Abstract
Two series of thermosensitive hydrogels were synthesized by copolymerizing N-isopropylacrylamide (NIPAAm) with various contents of novel hydrophobic crosslinkers, curcumin multiacrylate (CMA) and quercetin multiacrylate (QMA). The compositions of the resulting hydrogels were characterized using solid state-NMR (ss-NMR), and the temperature dependent swelling behavior and lower critical solution temperature (LCST) were characterized using swelling studies and differential scanning calorimetry (DSC). Increasing the crosslinker content resulted in a significant decrease in the LCST and swelling ratio of hydrogels, which could be attributed to the increased hydrophobicity introduced by CMA or QMA. All of the hydrogels demonstrated temperature responsive swelling with the extent of swelling decreasing with increasing crosslinker content. The lower crosslinker content gels displayed sharper phase transitions, while the high crosslinker content gels had broader phase transitions.
1. Introduction
Poly (N-isopropylacrylamide) (PNIPAAm) has received extensive attention as a temperature responsive polymer due to its sharp phase transition at its lower critical solution temperature (LCST) around 33°C [1–3]. NIPAAm based hydrogels are three-dimensional crosslinked polymeric networks, which are usually of interest for creating “smart” materials for application in biomedicine, drug delivery, tissue engineering, environmental treatment, etc. [4–10]. Generally, homopolymer NIPAAm gel exhibits a reversible phase change from hydrophilic to hydrophobic state at its LCST [4, 11]. When the temperature is below LCST, the polymer structure expands due to the hydration process of PNIPAAm chains, which results in hydrogel swelling. Conversely, at temperatures above LCST, the polymer network collapses [12–14]. Such phenomenon has been previously reported as a result of hydrogen bond forming/breaking between water molecules with their neighboring amide group from NIPAAm [15–18].
The LCST of NIPAAm can be controlled by adjusting the hydrophilic/hydrophobic balance in the polymer chain [19]. Various studies have shown that copolymerizing NIPAAm with another monomer could modify its LCST [1, 20–22]. Previously, it has been reported that the LCST can either be decreased through incorporating hydrophobic co-monomers or increased by incorporating hydrophilic co-monomers [19, 23–25]. An extensive number of hydrophobic/hydrophilic molecules have been explored for modifying the LCST, including acrylic acid (AA), methacrylic acid (MAA), butyl methacrylate (BMA) and di-n-propylacrylamide (DPAM) [1, 15, 18, 25–28]. In this study, our focus is on novel crosslinkers based on hydrophobic polyphenolic compounds for the modulation of the LCST of NIPAAm within a crosslinked matrix. Curcumin is a naturally derived hydrophobic polyphenol and is known for its numerous biological activities (e.g., demonstrated anti-cancer, anti-oxidation and anti-inflammatory properties) [29, 30]. Clinical trials suggested that curcumin was well tolerated by the human body, even at relatively high dosages (12 g/day) [31–33]. As a biomedical agent, curcumin have been studied for its therapeutic use in cancer treatment through inhibiting cells proliferation and promoting apoptosis [34, 35]. Additionally, curcumin has shown significant impact in reducing oxidative stress induced by environmental pollutants such as metal nanoparticles, polychlorinated biphenyls (PCBs), etc. [36, 37]. Furthermore, our recent efforts found that curcumin, and potentially other polyphenols (e.g., quercetin), have shown binding affinities to environmental contaminants (e.g., PCBs).
From our previous studies [38], we have reported a method to synthesize acrylate functionalized polyphenolic molecules, including curcumin diacrylate (CDA) and quercetin multiacrylate (QMA). However, our recent studies have suggested that the polymerization reaction results in formation of curcumin multiacrylate (CMA), which has a mixture of mono-, di-, and tri-acrylate, instead of previously reported CDA. In the current study, these designed polyphenolic acrylates were used as crosslinkers for NIPAAm to develop novel temperature responsive hydrogels. Herein, we report the influence of changes in the CMA or QMA content on the characteristics of NIPAAm-based hydrogels. The temperature dependent swelling ratios in deionized water were measured as a function of hydrogel composition. Swelling and deswelling behavior caused by a pulsing temperature change was characterized in reversible swelling study. The goal of this study was to synthesize and characterize NIPAAm-based hydrogel systems crosslinked with novel acrylated polyphenols. These unique T-responsive hydrogels have various potential applications including use in environmental remediation.
2. Experimental
2.1. Materials
The monomer N-Isopropylacrylamide (NIPAAm, 97%), initiator ammonium persulfate (APS, ≥98%), quercetin, curcumin, acryloyl chloride were purchased from Sigma-Aldrich Corporation (St. Louis, USA). Poly (ethylene glycol) 400 dimethacrylate (PEG400DMA) was purchased from Polysciences, Inc (Warrington, USA). All organic solvents were obtained from Sigma-Aldrich and Fisher Scientific (Hampton, USA). Agents mentioned above were used as received without further purification. No commercial crosslinker was used in this work; the acrylated polyphenols (CMA and QMA) synthesized by our group were used as hydrophobic crosslinkers. Both CMA and QMA have multiple acrylate groups that provided enough functional moieties for creating crosslinked network with NIPAAm.
