Abstract
Metabotropic GABAB receptors are known to modulate the activity of voltage-dependent calcium channels. Previously, we have shown that GABAB receptors couple to a non-Gi/o G-protein to enhance calcium influx through L-type calcium channels by activating-protein kinase C in neonatal rat hippocampal neurons. In the current study, the components of this signaling pathway were investigated further. Gαq was knocked down using morpholino oligonucleotides prior to examining GABAB-mediated enhancement of calcium influx. When Gαq G-proteins were eliminated using morpholino-mediated knockdown, the enhancing effects of the GABAB receptor agonist baclofen (10 μM) on calcium current or entry were eliminated. These data suggest that GABAB receptors couple to Gαq to regulate calcium influx. Confocal imaging analysis illustrating colocalization of GABAB receptors with Gαq supports this hypothesis. Furthermore, baclofen treatment caused translocation of PKCα (protein kinase C α) but not PKCβ or PKCε, suggesting that it is the α isoform of PKC that mediates calcium current enhancement. Inhibition of calcium/calmodulin-dependent kinase II (CaMKII) did not affect the baclofen mediated enhancement of calcium levels. In summary, activation of GABAB receptors during development leads to increased calcium in a subset of neurons through Gαq signaling and PKCα activation without the involvement of CaMKII.
Keywords: GABAB receptor, L-type calcium channel, protein kinase C, calcium/calmodulin-dependent protein kinase II, G-protein, Gαq
Graphical Abstract
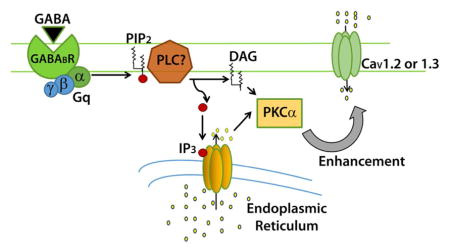
Activation of GABAB receptors in the neonatal rat hippocampus enhance voltage dependent calcium currents independently of Gi/o. In the present study, knockdown of Gαq with morpholino oligonucleotides abolished enhancement of calcium influx and protein kinase Cα was activated by GABAB receptors. Therefore, we hypothesize that GABAB receptors couple to Gq to activate PKCα leading to enhancement of L-type calcium current.
Introduction
GABAB receptors are members of a large group of class C 7 transmembrane G-protein coupled receptors, sharing structural similarities with metabotropic glutamate receptors (Kaupmann et al., 1997). Uniquely, these receptors form heteromers, composed of a GABAB1 and GABAB2 subunit, which dimerize within the ER to be trafficked to the cell membrane. While there are allosteric interactions between the subunits during activation, the ligand binds the GABAB1 subunit, and the G-protein interacts with GABAB2. Functionally, GABAB receptors may be either pre- or postsynaptic, and their activation is considered inhibitory via coupling to Gαi/o G-proteins. There are 3 well-described mechanisms of this inhibition: first, the α subunit of Gαi/o signaling inhibits adenylyl cyclase, reducing cAMP levels and/or protein kinase A (PKA) activation (Nishikawa et al., 1997). Second, calcium channels are directly inhibited by the βγ subunit. This reduces presynaptic neurotransmitter release and postsynaptic dendritic calcium spikes (Chalifoux and Carter, 2011). Finally, GABAB receptor stimulation activates G-protein-coupled inwardly-rectifying potassium (GIRK) channels, again through direct interaction with the βγ subunit (Reuveny et al., 1994; Wickman et al., 1994). In this case, potassium efflux generates inhibitory post-synaptic potentials which hyperpolarize the cell.
While cases of GABAB receptor activation leading to inhibition have been well documented, there are reports demonstrating an enhancement of voltage-dependent calcium channel activity (Shen and Slaughter, 1999; Carter and Mynlieff, 2004; Bray and Mynlieff, 2009, 2011; Park et al., 2010; Im and Rhim, 2012). In the hippocampus, long lasting (L-type) current is facilitated in a subset of cells when GABAB receptors were activated by the GABAB receptor-specific ligand baclofen (Carter and Mynlieff, 2004). This phenomenon is observed early in development, peaking at postnatal day 6 (P6) to P8 (Bray and Mynlieff, 2009) in rats. Furthermore, this GABAB receptor-mediated enhancement of calcium current was not sensitive to pertussis toxin, a known inhibitor of Gαi/o-mediated processes (Bray and Mynlieff, 2011). Our laboratory demonstrated that this enhancement is dependent on protein kinase C (PKC) activation. Enhancement of calcium current was eliminated when global PKC inhibitors were used and treatment of cells with the PKC activator phorbol 12-myristate 13-acetate (PMA) mimicked the effect of activating GABAB receptors. However, participants in the signaling pathway, such as specific G-proteins and isoforms of PKC, have not been previously investigated.
The present study sought to identify components of the GABAB receptor mediated increase in calcium current. Because the effect of calcium current enhancement was not sensitive to pertussis toxin, we hypothesized that GABAB receptors may couple to another G-protein besides Gαi/o. It is not likely a Gαs G-protein, because this pathway initiates PKA signaling. Instead, we hypothesized that the G-protein involved in this signaling pathway was a member of the Gαq family of G-proteins (Gαq, Gα11, Gα14 Gα15/16), which are known to activate PKCs through activation of phospholipase Cβ. To test this hypothesis, a morpholino-induced knockdown strategy was used to inhibit signaling of specific G-proteins.
Next, the identity of the specific isoform of PKC that is involved in the signal transduction pathway was investigated. There are at least 15 isoforms of PKC, separated into 3 families based on their requirements for activation. Conventional PKCs require calcium ions, diacylglycerol (DAG), and a phospholipid such as phosphotidylserine. Novel PKCs require DAG and a phospholipid, but not calcium ions; atypical PKCs require neither DAG nor calcium ions for activation. Since PMA treatment mimics GABAB receptor activation, the isoform was not likely to be atypical. Furthermore, the highest proportion of neurons demonstrating current enhancement occurs at P7. Different PKC isoforms demonstrate different temporal expression, so those isoforms which are highly expressed at P7, and also not of the atypical family, were examined using translocation to the membrane as a measure of activation.
Finally, the involvement of calcium/calmodulin-dependent kinase II (CaMKII) as a component of the signaling cascade was investigated. It has been shown previously that CaMKII is required in other G-protein pathways which result in calcium current enhancement (O-Uchi et al., 2008). In addition, CaMKII is known to phosphorylate L-channels, thereby increasing current (Hudmon et al., 2005; Abiria and Colbran, 2010). Thus, we hypothesized that PKC may activate CaMKII, which in turn phosphorylates the calcium channel. Results presented here provide evidence that the GABAB receptor couples to Gαq in neonatal hippocampal neurons, causing to PKCα translocate to the cell membrane, where it putatively interacts with L-type calcium channels, bypassing the involvement of CaMKII.
Methods
Isolation of hippocampal neurons
All animal protocols were approved by the Marquette University Institutional Animal Care and Use Committee according to guidelines set forth by the National Research Council in the Guide for the Care and Use of Laboratory Animals. Hippocampal neurons were obtained from P6 to P8 Sprague-Dawley rat pups of either sex (Onsite breeding colony, parents from Charles River Laboratories; Mynlieff, 1997). Briefly, pups were anesthetized with 100% CO2 and decapitated. Using sterile technique, the superior regions of the hippocampi were dissected, diced, and incubated in oxygenated PIPES-buffered saline with 0.5% Trypsin XI and 0.01% DNase I (Sigma-Aldrich, St. Louis MO, USA). After a 30 minute incubation at room temperature, the tissue was placed in a 35°C water bath for 60 minutes. After a series of rinses, the tissue was triturated and the cell suspension was plated onto poly-L-lysine coated dishes or glass coverslips (MW 38,500–60,000; Sigma Aldrich, St. Louis, MO, USA) and transferred to a 37°C 5% CO2 water-jacketed incubator. Cultures were maintained a minimum of 20 hours before experiments to allow recovery from enzymatic digestion.
