Abstract
Vaccinia-based systems have been extensively explored for the development of recombinant vaccines. Herein we describe an innovative vaccinia virus (VACV)-derived vaccine platform technology termed Sementis Copenhagen Vector (SCV), which was rendered multiplication-defective by targeted deletion of the essential viral assembly gene D13L. A SCV cell substrate line was developed for SCV vaccine production by engineering CHO cells to express D13 and the VACV host-range factor CP77, because CHO cells are routinely used for manufacture of biologics. To illustrate the utility of the platform technology, a SCV vaccine against chikungunya virus (SCV-CHIK) was developed and shown to be multiplication-defective in a range of human cell lines and in immunocompromised mice. A single vaccination of mice with SCV-CHIK induced antibody responses specific for chikungunya virus (CHIKV) that were similar to those raised following vaccination with a replication-competent VACV-CHIK and able to neutralize CHIKV. Vaccination also provided protection against CHIKV challenge, preventing both viremia and arthritis. Moreover, SCV retained capacity as an effective mouse smallpox vaccine. In summary, SCV represents a new and safe vaccine platform technology that can be manufactured in modified CHO cells, with pre-clinical evaluation illustrating utility for CHIKV vaccine design and construction.
Keywords: vaccine, chikungunya, vaccinia virus, live vectored vaccines, poxvirus, antibody, vaccine platform
Graphical Abstract
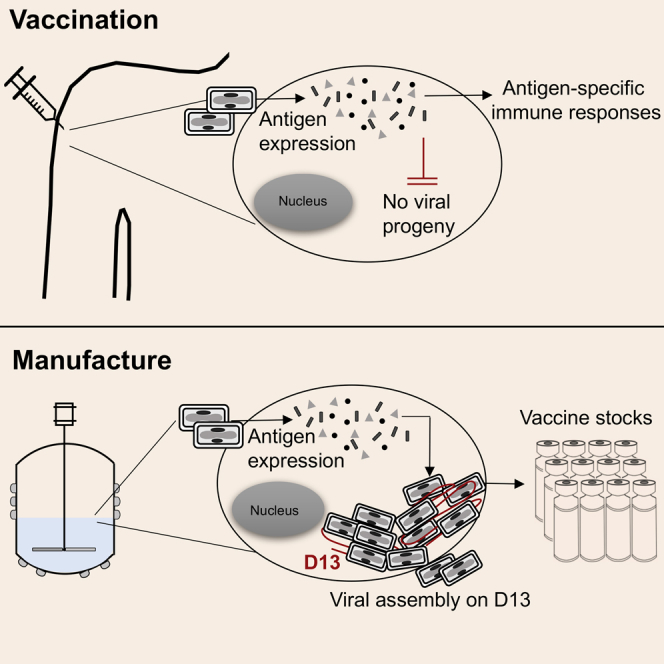
Eldi et al. report the development of the Sementis Copenhagen Vector technology, a multiplication-defective poxvirus-derived vaccine platform with a complementing CHO-based cell substrate line designed for large-scale vaccine stock manufacture. The utility of the technology was illustrated by the development of a safe and protective vaccine against chikungunya virus challenge.
Introduction
Vaccinia virus (VACV) vaccination successfully eradicated smallpox, and recombinant VACV-based vaccine vectors have subsequently been developed to target a number of diseases.1, 2, 3 The successful eradication of sylvatic rabies in northern and western Europe with the use of recombinant vaccinia rabies vaccine4, 5 highlights the utility and immunogenicity of recombinant vaccinia-based vaccines. The advantages of VACV systems include a large transgene payload capacity (up to 25 kb), minimal risk of host genome integration,6 induction of robust and long-lived cell-mediated and humoral immune responses,7, 8 and an established manufacturing process with cold chain-independent distribution capacity.9 However, replication-competent VACV is not considered safe by contemporary standards, with adverse responses ranging from mild to life-threatening manifestations. The latter includes eczema vaccinatum, post-vaccinal encephalitis, and progressive vaccinia, with immunocompromised individuals particularly at risk.10 Subsequent generations of VACV vaccine vectors addressed these safety concerns and include the passage-attenuated modified vaccinia Ankara (MVA)1, 11 and the genetically modified New York vaccinia (NYVAC).3, 12 Although these highly attenuated vaccinia-based vaccine vectors have been broadly classified as safe and immunogenic, some issues remain, including evidence of viral multiplication in some human cell lines13, 14 and current dependence on primary chicken embryo fibroblasts (CEFs) for vaccine production (IMVANEX15). However, efforts to bypass the need for primary cell lines are being pursued. These include the use of designer cell lines such as immortalized duck cells.16, 17
Herein we describe the development of the Sementis Copenhagen Vector (SCV) vaccine platform technology that comprises (1) a SCV vaccine vector system that is unable to produce infectious viral progeny in vaccine recipients and (2) a SCV cell substrate (SCS) cell line, based on Chinese hamster ovary (CHO) cells, that can be used for SCV vaccine production. The SCV vaccine was generated by targeted deletion of the D13L gene, which encodes an essential viral assembly protein.18, 19 This approach to VACV attenuation was chosen, instead of targeting the viral genome replication machinery,20 to preserve genome amplification, thereby permitting late-phase expression of vaccine antigens from the amplified genomes. Production of SCV vaccines in the SCS line was enabled by the in trans provision of D13 and the host-range protein CP77, which provides VACV multiplication capability in CHO cells.21 CHO cells are widely used for the manufacture of biologics,22, 23 with a CHO-based system for vaccine manufacture avoiding some issues associated with primary CEFs, such as risk of contamination, inherent batch-to-batch variation, lack of cell banking options, and restricted scale-up capacities.24 In addition, CHO cells allow for manufacture using suspension cultures, which permits rapid scale-up of production in bioreactors. Many licensed viral vaccines are made in adherent cells lines (MCR-5, WI-38, and Vero), in which scale-up requires multiple rounds of passaging and reseeding.
The utility of the SCV vaccine technology was illustrated by the development and testing of a SCV vaccine for chikungunya virus (CHIKV). The largest outbreak of CHIKV ever recorded began in 2004, with this mosquito-borne virus initially spreading from Africa to Asia (with small outbreaks in Europe). In 2013, the epidemic reached the Americas and is spreading through South America, where millions of cases have been reported.25, 26 The primary disease manifestation of CHIKV is acute and chronic polyarthritis or polyarthralgia,27, 28 with a number of severe disease manifestations recognized that result in hospitalization rates of 2.3%–13% and mortality rates of 0.01%–0.1%.29, 30 Herein we show that a SCV vaccine expressing the structural polyprotein cassette (C-E3-E2-6K-E1) of the Réunion Island isolate (LR2006 OPY1) of CHIKV (a SCV vaccine against CHIKV [SCV-CHIK]) provided, after a single vaccination, robust and durable neutralizing antibody responses and complete protection against both CHIKV and poxvirus (ectromelia virus [ECTV]) challenge. SCV-CHIK was unable to produce infectious viral progeny either in a panel of human cell lines or in immunocompromised mice. This study thus demonstrates the utility and effectiveness of the new SCV vaccine platform and illustrates its application to CHIKV vaccine design and construction.
