Abstract
Background
Non-small cell lung cancer (NSCLC) presentation, treatment, and outcomes vary widely according to socioeconomic factors and other patient characteristics. To determine whether medical comorbidities account for these observations, we incorporated a validated medical comorbidity index into an analysis of patients diagnosed with stage I–III NSCLC.
Patients and Methods
We performed a retrospective analysis of consecutive patients diagnosed with stage I–III NSCLC. Demographic, tumor, and comorbidity data were obtained from hospital tumor registries and individual patient records. The association between variables was assessed through multivariate logistic regression and survival analysis.
Results
A total of 454 patients met criteria for analysis. Median age was 65 years, and 51% were men. Individuals with a higher Charlson Comorbidity Index (CCI) were significantly more likely to present with early stage (stage I–II) NSCLC than were patients with lower CCI (OR 1.72; 95% CI, 1.14 to 2.63; P=0.01), although this association lost statistical significance (P=0.21) in a multivariate model. In multivariate logistic regression, overall survival remained associated with all variables: age, gender, race, insurance type, stage, histology, and CCI (P=0.0007). The CCI was associated with survival for patients with both early stage (P=0.02) and locally advanced (P=0.02) disease.
Conclusions
In this cohort of patients with stage I–III NSCLC, increasing comorbidity burden had a non-significant association with diagnosis at earlier disease stage. Although comorbidity burden was significantly associated with outcome for both early stage and locally advanced disease, it did not account for survival differences based on multiple other patient and disease characteristics.
Keywords: Lung cancer, comorbidities, disparities, Charlson Index, locally advanced, early stage, treatment, outcomes
Background
The presentation, treatment, and outcomes of non-small cell lung cancer (NSCLC) vary widely according to social factors and other patient characteristics.1–4 Earlier work at our institution has demonstrated that the reason for initial imaging and diagnosis, timeliness of diagnostic evaluation and therapy, treatment selection, and overall survival in patients with early stage and locally advanced disease differ according to socioeconomic status.5–8 Reasons for these disparities remain unclear. Potential explanations include system-level factors, provider preferences, patient preferences, and patient fitness for therapy.
Fitness for therapy is a key factor in the treatment and outcome of lung cancer. Due to advanced age at diagnosis and the prevalence of prior or ongoing smoking, many patients suffer from medical comorbidities.9,10 In published series, comorbidity rates in lung cancer populations range from approximately 20% to 50%.11–13 These estimates are considerably higher than rates reported for other common malignancies such as breast, gastrointestinal, and prostate cancer.14,15 Nevertheless, at least some of these rates might be underestimates. A number of studies have examined lung cancer populations enrolled in therapeutic clinical trials, a setting in which eligibility requirements may exclude individuals with major medical problems.16,17 Additionally, Medicare-derived data—the principal source of comorbidity data in national samples—may have only modest concordance with hospital records.18
To evaluate the impact of medical comorbidities on lung cancer presentation and outcomes in a contemporary real-world population, we evaluated consecutive patients with stage I–III NSCLC treated at the University of Texas Southwestern Medical Center (UT Southwestern). At UT Southwestern, a single medical faculty provides care to a diverse patient population, including a large urban safety net system. We focused on early stage and locally advanced NSCLC due to greater variation in treatment options and clinical outcomes than seen in metastatic disease. We employed the Charlson Comorbidity Index, a commonly used measure of medical comorbidity that has been studied extensively in lung cancer populations.19–22
Patients and Methods
This study was approved by the UT Southwestern Institutional Review Board. Data was obtained from the American College of Surgeons-approved UT Southwestern and Parkland Health and Hospital System Tumor registries. These registries draw patients from University Hospital, a 429-bed facility that serves a tertiary regional medical and surgical referral center, and Parkland Health and Hospital System, a 968-bed hospital and associated community clinics that is the primary safety net health care system for Dallas County. Dallas County has a population of 1.2 million people of whom 42% are Hispanic, 25% black and 29% non-Hispanic whites.23 We obtained additional comorbidity data from hospital administrative databases and through review of individual patient paper and electronic medical records.
