point (i.e., current serum creatinine value and current slope).
The effect of irbesartan compared with placebo on the RRT end point and SCr6 end point were estimated from Cox proportional hazard models. Cox proportional hazard models were conducted on the basis of the intention to treat principle, and survival time to the first relevant end point was used in each analysis. Because RRT is a competing risk for the SCr6 end point, an additional analysis was conducted accounting for the competing event of RRT. The subhazard ratio of the treatment effect was calculated using the Fine and Gray model (11), which extends the Cox proportional hazard model to competing risk data by taking into account the subdistribution hazard. Mean and SDs are provided for normally distributed data, and median and 25th–75th percentiles are provided for skewed data. Analyses were conducted with R statistical software version 2.15.3 (www.R-project.org; the JM package in R was used to implement the joint model).
Results
Comparison between the Duration to Reach RRT and eGFR11
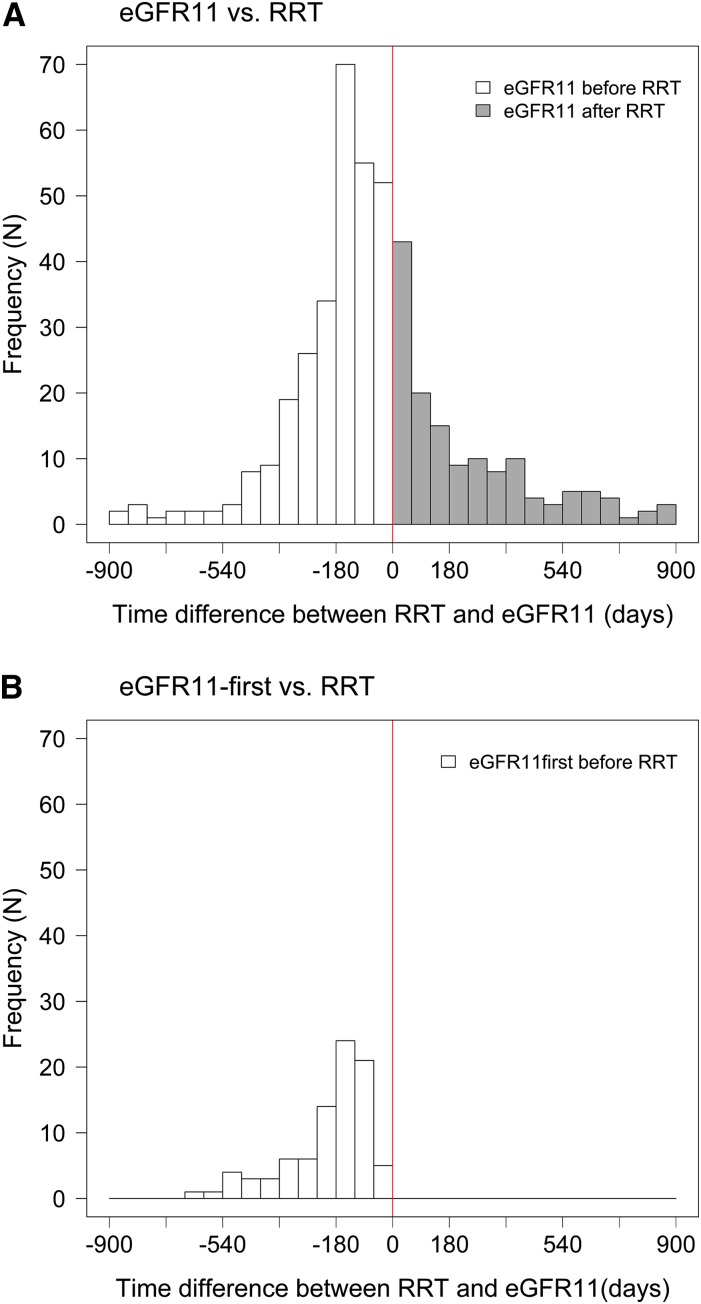
A total of 3055 patients with at least three eGFR measurements during follow-up were included in this analysis. Their baseline characteristics are shown in Supplemental Table 1. Of these 3055 patients, 448 (15%) initiated RRT during the trial period. The median time to RRT was 779 days. Median time to eGFR11 was 678 days (difference from RRT of 101 days; P=0.01). A large variation was observed in the time to eGFR11 and RRT (Figure 2). Among the 288 patients who reached eGFR11 before RRT initiation, the median time difference between eGFR11 and RRT was 150 days, whereas in the 160 patients who reached eGFR11 after initiation of RRT, the median time difference was 204 days (Table 1). The accuracy, defined as the percentage of patients with eGFR11 measurements within 90 days of the actual time to RRT, was 31% (Table 1).
