Abstract
The pre-metastatic niche — the accumulation of aberrant immune cells and extracellular matrix proteins in target organs — primes the initially healthy organ microenvironment and renders it amenable for subsequent metastatic cell colonization. By attracting metastatic cancer cells, mimics of the pre-metastatic niche offer both diagnostic and therapeutic potential. However, deconstructing the complexity of the niche by identifying the interactions between cell populations and the mediatory roles of the immune system, soluble factors, extracellular matrix proteins, and stromal cells has proved challenging. Experimental models need to recapitulate niche-population biology in situ and mediate in vivo tumour-cell homing, colonization and proliferation. In this Review, we outline the biology of the pre-metastatic niche and discuss advances in engineered niche-mimicking biomaterials that regulate the behaviour of tumour cells at an implant site. Such oncomaterials offer strategies for early detection of metastatic events, inhibiting the formation of the pre-metastatic niche, and attenuating metastatic progression.
1. Introduction
The hypothesis that tumour cells exhibit preferences when metastasizing to organs dates to 1889, when Steven Paget posited in his ‘seed-and-soil’ hypothesis that the spread of tumour cells is not random but governed by regulated processes and is pre-determined1. For example, in breast cancer, metastases tend to form primarily in bone, liver, lung, and brain tissues, which indeed indicates a tropism for specific microenvironments2. This ‘primed’ microenvironment, also known as the pre-metastatic niche (Box 1 and Fig. 1), is involved in promoting tumour cell homing, colonization and subsequent growth at the target organ. Once metastases form at niche sites, the clinical conversation typically changes from curative treatments to the prolongation of progression-free survival. Complications from metastasis are ultimately responsible for 90% of cancer-associated deaths1,2.
Box 1. The Pre-Metastatic Niche.
Kaplan et al first described the formation of a pre-metastatic niche mediated by VEGFR1+ bone marrow-derived hematopoietic progenitor cells4. They also found that in addition to the arrival of VEGFR1+ BMDCs, TSFs increase the proliferation of fibroblast-like stromal cells, which contribute to local deposition of fibronectin. VEGFR1+ niche cells express VLA-4 that binds to fibronectin and allows them to assemble at the site. Most notably, the VEGFR1+ niche cells act as harbingers of organ-specific carcinoma spread. This study was the first demonstration of a microenvironment designed to attract tumour cells to a target organ, and set the stage for future work to discover additional factors that contribute to niche formation.
Different types of metastasizing cancers have preferences for specific organ targets, implying that certain types of cancer are more likely to migrate to and flourish in specific microenvironments 132–134. Metastatic breast cancer cells often populate metastatic niches located at the lungs135, liver136, brain137, bone138, and lymph nodes139, with each tissue featuring various characteristics that promote tumour cell homing, adhesion, and growth. Aberrantly accumulated proteins produced by tumour-subverted stroma (including organ fibroblasts and endothelial cells) such as fibronectin, collagen IV, tenascin, and periostin promote tumour cell adhesion at metastatic sites107,140. Recently, exosomes from pancreatic ductal adenocarcinomas were shown to promote liver pre-metastatic niche formation and increase metastatic burden, demonstrating a role for exosomes in establishing the niche13. Additionally, macrophage-like Kupffer cells present at the liver uptake exosomes and subsequently increase TGF-β and fibronectin expression to recruit BMDCs. The ability for exosomes to interact with resident cells to determine the organotropism at target organs was further demonstrated with specific integrins shown to enable tissue targeting14.
The relative importance and interplay between players of the pre-metastatic niche have yet to be fully understood. This paucity of knowledge is partially due to the young age of the field; however, a significant challenge is posed when attempting to modify the pre-metastatic or metastatic site without experiencing off-target effects. Implanted biomaterials provide an ectopic location that enables deconstruction of the individual cues leading to pre-metastatic niche formation, tumour cell homing, colonization, and proliferation.
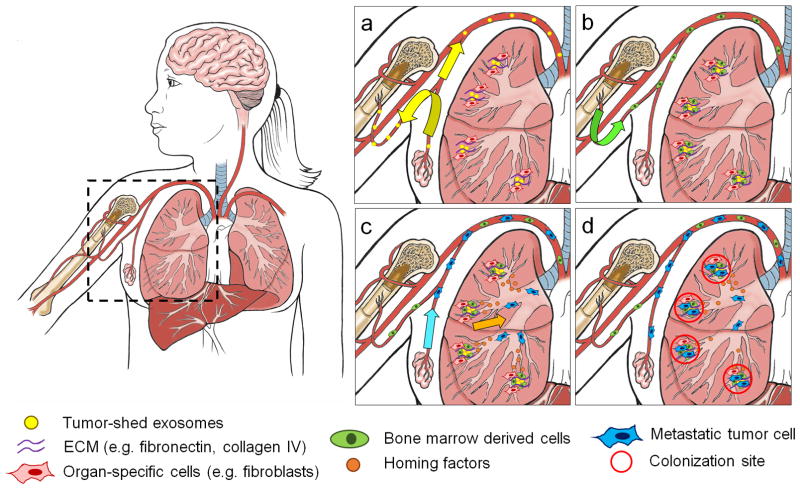
Figure 1.
Formation of the pre-metastatic niche. (a) Hypoxic tumour sheds exosomes (yellow dots) to simultaneously prepare the niche at a target organ by fusing to organ-specific cells (red cells, e.g. fibroblasts) and to stimulate mobilization of BMDCs. Other tumour-secreted factors (e.g. lysyl oxidase) crosslink ECM proteins (purple curves). (b) BMDCs (green cells) accumulate at conditioned sites, adhering to accumulated ECM. (c) BMDCs and other immune cells (e.g. myeloid derived suppressor cells) secrete factors (orange dots) to induce metastatic cell (blue cells) homing to niche sites. (d) Metastatic cells colonize and proliferate at metastatic niche sites. Core illustrations courtesy of Katie Aguado.
The pre-metastatic niche consists of a complex microenvironment that includes inflammatory immune cells, stromal cells, extracellular matrix (ECM) proteins, tumour-secreted exosomes, and homing factors. Tumour-secreted factors and tumour-derived exosomes (Fig. 1a) mobilize and recruit bone-marrow-derived cells (BMDCs) to niches in secondary organs (Fig. 1b), where they interact with the local stroma to create permissive and attractive sites for metastatic cells (Fig. 1c, d)3. The arrival of VEGFR1+ BMDCs to the pre-metastatic site preceded and predicted the arrival of tumour cells4. Other BMDC populations that have also been implicated in the formation of the pre-metastatic niche include CD11b+ myeloid cells, myeloid derived suppressor cells (MDSCs), neutrophils, tumour-associated macrophages, and regulatory T cells5–12. Tumour-secreted factors and exosomes can also directly modify the host stroma to establish a supportive microenvironment13,14. Additionally, fibroblasts, endothelial cells and lung epithelial cells have been associated with the establishment of the pre-metastatic niche via secretion of inflammatory cytokines and chemokines6,9,15. The compelling evidence that pre-metastatic niche formation is required for metastases (Box 1) has prompted biologists and biomedical scientists to elucidate the individual and combinatorial cues that affect cell-niche behaviour, with the ultimate aim of developing effective therapeutic interventions.
Because of the complex molecular pathways promoting metastasis, and their overlap with primary tumour progression, the study of the relative contributions of each pathway in vivo has been challenging. Strategies based on engineered biomaterials have enabled the deconstruction of these complex environments and the study of distinct processes such as primary tumour formation16–18, invasion19, and extravasation20,21, as well as metastatic cell homing22, colonization23 and proliferation24. Studying these processes by using engineered ectopic sites in vivo can therefore provide key information that can ideally complement insights obtained by genetic modification of the tumour or the host (Table 1). Moreover, the design of artificial biomaterials that mimic the pre-metastatic niche opens up translational opportunities, such as the diversion of metastatic cells away from target organs and the development of early detection strategies25,26 that had been unattainable with conventional approaches27.
Table 1.