2.2. Synthesis of acrylated polyphenolic crosslinkers
The synthesis of hydrophobic crosslinkers, CMA and QMA, was conducted based on previously reported literature. In brief, pure curcumin and quercetin were reacted with acryloyl chloride in tetrahydrofunran (THF) and triethylamine (TEA). The reaction was allowed to proceed overnight at room temperature, followed by vacuum filtration and distillation to remove any side products, unreacted reactants and excess solvents. The resulting CMA or QMA were in powder form and stored at −20°C until use. The average molecular weight of CMA and QMA were determined by gas chromatography-mass spectrometry (GC-MS), and they were 449.5g/mol and 518.0g/mol respectively. CMA and QMA structures (full acrylated forms) are given as follow:
2.3. Preparation of the copolymerized hydrogels and microparticles
Three different compositions of gels (0.9%, 1.8% and 2.7% of CMA or QMA) were synthesized through free radical polymerization. 0.9% PEG400DMA gels were prepared as the control. For a typical synthesis of 0.9% CMA gel, 0.020 g of CMA and 0.561 g of NIPAAm were mixed with 2 mL of DMSO, vortexed for a few minutes until the monomers were well dissolved. APS was dissolved in deionized water and then added to the monomer mixture at 4.0 weight % of combined weight of NIPAAm and CMA. Then, the mixture was transferred to an aluminum pan and the reaction was carried out at 80°C preheated oven for 3 hours. The gel was washed 3 times in an excess amount of acetone followed by 3 times in an excess amount of DI water to ensure the unreacted monomers were maximally leached out. After washing, CMA gel was cut into 5 mm diameter disks and freeze dried for at least 24 hours until no further mass change. Preliminary experiments were completed to determine the appropriate reaction conditions, which are summarized in Table 1. The dry gels were cryoground into microparticles through Cryo-mill SPEX6770 freezer/mill (Haan, Germany) in a set process to ensure the products in fine dried powder form. The morphology, size and shape of the gel microparticles were characterized by scanning electron microscope (SEM) using Hitachi S4300 microscope (Tokyo, Japan). All samples for SEM were sputtered with a thin layer of gold at 20 mA for about 3 min under argon gas. Size distribution, mean size ± standard deviation of the microparticles were measured by Shimadzu SALD-7101 nanoparticle size analyzer (Kyoto, Japan).
Table 1.
Feed composition and reaction time of the polyphenolic gels
| Sample | NIPAAm mol% | CMA mol% | QMA mol% | APS (wt %) | Time (hrs) |
|---|---|---|---|---|---|
| CMA0.9 | 99.1 | 0.9 | – | 4 | 3 |
| CMA1.8 | 98.2 | 1.8 | – | 4 | 3.5 |
| CMA2.7 | 97.3 | 2.7 | – | 4 | 4 |
| QMA0.9 | 99.1 | – | 0.9 | 4 | 3 |
| QMA1.8 | 98.2 | – | 1.8 | 4 | 3.5 |
| QMA2.7 | 97.3 | – | 2.7 | 4 | 4 |
2.4. Solid state-NMR (ss-NMR) analysis
Room temperature solid-state NMR spectra were acquired using a Tecmag Redstone spectrometer (Tecmag, Inc., Houston, TX) operating at 75.6 MHz for 13C (7.05 T static magnetic field). Samples were packed into 7.5 mm zirconia rotors and sealed with Teflon or Kel-F end caps (Revolution NMR, LLC, Fort Collins, CO). Experiments were performed using a 7.5 mm double resonance MAS probe (Varian, Palo Alto, CA). All 13C spectra were acquired using magic-angle spinning (MAS) [39] at 4 kHz, using ramped cross polarization (CP) [40], total sideband suppression (TOSS) [41], and Spinal 64 decoupling [42] with a 1H decoupling field of about 63 kHz. A 2 millisecond contact time was used in all experiments. 3-methyl glutaric acid (MGA) used for optimizing spectrometer and as an external standard, with the methyl peak referenced to 18.84 ppm [43]. Spectra were acquired with a 5 second pulse delay and a 25.6 millisecond acquisition time. All spectra were processed with 30 Hz of exponential line broadening.
2.5. Temperature dependent swelling study
The temperature dependent swelling was carried out in an isothermal water bath with the temperature range of approximately 22°C (room temperature) to 55°C. 8mm diameter gel pieces were measured by dry mass and then immersed in deionized water for at least 24 hours at room temperature. The temperature was then increased at a 5°C increments after each measurement. The equilibrium swollen mass was measured gravimetrically in a balance after the surface water being gently wiped off. The weight swelling ratio (q) was defined as:
| (1) |
Where Mswollen is the equilibrium swollen mass at each temperature point and Mdry is the dry mass of the gel after being freeze dried. Three measurements were taken for each sample.