Results
Design Rationale for the SCV Vaccine Technology
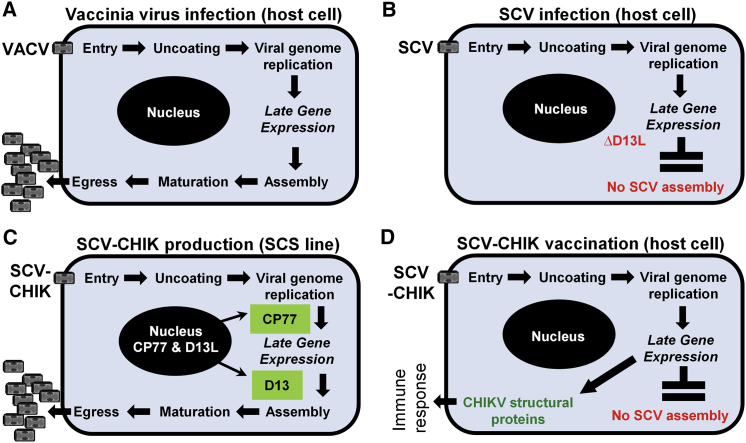
The Copenhagen strain of VACV, the parental virus used for the development of the SCV vaccine platform technology, was originally used as a smallpox vaccine in Denmark and the Netherlands and is replication-competent31 (Figure 1A). After entry, the viral genome is amplified (to ∼10,000 copies32, 33) and late gene expression is followed by virion assembly, maturation, and egress of VACV progeny (Figure 1A). Generation of infectious VACV progeny depends on the assembly protein D13, which provides a mechanical scaffold to facilitate cytoplasmic assembly of the developing virions (reviewed in Liu et al.19). The SCV vaccine platform incorporates a targeted deletion of the D13L gene in VACV to prevent viral assembly, thereby rendering SCV unable to generate infectious progeny in normally permissive cell lines. However, amplification of the SCV genome is retained (Figure 1B). CHO cells were engineered to constitutively express D13 and the cowpox virus host-range protein CP77. Without CP77, SCV DNA processing is blocked in CHO cells by host proteins.34 Expression of D13 and CP77 in the SCS line thus permits production of SCV vaccines (Figure 1C). Upon vaccination of mice or humans, a SCV vaccine (for example, SCV-CHIK) enters a host cell and, due to the absence of D13, is unable to generate viral progeny. SCV genome amplification results in the accumulation of a large number of genome copies, from which vaccine antigens (in this case, CHIKV structural proteins) are produced via late gene expression to instigate an effective immune response (Figure 1D). The SCV vaccine technology thus provides for a vaccinia-based vaccine vector platform that, in vaccine recipients, is unable to produce progeny or infectious progeny (hereafter referred to as multiplication-defective) and can be manufactured in a CHO-based cell substrate line.
Figure 1.
Rationale for the SCV Vaccine Platform Technology
(A) VACV multiplication in the cytoplasm of host cells is able to produce infectious viral progeny as VACV encodes the essential assembly protein D13. (B) Targeted deletion of D13L in SCV prevents virion assembly, rendering SCV multiplication-defective (unable to generate infectious progeny). (C) In trans provision of D13 in the CHO-based SCS line rescues viral assembly, allowing production of progeny for vaccine manufacture. Expression of the host-range protein CP77 allows SCV (or VACV) multiplication in CHO cells. (D) After delivery of SCV-CHIK to a vaccine recipient, the genome of SCV-CHIK is amplified and late gene expression drives production of the CHIKV structural proteins (vaccine antigen). In the absence of D13 protein, no SCV-CHIK viral progeny are generated.
Construction of SCV-CHIK
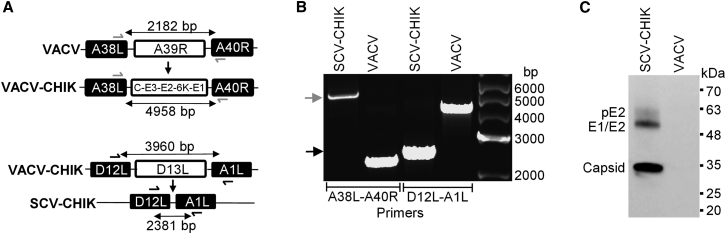
SCV-CHIK was constructed sequentially. Homologous recombination and positive selection were used to insert the structural CHIKV gene expression cassette (encoding the CHIKV S26 polyprotein sequence C-E3-E2-6K-E1) while deleting A39R, an immunomodulatory gene,35 to generate a VACV vaccine against CHIKV (VACV-CHIK). The D13L gene was then deleted to render the resultant SCV-CHIK multiplication-defective (Figure 2A). The insertion of the CHIKV gene cassette and the deletion of the D13L gene were confirmed by PCR (Figure 2B) and DNA sequencing of the resultant PCR products (data not shown). The CHIKV S26 polyprotein is processed by first releasing the capsid protein via autoproteolysis, mediated by a protease sequence found in the C-terminal end of the capsid protein sequence.36 The remaining polyprotein is then digested by cellular Signalase and Furin proteases to release E1, E2, E3, and 6K envelope proteins.37, 38 The expression of authentically processed polyprotein-derived CHIKV antigens was confirmed by immunoblot analysis of SCV-CHIK-infected SCS lysates using polyclonal mouse serum raised against inactivated CHIKV (Figure 2C).
Figure 2.
Construction and In Vitro Characterization of SCV-CHIK
(A) Schematic representation of SCV-CHIK construction. The CHIKV structural polyprotein cassette (C-E3-E2-6K-E1) was inserted into the A39R locus of VACV to generate VACV-CHIK. The D13L gene was deleted from VACV-CHIK to generate SCV-CHIK. (B) Viral DNA from parental VACV and SCV-CHIK was subjected to PCR analysis using site-specific primers shown in (A) to confirm the insertion of C-E3-E2-6K-E1 (green arrow) and deletion of D13L (blue arrow). (C) Immunoblot analysis of VACV- and SCV-CHIK-infected SCS cells using mouse polyclonal anti-CHIKV antisera confirmed expression of processed CHIKV antigens: pE2 (E3-E2), E1 and E2 (which co-migrate), and capsid.
Production of SCV-CHIK in the CHO-Based SCS Line
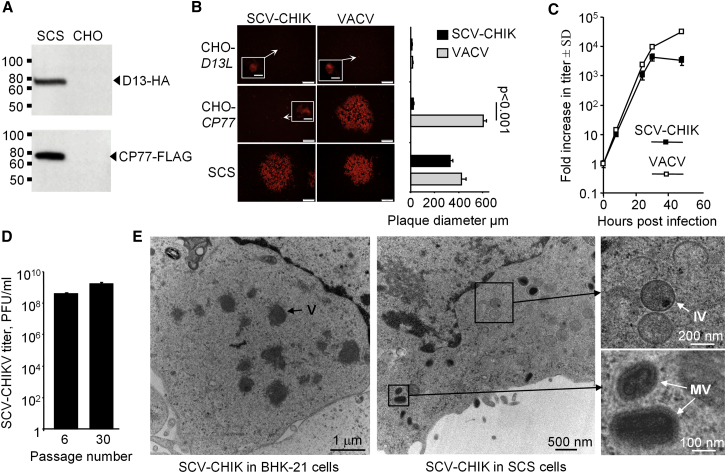
A proof-of-concept SCS line for the production of SCV vaccines was developed by transfecting CHO cells with plasmids encoding D13L (hemagglutinin [HA]-tagged) and CP77 (FLAG-tagged) genes under the control of the constitutive human elongation factor-1 alpha (EF-1a) promoter. Both genes were Chinese hamster (Cricetulus griseus) codon-optimized and integrated into the CHO genome using Piggybac transposon-mediated gene transfer technology.39 A monoclonal cell line was then established from stable polyclonal SCS cultures using flow cytometric-assisted single-cell sorting and expansion under antibiotic selection. Expression of D13 and CP77 was confirmed by immunoblotting of cell lysates using α-HA and α-FLAG antibodies, respectively (Figure 3A). No overt differences in the cellular morphology (data not shown) or growth kinetics (Figure S1) were observed between the SCS line and the parental CHO cells.