Strategies for characterizing the pre-metastatic niche and metastasis formation
| Strategy | Advantages | Disadvantages |
|---|---|---|
| Biomaterial pre-metastatic niche mimic |
|
|
| High risk tissue bed biopsy |
|
|
| Tumour cell modification |
|
|
| Genetically engineered mouse models (GEMM) |
|
|
Here, we review strategies for the design and implementation of engineered biomaterials as pre-metastatic niche mimics. We discuss the choice of synthetic or natural materials, the fabrication method, the inclusion of bioactive cues, and material properties such as degradability and porosity, and examine how biomaterials have been used to probe tumour-cell recruitment to an engineered niche and tumour cell behaviour upon arrival to the niche. We also describe how engineered niches may be used as novel detection and therapeutic strategies.
2. Cancer Cell Recruitment to an Engineered Niche
Cancer cells migrate from a primary tumour to a secondary target organ via a progressive cascade of events, including microenvironmental remodelling processes at each stage of disease progression2,28–30. Following the degradation of the tumour basement membrane, cells invade and gain access to the vasculature to become circulating tumour cells (CTCs)31. CTCs respond to chemokine gradients and “home” toward niche microenvironments at a target organ by escaping the vasculature via a process known as extravasation, at which point it is classified as a disseminated tumour cell (DTC)2,30. DTCs may be capable of adhering and colonizing the site, provided they have access to a permissible niche.
Tissue engineering approaches have been used to create biomaterial platforms that mimic properties of the pre-metastatic niche (Table 2). Material options include synthetic degradable materials (e.g. poly(lactic-co-glycolic acid), PLG), synthetic non-degradable materials (e.g. polyacrylamide), and natural materials (e.g. silk)32. Each of these materials can be formed into a porous scaffold structure that supports the retention of loaded factors or cells, integrates within a host tissue upon implantation, facilitates the formation of a defined microenvironment in vivo, and provides an ectopic site for the recruitment of metastatic tumour cells. The choice of material depends on the desired application and feature of the pre-metastatic niche to mimic. For example, in applications where the desired goal is to simulate the bone microenvironment, relatively stiff biomaterials with similar mechanical properties to bone may be advantageous33. These materials can be combined with factors to model the properties of the target organ34 and evaluate the contribution of each factor during homing and colonization27,35.
Table 2.
Materials and biological modifications to engineer the pre-metastatic niche
| Source | Material | Structure | Fabrication Method | Bioactive Modifications | Reference(s) |
|---|---|---|---|---|---|
| Synthetic | Poly(lactic-co-glycolic acid) | Scaffold | Gas foaming | CCL22, MDSCs | 27 |
| Poly(lactic-co-glycolic acid) | Layered scaffold | Microspheres pressed in gas foamed scaffold | Haptoglobin | 35 | |
| Poly(ε-caprolactone) | Scaffold | Gas foaming | None | 26 | |
| Poly(ε-caprolactone) | Scaffold | Gas foaming | Exosomes | 25 | |
| Poly(ε-caprolactone) | Scaffold | Electrospinning | Osteoblasts | 33 | |
| Poly-L-lactic acid | Microparticles | Precipitation | EPO, SDF-1 | 22 | |
| Hydroxyapatite | Nanoparticles in PLG scaffold | Precipitation, gas foaming | Serum protein | 56 | |
| Polyacrylamide | Porous gel | Microfabrication | Collagen I, BMSCs | 23,85 | |
| Polyurethane | Scaffold | Commercially available | MSCs | 86 | |
| Polyallyamine/polystyrene | Microparticles | Layer-by-layer coating | CAFs | 93 | |
| Natural | Bone fragments | Human/mouse sources | Direct harvest | None | 128,129 |
| Silk | Scaffold | Salt leaching | BMP-2 | 34,87,130 | |
| Lung/liver matrix | Coatings | Decellularization | None | 77 | |
| Osteoblast matrix | Mineralized sheets | Decellularization | None | 84 | |
| Collagen | Bulk gel | Embedded in microfluidic chamber | Osteo-differentiated MSCs, Endothelial cells | 109 |
2.1. Immune cell trafficking
Immune cells such as MDSCs36,37, macrophages38, T-cells39,40 and monocytes11 all contribute to niche formation and tumour cell homing. For instance, hypoxic tumour cells secrete lysyl oxidase which crosslinks collagen IV in the lung and facilitates the accumulation of CD11b+ monocytes for niche formation6,41. Purified populations of hematopoietic stem and progenitor cells (HSPCs) were also tracked in vivo using an orthotopic E0771 adenocarcinoma breast tumour model, and shown to differentiate readily into immunosuppressive myeloid cells7. Once immune cells accumulate at distal organs, they secrete a multitude of factors, facilitating the subsequent recruitment and colonization of DTCs42,43. Intravital imaging has been used to show the real-time interactions between immune cells and DTCs undergoing colonization, further elucidating the role of myeloid cell populations in providing a primed harbour for tumour cells at target organs44. While these studies identify the importance of immune cells in the pre-metastatic niche, few studies have investigated the interplay between tumour and immune cells within the niche itself, at least in part due to a lack of suitable research tools.
The host response to an implanted biomaterial includes several blood-material interactions, including the formation of a fibrous capsule consisting of inflammatory immune cells and fibroblasts around the border of the implant45. Although the overall inflammatory response to implanted biomaterials45–48 must be considered, recent studies have elucidated a connection between the immune cells recruited to a biomaterial in the context of cancer and those required to establish a pre-metastatic site (Fig. 2). For example, in an immune competent Balb/C mouse, a variety of inflammatory immune cell populations were recruited to a subcutaneously implanted poly(ε-caprolactone) (PCL) micro-porous scaffold (Fig. 2a, b). During a four-week implantation period, prior to 4T1 breast tumour cell inoculation, Ly6C+F4/80− inflammatory monocytes and CD11c+F4/80− dendritic cells accumulated at the implant site. However, following tumour inoculation, inflammatory monocytes further increased and Gr1hiCD11b+Ly6C− MDSCs accumulated at the scaffold (Fig. 2c), while dendritic cell and F4/80+CD11b+ macrophage populations decreased, thus recapitulating elements of the pre-metastatic niche and enabling tumour cell recruitment (Fig. 2d)26. In a separate study, poly-L-lactic acid (PLA) microspheres have been shown to recruit CD11b+ monocytes to the implant, which subsequently led to enhanced B1F10 melanoma cell homing at the implant site22. These studies indicate tumour cells can home to an implant due to the local foreign body response alone. Importantly, the composition of the immune cells in the foreign body response may differ in tumour-bearing relative to healthy animals, with the foreign body response in tumour-bearing hosts facilitating formation of a pre-metastatic niche at an ectopic location26. Therefore, the emerging mediatory role of the immune system for tumour cell recruitment to an implanted biomaterial has significant implications in the study of metastatic cell trafficking, as well as enabling detection and modulation of tumour cells at user-defined, ectopic locations.
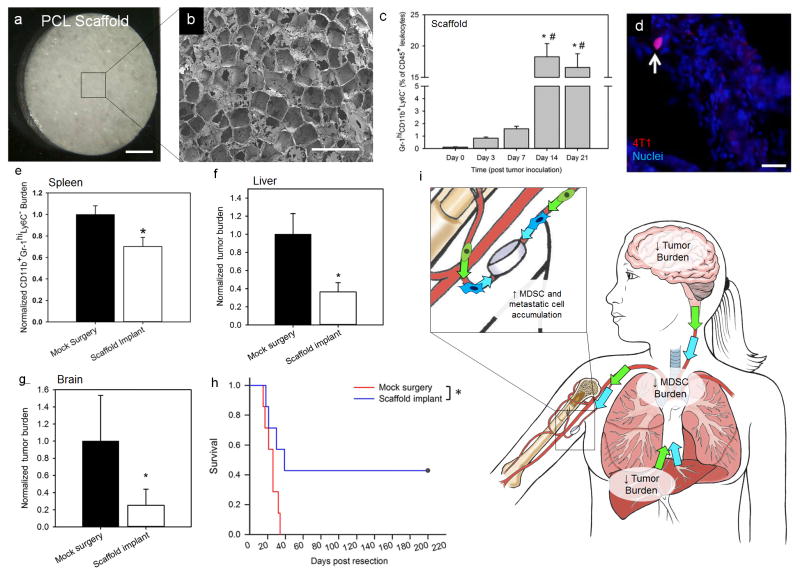
Figure 2.