Figure 2.
Large variability between the time to reach RRT and eGFR11. The solid vertical line at 0 represents the time to RRT (779 days). The white bars show patients who reached eGFR11 before RRT and gray bars show patients who reached eGFR11 after RRT. (A) Time to eGFR11 calculated on the basis of the individual eGFR slope. (B) Time to eGFR11 on the basis of the time to first confirmed eGFR measurement of 11 ml/min per 1.73 m2. During patient follow-up, 228 patients reached eGFR11 but not RRT. A total of 18 patients had an estimated time to eGFR11 beyond the range of the histogram. The time interval extended to 1 year beyond the histogram for eight patients, 1 and 2 years for three patients, 2 and 4 years for two patients, and beyond 4 years for five patients. eGFR11, time to a predefined eGFR level of 11 ml/min per 1.73 m2.
Table 1.
Difference in the time to RRT and eGFR11 in patients who reached eGFR11 either before or after RRT
| End Point | eGFR11 before RRT | eGFR11 after RRT | Accuracy P90, % | ||
|---|---|---|---|---|---|
| Patients with RRT, N | Median [25th–75th Percentile] Time Difference, d | Patients with RRT, N | Median [25th–75th Percentile] Time Difference, d | ||
| eGFR11 versus RRT | 288 | 150 [78–251] | 160 | 204 [51–495] | 31.0 |
| eGFR11-first versus RRT | 88 | 160 [115–266] | N/A | N/A | 13.6 |
| eGFR11 versus eGFR11-first | 56 | 48 [26–66] | 33 | 42 [20–81] | 82.0 |
The left part of the table shows the number of patients who reached eGFR11 before RRT was initiated and the time difference between eGFR11 and RRT. The right part of the table shows the number of patients who reached eGFR11 after RRT and the time difference (on the basis of extrapolation of the eGFR slope). eGFR11, time to a predefined eGFR level of 11 ml/min per 1.73 m2 on the basis of the individual’s eGFR slope; P90, proportion of patients in whom RRT was initiated within 90 d of reaching eGFR11; eGFR11-first, time to first measurement of predefined eGFR level of 11 ml/min per 1.73 m2.
To account for potential nonlinear eGFR declines, we also compared the time to RRT and time to first confirmed eGFR11. Because eGFR was not recorded after a patient had reached RRT, we could only calculate the time difference if RRT was initiated after eGFR11. In the 88 patients who reached RRT after eGFR11, the median time difference between first confirmed eGFR11 and RRT was 160 days, and 14% initiated RRT within 90 days of reaching eGFR11 (Table 2). To assess internal validity, we compared the time to eGFR11 on the basis of the individual eGFR slope and first eGFR11 measurement. The time difference was substantially smaller and accuracy was higher than the comparison of either of these eGFR metrics with RRT (Table 1). We also observed a significant difference in the time to SCr6 and RRT in the IDNT Trial (median time of 584 [382–902, 25th to 75th percentile] versus 688 [409–1011, 25th to 75th percentile] days, respectively; P<0.01).
Table 2.
