Main Text
Glial cell line-derived neurotrophic factor (GDNF) protein and gene therapy are currently under clinical investigation in Parkinson’s disease (PD) patients. GDNF has profound protective effects on dopamine neurons in numerous neurotoxicant PD models in rats and non-human primates.1, 2 However, in another PD model generated by recombinant adeno-associated virus (rAAV) vector transduction of human alpha-synuclein (α-syn) into the midbrain of the rat GDNF reportedly failed to exert robust neuroprotection.3 The marked discrepancy in preclinical therapeutic efficacy between these models of PD may have relevance to the ongoing clinical studies in sporadic PD.
In a recent rat study, Decressac et al.4 tested whether nigrostriatal overexpression of α-syn impairs GDNF signaling, rendering nigral dopamine (DA) neurons insensitive to its trophic effect. The authors used a rAAV rat model overexpressing human wild-type α-syn to levels at least 4-fold higher than endogenous rat α-syn levels.4, 5 The authors reported that the intracellular signaling response to GDNF was blocked in nigral DA neurons. The transcription factor nuclear receptor related 1 (Nurr1) and downstream target GDNF receptor tyrosine kinase (RET) were downregulated at both the transcriptional and translational levels in the rat midbrain. Overall, these data suggest that markedly increased α-syn in the rat is toxic and may result in the disruption of GDNF signaling.
Caution must be used in translating these results to clinical PD studies. The predictive value of the rAAV α-syn overexpression rat model to sporadic PD is unclear. For example, the marked overexpression of α-syn in the rAAV model fails to reproduce the pathological state of sporadic PD. Indeed, two studies of human tissue reported that α-syn mRNA expression was decreased in sporadic PD patients compared to age-matched controls.6, 7 The decrease of α-syn mRNA expression was due to lower mRNA expression in individual nigral DA neurons rather than to reduction in nigral cell numbers. Kinsbury et al.6 further showed that α-syn expression decreased gradually as the disease progressed. Further, preclinical models in which synucleinopathy is induced by intracerebral injection of pre-formed α-syn fibrils similarly report a decrease in soluble α-syn in neurons possessing Lewy body (LB)-like α-syn inclusions.8 There is also precedence for discordant observations to be made when modeling PD in rats versus primates. Viral delivery of GDNF to the nigrostriatal pathway decreases tyrosine hydroxylase (TH) gene expression in rats9 but increases TH in primates.1
Herein we evaluate the association of α-syn gene SNCA with GDNF signaling molecules in PD patient brain samples, α-syn transgenic mice, and AAV-mediated α-syn transgenic rats. We demonstrate that α-syn mRNA is not increased in sporadic PD and α-syn accumulation does not block expression of GDNF signaling molecules in PD and disease models.
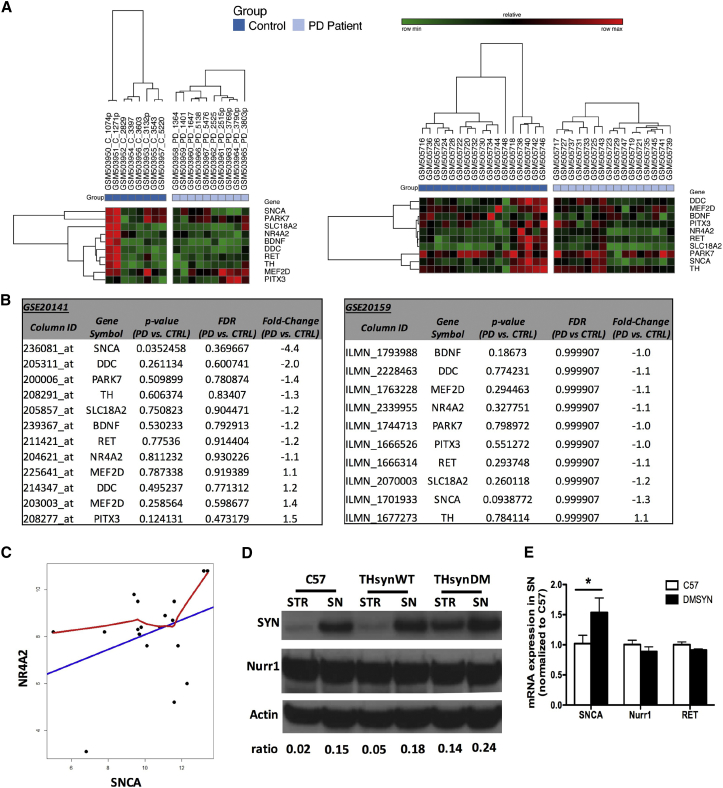
We observe no increase in α-syn gene (SNCA) expression in DA neurons laser-captured from the substantia nigra (SN) of 10 PD subjects and 8 age-matched control subjects (Gene Expression Omnibus [GEO]: GSE20141) (Figure 1A, left panel). Laser-captured samples eliminate the differences in DA neuron numbers between patients and controls. In this microarray dataset, α-syn gene (SNCA) expression (column ID: 236081; Figure 1B, left table) was significantly downregulated in PD subjects compared to controls (p = 0.035, false discovery rate [FDR] = 0.369, fold change = −4.4).