We collected data on patients diagnosed with stage I–III NSCLC between January 1, 2000 and December 31, 2005. Our focus was limited to stage I–III NSCLC because (1) there is greater variation in treatment and outcome than seen in stage IV disease and (2) previous studies have indicated that medical comorbidities are likely to have a greater effect on treatment variation and survival compared to stage IV disease.24,25 We excluded cases with malignant effusions (“wet IIIB”; stage IV in the American Joint Committee on Cancer [AJCC] 7th Edition26) because treatment (chemotherapy-based) and survival are distinct from other stage III cases and more closely resemble stage IV disease. In our analyses, we grouped stage I and II disease together because of the small number of stage II patients and the largely similar treatment paradigms for both stages. The time period from 2000–2005 was selected for the following reasons: (1) sufficient tumor registry data was first available for patients diagnosed in 2000, and (2) the 2005 cutoff allowed for sufficient follow-up at the time of outcome analysis.
Using these data, we calculated the Charlson Comorbidity Index (CCI) for each patient.19 The CCI incorporates 19 chronic diseases weighted according to association with mortality. We grouped patients according to total score into the following established categories: 0 (no comorbidity) 1–2 (average), 3–4 (moderate), and ≥ 5 (severe). We selected the CCI for this study because it is the most commonly used, validated comorbidity index in the medical literature.13,19,20
Univariate and multivariate Cox regression were used to determine the association between baseline characteristics (age, gender, race, insurance, histology, cancer stage, CCI score) and clinical outcomes. All variables included in univariate analyses were retained in multivariate models. Kaplan-Meier survival curves were drawn and log-rank tests were used to test survival differences according to baseline characteristics. All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, NC) in Microsoft Windows.
Results
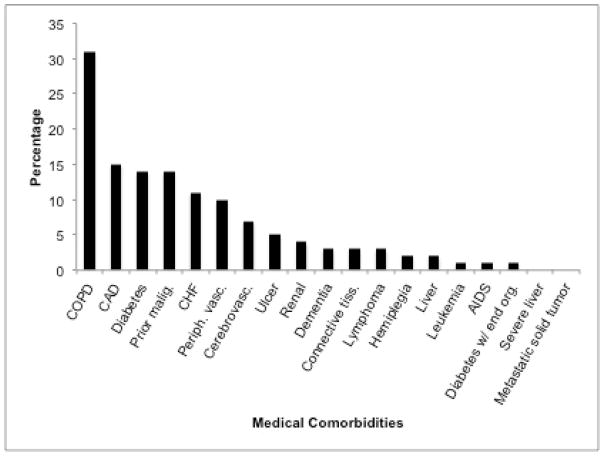
We identified 454 patients with stage I–III NSCLC diagnosed between January 1, 2000, and December 31, 2005. Baseline patient characteristics are shown in Table 1. Mean age was 65 years, 51% were male, and 62% were non-Hispanic white. Using CCI categories, 37% of patients had no comorbidity, 39% had average comorbidity, 18% had moderate comorbidity, and 6% had severe comorbidity. Specific medical comorbidities identified are listed in Figure 1. The most prevalent comorbid illnesses (each >5% of population) were chronic obstructive pulmonary disease (31%), coronary artery disease (15%), diabetes (14%), prior malignancy (14%), chronic heart failure (11%), peripheral vascular disease (10%), and cerebrovascular disease (7%). Despite the prevalence of diabetes and cerebrovascular disease, severe manifestations of these conditions were quite rare: only 1% had diabetes-related end-organ damage and only 2% had hemiplegia.
Table 1.