The association between time-varying eGFR is stronger with eGFR11, eGFR15, or serum creatinine ≥6.0 mg/dl compared with the association with RRT
| End Points | N | HR [95% CI] | P Valuea |
|---|---|---|---|
| Combined RENAAL and IDNT trials | |||
| RRT | 258 | 1.25 [1.11 to 1.41] | <0.01 |
| eGFR11-first | 317 | 1.53 [1.45 to 1.62] | |
| RRT | 153 | 1.22 [1.12 to 1.32] | <0.01 |
| eGFR15-first | 570 | 1.42 [1.37 to 1.48] | |
| IDNT Trial | |||
| RRT | 93 | 1.23 [1.11 to 1.69] | 0.08 |
| SCr6≥6.0 mg/dl | 84 | 1.56 [1.33 to 1.82] | |
| RRT | 64 | 1.18 [1.01 to 1.41] | 0.01 |
| eGFR11-first | 124 | 1.50 [1.38 to 1.63] | |
| RRT | 39 | 1.15 [1.03 to 1.37] | <0.01 |
| eGFR15-first | 216 | 1.49 [1.38 to 1.62] | |
| RENAAL Trial | |||
| RRT | 194 | 1.29 [1.09 to 1.51] | 0.03 |
| eGFR11-first | 193 | 1.57 [1.45 to 1.70] | |
| RRT | 114 | 1.15 [1.06 to 1.24] | <0.01 |
| eGFR15-first | 354 | 1.30 [1.26 to 1.35] | |
The HRs indicate the association between the RRT, eGFR11, eGFR15, and SCr6 end points with 1 ml/min per 1.73 m2 decline in eGFR, as calculated with the joint model. The trials were jointly and separately analyzed. N, number of events; HR, hazard ratio; 95% CI, 95% confidence interval; RENAAL, Reduction of End points in Non-insulin dependent diabetes mellitus with the Angiotensin II Antagonist Losartan Trial; IDNT, Irbesartan Diabetic Nephropathy Trial; eGFR11-first, time to first unconfirmed measurement of eGFR level of 11 ml/min per 1.73 m2; eGFR15-first, time to first unconfirmed measurement of eGFR level of 15 ml/min per 1.73 m2; SCr6, serum creatinine ≥6 mg/dl.
P value, comparing HR of RRT with SCr6, eGFR11-first, or eGFR15-first.
A joint model analysis showed that the hazard ratio for the association between eGFR level and RRT was significantly lower compared with the association between eGFR level and time to first eGFR11 or SCr6 (Table 2).
Results were similar in a sensitivity analysis of patients in whom the most optimal fit of the individual eGFR regression line could be fitted (Supplemental Table 2) or when eGFR<15 ml/min per 1.73 m2 was used to define kidney failure (Supplement Table 3).
Effect of Irbesartan on RRT and SCr6
The effects of irbesartan on a filtration-based end point (time to a sustained SCr6) and RRT were different. The SCr6 end point occurred in 58 patients in the placebo group and 36 patients in the irbesartan group, representing a hazard ratio of 0.60 (95% confidence interval [95% CI], 0.39 to 0.91; P=0.01). The RRT end point occurred in 90 patients in the placebo group and 74 patients in the irbesartan group, representing a nonsignificant hazard ratio of 0.78 (95% CI, 0.58 to 1.07; P=0.12). The effect of irbesartan on the eGFR11 end point was similar to the SCr6 end point (hazard ratio, 0.64; 95% CI, 0.45 to 0.91; P=0.01). Results were not different in a competing risk analysis (subhazard ratio for SCr6, 0.65; 95% CI, 0.42 to 1.01; P=0.06, and subhazard ratio for RRT, 0.86; 95% CI, 0.57 to 1.29; P=0.46). The competing risk subhazard ratio for the treatment effect of irbesartan on the eGFR11 versus RRT end point was 0.65 (95% CI, 0.45 to 0.92; P=0.02) versus 0.97 (95% CI, 0.60 to 1.58; P=0.91). In the joint model, after taking into account the patient’s eGFR trajectory, the treatment effect of irbesartan was 0.85 (95% CI, 0.20 to 3.61).
Discussion
In this study we found a large discrepancy between the time to eGFR11 and the time to RRT. This suggests that, although RRT is a hard end point in trials of kidney disease progression, the decision of RRT initiation appears not only to be driven by the filtration capacity of the kidney (i.e., serum creatinine or eGFR) but also by other factors. This finding may affect the evaluation of drug efficacy in CKD trials.
How do these findings effect on the current use of DSCr (or predefined serum creatinine or eGFR level) and initiation of dialysis as measures for RRT? These data have to be substantiated by other, preferably prospective studies. More importantly, we need to understand whether we want to analyze the renoprotective potential of interventions on the basis of their ability to attenuate, halt, or even improve GFR decline, or whether we want to establish if the drug postpones the need for RRT initiation. Potentially, a drug could not affect GFR change at all, but just improve the “tolerance” of the patient to withstand the sequelae of reduced kidney function, thus delaying the decision of the physician to start dialysis. Although the latter is clearly of importance (both for the patient and from a health care payer perspective) it may not be labeled as kidney protection, which is mainly defined as drugs that slow eGFR progression, delay overall structural kidney function loss, and/or reduce the sequelae of reduced filtration capacity. Our data supports that reaching a predefined level of serum creatinine or eGFR should always be included as a component of a hard kidney end point, as already occurs in most kidney outcome trials.