Figure 1.
Nurr1 and Associated Genes Expression in Sporadic PD Patients and α-syn Transgenic Mice
(A) Hierarchical clustering heatmaps for microarray datasets GEO: GSE20141 (left panel) and GSE20159 (right panel). (B) Expression of genes of interest in the microarray datasets (GEO: GSE20141, left table; GEO: GSE20159, right table). The p values as well as Benjamini-Hochberg false discovery rates (FDRs) and fold change were reported for every single gene. (C) The scatterplot was drawn from the robust multi-array average (RMA)-processed and logarithm 2-transformed microarray intensity data of 18 samples. The x axis represents SNCA as an independent variable, and the y axis represents NR4A2 as a dependent variable. The straight blue line is the linear regression result between the two genes SNCA and NR4A2, and the red line is gained by the local weighted scatterplot smoothing (LOWESS) regression analysis. The correlation coefficient is 0.4084 with p = 0.1442. (D) α-syn and Nurr1 protein expression in the striatum and nigra of C57, THsynWT, and THsynDM mice. Striatum (STR) and nigra (SN) tissues were microdissected from 6-month-old transgenic mice with expression of wild-type synuclein (THsynWT) or doubly mutated α-syn (A30P & A53T; THsynDM) or age-controlled C57. The expression of α-syn and Nurr1 was determined by western blot analysis. The signal density ratio of α-syn over action was quantified. (E) α-syn, Nurr1, and RET gene expression in the SN were determined by qRT-PCR.
To assess whether SN neurons with high α-syn expression might selectively degenerate in the early stages of the disease compared to neurons with reduced expression represent, we analyzed another set of microarray data with samples from early preclinical PD subjects (GEO: GSE20159). These SN samples consisted of 16 cases with subclinical, PD-related, α-syn-positive, incidental LB disease and 17 age-, sex-, and postmortem interval-matched controls. In this dataset, we again failed to detect an increase in α-syn gene expression in the subclinical PD group compared to control (Figures 1A, right panel, and 1B, right table). The subclinical PD microarray analysis indicates that SN neurons with high α-syn gene expression are not present in the early stages of the disease.
Decressac et al.4 showed a downregulation of Nurr1 and RET at the transcriptional and translational level in the rAAV-α-syn-transduced rats, which contributed to the disruption of GDNF signaling. However, by analyzing the microarray data (GEO: GSE20159 and GSE20141), which covered subclinical and clinical stages of diseases; separately, we failed to detect any significant decrease in gene expression levels for Nurr1, RET, and other associated genes (PARK7, SLC18A2, BDNF, DDC, TH, MEF2D, and PITX3) in the sporadic PD patients (Figures 1A and 1B). Furthermore, we analyzed the association between NR4A2 (Nurr1) and SNCA gene expression in individual PD patients and did not find any significant correlation between the two genes (Figure 1C), suggesting that SNCA may not affect NR4A2 expression at the transcriptional level.