Patient Baseline Characteristics
| Baseline Characteristic | Number (%) or mean (± SD) |
|---|---|
| Total patients | 467 |
| Age (years) | 65±11 |
| Gender | |
| Male | 243 (51) |
| Female | 224 (49) |
| Race | |
| Non-Hispanic white | 290 (62) |
| Black | 135 (29) |
| Hispanic | 25 (5) |
| Other | 17 (4) |
| Insurance | |
| Private | 134 (29) |
| Medicare | 199 (43) |
| Medicaid/County | 58 (12) |
| None | 61 (13) |
| Unknown | 15 (3) |
| Histology | |
| Adenocarcinoma | 230 (49) |
| Squamous | 160 (34) |
| Other | 77 (17) |
| Stage | |
| I–II | 246 (53) |
| III | 221 (47) |
| Charlson Comorbidity Index score | |
| 0 | 173 (37) |
| 1–2 | 183 (39) |
| 3–4 | 82 (18) |
| 5 | 29 (6) |
Figure 1.
Patients’ medical comorbidities and their prevalence.
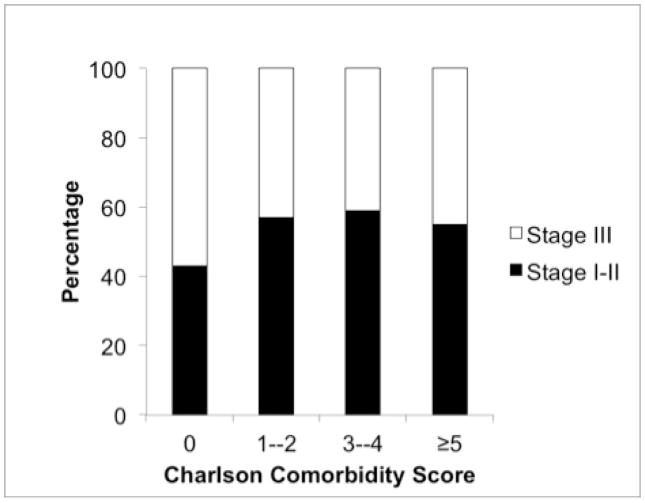
A number of patient and tumor characteristics were associated with comorbidity burden (see Table 2). In univariate analysis, the following factors were significantly associated with a higher Charlson Comorbidity Index (CCI): older age, male gender, stage I/II disease, and squamous histology. Having no medical insurance was significant associated with having a lower CCI. The relationship between CCI and disease stage is also shown in Figure 2. In general, the absence of medical comorbidities was associated with diagnosis at earlier disease stage. Among patients with a CCI of 0, 43% had stage I–II disease and 57% had stage III disease. In contrast, among patients with a CCI of 1–2, 57% had stage I–II disease and 43% had stage III disease, with similar proportions noted for patients with CCI 3–4 and CCI ≥ 5. In multivariate analysis performed without model selection (Table 2), CCI remained significantly associated with age and gender. There was a non-significant trend toward association with disease stage (HR 0.78; P=0.21). When independent variables with P>0.2 in univariate analysis were removed from the analysis, age and gender remained significant predictors of CCI, with disease stage again having a non-significant trend toward association (HR 0.77; P=0.16).
Table 2.
Association between baseline characteristics and comorbidity burden
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | Overall P Value | OR (95% CI) | P Value | Overall P Value | |
| Age (years) | <0.001 | 0.006 | ||||
| < 65 | 0.36 (0.26–0.51) | <0.001 | 0.46 (0.26–0.80) | 0.006 | ||
| ≥ 65 | Reference | Reference | ||||
| Gender | 0.03 | 0.004 | ||||
| Male | Reference | Reference | ||||
| Female | 0.68 (0.49–0.96) | 0.03 | 0.58 (0.40–0.84) | 0.004 | ||
| Race | 0.21 | 0.72 | ||||
| White | Reference | Reference | ||||
| Non-white | 0.80 (0.57–1.13) | 0.21 | 0.93 (0.62–1.40) | 0.72 | ||
| Stage of cancer | 0.002 | 0.21 | ||||
| I–II | Reference | Reference | ||||
| III | 0.58 (0.41–0.82) | 0.002 | 0.78 (0.53–1.15) | 0.21 | ||
| Insurance | <0.001 | 0.18 | ||||
| Private | Reference | Reference | ||||
| Medicare | 2.08 (1.37–3.14) | <0.001 | 1.19 (0.67–2.14) | 0.55 | ||
| Medicaid, County | 0.96 (0.54–1.70) | 0.89 | 1.24 (0.66–2.35) | 0.51 | ||
| No insurance | 0.52 (0.29–0.91) | 0.02 | 0.58 (0.30–1.12) | 0.10 | ||
| Histology | 0.07 | 0.22 | ||||
| Adenocarcinoma | Reference | Reference | ||||
| Squamous cell | 1.52 (1.04–2.22) | 0.03 | 1.33 (0.89–2.00) | 0.17 | ||
| Other | 0.92 (0.50–1.70) | 0.79 | 0.81 (0.43–1.53) | 0.52 | ||
OR > 1 indicates greater likelihood of higher Charslon Comorbidity Index
CI, confidence interval; OR, odds ratio
Figure 2.