Protein Knockdown
Knockdown of Gαq G-proteins in neuronal cultures was performed with morpholinos delivered by the Endo-Porter delivery system (Gene Tools LLC, Philomath OR, USA). Morpholino oligonucleotides were designed to sterically inhibit translation of Gαq mRNA (Entrez ID: 13591956 NM_031036, 5′ACGCCATGATGGACTCCAGAGTCAT); negative nonsense controls (5′AAACCCGGGTTTACG) were used to insure specificity of the target oligo. Morpholinos were synthesized with a 3′ carboxyfluorescein tag to visualize delivery. Endo-Porter peptide (final concentration 4 μM) and morpholino oligos (final concentration 2 μM) were added one time to the Neurobasal-A media in the culture dishes immediately after dissociation of the cells. Delivery of oligos and cell viability was assessed at 24, 48, and 96 hours post-delivery to insure cargo was delivered and cell health was not negatively impacted.
Western Blot Analysis
Whole hippocampal tissue or neurons from hippocampal cultures were obtained from rat pups age P6–P8. For whole tissue samples, hippocampi were dissected and homogenized in ice-cold buffer with protease inhibitors (250 mM sucrose, 10 mM Tris, 10 mM HEPES, 1 mM EDTA, 1 μg/mL pepstatin, 1 μg/mL leupeptin, 0.5 mg/mL Pefabloc; Sigma Aldrich, St. Louis MO, USA; pH 7.2 with HCl) followed by centrifugation at 3,287g for 10 minutes at 4°C. The supernatant was centrifuged at 41,473g for 30 minutes at 4°C, and the pellet was re-suspended in buffer and stored at −80°C. For cultured cell samples, neurons were scraped from culture dishes in homogenization buffer with protease inhibitors. The sample was centrifuged at 25,401g for 10 minutes at 4°C, and the pellet was re-suspended in 5% of the original volume of buffer to concentrate the sample. The suspension was centrifuged at 3,287g for 10 minutes, the supernatant was retained, and centrifuged at 41,473g for 30 minutes. The pellet was re-suspended in 15% of the original volume of homogenization buffer and stored at −80°C. Protein concentrations were measured at 280 nm with a Biophotometer (Eppendorf, Enfield CT, USA).
NuPAGE® lithium dodecyl sulfate (LDS) sample buffer and reducing agent (Life Technologies, Carlsbad CA, USA) were added to protein samples and the mixture was heated at 70°C for 10 minutes. The protein samples were run on a NuPAGE® Novex 12% Bis-Tris minigel and transferred to a polyvinylidene difluoride membrane (PVDF, 0.45 μM pore size) using NuPAGE® transfer buffer (Life Technologies, Carlsbad CA, USA). Membranes were washed with phosphate buffered saline (PBS, 134.4 mM NaCl, 4.36 mM KCl, 10.56 mM NaHPO4, 1.66 mM NaH2PO4, pH to 7.4 with HCl) and blocked for 2 hours in PBS containing 0.05% Tween, 5% nonfat dry milk, and 0.1% bovine serum albumin at room temperature. Proteins on membranes were labeled with polyclonal rabbit anti-Gαq (anti-GNAQ, 1:500–1:1000, GeneTex catalog# GTX114029, Irvine CA, USA) or polyclonal rabbit anti-Gα11 (1:500, GeneTex catalog# GTX118876, Irvine CA, USA) in blocking solution. After a 90 minute wash in PBS with 0.05% Tween, membranes were incubated with goat anti-rabbit HRP-conjugated secondary antibody (1:1000–1:2500, Pierce, Rockford IL, USA). The SuperSignal West Dura Extended Duration chemiluminescent enhancement kit (Pierce, Rockford IL, USA) was used to visualize the protein bands. The amount of protein loaded was verified by labeling the membranes with anti-β-tubulin antibodies (1:2000, Cell Signaling, Danvers MA). Quantification of bands was performed by measuring the integrated optical density (IOD) for each band using Labworks 4.6 software (UVP, Upland CA, USA). In experiments where expression was knocked down, data were normalized by dividing the IOD of the band labeled with anti-G-protein antibodies with the IOD of the band in the same lane visualized with anti-β-tubulin antibodies.
Electrophysiology
Calcium currents were measured in whole cell voltage clamp mode using a Dagan 3900A patch clamp amplifier (Dagan Corporation, Minneapolis MN, USA), Digidata 1322A acquisition setup, and pClamp 10.0 software (Molecular Devices, Sunnyvale CA). Extracellular recording solution (pH 7.4 with CsOH, 310–320 mOsm/L) contained 10 mM CaCl2, 145 mM tetraethylammonium chloride, 10 mM HEPES, and 1 μM tetrodotoxin (Tocris Bioscience, Ellisville MO, USA). Recording electrodes were pulled from borosilicate glass on a Flaming/Brown Micropipette Puller (model P87, Sutter Instrument Co., Novato CA, USA) to a resistance of 5–9 MT and filled with intracellular solution (140 mM Cs-aspartate, 5 mM MgCl2, 10 mM Cs2EGTA, 10 mM HEPES, 2 mM ATP-Na2, and 0.1 mM GTP; pH 7.4 with CsOH, 300–310 mOsm/L). Cells were held at −80 mV and depolarized to +10 mV with a 300 ms pulse. Whole cell currents were electronically filtered at 1 kHz and digitized at 2 kHz. Linear components of leak current were subtracted posthoc by the passive resistance protocol in pClamp 10.0. The GABAB agonist (RS)-baclofen (Tocris Bioscience, Ellisville MO, USA) was dissolved in HCl, diluted 1:1000 in recording solution to a final concentration of 10 μM and applied to neurons using a U-tube delivery system constructed with PE-10 polyethylene tubing housed in a glass tube. In experiments designed to test the effect of CaMKII inhibition, CaMKII inhibitors cell permeable autocamtide-2 related inhibitory peptide (Ant-AIP-II, IC50 4 nM, catalog#189485, EMD Millipore, Billerica, MA, USA; Ishida et al., 1998) or myristolated calmodulin kinase IINtide (IINtide, IC50 50 nM, catalog#208921, EMD Millipore, Billerica MA, USA; Chang et al., 1998) were also added to the baclofen solution and delivered via U-tube.
Calcium current measurements were taken at the end of the 300 ms depolarizing pulse to maximize the contribution of L-type channels to the total current while minimizing the contribution of N- and P/Q-type channels. A graph of elicited sustained current magnitude versus time was plotted, and a linear regression with 95% confidence interval was constructed to account for run-up or run-down of calcium current (Carter and Mynlieff, 2004; Bray and Mynlieff, 2011). A cell that showed an increase or decrease in sustained calcium current in response to baclofen displayed a current magnitude that fell outside the confidence interval.
Calcium Imaging
Cultured neurons were incubated for one hour in the dark at room temperature in Ringer’s solution (CIR, 154 mM NaCl, 5.6 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, 11 mM glucose, pH 7.4 with NaOH) with 5 μM Fura-2 acetoxymethyl ester (Fura-2 AM, Life Technologies, Carlsbad CA). Cells were rinsed and incubated for 30 minutes at room temperature in CIR without FURA-2 AM to allow for de-esterification. Neurons were depolarized by perfusion of a high potassium solution (100 mM NaCl, 50 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 11 mM glucose, pH to 7.4 with NaOH) to open voltage gated calcium channels. Baclofen (10 μM) in CIR was perfused onto the cells for 15 seconds, followed by baclofen in high potassium solution for 30 seconds. CaMKII inhibitors were added to the CIR/baclofen solution for pretreatment, then added to the high potassium/baclofen solution and applied via gravity. The fluorophore was excited at 340 and 380 nm, and emissions were measured at 510 nm using Slidebook 5.0 software (Intelligent Innovations, Denver CO, USA).
To determine inherent variability of the system, a group of control neurons were treated with high potassium solution 3 consecutive times (N=309). Response #1 and #3 were averaged as the “control” and the percent change with response #2 was calculated as the “pseudo” drug response. The response to the second application of high potassium gave an average of 101.5 ± 13.20 (standard deviation) percent change when compared to the average of the responses to application #1 and #3. During experiments, neurons were stimulated 3 times; the first and third stimulations were high potassium alone, and the middle stimulation was high potassium with another agent. To determine the effect of agent application during stimulation 2, its value was compared to the averaged value of stimulations 1 and 3 (high potassium control stimulations). The percent change with agent treatment when compared to the averaged before and after values was classified as an increase or decrease for a particular cell if the percent change with baclofen in 50 mM potassium solution was greater than two times the standard deviation (2 X 13.20) of the cellular response from the control neurons stimulated three times consecutively with high potassium solution alone.