To extend our analysis, we examined transgenic α-syn mice. We previously published microarray data from synuclein transgenic mice wherein a TH promoter drove overexpression of either wild-type α-syn (THsynWT) or doubly mutated (A30P and A53T) synuclein (THsynDM).10 We observed no significant downregulation in gene expression for Nurr1 and downstream target genes, including RET, PARK7, SLC18A2, BDNF, DDC, MEF2D, and PITX3, in the transgenic mice. We further validated the gene expression for Nurr1 and RET by RT-PCR and protein expression for Nurr1 by western blotting and again failed to detect significant decreases for Nurr1 and RET at the transcript level and Nurr1 at the protein level in the α-syn transgenic mice compared to wild-type controls (Figures 1D and 1E).
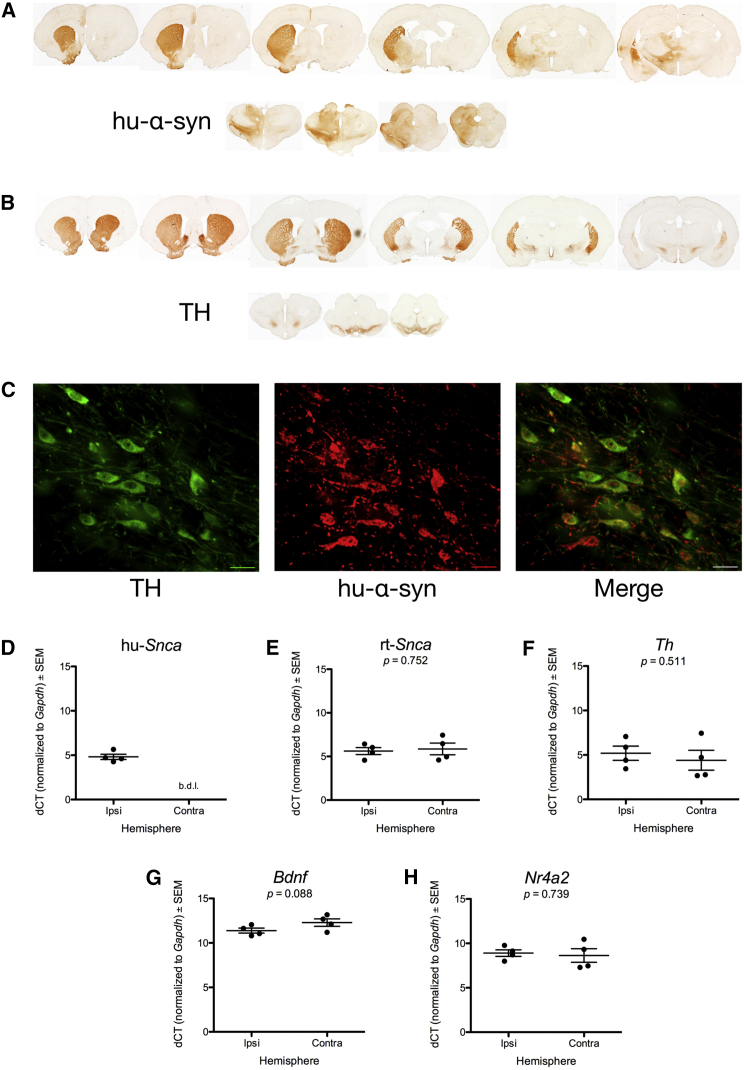
Finally, we also used rAAV to overexpress α-syn in a manner very similar to Decressac et al.4 rAAV expressing human wild-type α-syn (rAAV2/5-α-syn, 2.2 × 1012 genome copies per ml) was injected unilaterally into the SN as described previously.11 Eight weeks after nigral injection, brains were processed for immunohistochemistry for human wild-type α-syn (Figures 2A and 2C) or TH (Figures 2B and 2C). Stereological analysis of TH immunoreactive (THir) neurons in the SN pars compacta (SNpc) revealed a non-significant ∼10% loss of SNpc neurons at 8 weeks following injection. The ipsilateral SNpc possessed an estimated 12,626 ± 411 THir neurons compared to 11,401 ± 561 remaining THir neurons in the contralateral SNpc (t(8) = 1.764, p > 0.05). This modest level of degeneration is associated with an ∼50% increase in human α-syn protein in the striatum.11, 12 In contrast, Decressac et al.5 report an ∼80% SNpc loss with transduction parameters, which resulted in an ∼8-fold increase in α-syn protein in the striatum.