2.6. Reversible swelling study
Reversible swelling studies were conducted based upon the pulsatile temperature change. Equilibrium swelling ratios were obtained at temperatures alternated between room temperature and 55°C. The dry gels were initially swollen at room temperature until equilibrium. After mass being measured, the gels were quickly transferred to a 55°C water bath, allowed to swell for another 24 hours. After the mass was recorded, they were transferred back to room temperature bath. Exactly similar measurements were done for three cycles, where three measurements were taken for each sample.
2.7. LCST measurements
The LCSTs of the hydrogels were measured by differential scanning calorimetry (DSC Q200, TA instruments Inc., New Castle, USA). In a standard procedure, the hydrogel was immersed into DI water at room temperature for 24 hours to reach an equilibrium state. Then, the gel was cut into 2 mm diameter piece and placed in a T-zero pan with mass being carefully measured. The T-zero pan was then tightly sealed with a hermetic lid and placed into the furnace along with an empty sealed reference pan. All the samples were tested under dry nitrogen atmosphere with a flow rate of 50 mL/min, and heated from 10°C to 55°C at a rate of 2°C/min.
3. Results and discussion
3.1. Copolymerized hydrogel synthesis
The crosslinking mechanisms for the copolymer hydrogels are schematically illustrated in Fig. 2 (a) NIPAAm-co-QMA gels and (b) NIPAAm-co-CMA gels. The crosslinkers QMA or CMA were obtained by the reaction of curcumin or quercetin with acryloyl chloride. The multiple acrylate groups on QMA and CMA allow for crosslinking with the NIPAAm chain and the formation of branched network structures. Even without the presence of a normal crosslinker, CMA and QMA could still provide enough C=C to react with the vinyl group in NIPAAm. Due to this unique property, the network structures of the resulting copolymer hydrogels might be slightly different than normal hydrogel.
Fig. 2.
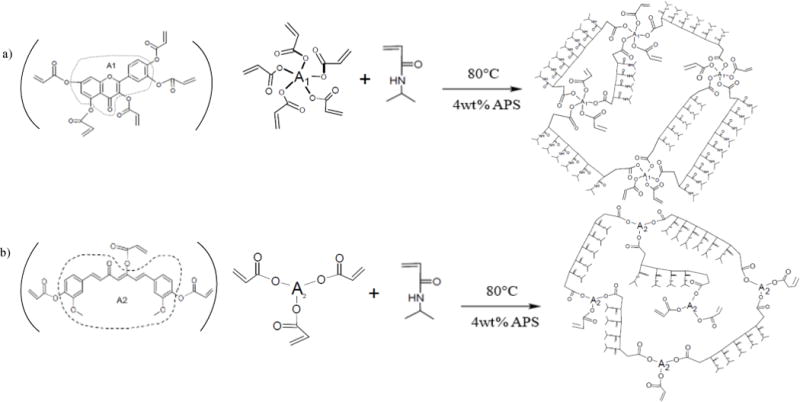
Schematic for the polymerization of (a) NIPAAm-co-QMA gels, (b) NIPAAm-co-CMA gels
For the reaction parameters studied, there were limited concentration ranges possible for the CMA and QMA incorporated into the hydrogel network. For CMA gels, once the crosslinker content was increased to 3.6 mol% or above, the gelation process was inhibited, resulting in a sticky product mixture instead of a hydrogel. A similar effect was observed with the QMA gels at higher crosslinker contents (i.e., greater than 3.6 mol%).
3.2. ss-NMR spectra
SSNMR spectra of pure NIPAAm and (a) CMA, copolymerized hydrogel with 0.9, 1.8 and 2.7 mol% of CMA content (b) QMA, copolymerized hydrogel with 0.9, 1.8 and 2.7 mol% of QMA content are given in Fig. 3. The spectrum of CMA copolymerized hydrogel showed peaks at 23 and 42 ppm, which are attributed to the n-isopropyl group (–CH(C2H6)) from NIPAAm. The peaks at 56 and 151 ppm are characteristics of the methoxy (–OCH3) and carbonyl (–C=O) groups from CMA, suggesting that CMA was successfully incorporated into the hydrogel matrix. Similarly, the presence of the peaks at 157 and 145 ppm in QMA copolymerized gel are the characteristics from QMA, and the peaks at 23 and 42 ppm are the characteristics from NIPAAm, which again suggests the successful crosslinking of QMA and NIPAAm monomers. Furthermore, CMA/QMA characteristic peak to NIPAAm characteristic peak ratios increased, which indicated that the content of CMA and QMA in the hydrogel increased.
Fig. 3.