The results of our study, using data from trials conducted 15–20 years ago, are in line with recent studies and contemporary practice, showing a wide variation as to when to initiate RRT (12). A web-based questionnaire conducted in 11 European countries among 433 nephrologists showed that only one third of all nephrologists considered eGFR as the most important factor in the decision to initiate RRT (13). Reasons for an earlier start of RRT included the clinical condition of the patient, such as uremic symptoms, whereas patient preference and lack of dialysis facilities delayed the start of RRT. Patients preferred initiation of dialysis over conservative treatment if they were able to dialyze during the day or evening rather than during the day only, if subsidized transport was available, and few hospital visits were required (14). Quality of life is another important factor that is taken into account when deciding to initiate RRT (15). A study from the United Kingdom established substantial variation among health care physicians in their likelihood to offer dialysis. The patient’s mental state appeared to be the most significant factor to affect the decision to offer dialysis (16). Taken together, the available data indicate that the decision of when to start RRT is multifactorial and is unlikely to be guided by a single parameter (for example, eGFR). Symptoms associated with uremia, which could drive the decision to initiate dialysis, are not systemically collected and analyzed in clinical trials and should be recorded in future (drug) trials to obtain more insight into which factors drive RRT initiation, and examine which of these factors mediate a potential renoprotective effect.
The variation in the time between the decision to offer dialysis and reaching a fixed serum creatinine or eGFR threshold may affect evaluation of drug efficacy. The effect of irbesartan on the RRT and SCr6 end point seemed to differ but the confidence intervals of the effect of irbesartan on both end points were overlapping. Additionally, the joint model analysis showed that after taking into account the patient’s eGFR trajectory, the treatment effect of irbesartan on the RRT end point was not statistically significant. Apparently, for angiotensin receptor blockers, a large part of the protective effect is mediated by their effect on slowing eGFR decline, and this may overwhelm potential effects that affect RRT decisions. Yet, as mentioned above, other interventions may influence RRT decisions through effects independent of filtration. In this respect, another trial has shown different effects of the intervention on the DSCr and RRT end point. In the Evaluation Prevention of Progression in CKD-2 Trial, AST-120 showed a trend toward a risk reduction for RRT of 18%, whereas no effect on DSCr was observed. The reverse was observed in the Evaluation Prevention of Progression in CKD-1 Trial. The authors suggested that regional differences across practices in when to initiate dialysis could explain these different results (17).
This study has limitations. First, the assessment of the timing between reaching eGFR11 and RRT was conditional on the occurrence of RRT. A total of 228 patients reached eGFR11 during patient follow-up but did not reach RRT. The censoring for RRT may have given a biased assessment of the time difference. However, this is probably a conservative bias because the time difference between eGFR11 and RRT will likely shift upwards if the censoring of RRT is taken into account. Second, we calculated the time to eGFR11 on the basis of linear interpolation or extrapolation of the eGFR slope. Spontaneous fluctuations in eGFR decline have been demonstrated (18), and may affect the prediction of time to eGFR11. However, to account for periods of accelerated kidney function decline, we performed an additional analysis comparing the time to the first eGFR measurement of 11 ml/min per 1.73 m2 and the time to RRT. This analysis confirmed the results of our main analysis. Third, we recognize that estimating GFR on the basis of creatinine level rather than relying on measured GFR can misclassify patients, which would bias the time to eGFR11 end point (19). Additionally, random noise in the measurement of serum creatinine may also account for the variation in timing between eGFR11 and RRT rather than factors influencing the RRT decision. Fourth, the number of SCr6 and RRT end points in the IDNT Trial was relatively small, which limited the power of analyses when comparing irbesartan treatment effects. Finally, the results can only be generalized to those who share the characteristics of the RENAAL and IDNT populations.
In conclusion, this study shows that the initiation of RRT cannot be explained by serum creatinine alone but likely also depends on other factors. If we agree that renoprotection of a drug should be expressed as the slowing, halting, or improvement of filtration capacity, one should only include filtration-based measures (fixed eGFR threshold of 15 ml/min per 1.73 m2 and/or a DSCr) in a kidney end point. Alternatively, one could dissect the RRT decision by recording the physician’s reasons for dialysis initiation. In any case, we should be clear as to which drug/intervention effect we want to detect when using the end point of RRT: renal filtration, patient wellbeing, or both.