Figure 2.
Moderate Viral Vector-Mediated α-syn Overexpression in the Rat Nigrostriatal System Does Not Decrease rat α-syn, BDNF, TH, or Nurr1 in the SN
Unilateral intranigral injections of recombinant rAAV2/5-α-syn results in human wild-type α-syn immunoreactivity in the nigrostriatal system. (A) Representative coronal sections demonstrating immmunoreactivity to human wild-type α-syn in the nigrostriatal hemisphere ipsilateral to injection. (B) Both hemispheres were labeled with antisera to tyrosine hydroxylase (TH). (C) Dual-label immunofluorescence identifies THir neurons of the SNpc that co-express human α-syn. A non-significant ∼10% loss of SNpc neurons was observed at 8 weeks following injection. (D) SN tissue punches analyzed using qPCR demonstrate human α-syn transgene (hu-Scna) in the ipsilateral, but not the contralateral, SN. (E–H) No differences between the ipsilateral and contralateral SN were detected for rat α-syn (rt-Snca) (E), BDNF (Bdnf) (F), TH (Th) (G), or Nurr1 (Nr4a2) (H). Scale bars, 25 μm.
In a separate cohort of identical rAAV2/5-α-syn-injected rats, SN tissue ipsilateral and contralateral to rAAV2/5-α-syn were examined for human wild-type α-syn transcript levels (hu-Snca). Expression of human wild-type α-syn transcript was only evident in the ipsilateral SN, with none detected in the contralateral SN (Figure 2D). The SN was also examined for levels of endogenous rat wild-type α-syn (rt-Snca), brain-derived neurotrophic factor (Bdnf), TH (Th) and Nurr1 (NR4A2). No statistically significant differences were observed due to α-syn transduction and overexpression compared to the contralateral, non-transduced, control hemisphere.
The analysis of PD brain tissue, transgenic mouse, and rAAV-transduced rat data indicate that expression of the α-syn gene (SNCA) is not increased in sporadic PD and α-syn accumulation does not block GDNF signaling in PD and disease models.
The analysis of public human datasets indicates that expression of the α-syn gene (SNCA) is not increased in the nigral dopaminergic neurons in patients with sporadic PD, which aligns with the previous studies showing no increase of SNCA gene expression in sporadic PD enteric neurons,13 CSF,14 and blood.15 Thus, accumulation of aggregated α-syn protein in sporadic PD is unlikely due to the enhanced SNCA gene expression, but, rather, is mediated by downstream failure to clear the protein, owing to either a breakdown in normal protein degradation processes and/or aberrant protein misfolding/post-translational processes that render these conformers resistant to normal degradation processes. The analysis of public human datasets also shows no change in the transcription level of Nurr1, RET, and other associated genes (PARK7, SLC18A2, BDNF, DDC, TH, MEF2Ds and PITX3) in the sporadic PD patients as well as no positive correlation of expression of SNCA and the Nurr1 gene, NR4A2. These findings at least suggest that, at the transcriptional level, GDNF signaling molecules Nurr1, RET, and other associated genes are not affected in sporadic PD.
It is also important to assess the GDNF signaling molecule Nurr1 expression at the translational level in PD patients. Chu et al.16 reported that SN neurons lacking α-syn inclusions from sporadic PD subjects displayed Nurr1 immunofluorescence optical density (OD) measurements that were similar to age-matched controls, whereas nigral neurons with α-syn LBs exhibited significantly decreased Nurr1 measurements. However those LB-bearing neurons were only, on average, representing 3.5%–15% of total SN neurons in sporadic PD patients, as shown by recent studies.17 It therefore appears that the majority (>85%) of SN neurons from sporadic PD contain normal protein levels of Nurr1.