Association between Charlson Comorbidity Index Score and NSCLC stage
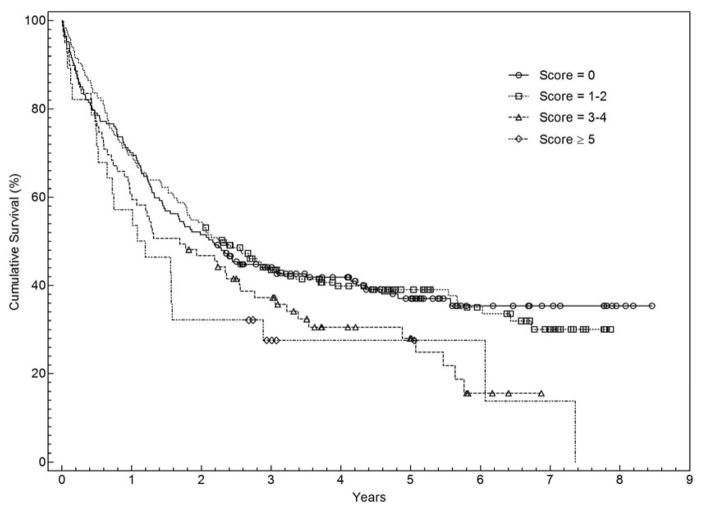
Associations between baseline patient/tumor characteristics and overall survival are shown in Table 3. Overall survival was significantly associated with gender, race, stage, histology, insurance type, and comorbidity index. Longer survival was seen among women, white patients, stage I–II disease, patients with private insurance, and adenocarcinoma histology. Patients with higher CCI had worse clinical outcomes; median overall survival of patients with CCI of 0 or CCI of 1–2 was almost twice that of patients with CCI of ≥ 5 (Figure 3). In a multivariable model, all baseline characteristics remained independently associated with survival (Table 3).
Table 3.
Association between baseline characteristics and overall survival
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Median survival (95% CI) (years) | HR (95% CI) | P Value | Overall P Value | HR (95% CI) | P Value | Overall P Value | |
| Age (years) | 0.14 | 0.08 | |||||
| < 65 | 2.25 (1.77–2.84) | 0.84 (0.67–1.06) | 0.73 (0.51–1.04) | 0.08 | |||
| ≥ 65 | 1.69 (1.28–2.44) | Reference | 0.14 | Reference | |||
| Gender | <0.001 | <0.001 | |||||
| Male | 1.34 (1.06–1.75) | Reference | Reference | ||||
| Female | 3.22 (2.44–5.67) | 0.57 (0.45–0.72) | <0.001 | 0.63 (0.49–0.82) | <0.001 | ||
| Race | 0.001 | 0.02 | |||||
| White | 2.56 (1.93–3.50) | Reference | Reference | ||||
| Non-white | 1.52 (1.13–1.99) | 1.47 (1.17–1.85) | 0.001 | 1.39 (1.05–1.83) | 0.02 | ||
| Stage of cancer | <0.001 | <0.001 | |||||
| I–II | 5.64 (3.92–6.73) | Reference | Reference | ||||
| III | 1.00 (0.79–1.18) | 3.01 (2.38–3.81) | <0.001 | 3.75 (2.86–4.90) | <0.001 | ||
| Charlson Comorbidity Index | 0.03 | <0.001 | |||||
| 0 | 2.14 (1.45–3.08) | Reference | Reference | ||||
| 1–2 | 2.30 (1.77–3.19) | 0.98 (0.75–1.28) | 0.88 | 1.13 (0.83–1.54) | 0.44 | ||
| 3–4 | 1.31 (0.97–2.55) | 1.38 (1.01–1.90) | 0.05 | 1.99 (1.37–2.89) | <0.001 | ||
| ≥ 5 | 1.14 (0.52–1.58) | 1.64 (1.03–2.60) | 0.04 | 1.97 (1.19–3.26) | 0.008 | ||
| Insurance | <0.001 | 0.05 | |||||
| Private | 4.23 (2.44–5.67) | Reference | Reference | ||||
| Medicare | 1.77 (1.27–2.56) | 1.47 (1.11–1.95) | 0.008 | 1.40 (0.97–2.04) | 0.07 | ||
| Medicaid, County | 1.81 (1.32–NR) | 1.31 (0.87–1.95) | 0.19 | 1.16 (0.73–1.83) | 0.53 | ||
| No insurance | 1.09 (0.67–2.17) | 2.11 (1.46–3.04) | <0.001 | 1.69 (1.10–2.61) | 0.02 | ||
| Histology | 0.02 | 0.005 | |||||
| Adenocarcinoma | 2.86 (2.11–4.39) | Reference | Reference | ||||
| Squamous cell | 1.49 (1.07–2.23) | 1.36 (1.06–1.75) | 0.02 | 1.15 (0.87–1.50) | 0.33 | ||
| Other | 0.98 (0.65–1.83) | 1.55 (1.02–2.30) | 0.03 | 2.01 (1.33–3.05) | 0.001 | ||