Immunostaining
To analyze the colocalization of GABAB receptors and Gαq, hippocampi from P6–8 rats were dissected, fixed, frozen and sliced into 20 μM sections. Sections were permeabilized with 0.5% Triton X-100 in PBS for 20 minutes and blocked with 10% goat serum (Invitrogen, Carlsbad, CA) in PBS with 0.05% Triton X-100. The sections were incubated for 2 hours with polyclonal rabbit anti-GNAQ (1:1000, Genetex catalog# GTX114029, Irvine, CA) and monoclonal mouse anti-GABABR1 (1:15, UC Davis Neuromab, catalog# 73–183, Davis CA, USA) in PBS with 0.05% Triton X-100 and 0.1% goat serum. After rinsing, sections were incubated with Dylight® 488-conjugated goat anti-rabbit IgG and Dylight® 550-conjugated goat anti-mouse IgG (Thermo Scientific, Rockford, IL) in PBS with 0.05% Triton X-100 and 0.1% goat serum. After rinsing, mounting medium with DAPI (4′,6-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA) was applied to stain nuclei; the sections were covered with a glass coverslip and sealed. Sections were imaged with a Nikon Perfect Focus Ti-E confocal microscope and NIS Elements imaging software (Nikon Instruments, Melville, NY).
To quantify the colocalization of GABAB receptors and Gαq G-proteins, regions of interest (ROI’s) in the stratum oriens, pyramidal cell layer, and stratum radiatum were drawn for each image. A Pearson’s correlation coefficient (PCC) was determined to quantify the degree of colocalization of the two proteins in each ROI. The PCC measures overlap of pixels for the two fluorophores; the higher the PCC, the more likely that the two proteins are overlapping in that ROI. A PCC was determined for the 3 ROI’s in each image (16 sections taken from 3 animals), then averaged across sections, such that there was a single PCC value for the stratum oriens, pyramidal cell layer, and stratum radiatum, respectively.
To analyze the translocation of PKC, neuronal cultures were treated with 1 μM phorbol-12-myristate-13 acetate (PMA, Calbiochem, La Jolla CA) or 10 μM baclofen for 10 minutes prior to fixation. Cells were fixed in a solution of 4% paraformaldehyde in PBS for 30 minutes at room temperature, permeabilized in PBS with 0.5% Triton X-100 for 20 minutes and blocked with 10% goat serum in PBS with 0.05% Triton X-100 for 45 minutes. The cells were incubated with either monoclonal rabbit anti-PKCα (1:500 dilution, catalog# GTX61153, Genetex, Irvine, CA), polyclonal rabbit anti-PKCβ (1:200 dilution, catalog# E021184, EnoGene Biotech Co., Ltd., New York, NY) or polyclonal rabbit anti-PKCε (1:1000 dilution, catalog# GTX 109028, Genetex, Irvine, CA) overnight at 4°C. Following two washes with PBS and 0.05% Triton X-100, cells were incubated with Dylight® 488-conjugated goat anti-rabbit secondary antibodies (1:500 dilution) for one hour at room temperature. Mounting medium with DAPI was applied for visualization of nuclei, and cells were imaged on a Nikon Perfect Focus Ti-E confocal microscope and NIS Elements imaging software (Nikon Instruments, Melville, NY).
Statistics
Comparisons of the number of cells that exhibited either enhancement of calcium entry with ratiometric imaging or enhancement of calcium current with electrophysiology in cells treated with morpholinos or CaMKII inhibitors were analyzed by a Chi square test. A one-way ANOVA was performed on PCC values obtained for the different regions of the hippocampus using confocal image analysis of protein colocalization followed by Holm Sidak pairwise comparisons. Differences in the number of cells demonstration translocation of different PKC isoforms were analyzed with Fisher’s exact test.
Results
Morpholino oligos inhibit Gαq protein expression
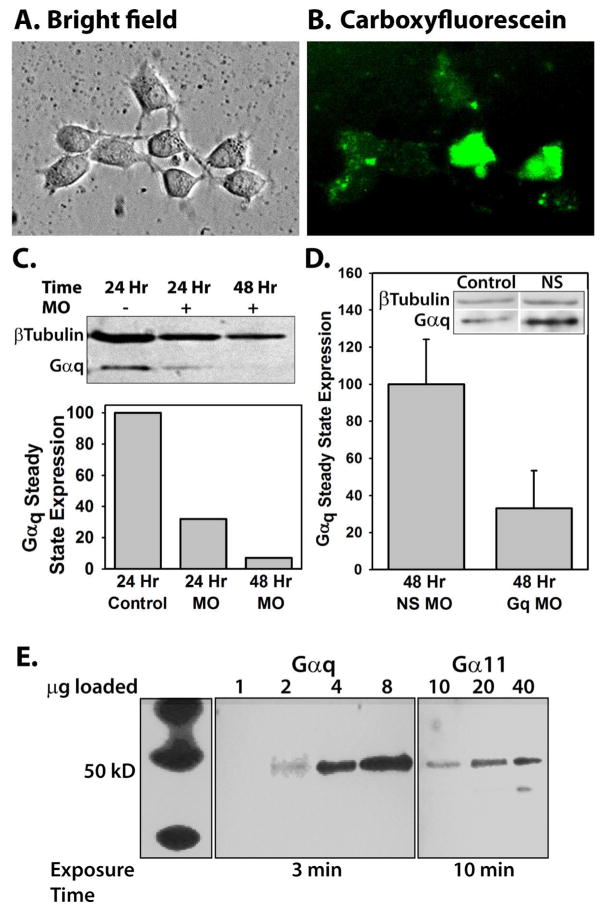
To verify that morpholino oligos were able to penetrate the cell membrane and were not detrimental to cell viability, cultures were treated with Endo-Porter and fluorescein-labeled morpholinos and examined after 24, 48, and 96 hours of incubation. Fig. 1A shows cultures after 96 hour treatment. Cells maintained their characteristic shapes and exhibited growing processes. Fig. 1B illustrates that essentially every cell exhibited some level of cytoplasmic fluorescence. Although fluorescence levels vary, no punctate fluorescence is seen, which would indicate morpholinos unable to exit endosomes. Visible fluorescence requires ten-fold higher concentrations of morpholino than are necessary to produce significant protein knockdown (Gene-tools, 2015). At concentrations ≥ 4 μM of oligos or ≥ 6 μM Endo-Porter, morpholinos began to negatively affect growth and viability of neuronal cultures (data not shown). Thus, 2 μM morpholino and 4 μM Endo-Porter were used in all experiments. To determine the effectiveness of morpholino-induced protein knockdown, neurons were treated with Endo-Porter alone, nonsense morpholinos, or morpholinos against Gαq and Western blotting was performed at various time points after the treatment. Figures 1C and D show the timecourse of Gαq knockdown. As demonstrated in Fig. 1D, preparations treated for 48 hours with morpholinos against Gαq appeared to have less Gαq than preparations treated with nonsense morpholinos. Cultures treated with Endo-Porter alone or nonsense morpholinos demonstrated normal levels of Gαq protein expression (for example, see inset in Fig. 1D). Thus, using the Endo-Porter delivery system, Gαq protein expression in neuronal cultures was reduced without negatively impacting cell health or viability. Due to the relatively long half-life of G-proteins (Derrien et al., 1996), all electrophysiological and calcium imaging experiments were performed following 48 hours incubation with Gαq morpholinos to insure sufficient protein knockdown.
Fig. 1.
A) Differential interference contrast image of a hippocampal culture from a postnatal day 4 rat pup treated with 10 μM control morpholino oligos and 2 μM Endo-Porter peptide, and maintained in culture for 96 hours. B) Fluorescent image of A; oligos were tagged with carboxyfluorescein for visualization. C) Western blot of proteins isolated from either a control culture (lane 1), a culture treated for 24 hours (lane 2) or 48 hours (lane 3) with 2 μM Gαq oligos. Protein bands were visualized with Gαq antibodies and β-tubulin antibodies as a loading control. Gαq band densities were calculated by dividing the integrated optical density of the Gαq band by the integrated optical density of the β tubulin band to control for loading and expressed as percent of control. D) Western blot analysis of 6 different preparations from cultured neurons treated for 48 hours with nonsense oligos (N=3, NS MO) or Gαq oligos (N=3, Gq MO). The data were analyzed as in C, with the average of the nonsense oligo treated preparations defined as 100%. Data are expressed as mean±SEM. The inset is an example of Gαq expression in control tissue compared to cells treated with nonsense morpholinos to demonstrate that the nonsense morpholinos do not cause nonspecific knockdown of Gαq. E) Western blot analysis of Gαq and Gα11 G-protein in the neonatal hippocampus. Analysis was performed using tissue from the superior region of the hippocampus and antibodies against either Gαq or Gα11 proteins. The left panel shows molecular weight markers (same experiment but exposed to film for 1 sec). To visualize bands labeled with Gα11 antibody, 10 times more protein was loaded (right panel) than for lanes labeled with Gαq antibodies (center panel) and the exposure time was increased to 10 minutes, compared with 3 minute exposure for bands labeled with Gαq antibodies.