The variability of rAAV α-syn gene transfer to the rat nigrostriatal pathway to elicit changes in SN dopamine neuron numbers, alterations in signaling molecules and neurobehavioral changes suggests that vector type, particle number, packaging methods, and purifications are potential contributors. Systematic evaluation of each is required to delineate which of these may be the most important determinant of the observed variability. Because these studies all require forced expression of a gene product from a virus vector administrated intracerebrally, the clinical relevance of any such models to sporadic PD is limited, if at all relevant.
Based on our PD human data, the gene expression of GDNF signaling molecules, including RET and NUR1, are not downregulated disregarding α-syn accumulation. It should be noted that Hadaczek et al.18 reported attenuated GNDF signaling as demonstrated by decreased phosphorylated RET (pRET) in neuronal cell lines and animals deficient in ganglio-series gangliosides. Whether pRET was decreased in PD brain remains to be investigated. Interestingly, AAV2-GDNF treatment was able to restore nigral TH-positive neurons and improve behavioral dysfunction in animals with ganglio-series gangliosides deficiency, suggesting that excess GDNF may suffice to maintain effective neuroprotective signaling, regardless of decreased pRET.
In summary, there are several important conclusions from these human transgenic mice and rAAV-transduced rat data. First, α-syn gene expression levels are not increased in the early stage of PD or in association with disease progression. Second, the majority of SN neurons in PD contain normal levels of Nurr1. Third, transgenic overexpression of human α-syn in mice did not result in downregulation of Nurr1 or RET. Fourth, rAAV transduction of rat SN producing a moderate increase in human α-syn did not result in downregulation of Nurr1, TH, or BDNF. We conclude that forced and marked overexpression of α-syn, as described in the rat rAAV model by Decressac et al.,4 is not a relevant model for human sporadic PD. Given there is no evidence to indicate that patients with sporadic PD will be refractory to GDNF therapy, clinical equipoise is warranted for ongoing GDNF therapeutic trials.
Footnotes
Supplemental Information includes Supplemental Materials and Methods and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.04.018.
Supplemental Information
References
- 1.Kells A.P., Eberling J., Su X., Pivirotto P., Bringas J., Hadaczek P., Narrow W.C., Bowers W.J., Federoff H.J., Forsayeth J., Bankiewicz K.S. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J. Neurosci. 2010;30:9567–9577. doi: 10.1523/JNEUROSCI.0942-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirik D., Rosenblad C., Bjorklund A., Mandel R.J. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J. Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Decressac M., Ulusoy A., Mattsson B., Georgievska B., Romero-Ramos M., Kirik D., Björklund A. GDNF fails to exert neuroprotection in a rat α-synuclein model of Parkinson’s disease. Brain. 2011;134:2302–2311. doi: 10.1093/brain/awr149. [DOI] [PubMed] [Google Scholar]
- 4.Decressac M., Kadkhodaei B., Mattsson B., Laguna A., Perlmann T., Björklund A. α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci. Transl. Med. 2012;4:163ra156. doi: 10.1126/scitranslmed.3004676. [DOI] [PubMed] [Google Scholar]
- 5.Decressac M., Mattsson B., Lundblad M., Weikop P., Björklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons. Neurobiol. Dis. 2012;45:939–953. doi: 10.1016/j.nbd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Kingsbury A.E., Daniel S.E., Sangha H., Eisen S., Lees A.J., Foster O.J. Alteration in alpha-synuclein mRNA expression in Parkinson’s disease. Mov. Disord. 2004;19:162–170. doi: 10.1002/mds.10683. [DOI] [PubMed] [Google Scholar]