Figure 3.
Production and Morphology of SCV-CHIK in SCS Cells
(A) Immunoblot analysis confirmed expression of the HA-tagged D13 (assembly protein; 56 kDa) and FLAG-tagged CP77 (host-range protein; 77 kDa) in the SCS line using anti-HA and anti-FLAG antibodies, respectively. (B) Monolayers of CHO-D13L, CHO-CP77, and the SCS line were infected with VACV or SCV-CHIK (MOI, 0.001 PFU/cell). After 48 hr, the cells were fixed and viral plaques were examined by immunofluorescent staining using polyclonal anti-VACV antibody. Representative plaque images (scale bars, 200 μm) and single infected cells (inserts; scale bars, 10 μm) are shown. Bar chart shows mean plaque size (micrometers ± SD; n = 16–25) for the two viruses in the corresponding three cell lines. Statistics by Kolmogorov-Smirnov test. (C) SCS monolayers were infected with SCV-CHIK and VACV (MOI, 0.01 PFU/cell) and at the indicated times, virus titers were determined by plaque assay. Data are represented as the fold increase in the viral titer over the original inoculum (PFU ± SD; n = 4). (D) SCS cells, passaged 6 or 30 times, were infected with SCV-CHIK (MOI, 0.01 PFU/cell), and after 48 hr, titers were determined (n = 4 replicates). (E) Electron microscopy examination of virus morphology in SCV-non-permissive BHK-21 and SCV-permissive SCS cells infected with SCV-CHIK (MOI, 2 PFUs/cell). SCV-CHIK production was blocked at the viroplasma stage in BHK-21 cells, whereas virion assembly and maturation were observed in the SCS line. V, viroplasma; IV, immature virions; MV, mature virions.
To confirm that both D13 and CP77 proteins were required to rescue multiplication of SCV-CHIK, CHO cells expressing only D13 (CHO-D13L) or CP77 (CHO-CP77) were generated. Monolayers of CHO-D13L, CHO-CP77, and the SCS line were infected with the multiplication-competent VACV or SCV-CHIK, and plaque morphology was assessed by immunofluorescent staining using an anti-VACV antibody. Cells expressing D13 displayed single-cell foci of infection for both VACV and SCV-CHIK, with no evidence of plaque formation, consistent with the inherent non-permissive nature of CHO cells to VACV multiplication (Figure 3B, top panels). Provision of CP77 alone only supported VACV multiplication, as shown by formation of viral plaques indicative of cell-to-cell spread (Figure 3B, middle panels). Expression of both D13 and CP77 in the SCS line facilitated SCV-CHIK multiplication with plaque morphology similar to VACV (Figure 3B, bottom panels). Infection of SCS monolayers with VACV or SCV-CHIK also sustained viral multiplication, with 3.6- to 4-fold log increases in titers observed over a 30 hr period (Figure 3C).
The ability of the SCS line to support SCV-CHIK multiplication following extended sub-culturing was evaluated, because this represents an essential criterion for commercial vaccine manufacture. Similar virus yields were obtained at early and late passages (6 and 30 passages) (Figure 3D).
Examination by electron microscopy of SCV-CHIK morphology in BHK-21 cells (non-permissive for SCV and permissive for VACV) clearly indicated that SCV multiplication was arrested at the viroplasma stage, as expected in the absence of D13 (Figure 3E, left panel). In contrast, the SCV-permissive SCS line showed evidence of active viral multiplication, illustrated by the presence of viroplasma, and immature and mature virions (Figure 3E, right panel). Collectively, these results confirm that the CHO-derived SCS line (expressing D13 and CP77) can support production of SCV vaccines and can be used as a cell substrate for SCV vaccine manufacture.
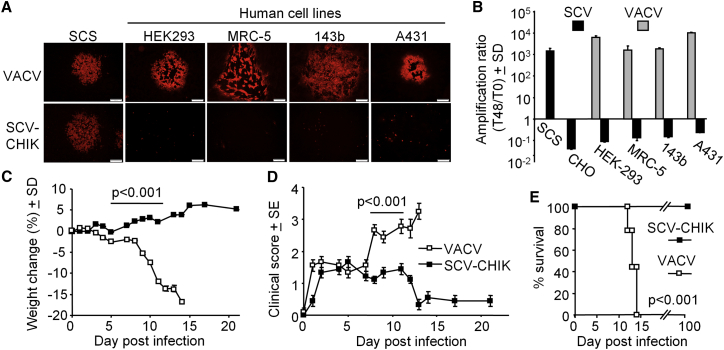
SCV-CHIK Does Not Replicate in Human Cell Lines
Deletion of D13 is designed to prevent SCV from producing infectious viral progeny in mammalian cells. To illustrate this multiplication-defective phenotype, monolayers of a panel of human cell lines permissive to VACV infection were infected with SCV-CHIK or VACV and examined for plaque formation by immunofluorescent antibody staining. In the SCS line, both VACV and SCV-CHIK infection produced plaques as expected (Figure 4A, SCS), indicating virus multiplication by cell-to-cell spread. In all other human cell lines tested, only VACV was able to generate plaques. SCV-CHIK infection was only observed in individual cells with no evident plaque formation, confirming that SCV-CHIK could infect individual cells but could not multiply and spread to neighboring cells (Figure 4A, human cell lines).
Figure 4.