Gαq protein is more highly expressed than Gα11 in neonatal hippocampus
Gα11 shares 90% sequence similarity with Gαq, and both G-proteins have a ubiquitous distribution pattern (Mizuno and Itoh, 2009). Therefore, it was important to determine whether there could be nonspecificity in the morpholino knockdown of Gαq that may decrease Gα11 as well. Protein preparations from cultured cells treated with Gαq or nonsense morpholinos were analyzed by Western blotting using Gα11 antibodies. If the morpholinos were specific to Gαq, there would be no change in the level of Gα11. However, no Gα11 signal was detected, even in control cultures not treated with morpholinos or Endo-Porter (data not shown). This result was suggestive that either the basal level of Gα11 was too low to detect with Western blotting in preparations of cultured neurons, or that the Gα11 antibody did not recognize its target. To determine if the Gα11 antibody could recognize Gα11 G-protein, protein samples were prepared from the superior region of fresh whole hippocampus so that the amount of protein loaded on the gel could be increased. The Gα11 antibody recognizes its target, but only if 10 times the amount of protein was loaded and the membrane was exposed to film for a longer period of time (Fig. 1E). When a lane loaded with 2 μg of protein was probed with Gαq antibody, the IOD was 7.2. When a lane loaded with 20 μg of protein was probed with Gα11 antibody, the IOD was 5.0 on the same film at the same exposure. These data suggest that the Gαq antibody recognizes its protein target without detecting any Gα11 protein. Thus, while it cannot be ruled out that the Gαq morpholino may nonspecifically knock down Gα11 G-protein, a more likely explanation is that while both G-proteins are present, only Gαq G-protein is knocked down.
Inhibiting Gαq expression abolishes baclofen-mediated enhancement of voltage-dependent calcium entry or current
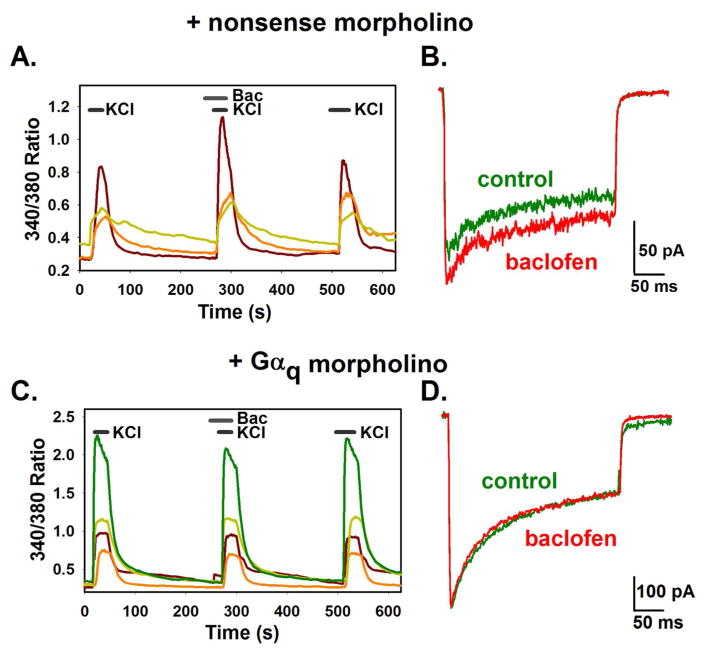
Despite a lack of precedence for the GABAB receptor coupling to a Gαq family member, downstream actions, including PKC activation, were consistent with the actions of a Gαq type G-protein. Therefore, we chose to knock down Gαq and assess whether application of the GABAB receptor agonist baclofen (10 μM) could still lead to calcium current enhancement. Hippocampal neurons were treated with either nonsense or Gαq morpholinos, and enhancement of calcium entry or current was assessed with calcium imaging and electrophysiology. In the nonsense morpholino-treated cells, 6 out of 64 cells (9.37%) demonstrated an enhancement of high Potassium induced calcium entry with baclofen treatment using calcium imaging (Fig. 2A). Of the 207 cells treated with Gαq morpholinos, no cells demonstrated an enhancement of high Potassium induced calcium entry with baclofen (Fig. 2C, Chi square, P < 0.001). Electrophysiology verified this result; in control cells treated with nonsense oligos, 3 out of 25 cells demonstrated an enhancement of sustained calcium current when baclofen was applied (Fig. 2B). In contrast, no cells out of 15 treated with Gαq morpholino oliogos for 48 hours showed any calcium current enhancement (Fig. 2D). This set of experiments demonstrates that knockdown of the Gαq G-protein abolished enhancement of calcium current or entry upon GABAB receptor activation, linking a Gαq family member to this pathway and thus the GABAB receptor itself.
Fig. 2.
Effect of 48 treatment with Gαq morpholino oligos on calcium entry in hippocampal neurons. Neurons were treated for 48 hours with nonsense morpholino oligos (A and B) or Gαq morpholino oligos (C and D). A and C) Increases in intracellular calcium were measured with ratiometric calcium imaging and reported as the 340/380 ratio. Neurons were stimulated with high Potassium, followed by a pretreatment with 10 μM baclofen and baclofen in high potassium (see methods). Each colored line represents a single cell. B and D) Calcium currents were elicited by a 300 ms depolarizing pulse to +10 mV from a holding potential of −80 mV in the absence (green trace) and presence of 10 μM baclofen (red trace).
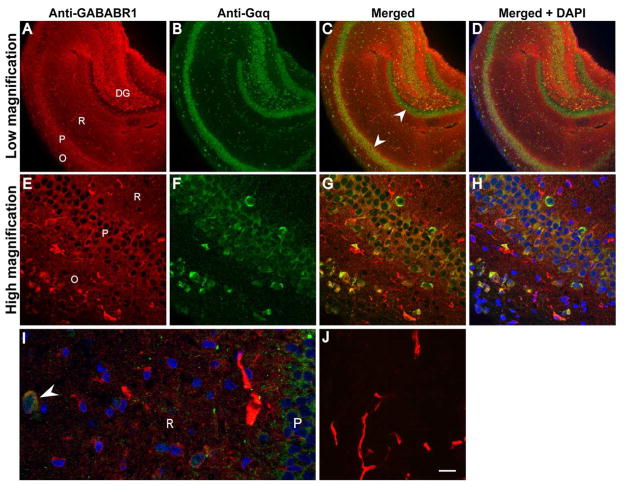
GABAB receptors colocalize with Gαq G-proteins
When hippocampal sections were labeled with anti-GABAB receptor antibodies (red), nearly all areas were labeled, with the exception of the granule cells in the dentrate gyrus (Fig. 3A). At high magnification, there was an absence of nuclear labeling with GABAB antibodies, with a clear delineation between the nuclei and the labeling (Fig. 3E, H). Antibodies against Gαq labeled cells within both the pyramidal cell layer and the granule cell layer (Fig. 3B, F). In addition, there appeared to be individual cell bodies labeled within the stratum oriens and stratum radiatum without any diffuse labeling throughout these layers, as seen with GABAB antibodies. At low magnification, colocalization of the two fluorphores was most evident in the pyramidal cell layer and is absent from the dentate gyrus granule cell layer (see white arrows indicating pyramidal cell layer and granule cell layer; Fig. 3C, D). Quantitative analysis done at high magnification in the superior region of the hippocampus supports qualitative evidence seen at low magnification (Fig. 3C, G). The average Pearson’s Correlation Coefficient (PCC), used to determine colocalization, was 0.125 ± 0.03 (SEM) in the stratum oriens. The PCC was 0.476 ± 0.03 in the pyramidal cell layer and 0.102 ± 0.01 in the stratum radiatum. The colocalization of GABAB receptors and Gαq is highest in the pyramidal cell layer, as suggested by the PCC value; the PCC is significantly higher in the pyramidal cell layer than either the stratum oriens or stratum radiatum (Fig. 3G; One-way ANOVA followed by Holm-Sidak pairwise comparison, P < 0.001). A small number of neurons in both the stratum radiatum and the stratum oriens were labeled by antibodies against Gαq, and some of these neurons also appeared to be labeled with GABAB receptor antibodies (Fig. 3I, arrowhead). These neurons had a relatively high (0.5–0.75) PCC value. The functional significance or physiological identity of these neurons is unknown.