Myeloid derived suppressor cell (MDSC) and metastatic cell trafficking in a breast tumour bearing mouse implanted with a biomaterial scaffold. (a) Photographic (scale = 1 mm) and (b) scanning electron microscope images (scale = 1 mm) of a microporous PCL scaffold. (c) Tumour progression influences Gr1hiCD11b+Ly6C− MDSCs accumulation at the PCL scaffold implanted subcutaneously in a Balb/C mouse inoculated with 4T1 triple negative breast tumour cells. (d) White arrow indicates tdTomato+ 4T1 cell among a cluster of cells localized to the scaffold. (e) PCL scaffolds reduce MDSC burden in the spleen, which indicates a reduction in systemic MDSC burden. Reduced tumour burden in mice receiving a scaffold implant is observed in the (f) liver and (g) brain (*P < 0.05). (h) Improved survival for tumour-resected mice receiving a scaffold implant relative to mice undergoing a mock surgery (n=7 for each group, *P < 0.05). (i) Proposed mechanism for MDSC and metastatic cell trafficking after scaffold implantation and tumour resection, with reduced MDSC burden in the circulation and reduced tumour burden in the liver and brain, with subsequent increased MDSC and metastatic cell accumulation at the scaffold. Figures reproduced with permission from the American Association for Cancer Research26. Core illustrations courtesy of Katie Aguado.
2.2. Soluble factors
Chemokines and cytokines that actively influence both immune and metastatic cell behaviour play an important role in niche formation (Supplementary Table 1). For example, secreted factors from stromal cells have been implicated in recruiting immune cells associated with the pre-metastatic niche, including SDF-1, TGF-β, S100A-8/9, IL-1, and caveolin-149. Similarly, immune factors including VEGF, IL-6, IL-1, TNFα, CCL17, G-CSF, Bv8, S100 proteins, CCL2 and CCL22 were shown to be overexpressed at target organs during metastatic progression, suggesting a role in pre-metastatic niche formation and tumour cell recruitment10,43,49. Also, VEGF was found to recruit VEGFR1+ BMDCs4, G-CSF mobilizes MDSCs5, Bv8 promotes angiogenesis and mobilization of myeloid cells8, IL-6 is responsible for tumour promoting inflammation50, CCL2 recruits monocytes and BMDCs and facilitates the extravasation of cancer cells12 and TNFα induces S100A8/9 expression which in turn attracts Mac1+ myeloid cells and tumour cells51,52. Additionally, inflammatory Mac1+ monocytes and lung endothelial cells are known to secrete calcium-binding S100A8 and S100A9 factors in the presence of a primary tumour, which initiates the recruitment of additional monocytes to pre-metastatic sites15,51. S100A8 and S100A9 are known to increase formation and activation of invadopodia via p38 signaling, which may promote tumour cell adhesion53. Immature Gr1+CD11b+ MDSCs are responsible for suppressing IFN-γ and increasing inflammatory cytokine expression, and induce the expression of MMP9 in cells to allow for matrix remodeling at the niche54.
3D scaffolds have been used to recruit metastatic melanoma tumour cells in vivo22 and to characterize the role of soluble factors in mediating metastasis to bone tissue in vitro, where tumour cells actively prepare the site for colonization through the release of cytokines such as IL-855,56. Colonizing breast tumour cells produce osteoclast-activating factors, including IL-6, IL-11, and TNFα, to initiate bone resorption and create space for a metastatic lesion57. Subcutaneously implanted chemokine releasing scaffolds have instead been used to compare two factors implicated in melanoma metastasis, SDF-1 and erythropoietin (EPO), with EPO scaffolds having increased tumour cell recruitment22 (Fig. 3a).
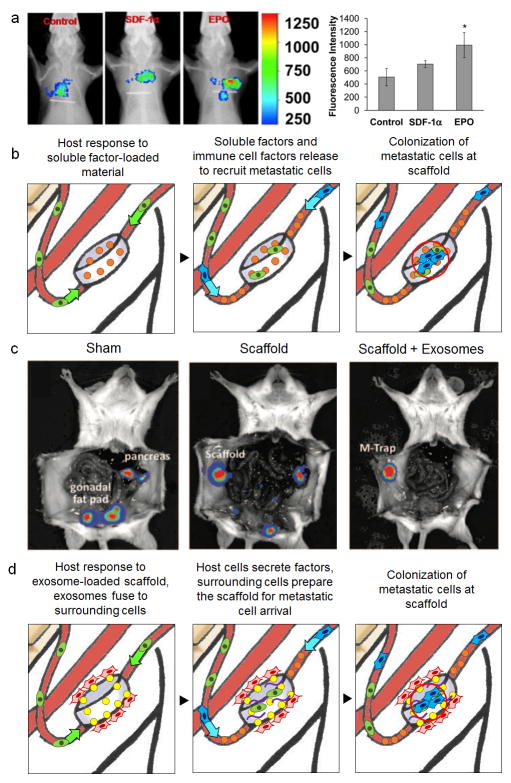
Figure 3.
Biomaterials loaded with soluble factors and exosomes mediate tumour cell homing. (a) Control, EPO and SDF-1α loaded scaffolds recruit labeled B16F10 melanoma cells, quantified using bioluminescence imaging (*P < 0.05). Figures reproduced with permission from Elsevier22. (b) Proposed mechanism for biomaterials pre-loaded with soluble factors of interest in mediating the recruitment of metastatic cells. (c) Exosome-laden scaffolds (M-trap) capture SKOV3 ovarian cancer cells delivered into the peritoneal cavity. Bioluminescence imaging shows control mice with metastasis to the pancreas and gonadal fat pads 1 week after inoculation. Blank scaffolds redirected tumour cells to the implant site, although abdominal metastases were still detected. M-trap scaffolds were able to recruit tumour cells with no visible metastases at 1 week after inoculation. (d) Proposed mechanism for exosomes in mediating preparation of the pre-metastatic niche at the scaffold. Figures reproduced with permission from Oxford University Press25.
A related strategy used virus delivery from biomaterials that encode for chemokines to modulate immune cell trafficking58. Similarly, PLG scaffolds with an immobilized lentivirus encoding for CCL22 modulated the immune cell composition within the scaffold27 resulting in an increase in MDSCs at the niche, which in turn enhanced tumour cell recruitment to the scaffold, similarly to that described in the natural niche36,59. These factors are thought to modulate the chemokines at the local environment; however, altering the trafficking of immune cells locally may potentially have an impact systemically. Collectively, these studies indicate that individual secreted factors have distinct cell recruitment abilities and direct release from the material may enable studies of immune and metastatic cell trafficking (Fig. 3b).
Silk biomaterial scaffolds have been developed to study the impact of BMP-2 on bone metastasis [60]60, since BMP-induced transcriptional pathways are activated during breast and prostate cancer invasion and bone metastasis61,62. Using a layered scaffold system, BMP-2 release stimulated the adhesion of PC3 prostate cancer cells to the scaffold and enhanced the expression of osteogenic markers. More recently, the immune cell secretome from a tumour-bearing mouse, thought to contain factors that mediate the attraction MDA-MB-231 breast cancer cells to engineered niches, was characterized with a combined approach of systems biology and biomaterial techniques35. Using mass spectrometry proteomics, 144 proteins were identified as uniquely secreted by the immune cells from diseased mice and were considered candidate mediators of metastatic cell homing. Using a complementary systems biology approach via measurement of large-scale transcription factor activity and subsequent computational network analysis, the list of candidate factors was narrowed to five. Haptoglobin, a secreted glycoprotein highly abundant in patients with inflammatory diseases and many types of cancer63–67, was identified as a critical mediator of homing. This key discovery then allowed PLG scaffolds to be engineered to specifically release haptoglobin at the site of implantation in orthotopic breast cancer mouse models. These protein-releasing scaffolds recruited significantly more metastatic tumour cells to the implant, compared to blank scaffolds, indicating a role for haptoglobin in breast cancer cell homing. Taken together, elucidation of the ability of secreted factors to recruit tumour cells to engineered niches indicates that these platforms can serve to validate components of the pre-metastatic niche and also facilitate the discovery of novel contributors to pre-metastatic niche formation and function.
