Summary
In 2001, WHO developed a pole for the administration of praziquantel without the use of weighing scales with encouraging results in African populations. In the present study, the pole was tested on height/weight data from 9,354 individuals from 11 non-African countries. In more than 98% of the individuals (C.I. 97.8-98-4) the pole estimated an acceptable dosage (30-60 mg/kg) - a performance not statistically different from the one observed in African populations. Reproducing the present pole in the form of a strip of paper and including it in each container of praziquantel would greatly facilitate the administration of the drug in large-scale interventions.
Keywords: Praziquantel administration, WHO dose pole
The regular treatment of vulnerable groups with praziquantel is WHO’s principal strategy for schistosomiasis control (WHO 2003). The drug administration is simple, but, as the individual dose of praziquantel is given according to body weight, reliable weighing scales are necessary in the field, which can be problematic and expensive. Hall et al (1999) therefore suggested the use of a pole, which estimates the number of tablets needed for treatment according to an individual's height. Three poles based on data from Ghana, Malawi and Tanzania were developed and tested in each country (n=1,803, n=2,069 and n= 2,078 respectively) with positive results (Hall et al. 1999). This idea was further developed by WHO and a single pole, developed from Guinea data, was tested with height and weight data from more than 25,000 individuals from 10 African countries. (Montresor et al. 2001)
It was found that the WHO dose pole estimates an acceptable dosage (30-60 mg/kg) for over 98% of the individuals (C.I. 97.7-98.1). Following field use in Uganda (Kabatereine N. personal communication), it was suggested to modify the pole to allow for its use for individuals shorter than 110 cm and taller than 178 cm, making it possible to use in non-school age populations as well.
The present study was conducted to investigate the validity of the WHO dose pole to indicate dosage of praziquantel in non-African populations. Our analysis was conducted on height-weight data previously randomly collected from 11 countries. The total number of records was 9,356, of which 7,453 were children aged between 6 and 15 years (school age).
Seven data sets originated from schistosomiasis-endemic countries (including all major non-African endemic countries). Four data sets, from non-endemic countries were, also included in the analysis to further confirm the worldwide validity of the WHO dose pole. Sixteen records (0.17%) were excluded from the analysis as suspected data entry errors since the weight was more than 3 standard deviations from the expected value for height.
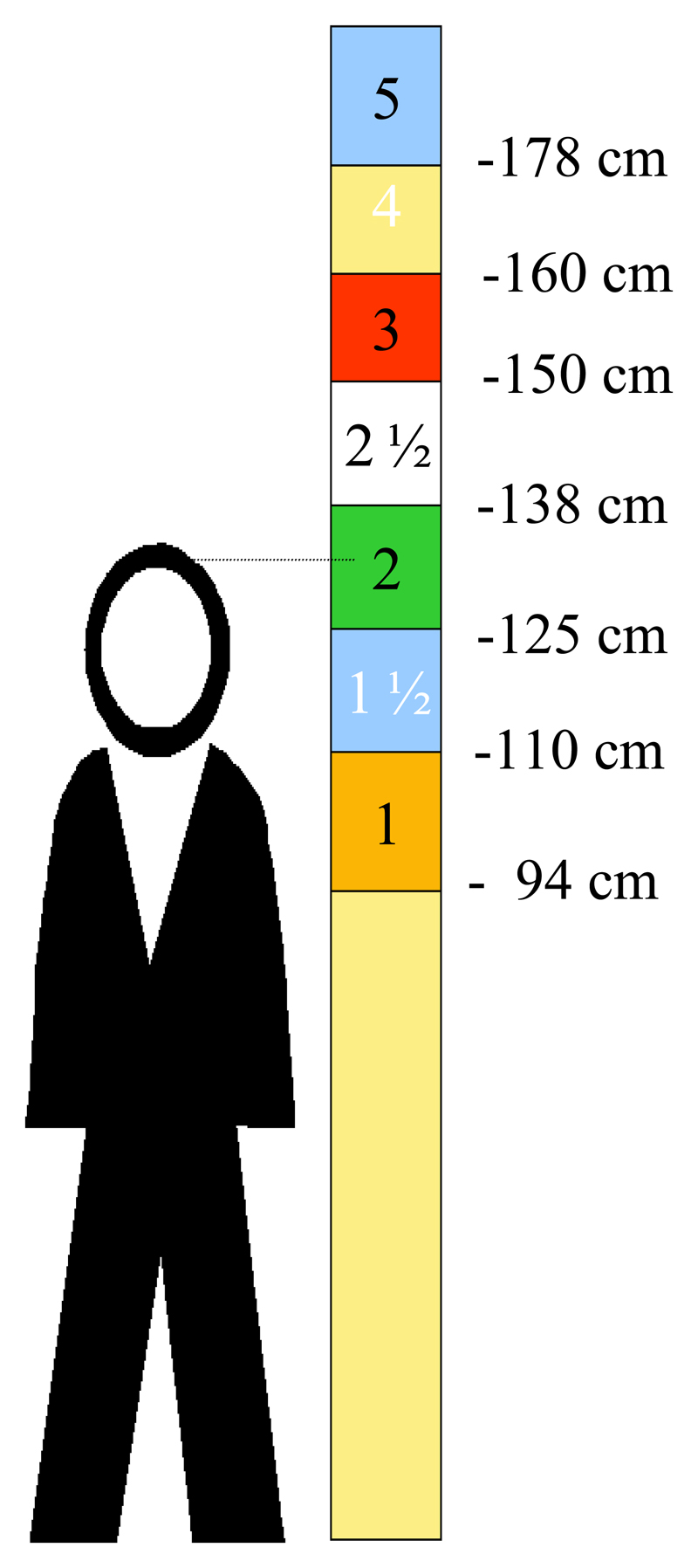
Two height intervals: between 94 cm and 110 cm (corresponding to 1 tablet) and over 178 cm (5 tablets) were added to the WHO pole (Figure 1). These new thresholds were calculated using the same equation used to define the original thresholds between 110 and 178 cm
Figure 1.