Fig. 3.
GABAB receptors colocalize with Gαq G-proteins in rat hippocampus. Hippocampal sections were labeled with polyclonal rabbit anti-GNAQ and monoclonal mouse anti-GABABR1 followed by Dylight® 488-conjugated goat anti-rabbit IgG (green) and Dylight® 550-conjugated goat anti-mouse IgG (red) for visualization. Nuclei were stained blue with DAPI. Colocalization of the red and green fluorophore appears yellow in the merged images (C, D, G, H, and I). A–D) Low magnification images showing the whole section of hippocampus. E–H) High magnification images showing the pyramidal cell layer (P), the stratum oriens (O), or the stratum radiatum (R). A small number of cells in both the stratum oriens and stratum radiatum appeared to show colocalization of GABAB and Gαq with a high PCC value. I) Expansion of a high magnification image demonstrating colocalization(white arrowhead). J) control sections (high magnification) processed without primary antibodies demonstrate autofluorescence of blood vessels. DG dentate gyrus. Scale bar in panel (high magnification) J = 10 μm,
CaMKII activity is not involved in GABAB receptor-mediated increases in intracellular calcium
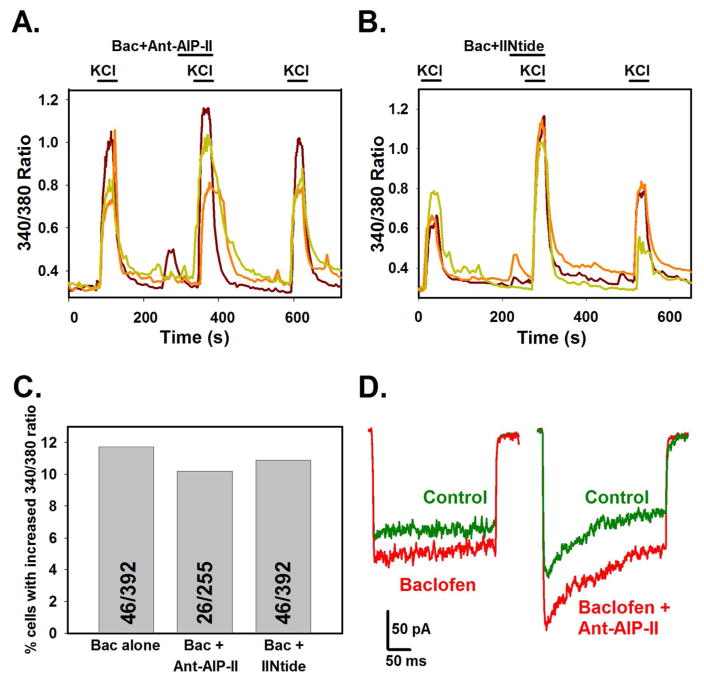
Cultured neurons were depolarized with 50 mM potassium solution, followed by a 50 mM potassium solution containing both 10 μM baclofen and a cell permeable CaMKII inhibitor, Ant-AIP-II (50 nM). Both baclofen and Ant-AIP-II were applied during a 15 second pretreatment period as well as during the high potassium application. Twenty-two of 255 (8.63%) cells imaged continued to demonstrate an increased 340/380 ratio, despite the presence of the inhibitor. When the inhibitor/baclofen solution was washed off, the response returned to original levels (Fig. 4A). Because there have been reports of nonspecific actions of CaMKII inhibitors on voltage-dependent calcium channels (Gao et al., 2006; Karls and Mynlieff, 2013), the result was confirmed using a second cell permeable inhibitor, IINtide (250 nM). A similar result was obtained, where 47 of 432 (10.88%) neurons showed a reversible increase in 340/380 ratio when baclofen was co-applied with IINtide (Fig. 4B). These values were not significantly different from control cells treated with baclofen alone, where 46 of 392 (11.73%) of cells showed increases in the high potassium-stimulated 340/380 ratio (Fig. 4C, Chi square). Electrophysiological recordings were performed in the presence and absence of 50 nM Ant-AIP-II and 10 μM baclofen. The calcium current enhancement displayed when Ant-AIP-II was co-applied with baclofen was not significantly different than that seen with baclofen alone (Fig. 4D). In these experiments, 4 of 23 (17.39%) cells treated with Ant-AIP-II and baclofen displayed a significant increase in calcium current. In control cells, 6 of 27 (22.22%) displayed a significant increase when treated with baclofen alone. Thus, since neither inhibitor blocked the enhancement of calcium entry or current, it is likely that CaMKII is not involved in the pathway where activation of GABAB receptors results in increased calcium entry via L-type channels.
Fig. 4.
The increase in calcium entry due to GABAB receptor activation with baclofen is not affected by CaMKII inhibitors. Neurons were stimulated with high potassium, followed by a pretreatment with 10 μM baclofen with or without a CaMKII inhibitor followed by baclofen and CaMKII inhibitor in high potassium for ratiometric calcium experiments. Each colored line in A and B represents a single cell. Enhancement of calcium influx by baclofen persists in the presence of CaMKII inhibitor Ant-AIP-II (A, 50 nM) or myristolated CaMKII inhibitor IINtide (B, 250 nM). Concentrations chosen were 5–10 fold higher than the reported IC50 values. C) There is no significant difference in number of cells demonstrating a baclofen mediated increase in calcium influx when either CaMKII inhibitor is present in comparison to control cultures without (Chi square, total N is inset in each bar). D) Electrophysiological recording shows persistent calcium current enhancement when baclofen (left) or baclofen and Ant-AIP-II (right) are present (red line) compared to a control trace (green line).
PKCα translocates to the membrane upon GABAB receptor activation
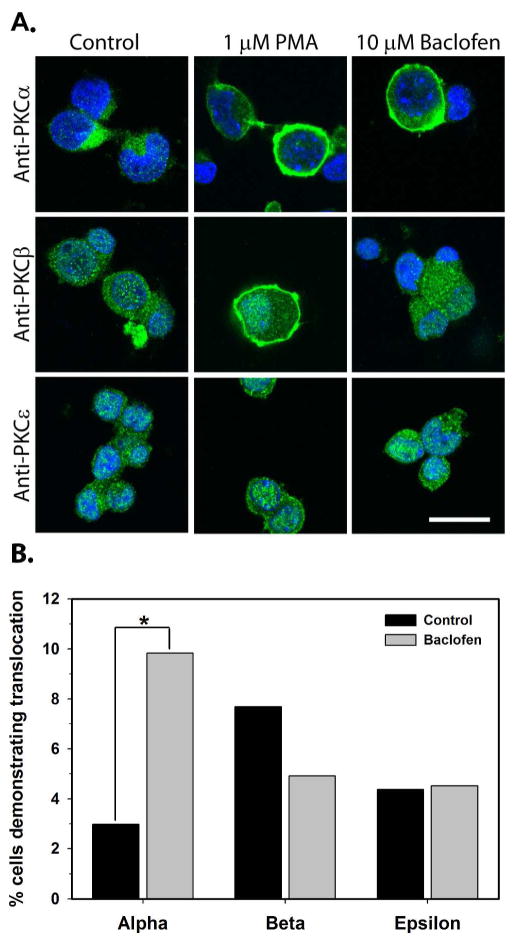
Analysis of immunohistochemical images with confocal microscopy was utilized to determine whether PKCα, PKCβ, or PKCε was translocated from the cytosol to the plasma membrane followed by 10 minutes treatment of cultured hippocampal neurons with baclofen (10 μM). A previous study demonstrated that activation of PKC by phorbol esters mimicked the effect of GABAB receptor stimulation on calcium currents in cultured hippocampal neurons (Bray and Mynlieff, 2011). Therefore, only phorbol ester sensitive isoforms of PKC that are expressed in the early neonatal period were tested (Tanaka and Nishizuka, 1994; Roisin and Barbin, 1997). Cells were either treated with no drugs (unstimulated) or with the phorbol ester PMA (1 μM) to verify that the translocation to the membrane was apparent in confocal images. Fig. 5A demonstrates that both treatment with PMA and baclofen caused translocation of PKCα from the cytosol to the plasma membrane. A small percent of cells in each culture demonstrated spontaneous translocation of all three isoforms of PKC examined in unstimulated cultures (Fig. 5B; 2.99–7.69%, N=104–568). All three isoforms translocated to the plasma membrane upon exposure to 10 minutes of 1 μM PMA in a subset of cells (50.17% of 291 cells for PKCα, 20.34% of 188 cells for PKCβ, 27.59% of 203 cells for PKCε). Only the percentage of cells demonstrating translocation of PKCα with baclofen treatment (9.83%, N=478) was significantly higher than the percent of cells demonstrating spontaneous translocation in unstimulated conditions (2.99%, N=568; Fisher’s exact test, P<0.0001).