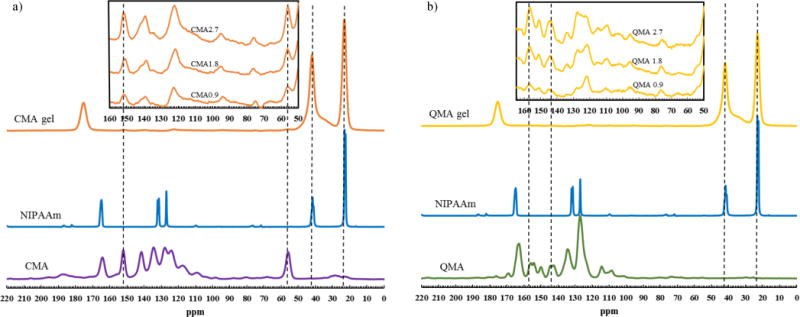
Solid state-NMR spectra of pure monomers and hydrogels at different composition (a) CMA gel system; (b) QMA gel system
3.3. Characterization of the morphology
NIPAAm copolymer hydrogels were successfully synthesized using the free radical polymerization with APS as the initiator. Additionally, hydrogel microparticles were obtained through cryogrinding. Hydrogel microparticles offer several advantages over the bulk gels including enhanced solvent transport resulting in faster swelling response, increased surface area, etc. Representative images of copolymerized hydrogels and gel microparticles are shown in Fig. 4 (a) CMA gel; (b) QMA gel; (c) CMA microparticles; (d) QMA microparticles. The designed CMA hydrogels were light orange/yellow color and the QMA hydrogels were of yellow/green color. The hydrogel color deepens and the texture became more rigid as more CMA/QMA was incorporated. The representative SEM images are shown in Fig. 5 (a) CMA; (b) QMA. It is observed that agglomerates were formed, suggesting the tendency of the microparticles to absorb moisture from the air very easily. From the SEM images, it is observed that the individual microparticles are approximately 5 μm in diameter. In addition, the size analyzer reported the mean diameter of the CMA and QMA microparticles as 19.8 ± 0.38 μm and 21.5 ± 0.39 μm, respectively. The size analyzer measured very small aggregates instead of separated microparticles, resulting in reported sizes larger than observed in the SEM.
Fig. 4.

Polyphenolic hydrogels and magnetic gels with the corresponding microparticles. (a)CMA gel; (b) QMA gel; (c) CMA gel microparticles; (d) QMA gel microparticles.
Fig. 5.
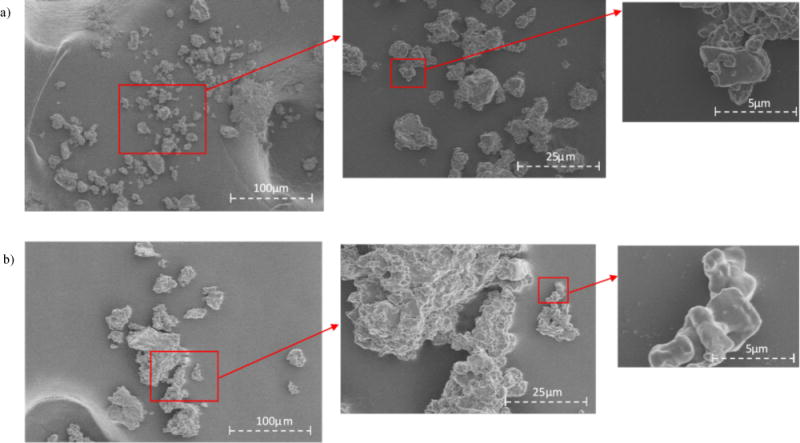
SEM images of (a) CMA hydrogel microparticles, (b) QMA hydrogel microparticles
3.4. Temperature responsive equilibrium swelling
The effect of crosslinkers content on hydrogel swelling behaviors are shown in Fig. 6. 0.9 mol% PEG400DMA gels were synthesized as the control. It can be noted that PEG 0.9 gels displayed the sharpest change in swelling ratio, and these gels showed the closest swelling behavior to homopolymer NIPAAm. Among the CMA/QMA gels, 0.9 content gels demonstrated the sharpest phase change, and the transition point occurred close to 33°C. The swelling transition became broader as more crosslinker was incorporated, which could be explained by the increased hydrophobicity introduced by CMA or QMA. The swelling ratios at room temperature decreased with increasing crosslinkers contents. As CMA or QMA content increased to 2.7 mol%, the gels had significantly reduced swelling ratios of 2.5 and 4, respectively. Zhang et al. reported this was a result of the dilution of the thermal response of NIPAAm with the incorporation of non-thermal responsive crosslinkers [44]. As expected, the swelling ratio of a hydrogel is decreased with increasing degree of crosslinking. The higher CMA or QMA content resulted in the higher degree of crosslinking. In addition, the broad phase transition noted in high CMA/QMA composition hydrogels could also be attributed to the high degrees of crosslinking.
Fig. 6.