2.3. Exosomes
Soluble factors that elicit dramatic changes in immune cell trafficking and the target organ ECM have similarly been characterized in exosomes. Typically 30–150 nm in diameter, exosomes are small membrane vesicles shed from cells68–71 and have been delivered locally as a means to promote tumour cell recruitment. The multi-vesicular bodies carry signalling molecules, secreted and internalized by different cell types, and participate in intracellular communication72,73. Exosomes were shown to prepare organs for tumour cell colonization and mobilize BMDCs to pre-metastatic niche sites3,14. For pre-metastatic niche formation in the lung, RNA molecules from tumour-shed exosomes activated the innate pattern recognition receptor TLR3 in alveolar type II cells, which stimulated neutrophil recruitment to a target site9. As such, tumour derived exosomes incorporated in engineered pre-metastatic niches may further elucidate their role during metastatic progression74. Applying this concept, exosomes in a 3D biomaterial scaffold can serve as a metastatic trap (M-Trap)25. The M-Trap device preferentially captured metastatic cells in both peritoneal and orthotopic models of ovarian cancer (Fig. 3c). As a result, mice implanted with M-Trap scaffolds survived significantly longer than those without implants, with improved overall survival demonstrated upon removal of the implant carrying the metastatic disease. As the collective understanding of how exosomes participate in the preparation of the niche expands13,14, biomaterials may serve as a novel tool to evaluate metastatic cell recruitment to a niche as a function of exosome presence (Fig. 3d).
2.4. Extracellular matrix
Tissue engineering strategies have been utilized to model organ-specific colonization, or organotropism, using in vitro mimics of the organ ECM. Tumour cell lines show a preference for the ECM according to integrin expression75, leading to the hypothesis that integrin binding dictates organotropism, with β1, α2, and α6 integrin subunit expression determining cellular adhesion to lung, liver, and brain ECM mimics76. By taking advantage of the cell surface receptors expressed on tumour cells, tissue-inspired biomaterials (such as bone, brain, and lung ECM) can recapitulate the integrin-mediated phenotypes and provide an “in vitro fingerprint” for cells with predictable metastatic targets. Further studies have determined that tumour-derived exosomes display distinct integrin patterns that preferentially bind to organ-specific cells, thus demonstrating that organotropism can be mediated through “packets” of extracellular signals14.
Tumour cell adhesion has also been tested using decellularized matrices to coat biomaterial scaffolds77. Organ decellularization is a commonplace tissue engineering method used to retain the active components of the matrix78 and has been recently used to assess tumour cell activity on primary tumour79, lung80, and bone-derived matrices81. Using this approach in vivo, decellularized lung and liver matrices obtained from tumour-bearing mice was used to coat micro-porous PCL scaffolds, and upon subcutaneous implantation, was shown to enhance tumour cell colonization at the scaffold (Fig. 4a, b). Interestingly, proteomics was used as a technique to evaluate the matrix composition and identify the unique components of organ-specific pre-metastatic niches77. In this example, myeloperoxidase, an enzyme that generates reactive oxygen species82,83, was determined and validated as a factor that mediates tumour cell colonization using an engineered myeloperoxidase-coated PCL scaffold. Another study investigated metastatic breast cancer cell colonization using scaffolds seeded with primary human osteoblasts to prepare a mineralized bone ECM mimic84. The myofibrillar network produced by seeded osteoblasts, investigated using scanning electron microscopy, was found to be comparable to the assembly of trabecular bone tissue. Atomic force microscopy was also used to measure the detachment force of various breast cancer cells as a measure of tumour cell adhesion to the engineered sites. Tumour cells seeded on the human bone mimic revealed gene expression changes in osteopontin, consistent with tumour cells colonizing bone tissue in vivo. Taken together, these results demonstrate the ability of combinatorial approaches to recapitulate elements of the in vivo niche84, and represent a highly controllable platform to study these interactions.
Figure 4.
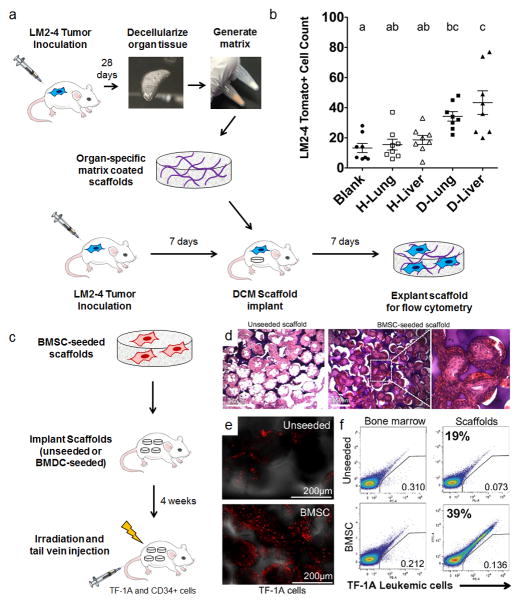
Modelling organotropism using ECM- or BMSC-functionalized scaffolds. (a) Decellularized lung and liver matrix from healthy and diseased mice inoculated with tdTomato-tagged LM-2 lung/liver targeting breast tumour cells was used to coat PCL scaffolds, and scaffolds were implanted subcutaneously in tumour-inoculated mice to detect differences in tumour cell colonization as a function of matrix coatings. Mouse image drawn by Katie Aguado and reproduced with permission from Nature Publishing Group35. (b) Matrix-coated scaffolds from diseased lungs and livers recruited more cells relative to blank and healthy coating controls as assessed by flow cytometry. Groups with different letters are significantly different (P < 0.05). Figures reproduced with permission from Elsevier77. (c) Delivery of multipotent BMSCs (CD44+, CD106+, CD14−, CD34−, CD45−, CD73+, and CD105+) on scaffolds recruit TF-1A leukemia cells to an implant site. (d) Images of H&E stained tissue sections of subcutaneously implanted 3D microfabricated polyacrylamide scaffolds (unseeded vs. BMSC seeded, scale bars = 250 μm). (e) Homing of intravenously transplanted human TF-1A cells to unseeded vs. BMSC seeded scaffolds. Confocal images of scaffolds show significantly more stained TF-1A cells arriving to BMSC-seeded scaffolds 6 hours after injection (scale bars = 250 μm). (f) Flow cytometric analysis of labeled TF-1A cells at the bone marrow vs. implanted scaffolds. FACS analysis suggests there were approximately twice as many cells at BMSC-seeded scaffolds relative to unseeded scaffolds. Figures reproduced freely under open access from the National Academy of Sciences85.
2.5. Manipulation of cell populations
A variety of cell types have been implicated in pre-metastatic niche formation, yet manipulation techniques (using, for example, transgenic strategies, antibody depletion, or adoptive transfer) can affect a population systemically. Alternatively, biomaterial scaffolds can be used as in vivo implants to recreate defined conditions. For example, the most successful approach, where cell transplantation has facilitated cancer cell recruitment, is a bone marrow niche mimic recreated by the transplantation of human bone marrow stromal cells on silk (Fig. 4c, d)34, BMDCs on polyacrylamide23,85, or mesenchymal stem cells on polyurethane86. These cells were initially cultured on the engineered scaffold (Table 2) in vitro and, upon implantation, the niches were able to recruit human breast cancer cells23,34,87, as well as erythroleukemia (Fig. 4e, f)85, acute myeloid leukemia 86, and prostate cancer cells23,88. Interestingly, studies suggest the frequency of capturing tumour cells using scaffolds seeded with BMDCs may correlate with the frequency of CTCs in the blood23. In sum, cell-laden materials are capable of capturing tumour cells at an ectopic site using animal models of both hematological and metastatic carcinoma origin.
Aside from bone marrow mimics, tissue engineered constructs have been used to deliver stromal cells (e.g. neutrophils, fibroblasts, lymphatic endothelial cells, or osteoblasts) at target organs where they provide a permissive microenvironment for human breast cancer cell colonization89,90. Local fibroblasts that participate in the formation of pre-metastatic niches become cancer-supportive through the secretion of growth factors and ECM remodeling proteins91. In a model of ovarian and colorectal peritoneal metastasis, cancer-associated fibroblasts (CAFs) were encapsulated within alginate/gelatin microparticles (500–700 μm in diameter), coated with a membrane composed of polyelectrolytes to retain the CAFs and prevent degradation. Once implanted in the intraperitoneal space of nude mice, CAFs and CAF-secreted ECM were found to be key in the formation of peritoneal niches for metastasis92. Injection of MP-CAFs into the peritoneal cavity redirected cancer cells to the microparticles and resulted in a biomimetic trap that prolonged animal survival93. Similarly, MDSCs have been harvested from spleens of mice and seeded onto PLG scaffolds prior to implantation in an orthotopic model of breast cancer27 using highly metastatic, brain-tropic MDA-MB-231BR cells94. MDSCs were retained on the scaffold after implantation, and recruited significantly more tumour cells to the implant site relative to blank scaffolds. Recent protocols have also been developed to engineer humanized bone tissue using electrospun PCL-tricalcium phosphate scaffolds seeded with human osteoblastic cells to mimic clinical bone metastases95. Using the bone mimic within humanized mouse models, the study modelled several stages of the human bone metastatic cascade, including spontaneous metastasis from orthotopic prostate tumours, systemic metastasis, and local bone colonization.