Fig. 5.
Activation of GABAB receptors causes translocation of PKCα. A) Cultured hippocampal neurons were treated with control media, the phorbol ester PMA (1 μM) in media, or baclofen (10μM) in media for 10 minutes before immunostaining. The scale bar in the lower right corner is 20 μm. B) The percent cells demonstrating translocation of PKCα, PKCβ, and PKCε in control cultures (black bars) was compared to the percent cells demonstrating translocation of PKCα, PKCβ, and PKCε following stimulation with baclofen (gray bars). N=568, 478, 104, 183, 183, and 177 for the PKCα control, PKCα baclofen, PKCβ control, PKCβ baclofen, PKCε control, and PKCε baclofen, respectively. *P<0.001, Fisher’s exact test.
Discussion
Previous findings in our lab have shown that in neonatal hippocampus, GABAB receptor activation decreases calcium current in a subset of neurons via Gαi/o and enhances L-type calcium current in other neurons via a non-Gi/o G-protein (Carter and Mynlieff, 2004; Bray and Mynlieff, 2011). Despite a variation in percentage of neurons demonstrating calcium current enhancement (approximately 10% of neurons in calcium imaging versus 25% in electrophysiology), knocking down Gαq produced a consistent result–that enhanced calcium entry could be abolished. The variation in number of cells which demonstrate enhancement is likely due to the sampling of every cell within a field during calcium imaging experiments, whereas sampling in electrophysiology allows the experimenter to pick only the healthiest, best looking cells. While this percentage may at first appear low, the hippocampus is composed of no less than 37 different types of inhibitory interneurons (Ascoli, 2013); thus a small percentage could encompass one or more of these 37 types, many of which have clearly delineated functions of their own. While the subset of cells demonstrating a reduction in calcium current has been attributed to inhibition of calcium channels by Gαi/o G-proteins, the pathway describing calcium current enhancement upon GABAB receptor activation has not previously been explored. The data obtained in the current study support a model in which GABAB receptors couple to Gαq activating PKCα to cause enhancement of voltage-dependent calcium current without the involvement of CaMKII (Fig. 6).
Fig. 6.
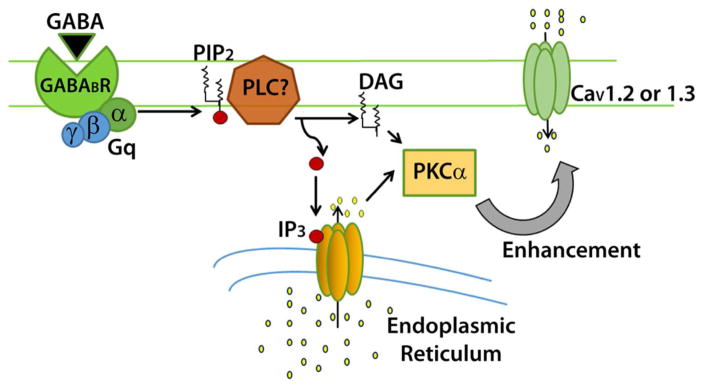
The current model for the pathway which begins with GABAB receptor activation and results in enhanced L-type calcium current. Binding of ligand to the GABAB receptor leads to activation of a Gαq G-protein, which putatively activates phospholipase C. The activity of PLC hydrolyses PIP2 into DAG and IP3, which can activate PKCα. PKCα bypasses CaMKII to lead to changes of the L-type calcium channel such that current is enhanced when the channel opens.
We chose to determine the involvement of Gαq in the pathway because a similar bidirectional pathway involving both Gαq/11 (mediating calcium current increase) and Gαi/o (mediating calcium current decrease) was observed in cardiac myocytes (O-Uchi et al., 2008). In addition, activation of Gαq family members (Gαq, Gα11, Gα14, Gα15/16) are known to activate PKC by stimulating β isoforms of phospholipase C that hydrolyze phosphatidylinositol bisphosphate (PIP2), forming inositol tris-phosphate (IP3) and diacylglycerol (DAG, for review see (Rhee, 2001). DAG and free calcium ions activate PKC, which then translocates from the cytosol to the cell membrane. Here, PKC may potentially phosphorylate the L-type calcium channel, either directly or indirectly through another second messenger. The phosphorylated channel putatively allows more calcium to enter when the cell is depolarized; biophysical changes to the channel have not yet been identified.
To test the model that Gαq is the G-protein that couples to the GABAB receptor, the translation of Gαq was inhibited by morpholinos, synthetic oligonucleotides that bind near the start codon of 5’ mRNA to inhibit binding of the ribosome. Morpholinos have several advantages over siRNA for knockdown of protein expression. siRNA are sensitive to intracellular enzymes, can trigger innate immune responses, and change the methylation state of DNA (Bayne and Allshire, 2005; Judge et al., 2005; Kawasaki and Taira, 2005; Marques and Williams, 2005). None of these things occur with morpholinos (Hudziak et al., 1996) and they are easily taken up into cultured cells through endocytosis stimulated by the Endo-Porter peptide. The lack of enzymatic degradation makes morpholinos particularly useful to knock down proteins with a relatively long half-life, such as G-proteins. Despite these advantages, there is only one report of Endo-Porter mediated morpholino knockdown in mammalian neurons (Chih et al., 2006). Validation of protein knockdown demonstrated here highlights the usefulness of this technique.
Western blot analysis indicated that the morpholinos were efficient in knocking down Gαq in primary hippocampal cultures, but it was important to verify the specificity of this knockdown. The most likely candidate for non-specific knockdown would be Gα11 due to high sequence homology with Gαq. The first 25 coding amino acids (the site of morpholino binding) are 92% identical. Data shown here suggest significantly higher expression of Gαq than Gα11 in neonatal hippocampus, consistent with other reports (Milligan, 1993; Ihnatovych et al., 2002). However, caution must be used in interpreting these results. Because different antibodies were used to detect each protein, it is possible that Gα11 antibodies simply give a weaker signal than Gαq antibodies, even if there is abundant protein. Despite this, we believe that Gαq is expressed to a relatively higher degree than Gα11, both due to previous expression data, and the temporal expression pattern of Gαq. The high expression of Gαq relative to Gα11 makes it the most likely target of the morpholinos. It is also noteworthy that Gαq is most highly expressed during the first postnatal week (Ihnatovych et al., 2002), which corresponds to the time when the highest percentage of neurons demonstrates calcium current enhancement with baclofen (Bray and Mynlieff, 2009).
Knockdown of Gαq eliminated the enhancement of calcium current by GABAB receptors supporting the hypothesis that Gαq is the G-protein that couples to the GABAB receptor. In addition, confocal imaging data demonstrated colocalization of Gαq and GABABRs. Mannoury la Cour et al. used an antibody-capture/scintillation proximity assay to show a lack of coupling between Gαq/11 and GABABRs (2008). However, this work reported the EC50 for baclofen at approximately 50 μM for Gαi/o coupling. These values are much higher than those used by others examining Gαi/o activation by GABABRs (Dolphin and Scott, 1986; Sodickson and Bean, 1996). Furthermore, the enhancing effect of baclofen is seen at high nanomolar concentrations while attenuation requires greater values (Shen and Slaughter, 1999). Thus, it is possible that the assay used by Mannoury la Cour et al. is not sensitive enough to show Gαq/GABABR coupling at low agonist concentrations.