Figure 3.
Kaplan Meier Curve for stage I–III NSCLC According to Charlson Comorbidity Index Score
Discussion
Medical comorbidities occur frequently among patients diagnosed with lung cancer, as the average age at diagnosis in the United States is over 70 years, and 85% of patients are current or former smokers. These comorbid conditions may impact treatment selection, toxicities, and outcomes.12,27,28 Specifically, patients with comorbidities have an increased likelihood of experiencing treatment-related adverse effects in addition to exacerbations of the comorbidity.9,29,30 For example, lung cancer patients with pre-existing pulmonary issues are more prone to experience radiation-associated toxicities such as pneumonitis or fibrosis.31 Due to comorbid conditions, these individuals may also be less likely to complete prescribed treatments, resulting in lower rates of disease control.32 Additionally, severe comorbidities may themselves limit life expectancy independent of the underlying malignancy.10,33
The current study provides further insight into the impact of medical comorbidities on the presentation and outcomes of lung cancer in a diverse, real-world population. In this setting, we found that medical comorbidities occur frequently, are associated with lung cancer diagnosis at earlier stage, and are associated with survival. Sixty percent of patients had one or more recorded medical comorbidity. This rate is considerably higher than that reported in many earlier studies, in which only 20–50% of patients had documented comorbidities.11–13 This difference most likely reflects differences in study populations and data sources. For instance, the study by Asmis and colleagues included patients who were enrolled on National Cancer Institute of Canada clinical trials and therefore had sufficient functional status and medical fitness to meet study eligibility criteria.12 Furthermore, our study represents a diverse population of whom many are medically underserved, which may contribute to increased burden of illness.34 The most common medical comorbidities observed (chronic obstructive pulmonary disease and coronary artery disease) share with lung cancer tobacco as a major risk factor.35 The concordance of chronic lung disease and lung cancer has substantial clinical implications, as it may render patients less likely to be candidates for potentially curative surgical interventions.36 Notably, 14% of patients in our cohort had a prior malignancy, many of which may have been tobacco-related. This relatively high rate of previous cancers also has implications on lung cancer clinical trial accrual, as most therapeutic clinical trials exclude patients with prior malignancy within a certain time of enrollment.