3. Tumour cell behaviour at engineered niches
Once a DTC adheres to and grows within a niche in the target organ, the cell is said to have colonized the organ. Colonization has been associated with specific genetic changes, including a mesenchymal-to-epithelial (MET) transition. In contrast to the EMT transition during invasion, MET is the process by which tumour cells return to their epithelial-like state to form a distant tumour mass. MET is typically characterized by gene expression studies, generally showing a return to E-cadherin expression and down-regulation of vimentin96. Metastatic colonization is also mediated by the activity of specific transcription factors, including decreased transforming growth factor β/mothers against decapentaplegic homolog 3 (TGFβ/SMAD3) canonical signalling activity, and the loss of the paired related homeobox factor (PRRX1) activity, both potent EMT inducers97,98. As DTCs successfully colonize the target organ, proliferation at the metastatic site may occur based on cues received from the pre-metastatic niche2. The cues involved are still largely unknown; however, there is evidence that the perivascular niche, as well as sprouting and stable endothelial networks, regulate dormancy through control of thrombospondin 1 (TSP-1), TGF-β, periostin, tenascin, versican, and fibronectin, all factors previously implicated in the pre-metastatic niche99. Without proper activation, tumour cells may undergo apoptosis at the target organ, remain dormant at the metastatic site for up to several years, or continue circulating through the body100–102,103.
The inability of DTCs to grow at a metastatic site104–106, a part of metastatic inefficiency, has been modelled using in vitro colonization experiments where the presence of specific ECM proteins can activate dormant CTCs back into a proliferative state. Using a 3D basement membrane culture system, solitary tumour cells can remain dormant due to cell cycle arrest through elevated abundance of cyclin dependent kinase inhibitor proteins p16 and p2724,107. In addition, the proliferation rates of a variety of breast cancer cell lines, measured using 2D and 3D basement membrane gels, pointed to signs of dormancy in the 3D culture in vitro. However, the introduction of fibronectin to the 3D culture environment enhanced proliferation rates of dormant cells and increased cytoskeletal rearrangements, consistent with a static to dynamic switch in phenotype.
The effect of tissue paracrine signalling on metastatic cells, as determined using 3D co-culture systems, can also be used to refine pre-metastatic niche models108. For instance, to recreate MDA-MB-231 tumour cell extravasation, the bone pre-metastatic niche was recently reproduced in 3D using a microfluidic platform consisting of osteo-differentiated mesenchymal stem cells embedded in a collagen gel lined with endothelial cells109. Likewise, 3D collagen gels containing human lung adenocarcinoma cells, lung fibroblasts, and macrophages were used to track matrix metalloproteinase 1 (MMP-1) and VEGF production in different culture conditions (e.g. hypoxia)110. Co-culture systems on a silk scaffold of human breast adenocarcinoma cells with osteoblast-like cells and mesenchymal stem cells have also resulted in enhanced migration, adhesion and drug resistance111. When compared to the same cells co-cultured in 2D on standard tissue culture plastic, the study further reported phenotypic changes in the niche osteoblasts, including decreased proliferation and mineralization, concomitantly to enhanced tumour cell activity111. A similar study was performed where the LNCaP cells were embedded in poly(ethylene glycol) (PEG) hydrogels and cultured with PCL scaffolds pre-seeded with human osteoblasts112. Following microarray analysis of cells obtained from two engineered scaffolds, the study revealed that paracrine signalling between cancer cells and osteoblasts altered the expression patterns of genes associated with homing and colonization (such as S100A6), compared to mono-culture controls.
4. Translational opportunities for pre-metastatic niche mimics
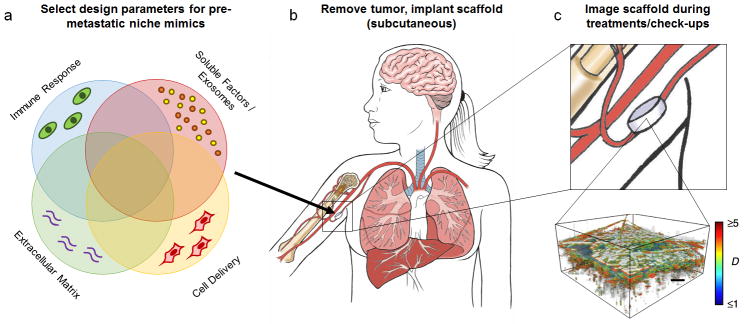
Implantable niches may serve as oncomaterials, a term we propose and define as biomaterials for oncology that enable the detection and the treatment of cancer metastasis (Fig. 5a, b). In a clinical setting, the probability of a tumour spreading to target organs has been shown to correlate with tumour size; for example, breast cancer tumours less than 1 cm in diameter have a lower risk of metastasis113. Detection strategies to map metastatic spread mostly rely on whole body imaging modalities such as Positron Emission Tomography (PET), x-ray Computed Tomography (x-ray CT) and Magnetic Resonance Imaging (MRI); however, the initial cell clusters are beyond the resolution of these imaging systems. These limitations are particularly problematic for highly aggressive cancers that follow a parallel progression model, where tumour cell dissemination and colonization occurs during the undetectable stages of the primary disease114.
Figure 5.
Proposed detection strategy for metastatic breast cancer. (a) Pre-metastatic niche oncomaterials may be designed from a variety of parameters, including the natural immune response to the implant, soluble factor delivery, extracellular matrix, and cell delivery. Parameters may be tuned depending on the cancer or the needs for a specific patient for designing the most effective oncomaterial. (b) After removal of the primary tumour, a biomaterial scaffold may be implanted subcutaneously, ideally before metastasis occurs. (c) Regular imaging at check-ups may be performed during the patient’s course of treatment. When using ISOCT, the shape factor (D) may be used to quantify microstructural alterations at the scaffold due to the arrival of metastatic tumour cells (scale bar = 200 μm). ISOCT image reproduced with permission from Nature Publishing Group27. Core illustrations courtesy of Katie Aguado.
4.1: Materials for metastatic cell detection
The early detection of rare CTCs in the blood may enable earlier treatments for metastatic cancer115, which has motivated the continued development of nanomaterials to isolate and characterize CTCs116. To date, genetic screening of tumour biopsy samples has been the most common approach for identifying biomarkers to developing personalized therapies. However, the progression of a cancer from a neoplastic or dysplastic lesion to metastasis is increasingly understood as the result of continued evolutionary pressure that dictates mutations of its genetic and molecular landscape. This dynamic behaviour is the primary factor responsible for the emergence of therapeutic-resistant clones and challenges the development of personalized therapies. For these reasons, techniques to capture, characterize, and culture CTCs are intended to complement primary tumour biopsy analysis and provide a comprehensive disease description for individual patients31,116,117. CellSearch for example, an FDA approved, commercially available CTC enrichment system, enables reliable detection of CTCs in blood samples from metastatic cancer patients118. Most notably, ex vivo culture of CTCs in conjunction with in vitro biomaterial mimics of the pre-metastatic niche have facilitated the capture, culture, and study of CTCs119–121.