The molecular interaction between the GABAB2 receptor subunit and Gαi/o involves both the second and third intracellular loops of GABAB2 (Duthey et al., 2002; Havlickova et al., 2002). This interaction occurs with the extreme C-terminus of the G-protein (Franek et al., 1999). The lack of sequence homology between Gαi/o and Gαq suggest that Gαq must couple to the GABAR at sites unique to those examined. Identifying the sites of direct interaction between the GABABR and Gαq will support the physiological and colocalization data presented here. Although this is the first example of Gαq coupling to GABAB receptors, coupling to more than one G-protein has been shown for other G-protein coupled receptors, including those that couple to both Gαq and Gαi/o (Offermanns and Simon, 1995; Hawes et al., 2000; Macfarlane et al., 2001). We believe GABAB receptors to have the same ability, coupling to both Gαi/o to mediate classic inhibitory responses and Gαq to mediate calcium current enhancement.
Enhancement of current by GABAB receptors may be mediated through a number of different mechanisms. It is possible that activation of GABAB receptors alone is sufficient to open L-type channels, as shown by Kuczewski et al. (Kuczewski et al., 2011). This report showed that application of 50 μM baclofen without depolarization caused an increase in calcium entry through L-type channels on the cell surface; however we were unable to replicate this effect using the same concentration of baclofen on our P6–P8 cultured hippocampal neurons (data not shown). GABAB receptors have been demonstrated to both increase and decrease potassium currents by interacting with different combinations of auxiliary potassium channel tetramerization domain-containing (KCTD) subunits (Schwenk et al., 2010; Hayasaki et al., 2012). Hayasaki et al. point out that while these auxiliary subunits are most likely responsible for the bidirectional response observed, several factors, including G-protein diversity, may lead to differential effects in GABAB receptor signaling. Based on data presented here, we believe GABAB mediated enhancement of L-type calcium current is due to its coupling to a Gαq G-protein through a novel pathway, rather than the canonical Gαi/o pathway. There are, however, other possible mechanisms for the observed effect. In airway smooth muscle, GABAB receptor stimulation led to activation of Gαi G-proteins and subsequent enhancement of calcium current; this study demonstrated that it was the βγ subunit of the G-protein which activated the same PLC/PKC pathway as Gαq (Mizuta et al., 2011). However, inhibiting Gαi/o signaling with pertussis toxin did not eliminate the observed enhancement in the hippocampus (Bray and Mynlieff, 2009). It is also unlikely that the Gαq βγ subunit may be mediating this effect, because βγ signaling requires a functional α subunit interaction for proper assembly (Smrcka, 2008). Therefore, while it is possible another mechanism exists in the GABAB receptor–L-type channel pathway, the most likely mechanism is GABAB coupling to Gαq.
GABAB receptor mediated activation of PKC is not the only possible pathway involved. Previous studies in our laboratory examined if calcium current enhancement could be mediated by PKA (Bray and Mynlieff, 2011). In this case, Gαs would likely couple to the GABAB receptor, leading to a cascade that would result in PKA, either in addition to or instead of PKC, phosphorylating the calcium channel. However, because enhancement was so clearly eliminated by knockdown of Gαq, and because baclofen caused PKC translocation, this G-protein is involved in mediating current enhancement. Furthermore, results of experiments designed to inhibit PKA with H-89 were ambiguous in determining a role for PKA in the pathway. Thus, while PKA (and therefore Gαs) may be involved, we believe Gαq is responsible for GABAB mediated calcium current enhancement.
The regulation of L-type calcium channels by PKC has been reported to both increase and decrease calcium current, and the effect on current seems to depend on the isoform of PKC involved. For example, phosphorylation by PKCε on the N terminus of CaV1.2 L-type calcium channels inhibits calcium current in cardiac cells (Yue et al., 2004). However, phosphorylation of the C terminus of CaV1.2 by multiple PKC isoforms in HEK293 cells, including PKCα, led to an increase in L-type channel activity (Yang et al., 2009). Stimulation of PKCβII and PKCε with PMA decreased activity of Cav1.3 L-type calcium channels (Baroudi et al., 2006). Thus, identifying the PKC isoform in GABAB-mediated L-type current enhancement provides more detailed information regarding isoform-specific regulation of calcium channels.
We have previously shown that activation of a phorbol ester-sensitive PKC is required for GABAB receptor mediated enhancement of calcium current (Bray and Mynlieff, 2011) and thus, here we sought to identify which isoform was involved. Of the 15 PKC isoforms identified only four have been shown to be robustly expressed in the early neonatal period and only three of these (α, β, and ε) fall into the categories of conventional or atypical PKCs that respond to phorbol ester stimulation. All three isoforms showed some basal level of translocation to the membrane (a measure of activation); however, only PKCα showed a significant increase in number of neurons showing translocation when treated with baclofen. Notably, the proportion of neurons that demonstrated this baclofen-mediated translocation of PKCα is approximately the same as the proportion that undergoes current enhancement.
In an effort to describe as complete a pathway as possible, we examined the involvement of CaMKII in the signal transduction cascade. Evidence of CaMKII activation by PKC and phosphorylation of L-type channels in cardiac myocytes (O-Uchi et al., 2008) suggested that CaMKII may be a component here; however, when neurons were treated with CaMKII inhibtors Ant-AIP-II (50 nM) or myristolated CaMKIIntide (250 nM) plus baclofen, a subset of cells still showed calcium enhancement when depolarized. Although we cannot be sure that the incubation period was sufficient for the inhibitors to penetrate the cells at the applied concentration, the data suggest that CaMKII is not part of the signaling pathway.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NS048900) and a grant from the National Science Foundation (0923041). Additionally, the authors would like to thank Amanda Larson for help with data collection.
Abbreviations used
- Ant-AIP-II
Antennapodia sequence-fused autocamtide-2 related inhibitory peptide II
- CaMKII
calcium/calmodulin-dependent protein kinase 2
- CREB
cAMP response element-binding protein
- DAG
diacylglycerol
- ERK1/2
extracellular signal-regulated kinase 1/2
- GIRK
G-protein coupled inwardly rectifying potassium channel
- IOD
integrated optical density
- IP3
inositol trisphosphate
- IIntide
myristolated calmodulin kinase IINtide
- KCTD
potassium channel tetramerization domain
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PCC
Pearson’s correlation coefficient
- PKA
protein kinase A
- PKC
protein kinase C
- ROI
region of interest
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Abiria SA, Colbran RJ. CaMKII associates with CaV1.2 L-type calcium channels via selected beta subunits to enhance regulatory phosphorylation. J Neurochem. 2010;112:150–161. doi: 10.1111/j.1471-4159.2009.06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA. Hippocampome.org. George Mason University; 2013. Jul 9, v1.0α R 3B. [Google Scholar]
- Baroudi G, Qu Y, Ramadan O, Chahine M, Boutjdir M. Protein kinase C activation inhibits Cav1.3 calcium channel at NH2-terminal serine 81 phosphorylation site. Am J Physiol Hear Circ Physiol. 2006;291:H1614–H1622. doi: 10.1152/ajpheart.00095.2006. [DOI] [PubMed] [Google Scholar]
- Bayne EH, Allshire RC. RNA-directed transcriptional gene silencing in mammals. Trends Genet. 2005;21:370–373. doi: 10.1016/j.tig.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Bray JG, Mynlieff M. Influx of calcium through L-type calcium channels in early postnatal regulation of chloride transporters in the rat hippocampus. Dev Neurobiol. 2009;69:885–896. doi: 10.1002/dneu.20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray JG, Mynlieff M. Involvement of protein kinase C and protein kinase A in the enhancement of L-type calcium current by GABAB receptor activation in neonatal hippocampus. Neuroscience. 2011;179:62–72. doi: 10.1016/j.neuroscience.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter TJ, Mynlieff M. GABAB receptors facilitate L-type and attenuate N-type Ca2+ currents in isolated hippocampal neurons. J Neurosci Res. 2004;76:323–333. doi: 10.1002/jnr.20085. [DOI] [PubMed] [Google Scholar]
- Chalifoux JR, Carter AG. GABAB receptor modulation of voltage-sensitive calcium channels in spines and dendrites. J Neurosci. 2011;31:4221–4232. doi: 10.1523/JNEUROSCI.4561-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang BH, Mukherji S, Soderling TR. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc Natl Acad Sci. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative Splicing Controls Selective Trans-Synaptic Interactions of the Neuroligin-Neurexin Complex. Neuron. 2006;51:171–178. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Derrien A, Langlois D, Saez JM. Expression and regulation of G alpha q and G alpha 11 mRNAs and proteins in bovine adrenal cells. Mol Cell Endocrinol. 1996;121:65–74. doi: 10.1016/0303-7207(96)03852-x. [DOI] [PubMed] [Google Scholar]
- Dolphin AC, Scott RH. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (−)-baclofen. Br J Pharmacol. 1986;88:213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duthey B, Caudron S, Perroy J, Bettler B, Fagni L, Pin JP, Prézeau L. A single subunit (GB2) is required for G-protein activation by the heterodimeric GABAB receptor. J Biol Chem. 2002;277:3236–3241. doi: 10.1074/jbc.M108900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franek M, Pagano A, Kaupmann K, Bettler B, Pin JP, Blahos J. The heteromeric GABA-B receptor recognizes G-protein alpha subunit C-termini. Neuropharmacology. 1999;38:1657–1666. doi: 10.1016/s0028-3908(99)00135-5. [DOI] [PubMed] [Google Scholar]
- Gao L, Blair LaC, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: Reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun. 2006;345:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Gene-tools.com. Assaying Delivery of Morpholinos into the Cytosol Using Fluoresceinated Oligos. 2015 Retrieved from http://www.genetools.com/sites/default/files/DeliveryDiscussion.pdf.