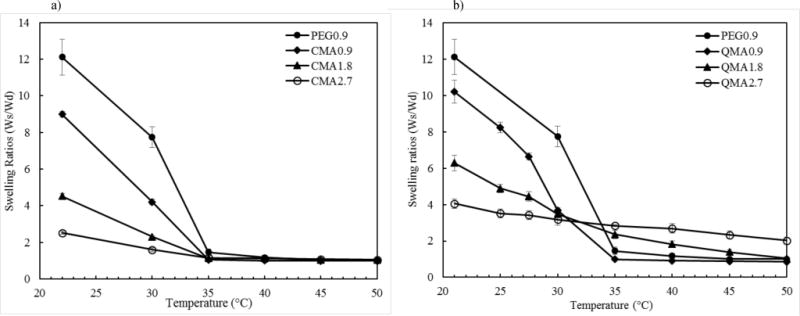
Weight swelling ratios of NIPAAm copolymer gels in response to temperature. a) CMA gels; (b) QMA gels. Data were plotted as mean±standard deviation and 3 measurements were finished by each sample.
The CMA gels showed no dependence on the content in high temperature region (40°C to 50°C), and all gels collapsed completely at 50°C. However, QMA gels displayed different degrees of swelling within the higher temperature region, and the QMA 2.7 gels showed a limited response to temperature change and did not collapse completely. This is likely due to the multifunctional nature of the QMA limiting the extent that the crosslink network can collapse. Similar phenomenon have been observed in Atta and Elsaeed’s work [45]. It is also interesting to note that QMA gels showed slightly higher swelling ratios than CMA gels. One possible explanation could be that the QMA gels did not reach the same conversion during the synthesis. As a result, QMA gels had a more loosely crosslinked network structure than CMA gels. Therefore, QMA gels were able to absorb more water at low temperature, which resulted in higher swelling ratios.
Gels in response to pulsatile temperature change were studied in order to confirm their reversible swelling/deswelling properties, as shown in Fig. 7. The temperature changes were designed to occur across the LCST of NIPAAm between room temperature and 55°C. The dramatic swelling ratios difference from low to high temperature could be explained by the hydrogel network collapse, followed by water being repelled from the crosslinked structure. The swelling ratio differences changed proportionally to the crosslinker composition, and the difference was getting smaller as more crosslinker being incorporated. CMA hydrogels showed completely reversible swelling properties at the chosen temperature range, and no decrease in reswelling ratios was observed, suggesting a good reversible swelling. However, QMA hydrogels showed slight decrease in reswelling ratios, and this was confirmed with a second study, so that the QMA reversible swelling data shows the average of 6 measurements. As discussed in the previous section, we suspect that the QMA gels were not fully reacted during the synthesis, so further crosslinking happened during the reversible heating process, which resulted in a decrease in hydrogel reswelling ratio.
Fig. 7.
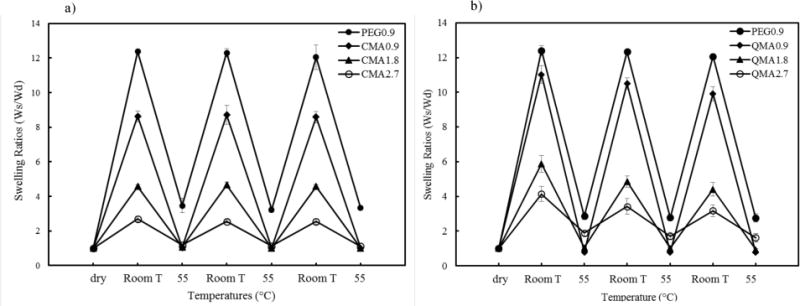
Weight swelling ratios of NIPAAm copolymer gels in response to pulsatile temperature changes between room temperature and 55°C. (a) CMA gels; (b) QMA gels
It is interesting to note that the PEG crosslinked NIPAAm gels did not completely collapse at 55°C in reversible swelling study. One possible explanation could be the forming of “skin” type barrier on the hydrogel surface. Some studies [44, 46] reported similar phenomenon and explained it was due to the hydrogel surface forming dense and less permeable skin layer once experiencing a sudden temperature change, which resulted in a water permeation rate difference between the surface and interior hydrogel. During the experiments, we observed water bubbles formed on the surface of PEG 0.9 gel, which could be due to the poor water-permeability of the skin barrier. However, such issues were not observed with CMA or QMA hydrogels, which is probably because they had higher crosslinking degree and lower water content, so they responded slower to sudden temperature change and the retained water was being diffused out more steadily.