CTCs are isolated from blood, whereas cells found within the pre-metastatic niche mimics have left the vasculature and may represent a distinct cell population with distinct prognostic value. Despite advances in ex vivo detection, CTCs may remain in the circulation for years, and those captured in blood samples may therefore not be representative of tumour cell populations capable of homing and colonization31,101,122, because the detection of CTCs does not indicate the existence of permissive niches. Recently, biomaterial scaffolds for the early detection of cancer metastasis27 were reported in an orthotopic mouse model of breast cancer. Micro-porous PLG scaffolds were implanted, either subcutaneously or in the intraperitoneal fat, and tumour cells populated these scaffolds prior to their colonization at common organ sites (i.e. lung, liver, and brain). Interestingly, using inverse spectroscopic optical coherence tomography (ISOCT)123, unique microstructural alterations were detected at the scaffold due to tumour cell arrival, allowing for a non-invasive and label-free detection method of metastatic colonization. This type of scaffold technology, coupled with ISOCT or other imaging techniques, may enable a viable method for early detection during low metastatic tumour burden (Fig. 5c). In a translational setting, these scaffolds alone, or modified with ECM proteins or cytokine delivery, could provide direct access to actively colonizing tumour cells for patient-specific phenotypic and genomic analyses.
4.2: Early intervention for metastatic cell capture can enhance survival
Implantable scaffolds have been shown to significantly increase survival in mouse models of metastasis. For instance, micro-porous PCL scaffolds have increased survival of immune competent mice inoculated with 4T1 metastatic breast cancer cells26. This scaffold provided a site for early detection and acted as a “sink” for metastatic tumour cells (Fig. 2d) and myeloid derived suppressor cells (Fig. 2e). As a result, the scaffold reduced the average tumour burden in the liver and brain (Fig. 2f, g). A post-surgical model of breast cancer metastasis was then used to investigate the impact of the scaffold on survival where the primary tumour was removed following the localization of tumour cells in the scaffolds. 40% of scaffold-implanted mice survived the tumour resection procedure past 200 days relative to sham controls where survival did not exceed 30 days (Fig. 2h). The study suggested that increased survival may result from a decreased burden of MDSCs present at the primary tumour and spleen of scaffold-implanted mice (Fig. 2i). Therefore, the study implicates that biomaterials designed to reduce the overall generation of MDSCs during metastatic disease progression or divert them to an ectopic location may impact survival. Similarly, exosome impregnated scaffolds drastically changed the pattern of peritoneal ovarian cancer metastasis by redirecting the vast majority of tumour cells to the implant (Fig. 3c)25, which resulted in a significant survival benefit for mice that received an implant (mean survival of ~200 days compared to ~120 days). Additionally, removal of the implant after focalization of the disease to the biomaterial further enhanced survival (~310 days mean survival).
4.3: Opportunities for metastasis detection platforms
Although recent evidence suggests that pre-metastatic niche models enable the early detection and treatment of metastatic disease27, open questions remain regarding the efficacy of these platforms when compared to other emerging metastasis detection technologies. Additional technologies for metastasis detection include exosome detection and CTC enumeration (Table 3). Both platforms are part of a larger initiative to utilize liquid biopsies to gain more information about a patient’s disease state, evolving molecular features, and response to therapy118,124. Advantages to liquid biopsy strategies for detection include the ease of sample collection and ability to collect multiple samples over the course of a patient’s treatment. While liquid biopsies have shown promise in these areas, they also have distinct disadvantages that could potentially be circumvented by pre-metastatic niche mimics. For example, exosome detection is likely to be less sensitive than CTC detection due to exosome heterogeneity and their presence in large numbers also in healthy patients68,125. Similarly, the presence of CTCs indicates the risk for metastasis but does not indicate the presence of permissive microenvironments in organs for these cells to home and colonize. These considerations show specific advantages for the use of pre-metastatic niche mimics (Table 3), however, there are also potential issues associated with the clinical use of these devices, such as the overall safety of creating a site for metastatic cells to home that will need to be evaluated thoroughly in clinical trials. Future methods may provide complementary implementations of these techniques to provide a more comprehensive evaluation on the metastatic state of a patient.
Table 3.
Risks and opportunities of detection platforms for metastasis
In a clinical setting, the choice of material (Table 2) is critical for designing a functional implantable device for recruiting and detecting metastatic cells. For example, materials such as PLG are susceptible to hydrolytic degradation, thus limiting the amount of time the material can remain in a patient. The scaffold should ideally maintain its structure for several months during a patient’s treatment, given that metastasis may occur on a timescale from months to years126. Polymer scaffold degradation is usually desired for tissue engineering applications where host cells eventually replace the material, but degradation is likely undesirable for long-term implantable metastatic detectors. Non- or semi-degradable materials could be used to fabricate implantable scaffolds less susceptible to hydrolytic degradation32. The material should also elicit an appropriate inflammatory response at the implant site to initiate the recruitment of metastatic cells, and should be amenable to harvesting intact populations of tumour cells for downstream analysis77. Additionally, scaffold porosity increases the interior surface area for blood vessel and immune cell infiltration to provide tumour cells access to the scaffold32. Material selection is paramount for successful translation of pre-metastatic niche mimics as oncomaterials.
Thus far, no clinical trials have been initiated for the application of biomaterial pre-metastatic niche mimics. Although several biomaterials utilized as pre-metastatic niche mimics are already FDA approved for use in human patients (Table 2), limitations in imaging tumour cell arrival at the implant remain. ISOCT is a practical approach for detecting the nanostructural alterations due to tumour cell arrival123, however, the penetration depth that is associated with this optical technique will need to be enhanced for translation to a clinical setting. Imaging technologies, such as ultrasound127, are already available in the clinic and may be implemented for tumour cell detection at a scaffold. Even though safety remains an open question, the future for pre-metastatic niche mimics as oncomaterials remains promising.
5. Opportunities and Conclusions
Pre-metastatic niche mimics offer the ability to identify and validate critical factors leading to metastatic cell colonization at an ectopic site. Roles of inflammatory immune cells, secreted factors, exosomes, ECM proteins, and delivered cells have been evaluated using niche mimics to determine contributions to metastatic cell homing and colonization. Furthermore, the capture of early metastatic cells at a pre-defined site may enable early detection of metastatic cell dissemination. The development of novel imaging modalities, or the engineering of probes to label colonizing tumour cells may enable real-time tracking of tumour cells or vascular leakiness at the niche during the evolution of the disease. Capturing tumour cells at an ectopic site could potentially reduce the burden of disease in solid organs and provide an extended window of time over which a therapeutic intervention may succeed. The use of oncomaterials supplemented with current therapeutic strategies such as surgery and chemotherapy may serve as a disruptive technique for combating metastasis. Extending beyond the concept of capturing tumour cells, scaffolds may be bioengineered to manipulate other types of circulating niche components, including exosomes and immune cells that reflect disease (e.g., MDSCs). Furthermore, future work in the genetic profiling of captured metastatic cells at implanted niches may lead to the identification of the types of cells arriving at the scaffold (e.g. tumour stem cells, EpCAM+ cells), which may in turn guide the discovery of targets to treat metastasis based on the disease biology. In conclusion, the successful integration of pre-metastatic niche components in biomaterials can enable the discovery of biomarkers and other molecular cues leading to metastasis, and could be developed further as diagnostic and therapeutic platforms.
Supplementary Material
Acknowledgments
We thank Katie Aguado for figure illustrations. B.A.A. and G.G.B. are both recipients of a National Science Foundation Graduate Research Fellowship. Financial support for this manuscript was provided by the National Institutes of Health and the National Cancer Institute (R01 CA173745).
Footnotes
Author Contributions
B.A.A., G.G.B., and L.D.S. wrote and edited the manuscript. B.A.A. prepared figures. B.A.A. and G.G.B. prepared tables. S.S.R. and J.S.J. edited and provided important consultations for the manuscript.