Disclosures
M.W. and P.M. report no conflicts of interest. J.P.D. reports research support from Nephrogenex, Eli-Lilly, and Keryx. D.d.Z. is consultant for and received honoraria (to his employer) from AbbVie, Astellas, Eli-Lilly, Chemocentryx, Fresenius, and Janssen. H.J.L.H. is consultant for and received honoraria from AbbVie, Astellas, Astra Zeneca, Boehringer Ingelheim, Fresenius, Janssen, and Merck, and has a policy of honoraria going to his employer.
Supplementary Material
Acknowledgments
We would like to thank Dr. D. Rizopoulos for providing statistical advice and expertise.
The authors did not receive direct financial support from third parties for the work described in this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Rethinking End Points in Clinical Trials of Renoprotective Medication,” on pages 1561–1562.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09590916/-/DCSupplemental.
References
- 1.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD; The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Lambers Heerspink HJ, Perkovic V, de Zeeuw D: Is doubling of serum creatinine a valid clinical ‘hard’ endpoint in clinical nephrology trials? Nephron Clin Pract 119: c195–c199, discussion c199, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, Grunfeld JP, Keane WF, Kurokawa K, McGill JB, Mitch WE, Parving HH, Remuzzi G, Ribeiro AB, Schluchter MD, Snavely D, Zhang Z, Simpson R, Ramjit D, Shahinfar S; RENAAL Study Investigators : The losartan renal protection study--Rationale, study design and baseline characteristics of RENAAL (Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan). J Renin Angiotensin Aldosterone Syst 1: 328–335, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Rodby RA, Rohde RD, Clarke WR, Hunsicker LG, Anzalone DA, Atkins RC, Ritz E, Lewis EJ; For the Collaborative Study Group : The Irbesartan type II diabetic nephropathy trial: Study design and baseline patient characteristics. Nephrol Dial Transplant 15: 487–497, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S; RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I; Collaborative Study Group : Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 8.U.S. Renal Data System, USRDS 2001 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2001 . [Google Scholar]
- 9.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3[Suppl]: 1–150, 2013 [Google Scholar]
- 10.Li L, Astor BC, Lewis J, Hu B, Appel LJ, Lipkowitz MS, Toto RD, Wang X, Wright JT Jr, Greene TH: Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 59: 504–512, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 12.Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, Finne P, Ioannidis GA, Salomone M, Traynor JP, Zurriaga O, Verrina E, Jager KJ: Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant 24: 3175–3182, 2009 [DOI] [PubMed] [Google Scholar]
- 13.van de Luijtgaarden MW, Noordzij M, Tomson C, Couchoud C, Cancarini G, Ansell D, Bos WJ, Dekker FW, Gorriz JL, Iatrou C, Garneata L, Wanner C, Cala S, Stojceva-Taneva O, Finne P, Stel VS, van Biesen W, Jager KJ: Factors influencing the decision to start renal replacement therapy: Results of a survey among European nephrologists. Am J Kidney Dis 60: 940–948, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Morton RL, Snelling P, Webster AC, Rose J, Masterson R, Johnson DW, Howard K: Factors influencing patient choice of dialysis versus conservative care to treat end-stage kidney disease. CMAJ 184: E277–E283, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foote C, Morton RL, Jardine M, Gallagher M, Brown M, Howard K, Cass A: COnsiderations of Nephrologists when SuggestIng Dialysis in Elderly patients with Renal failure (CONSIDER): A discrete choice experiment. Nephrol Dial Transplant 29: 2302–2309, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Kee F, Patterson CC, Wilson EA, McConnell JM, Wheeler SM, Watson JD: Stewardship or clinical freedom? variations in dialysis decision making. Nephrol Dial Transplant 15: 1647–1657, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Schulman G, Berl T, Beck GJ, Remuzzi G, Ritz E, Arita K, Kato A, Shimizu M: Randomized placebo-controlled EPPIC trials of AST-120 in CKD. J Am Soc Nephrol 26: 1732–1746, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah BV, Levey AS: Spontaneous changes in the rate of decline in reciprocal serum creatinine: Errors in predicting the progression of renal disease from extrapolation of the slope. J Am Soc Nephrol 2: 1186–1191, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Gaspari F, Ruggenenti P, Porrini E, Motterlini N, Cannata A, Carrara F, Jiménez Sosa A, Cella C, Ferrari S, Stucchi N, Parvanova A, Iliev I, Trevisan R, Bossi A, Zaletel J, Remuzzi G; GFR Study Investigators : The GFR and GFR decline cannot be accurately estimated in type 2 diabetics. Kidney Int 84: 164–173, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.