The pole should lean vertically against a wall. Each individual is classified in one of the height intervals which corresponds to a number of praziquantel tablets (600 mg). Everyone with a height over 178 cm should be treated with 5 tablets of praziquantel. In the illustrative diagram above, the person needs 2 tablets of praziquantel.
[y = 0.005x2 - 0.7909x + 44.647] .
Each person's height was classified using the pole's intervals and the number of tablets and the total dose (in mg) indicated by the pole was recorded. The individual dosage was then calculated by dividing the total dose by the weight of each individual registered in the data set (in mg/kg).
A dosage between 40 -60 mg/kg was considered optimal (WHO 2004) and a dosage between 30-60 mg/kg was considered acceptable for the significant activities demonstrated by praziquantel at this dosage (Taylor et al 1988).
The average dose provided by the WHO pole was 44.74 mg/Kg (range 42.06 mg/Kg – 47.06 g/Kg). The different dosages of praziquantel calculated using the pole are presented in Table 1.
Table 1.
Dosages of praziquantel that would have been administered to individuals (from 11 countries) using the WHO dose pole for praziquantel to classify their height.
| Data from | Country | Sample size | Age range | Within the pole interval | Average dose (SD) | <30 mg/kg | ≥30 - ≤60 mg/kg |
>60 mg/kg | Minimum dosage received | Maximum dosage received | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 <40 mg/kg | ≥40 ≤60 mg/kg | ||||||||||
| Endemic countries | Brazil | 353 | 7-14 | 353 | 44.5 (5.2) | - | 53 (15%) | 300 (85%) | - | 31 | 60 |
| Cambodia | 3451 | 1-64 | 3300 | 44.8 (6.5) | 18 (0·6%) | 670 (20·3%) | 2598 (78·7%) | 14 (0·4%) | 26 | 69 | |
| China | 532 | 6-12 | 532 | 42.1 (7.3) | 31 (5·8%) | 162 (30·5%) | 338 (63·5%) | 1 (0·2%) | 20 | 67 | |
| Indonesia | 639 | 6-14 | 639 | 43.0 (6.5) | 16 (2·5%) | 125 (19·5%) | 490 (77%) | 8 (1·3%) | 23 | 66 | |
| Malaysia | 712 | 1-10 | 472 | 43.8 (6.4) | - | 117 (24.7%) | 355 (75.3%) | - | 34 | 60 | |
| Oman | 523 | 9-11 | 523 | 45.5 (6.6) | 13 (2·4%) | 59 (11·3%) | 446 (85·3%) | 5 (1%) | 21 | 67 | |
| Yemen | 1461 | 5-18 | 1460 | 45.3 (6.3) | 3 (0·2%) | 191 (13·1%) | 1249 (85·6%) | 17 (1·1%) | 28 | 79 | |
| Sub total Endemic countries | 7671 | 7279 | 44.5 (6.8) | 81 (1·1%) | 1377 (18·9%) | 5776 (79·4%) | 45 (0·6%) | 20 | 79 | ||
| Non-endemic countries | Afghanistan | 198 | 10-12 | 198 | 43.0 (6.3) | 0 | 44 (22·2%) | 154 (77·8%) | 0 | 30 | 56 |
| Bhutan | 263 | 6-17 | 263 | 44.5 (6.5) | 1 (0·3%) | 47 (18·0%) | 209 (79·4%) | 6 (2·3%) | 26 | 67 | |
| Myanmar | 249 | 7-15 | 248 | 47.6 (6.1) | - | 17 (6·8%) | 225 (90·8%) | 6 (2·4%) | 32 | 83 | |
| Seychelles | 975 | 3-18 | 903 | 45.9 (6.9) | 12 (1·3%) | 192 (21·2%) | 687 (76·1%) | 12 (1·3%) | 24 | 64 | |
| Sub total Non-endemic countries | 1685 | 1612 | 45.6 (7.4) | 13 (0·8%) | 300 (18·6%) | 1275 (79·1%) | 24 (1·5%) | 24 | 83 | ||
| TOTAL | 9356 | 8891 | 44.7 (6.9) | 94 (1%) | 1677 (18·9%) | 7051 (79·3%) | 69 (0·8%) | 20 | 83 | ||
| 8728 98·2%* | |||||||||||
p>0.05 compared with result in African populations
The WHO pole estimated an optimal dosage in 79% (C.I. 78·4-80·1) and an acceptable dosage in 98% of cases (C.I. 97·8-98·4). In data from African population the optimal and the acceptable dosages estimated were 81.0% and 98.0% respectively. The results in African and non-African data sets were compared with the Large Sample Normal Test and resulted statistically similar (p>0.05).