Competing Financial Interests
No conflicts of interest exist.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 2.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 3.Peinado H, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chafe SC, et al. Carbonic anhydrase IX promotes myeloid-derived suppressor cell mobilization and establishment of a metastatic niche by stimulating G-CSF production. Cancer Res. 2015;75:996–1008. doi: 10.1158/0008-5472.CAN-14-3000. [DOI] [PubMed] [Google Scholar]
- 6.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giles AJ, et al. Activation of Hematopoietic Stem/Progenitor Cells Promotes Immunosuppression Within the Pre-metastatic Niche. Cancer Res. 2016;76:1335–1347. doi: 10.1158/0008-5472.CAN-15-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowanetz M, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci U S A. 2010;107:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, et al. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell. 2016;30:243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Olkhanud PB, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian BZ, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sceneay J, et al. Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72:3906–3911. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 13.Costa-Silva B, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 16.Gill BJ, West JL. Modeling the tumor extracellular matrix: Tissue engineering tools repurposed towards new frontiers in cancer biology. J Biomech. 2014;47:1969–1978. doi: 10.1016/j.jbiomech.2013.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Infanger DW, Lynch ME, Fischbach C. Engineered culture models for studies of tumor-microenvironment interactions. Annu Rev Biomed Eng. 2013;15:29–53. doi: 10.1146/annurev-bioeng-071811-150028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu L, Mooney DJ. Biomaterials and emerging anticancer therapeutics: engineering the microenvironment. Nat Rev Cancer. 2016;16:56–66. doi: 10.1038/nrc.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CC, George SC. A three-dimensional in vitro model of tumor cell intravasation. Integr Biol (Camb) 2014;6:603–610. doi: 10.1039/c3ib40170g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeon JS, et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A. 2015;112:214–219. doi: 10.1073/pnas.1417115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeon JS, Zervantonakis IK, Chung S, Kamm RD, Charest JL. In vitro model of tumor cell extravasation. PLoS One. 2013;8:e56910. doi: 10.1371/journal.pone.0056910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko CY, et al. The use of chemokine-releasing tissue engineering scaffolds in a model of inflammatory response-mediated melanoma cancer metastasis. Biomaterials. 2012;33:876–885. doi: 10.1016/j.biomaterials.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bersani F, et al. Bioengineered implantable scaffolds as a tool to study stromal-derived factors in metastatic cancer models. Cancer Res. 2014;74:7229–7238. doi: 10.1158/0008-5472.CAN-14-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barkan D, et al. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Fuente A, et al. M-Trap: Exosome-Based Capture of Tumor Cells as a New Technology in Peritoneal Metastasis. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SS, et al. Enhanced Survival with Implantable Scaffolds That Capture Metastatic Breast Cancer Cells In Vivo. Cancer Res. 2016;76:5209–5218. doi: 10.1158/0008-5472.CAN-15-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azarin SM, et al. In vivo capture and label-free detection of early metastatic cells. Nat Commun. 2015;6:8094. doi: 10.1038/ncomms9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 29.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 31.Krebs MG, et al. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol. 2014;11:129–144. doi: 10.1038/nrclinonc.2013.253. [DOI] [PubMed] [Google Scholar]
- 32.Holzapfel BM, et al. How smart do biomaterials need to be? A translational science and clinical point of view. Adv Drug Deliver Rev. 2013;65:581–603. doi: 10.1016/j.addr.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Thibaudeau L, et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis Model Mech. 2014;7:299–309. doi: 10.1242/dmm.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreau JE, et al. Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 2007;67:10304–10308. doi: 10.1158/0008-5472.CAN-07-2483. [DOI] [PubMed] [Google Scholar]
- 35.Aguado BA, et al. Secretome identification of immune cell factors mediating metastatic cell homing. Sci Rep. 2015;5:17566. doi: 10.1038/srep17566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Edwards CM, Mundy GR. Gr-1+CD11b+ myeloid-derived suppressor cells: formidable partners in tumor metastasis. J Bone Miner Res. 2010;25:1701–1706. doi: 10.1002/jbmr.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma SK, et al. Pulmonary alveolar macrophages contribute to the premetastatic niche by suppressing antitumor T cell responses in the lungs. J Immunol. 2015;194:5529–5538. doi: 10.4049/jimmunol.1403215. [DOI] [PubMed] [Google Scholar]
- 39.Monteiro AC, et al. T cells induce pre-metastatic osteolytic disease and help bone metastases establishment in a mouse model of metastatic breast cancer. PLoS One. 2013;8:e68171. doi: 10.1371/journal.pone.0068171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan W, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox TR, et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature. 2015;522:106–110. doi: 10.1038/nature14492. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013;91:411–429. doi: 10.1007/s00109-013-1021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Headley MB, et al. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016 doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51:239–240. 242, 244. doi: 10.2144/000113754. passim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Franz S, Rammelt S, Scharnweber D, Simon JC. Immune responses to implants - a review of the implications for the design of immunomodulatory biomaterials. Biomaterials. 2011;32:6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 48.Mikos AG, McIntire LV, Anderson JM, Babensee JE. Host response to tissue engineered devices. Adv Drug Deliv Rev. 1998;33:111–139. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell. 2016;30:668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Rutkowski MR, et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hiratsuka S, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 52.Tomita T, Sakurai Y, Ishibashi S, Maru Y. Imbalance of Clara cell-mediated homeostatic inflammation is involved in lung metastasis. Oncogene. 2011;30:3429–3439. doi: 10.1038/onc.2011.53. [DOI] [PubMed] [Google Scholar]
- 53.Rafii S, Lyden D. S100 chemokines mediate bookmarking of premetastatic niches. Nat Cell Biol. 2006;8:1321–1323. doi: 10.1038/ncb1206-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan HH, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krishnan V, Vogler EA, Sosnoski DM, Mastro AM. In vitro mimics of bone remodeling and the vicious cycle of cancer in bone. J Cell Physiol. 2014;229:453–462. doi: 10.1002/jcp.24464. [DOI] [PubMed] [Google Scholar]
- 56.Pathi SP, Lin DD, Dorvee JR, Estroff LA, Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32:5112–5122. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang Y, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 58.Gower RM, et al. Modulation of leukocyte infiltration and phenotype in microporous tissue engineering scaffolds via vector induced IL-10 expression. Biomaterials. 2014;35:2024–2031. doi: 10.1016/j.biomaterials.2013.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon H, et al. Development of an in vitro model to study the impact of BMP-2 on metastasis to bone. Journal of Tissue Engineering and Regenerative Medicine. 2010;4:590–599. doi: 10.1002/term.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katsuno Y, et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene. 2008;27:6322–6333. doi: 10.1038/onc.2008.232. [DOI] [PubMed] [Google Scholar]
- 62.Feeley BT, et al. Overexpression of noggin inhibits BMP-mediated growth of osteolytic prostate cancer lesions. Bone. 2006;38:154–166. doi: 10.1016/j.bone.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 63.Andersen CB, et al. Structure of the haptoglobin-haemoglobin complex. Nature. 2012;489:456–459. doi: 10.1038/nature11369. [DOI] [PubMed] [Google Scholar]
- 64.Fujita K, et al. Serum fucosylated haptoglobin as a novel prognostic biomarker predicting high-Gleason prostate cancer. Prostate. 2014;74:1052–1058. doi: 10.1002/pros.22824. [DOI] [PubMed] [Google Scholar]
- 65.Pompach P, et al. Site-specific glycoforms of haptoglobin in liver cirrhosis and hepatocellular carcinoma. Mol Cell Proteomics. 2013;12:1281–1293. doi: 10.1074/mcp.M112.023259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun L, et al. Combination of haptoglobin and osteopontin could predict colorectal cancer hepatic metastasis. Ann Surg Oncol. 2012;19:2411–2419. doi: 10.1245/s10434-011-2177-2. [DOI] [PubMed] [Google Scholar]
- 67.Takeda Y, et al. Fucosylated haptoglobin is a novel type of cancer biomarker linked to the prognosis after an operation in colorectal cancer. Cancer. 2012;118:3036–3043. doi: 10.1002/cncr.26490. [DOI] [PubMed] [Google Scholar]
- 68.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thakur BK, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wendler F, et al. Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene. 2017;36:877–884. doi: 10.1038/onc.2016.253. [DOI] [PubMed] [Google Scholar]