- Havlickova M, Prezeau L, Duthey B, Bettler B, Pin JP, Blahos J. The intracellular loops of the GB2 subunit are crucial for G-protein coupling of the heteromeric gamma-aminobutyrate B receptor. Mol Pharmacol. 2002;62:343–350. doi: 10.1124/mol.62.2.343. [DOI] [PubMed] [Google Scholar]
- Hawes BE, Erin KIL, Green B, O’Neill KIM, Fried S, Graziano MP. The melanin-concentrating hormone receptor couples to multiple G proteins to activate diverse intracellular signaling pathways. Endocrinology. 2000;141:4524–4532. doi: 10.1210/endo.141.12.7833. [DOI] [PubMed] [Google Scholar]
- Hayasaki H, Sohma Y, Kanbara K, Otsuki Y. Heterogenous GABAB receptor-mediated pathways are involved in the local GABAergic system of the rat trigeminal ganglion: Possible involvement of KCTD proteins. Neuroscience. 2012;218:344–358. doi: 10.1016/j.neuroscience.2012.05.038. [DOI] [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudziak RM, Barofsky E, Barofsky DF, Weller DL, Huang SB, Weller DD. Resistance of morpholino phosphorodiamidate oligomers to enzymatic degradation. Antisense Nucleic Acid Drug Dev. 1996;6:267–272. doi: 10.1089/oli.1.1996.6.267. [DOI] [PubMed] [Google Scholar]
- Ihnatovych I, Novotny J, Haugvicova R, Bourova L, Mares P, Svoboda P. Opposing changes of trimeric G protein levels during ontogenetic development of rat brain. Dev Brain Res. 2002;133:57–67. doi: 10.1016/s0165-3806(01)00322-4. [DOI] [PubMed] [Google Scholar]
- Im BH, Rhim H. GABA(B) receptor-mediated ERK1/2 phosphorylation via a direct interaction with Ca(V)1.3 channels. Neurosci Lett. 2012;513:89–94. doi: 10.1016/j.neulet.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Ishida A, Shigeri Y, Tatsu Y, Uegaki K, Kameshita I, Okuno S, Kitani T, Yumoto N, Fujisawa H. Critical amino acid residues of AIP, a highly specific inhibitory peptide of calmodulin-dependent protein kinase II. FEBS Lett. 1998;427:115–118. doi: 10.1016/s0014-5793(98)00405-0. [DOI] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K, Maclachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Karls AS, Mynlieff M. Nonspecific, reversible inhibition of voltage-gated calcium channels by caMKII inhibitor CK59. Cell Mol Neurobiol. 2013;33:723–729. doi: 10.1007/s10571-013-9941-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupmann K, et al. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Taira K. Transcriptional gene silencing by short interfering RNAs. Curr Opin Mol Ther. 2005;7:125–131. [PubMed] [Google Scholar]
- Kuczewski N, Fuchs C, Ferrand N, Jovanovic JN, Gaiarsa JLL, Porcher C. Mechanism of GABAB receptor-induced BDNF secretion and promotion of GABAA receptor membrane expression. J Neurochem. 2011;118:533–545. doi: 10.1111/j.1471-4159.2011.07192.x. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-Activated Receptors. Pharm Rev. 2001;53:245–282. [PubMed] [Google Scholar]
- Mannoury La Cour C, Herbelles C, Pasteau V, De Nanteuil G, Millan MJ. Influence of positive allosteric modulators on GABAB receptor coupling in rat brain: A scintillation proximity assay characterisation of G protein subtypes. J Neurochem. 2008;105:308–323. doi: 10.1111/j.1471-4159.2007.05131.x. [DOI] [PubMed] [Google Scholar]
- Marques JT, Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- Milligan G. Regional distribution and quantitative measurement of the phosphoinositidase C-linked guanine nucleotide binding proteins G11 alpha and Gq alpha in rat brain. J Neurochem. 1993;61:845–851. doi: 10.1111/j.1471-4159.1993.tb03595.x. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Itoh H. Functions and regulatory mechanisms of Gq-signaling pathways. Neurosignals. 2009;17:42–54. doi: 10.1159/000186689. [DOI] [PubMed] [Google Scholar]
- Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA, Emala CW. G i-coupled γ-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol. 2011;45:1232–1238. doi: 10.1165/rcmb.2011-0088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynlieff M. Dissociation of postnatal hippocampal neurons for short term culture. J Neurosci Methods. 1997;73:35–44. doi: 10.1016/s0165-0270(96)02209-1. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Toker a, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997;272:952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Simon MI. G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem. 1995;270:15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- O-Uchi J, Sasaki H, Morimoto S, Kusakari Y, Shinji H, Obata T, Hongo K, Komukai K, Kurihara S. Interaction of alpha1-adrenoceptor subtypes with different G proteins induces opposite effects on cardiac L-type Ca2+ channel. Circ Res. 2008;102:1378–1388. doi: 10.1161/CIRCRESAHA.107.167734. [DOI] [PubMed] [Google Scholar]
- Park HW, Jung H, Choi KH, Baik JH, Rhim H. Direct interaction and functional coupling between voltage-gated CaV1.3 Ca2+ channel and GABAB receptor subunit 2. FEBS Lett. 2010;584:3317–3322. doi: 10.1016/j.febslet.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Reuveny E, Slesinger PA, Inglese J, Morales JM, Iñiguez-Lluhi JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein beta gamma subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisin MP, Barbin G. Differential expression of PKC isoforms in hippocampal neuronal cultures: modifications after basic FGF treatment. Neurochem Int. 1997;30:261–270. doi: 10.1016/s0197-0186(96)00095-2. [DOI] [PubMed] [Google Scholar]
- Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, Tarusawa E, Kulik A, Unger A, Ivankova K, Seddik R, Tiao JY, Rajalu M, Trojanova J, Rohde V, Gassmann M, Schulte U, Fakler B, Bettler B. Native GABA(B) receptors are heteromultimers with a family of auxiliary subunits. Nature. 2010;465:231–235. doi: 10.1038/nature08964. [DOI] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Metabotropic GABA receptors facilitate L-type and inhibit N-type calcium channels in single salamander retinal neurons. J Physiol. 1999;516(Pt 3):711–718. doi: 10.1111/j.1469-7793.1999.0711u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrcka AV. G protein βγ subunits: Central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci. 2008;65:2191–2214. doi: 10.1007/s00018-008-8006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodickson DL, Bean BP. GABA B Receptor-Activated Inwardly Rectifying Potassium Current in Dissociated Hippocampal CA3 Neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Wickman KD, Iñiguez-Lluhl JA, Davenport PA, Taussig R, Krapivinsky GB, Linder ME, Gilman AG, Clapham DE. Recombinant G-protein beta gamma-subunits activate the muscarinic-gated atrial potassium channel. Nature. 1994;368:255–257. doi: 10.1038/368255a0. [DOI] [PubMed] [Google Scholar]
- Yang L, Doshi D, Morrow J, Katchman A, Chen X, Marx SO. Protein kinase C isoforms differentially phosphorylate Ca(v)1.2 alpha(1c) Biochemistry. 2009;48:6674–6683. doi: 10.1021/bi900322a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Qu Y, Boutjdir M. Beta- and alpha-adrenergic cross-signaling for L-type Ca current is impaired in transgenic mice with constitutive activation of epsilonPKC. Biochem Biophys Res Commun. 2004;314:749–754. doi: 10.1016/j.bbrc.2003.12.155. [DOI] [PubMed] [Google Scholar]