- 7.Neystat M., Lynch T., Przedborski S., Kholodilov N., Rzhetskaya M., Burke R.E. Alpha-synuclein expression in substantia nigra and cortex in Parkinson’s disease. Mov. Disord. 1999;14:417–422. doi: 10.1002/1531-8257(199905)14:3<417::aid-mds1005>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Osterberg V.R., Spinelli K.J., Weston L.J., Luk K.C., Woltjer R.L., Unni V.K. Progressive aggregation of alpha-synuclein and selective degeneration of lewy inclusion-bearing neurons in a mouse model of parkinsonism. Cell Rep. 2015;10:1252–1260. doi: 10.1016/j.celrep.2015.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Georgievska B., Kirik D., Björklund A. Overexpression of glial cell line-derived neurotrophic factor using a lentiviral vector induces time- and dose-dependent downregulation of tyrosine hydroxylase in the intact nigrostriatal dopamine system. J. Neurosci. 2004;24:6437–6445. doi: 10.1523/JNEUROSCI.1122-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller R.M., Kiser G.L., Kaysser-Kranich T., Casaceli C., Colla E., Lee M.K., Palaniappan C., Federoff H.J. Wild-type and mutant alpha-synuclein induce a multi-component gene expression profile consistent with shared pathophysiology in different transgenic mouse models of PD. Exp. Neurol. 2007;204:421–432. doi: 10.1016/j.expneurol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Gombash S.E., Manfredsson F.P., Kemp C.J., Kuhn N.C., Fleming S.M., Egan A.E., Grant L.M., Ciucci M.R., MacKeigan J.P., Sortwell C.E. Morphological and behavioral impact of AAV2/5-mediated overexpression of human wildtype alpha-synuclein in the rat nigrostriatal system. PLoS One. 2013;8:e81426. doi: 10.1371/journal.pone.0081426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polinski N.K., Gombash S.E., Manfredsson F.P., Lipton J.W., Kemp C.J., Cole-Strauss A., Kanaan N.M., Steece-Collier K., Kuhn N.C., Wohlgenant S.L., Sortwell C.E. Recombinant adenoassociated virus 2/5-mediated gene transfer is reduced in the aged rat midbrain. Neurobiol. Aging. 2015;36:1110–1120. doi: 10.1016/j.neurobiolaging.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barrenschee M., Zorenkov D., Böttner M., Lange C., Cossais F., Scharf A.B., Deuschl G., Schneider S.A., Ellrichmann M., Fritscher-Ravens A., Wedel T. Distinct pattern of enteric phospho-alpha-synuclein aggregates and gene expression profiles in patients with Parkinson’s disease. Acta Neuropathol. Commun. 2017;5:1. doi: 10.1186/s40478-016-0408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mollenhauer B., Locascio J.J., Schulz-Schaeffer W., Sixel-Döring F., Trenkwalder C., Schlossmacher M.G. α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011;10:230–240. doi: 10.1016/S1474-4422(11)70014-X. [DOI] [PubMed] [Google Scholar]
- 15.Tan E.K., Chandran V.R., Fook-Chong S., Shen H., Yew K., Teoh M.L., Yuen Y., Zhao Y. Alpha-synuclein mRNA expression in sporadic Parkinson’s disease. Mov. Disord. 2005;20:620–623. doi: 10.1002/mds.20391. [DOI] [PubMed] [Google Scholar]
- 16.Chu Y., Le W., Kompoliti K., Jankovic J., Mufson E.J., Kordower J.H. Nurr1 in Parkinson’s disease and related disorders. J. Comp. Neurol. 2006;494:495–514. doi: 10.1002/cne.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkkinen L., O’Sullivan S.S., Collins C., Petrie A., Holton J.L., Revesz T., Lees A.J. Disentangling the relationship between lewy bodies and nigral neuronal loss in Parkinson’s disease. J. Parkinsons Dis. 2011;1:277–286. doi: 10.3233/JPD-2011-11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadaczek P., Wu G., Sharma N., Ciesielska A., Bankiewicz K., Davidow A.L., Lu Z.H., Forsayeth J., Ledeen R.W. GDNF signaling implemented by GM1 ganglioside; failure in Parkinson's disease and GM1-deficient murine model. Exp Neurol. 2015;263:177–189. doi: 10.1016/j.expneurol.2014.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.