- 72.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 73.Becker A, et al. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30:836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brinton LT, Sloane HS, Kester M, Kelly KA. Formation and role of exosomes in cancer. Cell Mol Life Sci. 2015;72:659–671. doi: 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barney LE, et al. A cell-ECM screening method to predict breast cancer metastasis. Integr Biol (Camb) 2015;7:198–212. doi: 10.1039/c4ib00218k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aguado BA, et al. Extracellular matrix mediators of metastatic cell colonization characterized using scaffold mimics of the pre-metastatic niche. Acta Biomater. 2016;33:13–24. doi: 10.1016/j.actbio.2016.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu WD, et al. Development of an Acellular Tumor Extracellular Matrix as a Three-Dimensional Scaffold for Tumor Engineering. Plos One. 2014;9 doi: 10.1371/journal.pone.0103672. ARTN e103672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mishra DK, et al. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann Thorac Surg. 2012;93:1075–1081. doi: 10.1016/j.athoracsur.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Villasante A, Marturano-Kruik A, Vunjak-Novakovic G. Bioengineered human tumor within a bone niche. Biomaterials. 2014;35:5785–5794. doi: 10.1016/j.biomaterials.2014.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kubala L, et al. The potentiation of myeloperoxidase activity by the glycosaminoglycan-dependent binding of myeloperoxidase to proteins of the extracellular matrix. Biochim Biophys Acta. 2013;1830:4524–4536. doi: 10.1016/j.bbagen.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 83.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ARS.2009.2538. [DOI] [PubMed] [Google Scholar]
- 84.Taubenberger AV, Quent VM, Thibaudeau L, Clements JA, Hutmacher DW. Delineating breast cancer cell interactions with engineered bone microenvironments. J Bone Miner Res. 2013;28:1399–1411. doi: 10.1002/jbmr.1875. [DOI] [PubMed] [Google Scholar]
- 85.Lee J, et al. Implantable microenvironments to attract hematopoietic stem/cancer cells. Proc Natl Acad Sci U S A. 2012;109:19638–19643. doi: 10.1073/pnas.1208384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaiselbuh SR, Edelman M, Lipton JM, Liu JM. Ectopic human mesenchymal stem cell-coated scaffolds in NOD/SCID mice: an in vivo model of the leukemia niche. Tissue Eng Part C Methods. 2010;16:1523–1531. doi: 10.1089/ten.tec.2010.0179. [DOI] [PubMed] [Google Scholar]
- 87.Seib FP, Berry JE, Shiozawa Y, Taichman RS, Kaplan DL. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials. 2015;51:313–319. doi: 10.1016/j.biomaterials.2015.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Holzapfel BM, et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials. 2014;35:4108–4115. doi: 10.1016/j.biomaterials.2014.01.062. [DOI] [PubMed] [Google Scholar]
- 89.Lee E, et al. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nature Communications. 2014;5 doi: 10.1038/ncomms5715. ARTN4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413-+. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 92.De Boeck A, et al. Differential secretome analysis of cancer-associated fibroblasts and bone marrow-derived precursors to identify microenvironmental regulators of colon cancer progression. Proteomics. 2013;13:379–388. doi: 10.1002/pmic.201200179. [DOI] [PubMed] [Google Scholar]
- 93.De Vlieghere E, et al. Tumor-environment biomimetics delay peritoneal metastasis formation by deceiving and redirecting disseminated cancer cells. Biomaterials. 2015;54:148–157. doi: 10.1016/j.biomaterials.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 94.Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 95.Martine LC, et al. Engineering a humanized bone organ model in mice to study bone metastases. Nat Protoc. 2017;12:639–663. doi: 10.1038/nprot.2017.002. [DOI] [PubMed] [Google Scholar]
- 96.Hugo H, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- 97.Ell B, Kang Y. Transcriptional control of cancer metastasis. Trends Cell Biol. 2013;23:603–611. doi: 10.1016/j.tcb.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ocana OH, et al. Metastatic colonization requires the repression of the epithelial-mesenchymal transition inducer Prrx1. Cancer Cell. 2012;22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 99.Ghajar CM, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 101.Meng S, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–8162. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 102.Naumov GN, et al. Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res. 2002;62:2162–2168. [PubMed] [Google Scholar]
- 103.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luzzi KJ, et al. Multistep Nature of Metastatic Inefficiency. The American Journal of Pathology. 1998;153:865–873. doi: 10.1016/s0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weiss L. Metastatic inefficiency. Adv Cancer Res. 1990;54:159–211. doi: 10.1016/s0065-230x(08)60811-8. [DOI] [PubMed] [Google Scholar]
- 106.Weiss L. Metastatic inefficiency: intravascular and intraperitoneal implantation of cancer cells. Cancer Treat Res. 1996;82:1–11. doi: 10.1007/978-1-4613-1247-5_1. [DOI] [PubMed] [Google Scholar]
- 107.Barkan D, Green JE, Chambers AF. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46:1181–1188. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Arrigoni C, Bersini S, Gilardi M, Moretti M. In Vitro Co-Culture Models of Breast Cancer Metastatic Progression towards Bone. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17091405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bersini S, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35:2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu XQ, Kiefl R, Roskopf C, Tian F, Huber RM. Interactions among Lung Cancer Cells, Fibroblasts, and Macrophages in 3D Co-Cultures and the Impact on MMP-1 and VEGF Expression. PLoS One. 2016;11:e0156268. doi: 10.1371/journal.pone.0156268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Talukdar S, Kundu SC. Engineered 3D Silk-Based Metastasis Models: Interactions Between Human Breast Adenocarcinoma, Mesenchymal Stem Cells and Osteoblast-Like Cells. Advanced Functional Materials. 2013;23:5249–5260. doi: 10.1002/adfm.201300312. [DOI] [Google Scholar]
- 112.Sieh S, et al. Paracrine interactions between LNCaP prostate cancer cells and bioengineered bone in 3D in vitro culture reflect molecular changes during bone metastasis. Bone. 2014;63:121–131. doi: 10.1016/j.bone.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 113.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 114.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 115.Esmaeilsabzali H, Beischlag TV, Cox ME, Parameswaran AM, Park EJ. Detection and isolation of circulating tumor cells: principles and methods. Biotechnol Adv. 2013;31:1063–1084. doi: 10.1016/j.biotechadv.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 116.Yoon HJ, Kozminsky M, Nagrath S. Emerging role of nanomaterials in circulating tumor cell isolation and analysis. ACS Nano. 2014;8:1995–2017. doi: 10.1021/nn5004277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature Reviews Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 118.Riethdorf S, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 119.Bichsel CA, et al. Diagnostic microchip to assay 3D colony-growth potential of captured circulating tumor cells. Lab Chip. 2012;12:2313–2316. doi: 10.1039/c2lc40130d. [DOI] [PubMed] [Google Scholar]
- 120.Lee J, Kohl N, Shanbhang S, Parekkadan B. Scaffold-integrated microchips for end-to-end in vitro tumor cell attachment and xenograft formation. Technology (Singap World Sci) 2015;3:179–188. doi: 10.1142/S2339547815500065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yu M, et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science. 2014;345:216–220. doi: 10.1126/science.1253533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bednarz-Knoll N, Alix-Panabières C, Pantel K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Research. 2011;13:1. doi: 10.1186/bcr2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yi J, Backman V. Imaging a full set of optical scattering properties of biological tissue by inverse spectroscopic optical coherence tomography. Opt Lett. 2012;37:4443–4445. doi: 10.1364/OL.37.004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126:1208–1215. doi: 10.1172/JCI81135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tauro BJ, et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12:587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frangioni JV. New technologies for human cancer imaging. J Clin Oncol. 2008;26:4012–4021. doi: 10.1200/JCO.2007.14.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nemeth JA, et al. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res. 1999;59:1987–1993. [PubMed] [Google Scholar]
- 129.Xia TS, et al. Bone metastasis in a novel breast cancer mouse model containing human breast and human bone. Breast Cancer Res Treat. 2012;132:471–486. doi: 10.1007/s10549-011-1496-0. [DOI] [PubMed] [Google Scholar]
- 130.Kwon H, et al. Development of an in vitro model to study the impact of BMP-2 on metastasis to bone. J Tissue Eng Regen Med. 2010;4:590–599. doi: 10.1002/term.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cristofanilli M, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 132.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017 doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- 135.Maru Y. The lung metastatic niche. J Mol Med (Berl) 2015;93:1185–1192. doi: 10.1007/s00109-015-1355-2. [DOI] [PubMed] [Google Scholar]
- 136.Azizidoost S, et al. Hepatic metastatic niche: from normal to pre-metastatic and metastatic niche. Tumour Biol. 2015 doi: 10.1007/s13277-015-4557-x. [DOI] [PubMed] [Google Scholar]
- 137.Winkler F. The brain metastatic niche. J Mol Med (Berl) 2015;93:1213–1220. doi: 10.1007/s00109-015-1357-0. [DOI] [PubMed] [Google Scholar]
- 138.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sleeman JP. The lymph node pre-metastatic niche. J Mol Med (Berl) 2015;93:1173–1184. doi: 10.1007/s00109-015-1351-6. [DOI] [PubMed] [Google Scholar]
- 140.Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22(Suppl 2):S66–72. doi: 10.1016/j.breast.2013.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.