SCV-CHIK Multiplication Defect in Human Cell Lines and Attenuated Pathology in Immunocompromised Mice
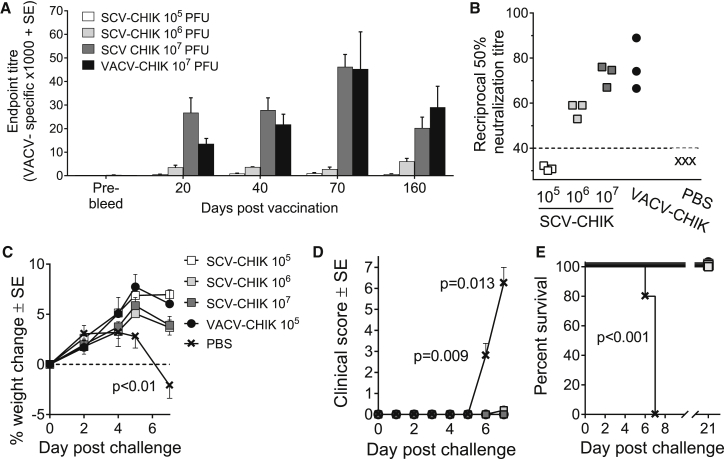
(A) Monolayers of the indicated cell lines were infected with VACV or SCV-CHIK (MOI, 0.001 PFU/cell) and fixed after 48 hr, and cells were examined by immunofluorescent staining using polyclonal anti-VACV antibody. Large plaques were observed in all lines infected with VACV. No plaques were observed in the human cell lines infected with SCV-CHIK. The human cell lines were HEK293, MRC-5 (human lung fibroblast), 143b (human osteosarcoma), and A431 (human epidermoid carcinoma). (B) The indicated cell lines were infected with VACV or SCV-dsRed (MOI, 0.01 PFU/cell), and viral titers 48 hr post-infection determined by plaque assay and the amplification ratio, or fold increase in viral titer from day 0 (T0) to 48 hr (T48), was calculated. In the human cell lines, SCV-dsRed showed an amplification ratio of <1, indicating decay of the input virus. (C) Groups of 6- to 8-week-old female SCID mice (n = 9 mice per group) were vaccinated i.p. with 107 PFUs of VACV or SCV-CHIK, and animal weights were measured over time. Statistics by repeated measures ANOVA for the indicated period when all mice were alive. (D) Clinical scores for the animals described in (C). Statistics as in (C). (E) Survival for the animals described in (C). Mice were euthanized upon losing ≥15% of their original body weight or pre-determined humane endpoints. Statistics by log rank (Mantel-Cox) test.
Using the same cells and a SCV construct expressing a different recombinant protein (dsRed), production of infectious viral progeny was assessed. Results were expressed as an amplification ratio, i.e., the ratio of the output virus titer 48 hr (T48) post-infection to the original input virus titer on day 0 (T0). As expected, VACV was able to multiply efficiently in all cell lines tested, as shown by >1,000-fold amplification of virus titers over 48 hr. In contrast, SCV-dsRed infection resulted in an amplification ratio of less than 1, confirming that SCV-dsRed was not able to produce infectious progeny in these cells (Figure 4B).
Altogether, these results demonstrate an inability of the SCV vaccine to produce viral progeny in different human cell lines (irrespective of the recombinant insert), an important safety consideration for future human use.
SCV-CHIK Fails to Replicate or Cause Disease in Immunocompromised Mice
The replication-competent VACV vaccine is associated with a risk of severe adverse reactions in immunocompromised individuals. Severe combined immunodeficiency (SCID) mice represent a model of lethal progressive vaccinia40 and were used to illustrate the safety profile of SCV-CHIK. As expected, SCID mice infected with VACV showed progressive weight loss (Figure 4C) and displayed significant clinical symptoms from day 6 post-infection (Figure 4D), with all mice reaching ethically defined disease-severity endpoints requiring euthanasia between days 12 and 14 post-infection (100% mortality) (Figure 4E). In contrast, the same dose of SCV-CHIK was well tolerated, with no weight loss or significant clinical symptoms and no mortality (up to 100 days post-infection) (Figures 4C–4E). Susceptibility of SCID mice to VACV infection was associated with significant viral dissemination and multiplication in various organs, whereas no infectious plaque-forming virus particles were detected in the organs of SCV-CHIK-vaccinated mice (Table S1). In addition, after 20 passages in the SCS line, SCV-CHIK remained multiplication-defective in SCID mice (data not shown). These results illustrated that, in SCID mice, SCV-CHIK did not cause adverse clinical responses and did not show any detectable reversion to multiplication competence.
SCV-CHIK Vaccination Induces VACV-Specific Antibody Responses
The parental VACV has been widely used as a smallpox vaccine. To determine whether SCV also has potential utility as a smallpox vaccine, anti-VACV antibody responses were evaluated in mice after a single vaccination with SCV-CHIK at different doses (105, 106, and 107 plaque-forming units [PFUs]). A dose- and time-dependent increase (107 > 106 > 105 PFUs) in anti-VACV immunoglobulin G (IgG) responses was observed after SCV-CHIK vaccination (Figure 5A). Mice vaccinated with a single 107 PFU dose of SCV-CHIK demonstrated similar levels of antibody responses to those of mice receiving 107 PFU VACV-CHIK. A dose-dependent increase in the anti-VACV neutralizing antibody titers was also observed following SCV-CHIK vaccination, with similar neutralization titers evident for SCV-CHIK and VACV-CHIK-vaccinated mice at a dose of 107 PFUs (Figure 5B). In addition to the antibody responses, mice vaccinated with SCV-CHIK generated poxvirus-specific CD8 T cell responses (Figure S2).
Figure 5.
SCV-CHIK Induces VACV-Specific Antibody Responses and Protects against Lethal Mousepox Challenge
(A) Groups of 6- to 8-week-old female C57BL/6 mice (n = 5 mice per group) were vaccinated i.p. with VACV-CHIK (107 PFUs) or SCV-CHIK (105, 106, and 107 PFUs) and bled at the time points indicated, and VACV-specific IgG levels were determined by endpoint ELISA. (B) At day 30 post-vaccination, serum VACV-specific neutralization activity was determined by PRNT50 assays (n = 3 mice per group). The dotted line indicates the limit of detection (1 in 40 serum dilution). (C) Groups of BALB/c mice (n = 5 per group) were vaccinated with SCV-CHIK or VACV-CHIK or mock vaccinated with PBS. After 4 weeks, mice were challenged with LD50 of ECTV and animal weights were monitored over time. The percentage of weight change post-challenge over time is presented. Statistics by t tests; the PBS mock-vaccinated group was significantly different from all other groups at day 7 post-challenge. (D) Mean clinical scores for animals in (C). Legend as in (C). Statistics by Kolmogorov-Smirnov test; the PBS mock-vaccinated group was significantly different from all other groups at days 6 and 7 post-challenge. (E) Survival post-challenge. Legend as in (C). Statistics by log rank (Mantel-Cox) test.
SCV-CHIK Vaccination Protects against Lethal Mousepox Virus Challenge
The ability of SCV-CHIK-vaccinated mice to resist poxvirus challenge was evaluated in a lethal ECTV challenge model using mousepox-susceptible BALB/c mice. Mice vaccinated with 107, 106, and 105 PFU SCV-CHIK or 105 PFU VACV exhibited similar levels of protection against weight loss (Figure 5C), clinical symptoms (Figure 5D), and mortality (Figure 5E). Dissemination of ECTV to multiple organs was also prevented in all vaccinated mice (Table S2). These results illustrate that a single vaccination with SCV-CHIK provides complete protection from a lethal poxvirus challenge, suggesting that the SCV retains utility as a smallpox vaccine.