The possible association between increasing comorbidity burden and earlier stage at lung cancer diagnosis may be due to the more intensive medical care received by these patients. Because a decreasing proportion of early stage lung cancers are initially detected due to symptoms,5 increasingly they are detected incidentally during radiographic evaluation performed for other reasons. Patients with medical comorbidities may be more likely to undergo such imaging studies,37 whether as a component of routine disease surveillance, preoperative screening for surgical procedures, or during acute hospitalizations. The impact of computed tomography-based screening for lung cancer38 on this trend is not yet known. Another potential explanation for this finding is that patients with greater medical comorbidities may have been less likely to undergo invasive disease assessments such as mediastinoscopy, resulting in understaging.
We observed a clear association between the CCI and overall survival. Median overall survival exceeded 2 years among patients with CCI 0 and CCI 1–2, compared to 1.1 years among patients with CCI ≥ 5. This finding persisted in multivariable analysis incorporating numerous patient and disease factors (HR for death 1.99 for CCI 3–4 and 1.97 for CCI ≥ 5 compared to CCI 0). Studies of the predictive ability of the CCI in lung cancer have had varying results. In early-stage disease, the CCI predicts higher perioperative mortality.21 It also predicts long-term survival, but may account for only a small proportion of survival variation.39 In advanced disease, results are mixed. Using a cut-off of CCI ≥ 1, Asmis and colleagues found an association with survival among patients enrolled in National Cancer Institute of Canada clinical trials.12 However, in a subset analysis of elderly patients treated on a clinical trial and in a recent single-center series, CCI did not predict survival.25,40 It seems likely that the relatively short survival in these studies (approximately 7 months) and in metastatic lung cancer in general may limit the discriminative capacity of the CCI in this disease setting. By contrast, we found that the CCI predicted survival in both early-stage (median survival 5.6 years) and locally advanced (median survival 1 year) disease.
The current analysis builds upon our previous study demonstrating that, even within a single medical center with a single medical staff, there are socioeconomic disparities in clinical outcomes among patients with stage I–III NSCLC.7 We had hypothesized that medical comorbidities might account for these differences. However, even when accounting for CCI, stage and other factors in a multivariable model, non-white and indigent patients (defined as having Medicaid, the county indigent insurance plan, or no insurance) have inferior survival. Indeed, both groups had a trend toward lower comorbidity compared to white patients and patients with private insurance (P=0.21 and P=0.13, respectively). It is possible that, due to less frequent medical care, these individuals may be more likely to have medical conditions that go unrecognized or unrecorded, resulting in underscoring of the CCI. Alternatively, there may be as yet unidentified social factors that impact survival in these populations.
Principal limitations of our study include its retrospective nature, relatively small sample size, and single-center setting. Although our center provides access to a diverse patient cohort, certain patient characteristics may not have be representative of the larger lung cancer population. For example, the median age of the study cohort (65 years) is approximately 5 years younger than the national average. The use of both administrative data41 and detailed review of individual patient medical records increased the likelihood of identifying medical comorbidities in the study population.
Conclusion
This study identifies the influence of medical comorbidity on the presentation and outcome in early stage and locally advanced NSCLC. In a real-world population, over half of lung cancer patients have medical comorbidities. Absence of medical comorbidities may be associated with more advanced disease stage at diagnosis. Although comorbidity index is associated with survival, it does not account for survival differences based on socioeconomic status. Further investigation is required to elucidate the etiology of these disparities.
Clinical Practice Points.
Medical comorbidities are frequent in lung cancer patients and are significantly higher than other common malignancies. It is not clear to what extent these comorbid conditions affect treatment selection and patient overall survival.
We evaluated medical comorbidities utilizing the Charlson Comorbidity Index (CCI) in patients with stage I–III NSCLC treated at a medical institution that provides care to a demographically diverse population.
Older age, male gender, and squamous histology were associated with higher CCI.