Only 1% (94) of the 8,886 individuals would have received a sub-curative dosage, and 0·8% (68) would have received over-dosage. The introduction of the additional intervals between 94 and 110 cm and over 178 cm allowed us to estimate a dose for 753 individuals that would have been excluded by the previous version of the pole.
Most of the individuals classified by the additional intervals where shorter that 94 cm.
This is consistent with the communication of Kabatereine evaluating that the 5-tablets interval was reached in approximately 1% of the cases.
We conclude that the WHO dose pole, which was developed from African data, performs equally well in all the endemic and non-endemic countries for which it was possible to obtain height-weight data sets. Because of the limited number of cases of under-dosage, over-dosage, the minimum and maximum dosage provided, and the safety of the drug at high dosage (Bittencourt et al 1990), we suggest the use of the WHO pole, as a mean to calculate the dose of praziquantel in areas where weighing facilities are not available or not reliable
The least accurate performance of the WHO dose pole was obtained in the data set from China (Iwata et al 2003) composed of data from children in Beijing, 20% of whom were over-weight (Body Mass Index over 95 percentile). Even in this data set the number of children receiving appropriate dose was 94% (C.I. 91·5-95·7) a performance statistically similar to the one obtained in data from Guinea (Montresor et al 2001) for which the pole was originally developed. The new intervals between 94 and 110 cm and over 178 cm now extend the use of the WHO pole to praziquantel distribution campaigns to communities.
Reproducing the present pole in the form of a strip of paper and including it in each container of praziquantel would enormously facilitate the administration of the drug in large-scale interventions.
Acknowledgements
We thank Dr R. Tsuyuoka (WHO Cambodia) and Dr E.M. Christophel (WHO Manila) for facilitating the contacts with the researchers in the Western Pacific, Dr M. Shaban (Ministry of Health, Oman), and Dr A. Sasongko (Yayasan Kusuma Buana, Jakarta, Indonesia), for supplying data from their countries, Dr M. Albonico (Ivo de Carneri Foundation, Milan, Italy) and Médecins Sans Frontières Switzerland for the data from Seychelles and Cambodia. We also thank all the personnel from NGOs, universities and ministries of health involved in the data collection in the 11 countries.
Footnotes
Conflict of interest statement
None declared.
References
- Bittencourt PR, Gracia CM, Gorz AM, Mazer S, Oliveira TV. High-dose praziquantel for neurocysticercosis: efficacy and tolerability. Eur Neurol. 1990;30:229–234. doi: 10.1159/000117352. [DOI] [PubMed] [Google Scholar]
- Hall A, Nokes C, Wen ST, Adjei S, Kihamia C, Mwanri L, Bobrow E, de Graft-Johnson J, Bundy D. Alternatives to bodyweight for estimating the dose of praziquantel needed to treat schistosomiasis. Trans R Soc Trop Med Hyg. 1999;93:653–658. doi: 10.1016/s0035-9203(99)90087-1. [DOI] [PubMed] [Google Scholar]
- Iwata F, Hara M, Okada T, Harada K, Li S. Body fat ratio in urban Chinese children. Pediat Int. 2003;45:190–192. doi: 10.1046/j.1442-200x.2003.01688.x. [DOI] [PubMed] [Google Scholar]
- Montresor A, Engels D, Chitsulo L, Bundy DAP, Brooker S, Savioli L. Development and validation of a “tablet pole” for the administration of praziquantel in sub-Saharan Africa. Trans R Soc Trop Med Hyg. 2001;95:542–544. doi: 10.1016/s0035-9203(01)90034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P, Murare HM, Manomano K. Efficacy of low doses of praziquantel for Schistosoma mansoni and S. haematobium. J Trop Med Hyg. 1988;91:13–17. [PubMed] [Google Scholar]
- WHO. Report of a WHO Expert Committee, Technical Report Series No.912. World Health Organization; Geneva: 2003. Prevention and Control of Schistosomiasis and Soil-Transmitted Helminthiasis. [PubMed] [Google Scholar]
- WHO. Model Formulary. World Health Organization; Geneva: 2004. [Google Scholar]