A Single Vaccination with SCV-CHIK Produces Robust and Durable Anti-CHIKV Antibody Responses
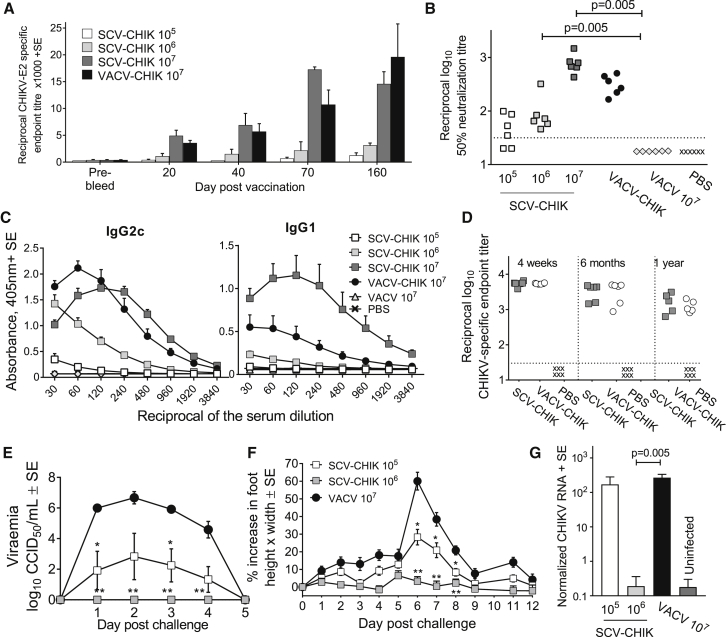
Antibodies are deemed to be critical for protection against CHIKV,41, 42, 43, 44, 45 with human and mouse antibodies directed at the E2 surface glycoprotein of CHIKV shown to mediate protection.46 Mice were given a single dose of vaccine (105, 106, and 107 PFUs of SCV-CHIK), and CHIKV E2-specific IgG responses were examined by ELISA. A dose- and time-dependent increase in CHIKV-E2-specific IgG levels was observed, with mice given 107 PFUs showing similar kinetics and magnitude of antibody responses to those induced by replication-competent VACV-CHIK-vaccinated mice (Figure 6A). This illustrated that multiplication-defective SCV-CHIK was able to generate antibody responses comparable to a recombinant immunogen as multiplication-competent VACV-CHIK.
Figure 6.
Antibody Responses and Protection against CHIKV Infection and Disease following a Single Vaccination with SCV-CHIK
(A) Groups of 6- to 8-week-old female C57BL/6 mice (n = 5 per group) were vaccinated with the indicated doses of SCV-CHIK or VACV-CHIK. At the indicated times, serum anti-CHIKV E2-specific IgG endpoint titers were determined. (B) At day 30 post-vaccination, CHIKV-neutralizing antibody responses were assessed by micro-neutralization assays. The dotted line indicates the limit of detection (1 in 30 serum dilution). Statistics by Kolmogorov-Smirnov test. (C) At day 30 post-vaccination, anti-CHIKV IgG2c and IgG1 antibody levels were measured (using inactivated CHIKV as the antigen) in mice vaccinated as in (A), with serum from mice vaccinated with VACV or mock vaccinated with PBS used as negative controls. (D) At the indicated times post-vaccination, anti-CHIKV IgG2c levels in mice vaccinated with SCV-CHIK or VACV-CHIK (both 107 PFUs), or mock vaccinated with PBS were examined by ELISA using inactivated virus as antigen. (E) Vaccinated mice (SCV-CHIK at 105 and 106 PFUs and VACV at 107 PFUs; for legend, see F) were challenged with CHIKV (Reunion Island isolate), and viremia was measured over time. Statistics by Kolmogorov-Smirnov test (n = 6 mice per group); *p = 0.031, **p = 0.005 compared with the VACV 107 PFU group. (F) Foot swelling after CHIKV challenge of mice described in (E). Statistics by Kolmogorov-Smirnov test; *p ≤ 0.034, **p ≤ 0.002 compared with VACV. (G) Persistence of CHIKV RNA in the feet of the vaccinated mice after challenge described in (E) was quantified by qRT-PCR (normalized against RPL13A) 30 days post-challenge. Statistics by Kolmogorov-Smirnov test.
Neutralizing antibody responses were detected in four of six mice receiving 105 PFUs of SCV-CHIK, whereas all six mice receiving 106 and 107 PFUs of SCV-CHIK generated neutralizing antibody responses (Figure 6B). At equivalent doses (107 PFUs), SCV-CHIK- and VACV-CHIK-vaccinated mice produced comparable neutralizing antibody responses (Figure 6B).
CHIKV-specific IgG1 and IgG2c levels were examined in serum of mice day 30 post-vaccination to provide an indication of the Th1/Th2 balance of the antibody responses. At 107 PFUs, SCV-CHIK stimulated IgG2c responses comparable to those induced by VACV-CHIK (Figure 6C, left panel). However, SCV-CHIK induced higher IgG1 responses than VACV-CHIK, suggesting the generation of a more balanced Th1 (IgG2c)/Th2 (IgG1) response by SCV-CHIK, with VACV-CHIK being more Th1 biased (Figure 6C, right panel).
To analyze the longevity of antibody responses induced by SCV-CHIK, anti-CHIKV antibody responses were examined 4 weeks, 6 months, and 1 year post-vaccination. A limited drop in anti-CHIKV antibody levels was observed over this period, with antibody levels remaining comparable between SCV-CHIK- and VACV-CHIK-vaccinated mice throughout (Figure 6D). In addition, CHIKV E2-specific antibody-secreting cells (ASCs) (Figure S3) and CHIKV E2/E1 and capsid-specific interferon gamma (IFN-γ)-producing cells (Figure S4) were detected 1 year post-vaccination in SCV-CHIK- and VACV-CHIK-vaccinated mice.
A Single Vaccination with SCV-CHIK Protects against CHIKV Challenge
The vaccination-CHIKV challenge model47, 48 was piloted by demonstrating that mice vaccinated with VACV-CHIK (107 PFUs) and challenged with CHIKV (Reunion Island isolate; LR2006-OPY1, 104 CCID50) were protected against viremia and arthralgia (Figure S5). Because SCV-CHIK induced neutralizing antibody responses comparable to those of VACV-CHIK at the same 107 PFU dose, the protective efficacy of SCV-CHIK was evaluated at lower doses (105 and 106 PFUs). Mice vaccinated with SCV-CHIK at 106 PFUs and challenged 40 days later (with the Reunion Island isolate; LR2006-OPY1, 104 CCID50) showed no detectable viremia (Figure 6E) or foot swelling (Figure 6F). Mice vaccinated with a 10-fold lower dose (105 PFUs) were partially protected, with an approximate 4-log reduction in viremia and significantly reduced foot swelling compared to control VACV-infected mice. Longevity studies confirmed that SCV-CHIK-vaccinated mice were protected against CHIKV challenge 1 year post-vaccination (Figure S6).
Persistence of CHIKV genomic RNA, particularly in the joint tissues, has been associated with chronic arthritic disease following CHIKV infection in humans49 and in the mouse model.48, 50 At day 30 post-challenge, mice vaccinated with 106 PFUs, but not 105 PFUs, of SCV-CHIK showed a significant reduction to background levels in the level of persistent viral RNA (Figure 6G), illustrating that SCV-CHIK vaccination can prevent establishment of persistent CHIKV RNA in joint tissues.