3.6. LCST measurement
The LCSTs were characterized by differential scanning calorimeter (DSC). The DSC thermograms of the two NIPAAm copolymer hydrogels (0.9%–2.7%) are shown in Fig. 8 (a) for CMA gels and (b) for QMA gels, numerical values were plotted and listed in Fig. 8 (c). The endothermic peaks were referred as LCSTs of the hydrogels. There appears to be distinct peaks at 32.2°C, 31.2°C, 30.4°C for CMA gels and 31.3°C, 30.4°C, 28.9°C for QMA gels, respectively. As expected, the LCSTs of the gels were shifted to lower temperatures as more CMA or QMA got incorporated. It is well known that NIPAAm has both hydrophobic isopropyl group and hydrophilic -NH-CO-group, and the hydrophilic group forms intramolecular hydrogen bonds with water at low temperature. The overall hydrophilic content was decreased, and less hydrogen bonds were formed thus lower temperature was needed to break the bonds. The LCST could either be decreased by incorporation of hydrophobic components or increased by addition of hydrophilic components [47, 48].
Fig. 8.
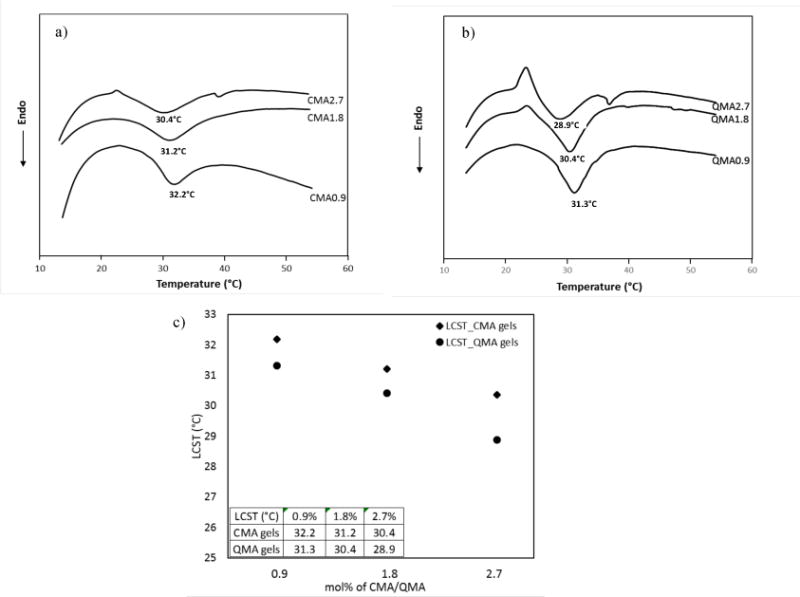
Typical DSC thermograms of the NIPAAm copolymerized hydrogels. (a) CMA0.9–2.7%; (b) and QMA 0.9–2.7%. (c) The numerical values of the LCSTs.
The LCST shifting in QMA gels was more distinct than CMA gels, besides, the CMA gels showed a broader peak shape than QMA gels. An explanation for this phenomenon could be that the QMA is slightly more hydrophobic, given the molecular structures of CMA and QMA showed in Fig. 1. Thus, the LCST was shifted more in QMA-co-NIPAAm gels due to more hydrophobic moieties being introduced into the hydrogel network.
Fig. 1.
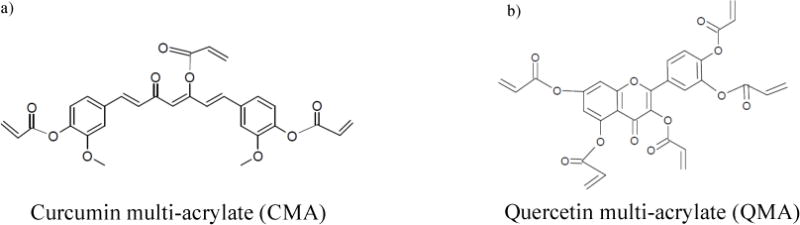
Structures of acrylated polyphenols, (a) CMA, (b) QMA.
4. Conclusion
A series of temperature responsive hydrogels with various crosslinker compositions were successfully synthesized through crosslinking NIPAAm with CMA or QMA. The resulting hydrogels exhibited distinct phase transition behaviors in a narrow temperature range and good responsiveness to reversible temperature change. The addition of QMA or CMA showed significant impact in controlling the swelling behavior and LCST of the hydrogels. The swelling ratios of the gels were decreased with increasing crosslinker loading, so did their temperature responsiveness. Meanwhile, the LCSTs of the copolymer gels showed a shifting to the lower temperatures with increasing contents of crosslinkers. The discussed polyphenolic NIPAAm gels can provide an attractive platform for a variety of applications, such as an intelligent water remediation strategy to remove environmental organic pollutants (e.g., PCBs).
Acknowledgments
The project described was supported by research grant from National Institute of Environmental Health Sciences (NIEHS) (Project No: P42ES007380).