Increasing comorbidity burden had a non-significant association with diagnosis at earlier disease stage.
Increasing comorbidity burden also predicted for worse overall survival. This association persisted even when controlling for multiple patient- and tumor-associated prognostic factors. Furthermore, socioeconomic status remained associated with clinical outcomes in this multivariable model, suggesting that further study to elucidate reasons for these disparities is required.
Acknowledgments
Funding provided by the National Institutes of Health CTSA Grant KL2RR024983 (North and Central Texas Clinical and Translational Science Initiative) (to D.E.G.), the National Cancer Institute Clinical Investigator Team Leadership Award (1P30 CA142543-01 supplement) (to D.E.G.), and an American Cancer Society and Simmons Cancer Center Grant (ACS-IRG-02-196) (to D.E.G.).
The authors thank Joan Cox and Alejandra Madrigales for providing data from the Parkland Health and Hospital System and UT Southwestern Tumor Registries. The authors thank Steven Boll for providing data from the Parkland Health and Hospital System discharge database.
Biostatistical support was provided by the Biostatistics Shared Resource at the Harold C. Simmons Cancer Center, University of Texas Southwestern Medical Center, Dallas, Texas, USA, which is supported in part by a National Cancer Institute Cancer Center Support Grant, 1P30 CA142543-01.
Footnotes
Conflicts of Interest:
All authors have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 2.Greenwald HP, Polissar NL, Borgatta EF, et al. Social factors, treatment, and survival in early-stage non-small cell lung cancer. Am J Public Health. 1998;88:1681–4. doi: 10.2105/ajph.88.11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park ER, Japuntich SJ, Traeger L, et al. Disparities between blacks and whites in tobacco and lung cancer treatment. Oncologist. 2011;16:1428–34. doi: 10.1634/theoncologist.2011-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lathan CS, Neville BA, Earle CC. The effect of race on invasive staging and surgery in non-small-cell lung cancer. J Clin Oncol. 2006;24:413–8. doi: 10.1200/JCO.2005.02.1758. [DOI] [PubMed] [Google Scholar]
- 5.Taiwo EO, Yorio JT, Yan J, et al. How have we diagnosed early-stage lung cancer without radiographic screening? A contemporary single-center experience. PLoS One. 2012;7:e52313. doi: 10.1371/journal.pone.0052313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yorio JT, Xie Y, Yan J, et al. Lung cancer diagnostic and treatment intervals in the United States: a health care disparity? J Thorac Oncol. 2009;4:1322–30. doi: 10.1097/JTO.0b013e3181bbb130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yorio JT, Yan J, Xie Y, et al. Socioeconomic disparities in lung cancer treatment and outcomes persist within a single academic medical center. Clin Lung Cancer. 2012;13:448–57. doi: 10.1016/j.cllc.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber DE, Rasco DW, Le P, et al. Predictors and impact of second-line chemotherapy for advanced non-small cell lung cancer in the United States: real-world considerations for maintenance therapy. J Thorac Oncol. 2011;6:365–71. doi: 10.1097/JTO.0b013e3181fff142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28:4086–93. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 10.Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 11.Earle CC, Venditti LN, Neumann PJ, et al. Who gets chemotherapy for metastatic lung cancer? Chest. 2000;117:1239–46. doi: 10.1378/chest.117.5.1239. [DOI] [PubMed] [Google Scholar]
- 12.Asmis TR, Ding K, Seymour L, et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group trials. J Clin Oncol. 2008;26:54–9. doi: 10.1200/JCO.2007.12.8322. [DOI] [PubMed] [Google Scholar]
- 13.Moro-Sibilot D, Aubert A, Diab S, et al. Comorbidities and Charlson score in resected stage I nonsmall cell lung cancer. Eur Respir J. 2005;26:480–6. doi: 10.1183/09031936.05.00146004. [DOI] [PubMed] [Google Scholar]
- 14.Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–7. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 15.Albertsen PC, Hanley JA, Gleason DF, et al. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA. 1998;280:975–80. doi: 10.1001/jama.280.11.975. [DOI] [PubMed] [Google Scholar]