Altogether, these findings demonstrate the ability of SCV-CHIK to elicit a robust, balanced, and durable CHIKV-specific antibody response and provide protection against viremia, acute arthritis, and persistence of viral RNA following CHIKV challenge.
Discussion
Herein we describe the development of a novel VACV-based SCV vaccine platform technology that is unable to generate infectious progeny (multiplication-defective) in vaccine recipients while retaining the ability to induce robust immune responses to a recombinant vaccine antigen. We also illustrate that SCV vaccines can be produced in a CHO-based system, a cell line widely used for manufacture of biologics in the biopharmaceutical industry due to their ability to be grown in chemically defined growth media as suspension cultures to high cell densities in industrial scale bioreactors.22, 23
A key design feature of the SCV vaccine platform is the targeted genetic deletion of the VACV D13L gene, which renders SCV vaccines unable to generate viral progeny. Loss of D13 expression prevents SCV virion assembly while preserving genome replication and late gene expression of vaccine antigens. Attenuation of the SCV vaccine was demonstrated in vitro in several human cell lines, including human lung and skin epithelial cell lines that are VACV permissive and are representative of sites of active virus multiplication in human infections. In addition, we have demonstrated that SCV can be safely administered to immunocompromised SCID mice, confirming attenuation in vivo. This multiplication defect suggests SCV will have a safety profile in humans similar to that of the host-range restricted MVA, which demonstrated an impeccable safety record during the end of the smallpox eradication campaign in which more than 120,000 people were vaccinated without serious adverse effects51 and has been shown to be safe and well tolerated in individuals with atopic dermatitis or HIV.52, 53
The CHO-based cell substrate system for the production of SCV vaccines provides a number of advantages over existing primary cell culture and diploid and continuous cell line-based vaccine production systems of registered viral vaccines. For instance, the widely used CEF cell culture is a primary cell substrate for which (1) supply logistics introduces a risk of contamination, (2) batch-to-batch variation is inherent because cell banking practices cannot be adopted, and (3) the need for specific pathogen-free stocks restricts the on-demand production capacity. Furthermore, most diploid and continuous cell lines used in manufacturing the vaccine have a finite culture life span and are growth anchorage dependent, with logistical and cost implications for scaled production. To circumvent these issues, we provide proof of principle that CHO cells, the most frequently used system for industrial manufacture of biologics,22, 23 can be engineered to express D13 and CP77 (the SCS line) in the construction and production of SCV vaccine stocks. We were able to demonstrate a robust 4-fold log amplification of the virus in the SCS line within 30 hr of infection, with viral yields stably maintained for more than 30 passages. We are in the process of developing a monoclonal suspension SCS line that can be grown and maintained in a chemically defined medium to facilitate current good manufacturing practice of vaccine stocks for future clinical trials.
The re-emergence of CHIKV, a mosquito-transmitted alphavirus responsible for millions of cases reported globally, highlights the need for an effective vaccine. In response, a number of CHIK vaccines have been developed (all using CHIKV structural glycoproteins as vaccine antigens), with efficacy studies ranging from pre-clinical testing to early human trials.41, 42, 43 To illustrate the utility of the SCV platform technology, SCV-CHIK was constructed and shown, after a single vaccination, to induce a robust and durable antibody response that mediates protection in a pre-clinical mouse challenge model of acute and chronic CHIKV arthritic disease48 up to 1 year post-vaccination. The protection conferred by SCV-CHIK against viremia and foot swelling is comparable to the only other VACV-vectored CHIK vaccine under investigation, the MVA-CHIKV vaccine, which has also been shown to be efficacious in mouse model studies.54, 55 VACV-based vaccines have a number of characteristics that make them potentially attractive for use in resource poor settings, primarily cold chain-independent distribution9 and long-lasting immunity,8 clearly desirable features for rapidly spreading epidemics of infectious diseases such as CHIKV.25, 26, 27 Rapid onset of immunity after a single vaccination is also a highly desirable feature in these epidemic situations, and the effectiveness of the SCV vaccine platform to consistently do this will be further characterized in planned primate studies.
The SCV vaccine platform technology also has the inherent capacity to function as a smallpox vaccine. Smallpox has been eradicated for more than 30 years, and routine vaccination has ceased. However, biotechnological advances have increased the threat of bioterrorism, and human outbreaks of re-emerging zoonotic poxvirus are possible following waning herd immunity; ergo, there remains a risk of either intentional or unintentional outbreaks of poxviral diseases in the general population.56 Marketing authorization for Bavaria Nordic’s MVA-based smallpox vaccine IMVAMUNE highlights that health agencies recognize the need for safe and effective vaccines against human orthopoxvirus infections, given that the side effects associated with the original vaccines are now considered unacceptable.40, 57 Our results demonstrate that following SCV-CHIK vaccination, mousepox-susceptible BALB/c mice generate a VACV-specific cellular and humoral immune response that mediates protection against a lethal heterologous ECTV challenge. Combined with evidence of in vivo attenuation in immunocompromised SCID mice and inherent cell substrate manufacturing advantages, SCV could also be used as a practical and effective poxvirus vaccine.
In conclusion, we report the development of a novel, attenuated SCV vaccine platform technology with an integrated CHO-based SCS line and scale-up capacity for vaccine manufacture. Our findings confirm that SCV-CHIK is multiplication-defective and can be safely administered to immunocompromised mice. A single administration of SCV-CHIK elicits a robust and durable neutralizing antibody response and provides complete protection to a subsequent CHIKV challenge. Furthermore, preliminary studies indicate that intramuscular vaccination with SCV-CHIK generates antibody titers equivalent to those observed after intraperitoneal (i.p.) administration (data not shown). We are evaluating these responses further to provide evidence for translation to human use.
Materials and Methods
Ethics Statement
All mouse work was conducted in accordance with the Australian Code for the Care and Use of Animals for Scientific Purposes as defined by the National Health and Medical Research Council of Australia. Animal experiments were conducted under protocols approved by the institutional animal ethics committees of University of South Australia and South Australian Health and Medical Research Institute (SAHMRI). CHIKV challenge studies were approved by the Queensland Institute of Medical Research (QIMR) Berghofer Medical Research Institute animal ethics committee and were conducted in a biosafety level-3 facility.
VACV-CHIK Construction
The origin and history of VACV (Copenhagen strain) are described by Glosser,31 and VACV was gifted to P.M.H. by Dr. Robert Drillien at the Institute of Virology (Strasbourg, France).
VACV-CHIK was constructed by homologous recombination to replace the A39R open reading frame (ORF) of VACV with a poxvirus expression cassette expressing the CHIKV 26S-structural polyprotein (GenBank: AM258992), under the control of VACV promoters,58 together with a poxvirus expression cassette for the expression of the E. coli guanine phosphoribosyltransferease (Ecogpt). Homologous recombination and positive selection of recombinant viruses containing the co-insertion of the Ecogpt expression cassette was undertaken as described (protocol 6),59, 60, 61 in which the homologous recombination arms flanking the CHIKV-26S and Ecogpt expression cassettes were designed to be homologous with the sequences flanking the A39R ORF.
After successful replacement of the A39R ORF with CHIKV-26S and Ecogpt expression cassettes, the Ecogpt expression cassette was removed by a second homologous recombination and counterselection against Ecogpt expression as described (protocol 7),59, 62 whereby the homologous recombination arms were designed to be homologous to the sequences flanking the Ecogpt expression cassette within the recombinant VACV-CHIK genome.