References
- 1.Xue W, Hamley IW. Thermoreversible swelling behaviour of hydrogels based on N-isopropylacrylamide with a hydrophobic comonomer. Polymer. 2002;43(10):3069–3077. [Google Scholar]
- 2.Hirokawa Y, Tanaka T. Volume phase transition in a nonionic gel. The Journal of chemical physics. 1984;81(12):6379–6380. [Google Scholar]
- 3.Ogata T, Nonaka T, Kurihara S. Permeation of Solutes with Different Molecular-Size and Hydrophobicity through the Poly(Vinyl Alcohol)-Graft-N-Isopropylacrylamide Copolymer Membrane. Journal of Membrane Science. 1995;103(1–2):159–165. [Google Scholar]
- 4.Hou Y, et al. Thermoresponsive nanocomposite hydrogels with cell-releasing behavior. Biomaterials. 2008;29(22):3175–84. doi: 10.1016/j.biomaterials.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 5.Vihola H, et al. Drug Release Characteristics of Physically Cross-Linked Thermosensitive Poly(N-vinylcaprolactam) Hydrogel Particles. Journal of Pharmaceutical Sciences. 2008;97(11):4783–4793. doi: 10.1002/jps.21348. [DOI] [PubMed] [Google Scholar]
- 6.Stile RA, Healy KE. Thermo-responsive peptide-modified hydrogels for tissue regeneration. Biomacromolecules. 2001;2(1):185–194. doi: 10.1021/bm0000945. [DOI] [PubMed] [Google Scholar]
- 7.Jing GH, et al. Recent progress on study of hybrid hydrogels for water treatment. Colloids and Surfaces a-Physicochemical and Engineering Aspects. 2013;416:86–94. [Google Scholar]
- 8.Kureha T, Sato T, Suzuki D. Relationship between Temperature-Induced Changes in Internal Microscopic Structures of Poly(N-isopropylacrylamide) Microgels and Organic Dye Uptake Behavior. Langmuir. 2014;30(29):8717–8725. doi: 10.1021/la501838c. [DOI] [PubMed] [Google Scholar]
- 9.Parasuraman D, Sarker AK, Serpe MJ. Recyclability of poly (N-isopropylacrylamide) microgel-based assemblies for organic dye removal from water. Colloid and Polymer Science. 2013;291(8):1795–1802. [Google Scholar]
- 10.Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics. 2008;68(1):34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo M, et al. Effect of polyelectrolyte on the lower critical solution temperature of poly (N-isopropyl acrylamide) in the poly (NIPAAm-co-acrylic acid) hydrogel. Polymer. 2000;41(15):5713–5719. [Google Scholar]
- 12.Ju HK, Kim SY, Lee YM. pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly (N-isopropylacrylamide) Polymer. 2001;42(16):6851–6857. [Google Scholar]
- 13.Schild HG. Poly (N-Isopropylacrylamide) - Experiment, Theory and Application. Progress in Polymer Science. 1992;17(2):163–249. [Google Scholar]
- 14.Kim SY, et al. Thermo- and pH-responsive behaviors of graft copolymer and blend based on chitosan and N-isopropylacrylamide. Journal of Applied Polymer Science. 2000;78(7):1381–1391. [Google Scholar]
- 15.Lue SJ, Chen CH, Shih CM. Tuning of Lower Critical Solution Temperature (LCST) of Poly(N-Isopropylacrylamide-co-Acrylic acid) Hydrogels. Journal of Macromolecular Science Part B-Physics. 2011;50(3):563–579. [Google Scholar]
- 16.Lue SJ, et al. Thermally on-off switching membranes of poly(N-isopropylacrylamide) immobilized in track-etched polycarbonate films. Journal of Membrane Science. 2007;301(1–2):142–150. [Google Scholar]
- 17.Dimitrov I, et al. Thermosensitive water-soluble copolymers with doubly responsive reversibly interacting entities. Progress in Polymer Science. 2007;32(11):1275–1343. [Google Scholar]
- 18.Rzaev ZM, Dincer S, Pişkin E. Functional copolymers of N-isopropylacrylamide for bioengineering applications. Progress in Polymer Science. 2007;32(5):534–595. [Google Scholar]
- 19.Bae YH, Okano T, Kim SW. On-Off Thermocontrol of Solute Transport .2. Solute Release from Thermosensitive Hydrogels. Pharmaceutical Research. 1991;8(5):624–628. doi: 10.1023/a:1015860824953. [DOI] [PubMed] [Google Scholar]
- 20.Zhao CW, et al. Synthesis of biodegradable thermo- and pH-responsive hydrogels for controlled drug release. Polymer. 2009;50(18):4308–4316. [Google Scholar]
- 21.Spizzirri UG, et al. Thermo-Responsive Albumin Hydrogels with LCST Near the Physiological Temperature. Journal of Applied Polymer Science. 2011;121(1):342–351. [Google Scholar]
- 22.Choi C, Chae SY, Nah JW. Thermosensitive poly (N-isopropylacrylamide)-b-poly (ε-caprolactone) nanoparticles for efficient drug delivery system. Polymer. 2006;47(13):4571–4580. [Google Scholar]
- 23.Yoshida R, et al. Modulating the Phase-Transition Temperature and Thermosensitivity in N-Isopropylacrylamide Copolymer Gels. Journal of Biomaterials Science-Polymer Edition. 1994;6(6):585–598. doi: 10.1163/156856294x00536. [DOI] [PubMed] [Google Scholar]