- 16.Fuks A, Weijer C, Freedman B, et al. A study in contrasts: eligibility criteria in a twenty-year sample of NSABP and POG clinical trials. National Surgical Adjuvant Breast and Bowel Program. Pediatric Oncology Group. J Clin Epidemiol. 1998;51:69–79. doi: 10.1016/s0895-4356(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 18.Klabunde CN, Harlan LC, Warren JL. Data sources for measuring comorbidity: a comparison of hospital records and medicare claims for cancer patients. Med Care. 2006;44:921–8. doi: 10.1097/01.mlr.0000223480.52713.b9. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2005;28:759–62. doi: 10.1016/j.ejcts.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 21.Wang CY, Lin YS, Tzao C, et al. Comparison of Charlson comorbidity index and Kaplan-Feinstein index in patients with stage I lung cancer after surgical resection. Eur J Cardiothorac Surg. 2007;32:877–81. doi: 10.1016/j.ejcts.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Birim O, Maat AP, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;23:30–4. doi: 10.1016/s1010-7940(02)00721-2. [DOI] [PubMed] [Google Scholar]
- 23.US Census Bureau. [Accessed: April 10th, 2013];State and County QuickFacts 2010. Available at http://quickfacts.census.gov/qfd/index.html.
- 24.Battafarano RJ, Piccirillo JF, Meyers BF, et al. Impact of comorbidity on survival after surgical resection in patients with stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2002;123:280–7. doi: 10.1067/mtc.2002.119338. [DOI] [PubMed] [Google Scholar]
- 25.Ganti AK, Siedlik E, Marr AS, et al. Predictive ability of Charlson comorbidity index on outcomes from lung cancer. Am J Clin Oncol. 2011;34:593–6. doi: 10.1097/COC.0b013e3181fe445b. [DOI] [PubMed] [Google Scholar]
- 26.Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694–705. doi: 10.1097/JTO.0b013e31812d05d5. [DOI] [PubMed] [Google Scholar]
- 27.Blanco JA, Toste IS, Alvarez RF, et al. Age, comorbidity, treatment decision and prognosis in lung cancer. Age Ageing. 2008;37:715–8. doi: 10.1093/ageing/afn226. [DOI] [PubMed] [Google Scholar]
- 28.Firat S, Pleister A, Byhardt RW, et al. Age is independent of comorbidity influencing patient selection for combined modality therapy for treatment of stage III nonsmall cell lung cancer (NSCLC) Am J Clin Oncol. 2006;29:252–7. doi: 10.1097/01.coc.0000217824.20290.ab. [DOI] [PubMed] [Google Scholar]
- 29.Farjah F, Wood DE, Varghese TK, et al. Health care utilization among surgically treated Medicare beneficiaries with lung cancer. Ann Thorac Surg. 2009;88:1749–56. doi: 10.1016/j.athoracsur.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Lee L, Cheung WY, Atkinson E, et al. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol. 2011;29:106–17. doi: 10.1200/JCO.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 31.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 32.Frasci G, Lorusso V, Panza N, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18:2529–36. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- 33.Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22:3099–103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 34.Albain KS, Unger JM, Crowley JJ, et al. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101:984–92. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dela Cruz CS, Tanoue LT, Matthay RA. Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 2011;32:605–44. doi: 10.1016/j.ccm.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–76. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulligan CR, Meram AD, Proctor CD, et al. Unlimited access to care: effect on racial disparity and prognostic factors in lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:25–31. doi: 10.1158/1055-9965.EPI-05-0537. [DOI] [PubMed] [Google Scholar]
- 38.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. Int J Cancer. 2003;103:792–802. doi: 10.1002/ijc.10882. [DOI] [PubMed] [Google Scholar]
- 40.Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865–72. doi: 10.1200/JCO.2005.02.527. [DOI] [PubMed] [Google Scholar]
- 41.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Med Care. 2002;40:675–85. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]