SCV-CHIK Construction
To generate SCV-CHIK, the D13L ORF was deleted from VACV-CHIK by homologous recombination, in which positive selection for successful deletion was undertaken by infection of CHO cells expressing only the D13 protein (CHO-D13L). Because CHO is non-permissive to VACV infection, positive selection for D13L-deleted virus was achieved by replacing the D13L ORF with an expression cassette coding for the cowpox virus CHO host-range protein CP77. A homologous recombination plasmid was constructed that consisted of a CP77 expression cassette and a dsRed fluorescent protein expression cassette flanked by left and right recombination arms that were homologous to sequences flanking the D13L ORF within VACV-CHIK. The dsRed expression cassette was added, because VACV-expressing CP77 does not form lytic plaques in CHO; therefore, infection was monitored by the presence of red fluorescence. To help in removal of the CP77 and dsRed expression cassettes that had replaced the D13L ORF, a repeat sequence of the left homologous recombination arm was placed downstream of the CP77 and dsRed expression cassettes but upstream of the right homologous recombination arm within the homologous recombination plasmid. Homologous recombination was performed by transfecting the D13L-deleting homologous recombination plasmid into CHO-D13L previously infected with VACV-CHIK at an MOI of 0.01 PFU/cell. SCV-CHIK was enriched from the homologous recombination infection by amplifying the virus in CHO-D13L, followed by infecting a fresh set of CHO-D13L cells with the amplified virus. These infected cells were recovered and made into a single-cell suspension by TrypLE Select digestion (Thermo Fisher Scientific) and then single-cell sorted so that one red fluorescent cell was seeded into 1 well of a 96-well plate containing cultured CHO-D13L cells using a FACSAria Fusion flow cytometer (BD Biosciences). The red fluorescent cell-seeded 96-well plate was then incubated at 37°C/5% CO2 until red fluorescent foci of infection could be seen in any of the wells of the 96-well plate. Wells containing a single focus of infection were harvested and resuspended as single-cell suspensions before single-cell sorting and seeding into 96-well plates of fresh CHO-D13L cells. This single-cell sorting process was repeated five times to eliminated trace contamination with the original VACV-CHIK and produce clonal SCV-CHIK. A number of clonally purified SCV-CHIK were amplified in CHO-D13L cells and then tested by PCR analysis for the presence of contaminating VACV-CHIK and to confirm D13L replacement by the CP77 and dsRed expression cassettes. The best clone was then amplified in the SCS line (expressing both CP77 and D13 proteins) to encourage intramolecular recombination between the left homologous recombination sequence and the repeat sequence, because the dependence of the viral expression of CP77 is no longer required, resulting in the deletion of the CP77 and dsRed expression cassettes from SCV-CHIK. The infected SCS cells were brought into single-cell suspension by digestion with TrypLE Select and cell sorted using a FACSAria Fusion flow cytometer, in which non-fluorescent cells were bulk sorted and retained. SCV-CHIK virus was further amplified to make seed stock by progressive scaled-up infections of SCS cells.
PCR Analysis and Sequencing Verification
Construction and homogeneity of SCV-CHIK was confirmed by PCR using primers spanning the inserted or deleted regions of the viral genome. Viral DNA from infected SCS monolayers was extracted using a DNeasy kit (QIAGEN). All PCR reactions were performed using KAPA HiFi polymerase (Kapa Biosystems) with primer pairs for the A39R locus (forward 5′-TAAGATTCCTAAATTAGTAGAAAA-3′ and reverse 5′-ACACGAATGCAGTTTGGGAGTAT-3′) and the D13L locus (forward 5′-CGACACCCGTTTCATGGAACAA-3′ and reverse 5′-GGACGACGAGATACGTAGAGTGT-3′). PCR products were run on a 0.8% agarose gel, visualized using GelRed (Biotium), and sequence confirmed by Sanger sequencing at the Australian Genome Research Foundation (AGRF).
CHO-D13L, CHO-CP77, and SCS Line Construction
CHO cells (ATCC CCL-61) cultured to 50%–70% confluence were transfected with 1 μg of pLL07 (HA-tagged CP77 expression plasmid) and/or pLL29 (FLAG-tagged D13 expression plasmid) using Effectene transfection reagent (QIAGEN) according to the manufacturer’s instructions. Transfected cells were maintained under antibiotic selection (hygromycin B, 500 μg/mL; G418, 1,000 μg/mL), and monoclonal CHO-D13L, CHO-CP77, and SCS lines were established by single-cell sorting (BD FACSAria Fusion), followed by further expansion under antibiotic selection. Expression of D13 and/or CP77 was confirmed by immunoblotting using anti-FLAG (1:500; Abcam ab49763) and anti-HA tag (1:500; Abcam ab1265) antibodies, respectively.
SCV-CHIK, VACV-CHIK, and VACV Production and Titration
VACV and VACV-CHIK stocks for mice vaccination studies were prepared by infecting BHK-21 cells (ATCC CCL-10) cultured in multiple T175 flasks at an MOI of 0.01 PFU/cell. Because VACVs are cell associated, infected cells were recovered by centrifugation to form a pellet after 90%–100% cytopathic effect. Virus was extracted from the resuspended cell pellet by sonication, with insoluble material removed by low-speed centrifugation. The clarified supernatant was then layered onto a 36% sucrose cushion and centrifuged at 3,200 × g for 18 hr at 10°C to pellet the virus. The viral pellet was resuspended in 10 mM Tri-HCl (pH 8) and stored at −80°C. SCV-CHIK was produced in the same manner except the SCS line was used as cell substrate for virus production.
Titration of VACV and VACV-CHIK were performed by counting plaques in infected BS-C-1 monolayers (ATCC CCL-26) stained with crystal violet as described.63 Titration of SCV-CHIK was carried out by infecting the SCS line, and because lytic plaques do not form in this cell line, immunohistochemical staining was used to reveal foci of infections that could be counted as plaques, as described for MVA in Kremer et al.64 Infected cell foci (plaques) were identified with horseradish peroxidase (HRP)-conjugated anti-vaccinia antibody (1:400 dilution; Alpha Diagnostic International) and stained using tetramethylbenzidine (TMB) substrate for membranes (Sigma-Aldrich).
Immunoblot Analysis of CHIKV Antigen Expression by SCV-CHIK
To confirm expression of CHIKV antigens by SCV-CHIK, monolayers of SCS were infected with either VACV or SCV-CHIK (MOI, 1 PFU/cell). Whole-cell extracts from 48 hr post-infection were separated by 12% SDS-PAGE and analyzed by immunoblotting using anti-CHIKV antibody generated from mice immunized with inactivated CHIKV (1:200 dilution).47 Anti-mouse IgG-HRP-conjugated secondary antibody (1:5,000; Sigma-Aldrich) and a chemiluminescence system (Clarity ECL western blotting substrate; Bio-Rad) were used for detection. Blots were imaged using a ChemiDoc XRS (Bio-Rad).