- 24.Kim S, Healy KE. Synthesis and characterization of injectable poly (N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules. 2003;4(5):1214–1223. doi: 10.1021/bm0340467. [DOI] [PubMed] [Google Scholar]
- 25.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Advanced drug delivery reviews. 2001;53(3):321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 26.Prior-Cabanillas A, et al. Influence of the swelling history on the swelling kinetics of stimuli-responsive poly [(N-isopropylacrylamide)-co-(methacrylic acid)] hydrogels. Polymer. 2005;46(3):685–693. [Google Scholar]
- 27.Galaev IY, Mattiasson B. ‘Smart’polymers and what they could do in biotechnology and medicine. Trends in biotechnology. 1999;17(8):335–340. doi: 10.1016/s0167-7799(99)01345-1. [DOI] [PubMed] [Google Scholar]
- 28.Dai H, et al. A temperature-responsive copolymer hydrogel in controlled drug delivery. Macromolecules. 2006;39(19):6584–6589. [Google Scholar]
- 29.Patil VS, Dziubla TD, Kalika DS. Static and dynamic properties of biodegradable poly(antioxidant beta-amino ester) networks based on incorporation of curcumin multiacrylate. Polymer. 2015;75:88–96. [Google Scholar]
- 30.Nguyen HTP, et al. Novel alginate-based nanocarriers as a strategy to include high concentrations of hydrophobic compounds in hydrogels for topical application. Nanotechnology. 2015;26(25) doi: 10.1088/0957-4484/26/25/255101. [DOI] [PubMed] [Google Scholar]
- 31.Lao CD, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng AL, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–900. [PubMed] [Google Scholar]
- 33.Shoba G, et al. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–6. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- 34.Teong B, et al. Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. Journal of Materials Science-Materials in Medicine. 2015;26(1) doi: 10.1007/s10856-014-5357-3. [DOI] [PubMed] [Google Scholar]
- 35.Anand P, et al. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4(6):807–18. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 36.Glauert HP, et al. Role of oxidative stress in the promoting activities of PCBs. Environmental Toxicology and Pharmacology. 2008;25(2):247–250. doi: 10.1016/j.etap.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tharappel JC, et al. Effect of antioxidant phytochemicals on the hepatic tumor promoting activity of 3,3′,4,4′-tetrachlorobiphenyl (PCB-77) Food and Chemical Toxicology. 2008;46(11):3467–3474. doi: 10.1016/j.fct.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wattamwar PP, et al. Synthesis and characterization of poly(antioxidant beta-amino esters) for controlled release of polyphenolic antioxidants. Acta Biomater. 2012;8(7):2529–37. doi: 10.1016/j.actbio.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 39.Andrew E, Bradbury A, Eades R. Removal of dipolar broadening of nuclear magnetic resonance spectra of solids by specimen rotation. 1959 [Google Scholar]
- 40.Pines A, Gibby M, Waugh J. Proton- enhanced NMR of dilute spins in solids. The Journal of Chemical Physics. 1973;59(2):569–590. [Google Scholar]
- 41.Dixon W, et al. Total suppression of sidebands in CPMAS C-13 NMR. Journal of Magnetic Resonance (1969) 1982;49(2):341–345. [Google Scholar]
- 42.Fung B, Khitrin A, Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. Journal of Magnetic Resonance. 2000;142(1):97–101. doi: 10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 43.Barich DH, et al. 3-Methylglutaric acid as a 13 C solid-state NMR standard. Solid state nuclear magnetic resonance. 2006;30(3):125–129. doi: 10.1016/j.ssnmr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Zhang XZ, et al. Synthesis and characterization of partially biodegradable and thermosensitive hydrogel. J Mater Sci Mater Med. 2004;15(8):865–75. doi: 10.1023/B:JMSM.0000036274.83104.fe. [DOI] [PubMed] [Google Scholar]
- 45.Atta AM, Elsayed AM, Shafy HI. Uses of electron- beam irradiation to prepare pH-and temperature- sensitive hydrogels from reactive poly (vinyl alcohol) grafts. Journal of applied polymer science. 2008;108(3):1706–1715. [Google Scholar]
- 46.Gutowska A, et al. Heparin release from thermosensitive hydrogels. Journal of controlled release. 1992;22(2):95–104. [Google Scholar]
- 47.Huang S, et al. Dual pH- and temperature- responsive hydrogels with extraordinary swelling/deswelling behavior and enhanced mechanical performances. Journal of Applied Polymer Science. 2015;132(9) [Google Scholar]
- 48.Bae YH, Okano T, Kim SW. “On–Off” thermocontrol of solute transport. I. Temperature dependence of swelling of N-isopropylacrylamide networks modified with hydrophobic components in water. Pharmaceutical research. 1991;8(4):531–537. doi: 10.1023/a:1015871732706. [DOI] [PubMed] [Google Scholar]