Immunofluorescent Staining of SCV-CHIK and VACV Plaques
Cell lines were infected with virus (VACV or SCV-CHIK; MOI, 0.001 PFU/cell), fixed with 1:1 solution of methanol/acetone, and air-dried before immunofluorescent staining with rabbit anti-VACV (1:400; Alpha Diagnostic International) and a secondary antibody, tetramethylrhodamine (TRITC)-conjugated anti-rabbit IgG F(ab)′2 fragment (1:400; Life Technologies). Images were taken using an Olympus IX51 inverted fluorescent microscope.
Electron Microscopy
SCV-CHIK- and VACV-infected cells (MOI, 2 PFUs/cell) were fixed in 4% paraformaldehyde and 1.25% glutaraldehyde with 4% sucrose in PBS, washed with PBS containing 4% sucrose, and post-fixed with 2% osmium tetroxide. Cells were dehydrated through an ethyl alcohol series and propylene oxide, followed by infiltration and embedding in epoxy resin (EMbed 812/Araldite 502 mixture; Emgrid Australia). Thin sections, cut using a Leica EM UC6 Ultramicrotome, were stained with 4% aqueous uranyl acetate and Reynolds lead citrate and imaged on the FEI Tecnai G2 Spirit transmission electron microscope.
The SCID Mouse Study
Groups of 6- to 8-week-old female SCID mice were inoculated with 107 PFUs/mouse of VACV or SCV-CHIK i.p. and monitored for 100 days. Mice were weighed, and clinical scoring was undertaken using a matrix, which included scores for coat quality, food intake, movement reluctance, weight loss, skin condition, and posture. Mice were euthanized when ethically defined endpoints were reached.
Viral loads in organs were determined in VACV- or SCV-CHIK-infected mice by viral plaque assay. To determine the VACV titers and revertant to multiplication-competent SCV-CHIK, serial dilutions of organ homogenates (FastPrep24-5G Homogenizer with 2.8 mm ceramic beads; MP Biomedicals) were plated onto confluent BS-C-1 monolayers, and the plaques were visualized by crystal violet staining after 48 hr.
Post-vaccination VACV Antibody Responses and ECTV Challenge
Inbred, specific pathogen-free 6- to 8-week-old female BALB/c purchased from the Animal Resources Center (Canning Vale) were vaccinated i.p. for accuracy and consistency of dosing with the indicated vaccines or PBS. The i.p. route also allowed higher-dose comparisons to be made while minimizing the impact on animal welfare. VACV-specific titers were determined by standard ELISA using heat-inactivated VACV (105 PFUs/well) as antigen and HRP-conjugated rabbit anti-mouse IgG antibody (Sigma-Aldrich) and TMB chromogen (Sigma-Aldrich) for detection. The endpoint titers were calculated when the optical density (OD) readings reached the mean absorbance values of the negative serum samples plus three times the SD.
VACV plaque reduction neutralization tests (PRNT50) were conducted as described.65 Briefly, sera were heat inactivated, incubated with 100 PFUs of VACV for 1 hr, transferred onto BS-C-1 monolayers for 1 hr, and incubated at 37°C for 2 days. Viral plaques were visualized by the addition of 0.4% crystal violet solution. PRNT50 titer was calculated as the reciprocal of sera dilution at which 50% of the input virus is neutralized and determined by a non-linear four-parameter curve fit of data using GraphPad Prism v.6.
Vaccinated and control mice were challenged with lethal dose 50 (LD50) of ECTV subcutaneously. Weight loss and mortality was monitored. Clinical disease was scored using a matrix that included scores for coat condition, eye condition, movement, limb condition, and hunching. Mice were euthanized when ethically defined endpoints were reached.
Recombinant CHIKV E2 Production
Recombinant E2 sequence (transmembrane domain deleted; amino acids 336–386) was cloned into the BamHI and HindIII restriction enzyme sites of the pQE30 vector (QIAGEN) using InFusion seamless cloning (Clontech) according to the manufacturer’s instructions. Recombinant protein expression in M15[pREP4] E. coli transformants was induced by 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG), and protein was purified under denaturing conditions using gravity-flow column purification with TALON resin (Clontech). Purified proteins were assessed using SDS-PAGE and protein concentration determined by bicinchoninic acid (BCA) assay (Pierce).
Post-vaccination CHIKV Antibody Responses and CHIKV Challenge
Groups of 6- to 8-week-old female C57BL/6 mice were vaccinated i.p. with the indicated vaccines or PBS. Serum antibody responses were determined by standard ELISA (1) measuring total IgG responses and recombinant E2 as antigen (see earlier) and (2) measuring IgG1 and IgG2c responses using whole inactivated CHIKV as antigens as described.66 CHIKV neutralization titers against the Reunion Island isolate (LR2006-OPY1) of CHIKV were determined as described.50
Vaccinated mice were challenged with 104 cell culture infectious dose 50% (CCID50) of the Reunion Island CHIK isolate injected subcutaneously into the ventral side of each hindfoot toward the ankle as described.47 Post-challenge viremia, foot swelling measurements, and qRT-PCR for CHIKV RNA were undertaken as described.47, 50
Statistics
Statistical analysis of experimental data was performed using IBM SPSS Statistics for Windows, v.19.0. Two-sample comparison using t test was performed when the difference in variances was <4, skewness was >−2, and kurtosis was <2. Non-parametric data with a difference in variances of <4 was analyzed using Mann-Whitney U test; if the difference of variances was >4, the Kolmogorov-Smirnov test was employed. The log rank (Mantel-Cox) test was used for statistical analysis of surviving proportions.
Author Contributions
P.M.H. conceived, commissioned, and directed this project; P.E., T.H.C., L.L., K.R.D., P.M.H., and J.D.H. designed the experiments; and P.E., T.H.C., and L.L. performed the experiments, analyzed the results, prepared the figures, and wrote the manuscript. N.A.P. planned, performed, and analyzed the chikungunya challenge experiments. A.S. planned and supervised the chikungunya challenge experiments and helped prepare the figures. All authors discussed the results and critically edited the manuscript. P.M.H., K.R.D., and J.D.H. supervised the project. The principal investigator is J.D.H.
Conflicts of Interest
This research was supported by funding from Sementis Ltd., in which P.M.H. and J.D.H. are shareholders. A.S. is an unpaid member of the Sementis Ltd. scientific advisory board and has undertaken contract research and development for Sementis Ltd.
Acknowledgments
We thank Jamie Zhang, Robyn Kievit, Melissa Tan, Pooja Patel, Fan Jia, and Gary Heinemann from the Experimental Therapeutics Laboratory, University of South Australia, for their technical assistance, and Dr. Cara Fraser and staff at the South Australian Health and Medical Research Institute Preclinical Imaging and Research Laboratories for their support and assistance in the ectromelia virus challenge studies.
This work was supported by Sementis Ltd., which acknowledges the support of its shareholders in the development of this technology, as well as the Australian Department of Industry, Enterprise Connect Researchers in Business Fellowships (L.L. and T.H.C.), a Science Industry Endowment Fund STEM+ Business Fellowship (T.H.C.), and Australian Research Council Linkage grant LP160100633. K.R.D. was supported by a National Health and Medical Research Training Fellowship APP1012386; N.A.P. was supported by an Advance Queensland Research Fellowship from the Queensland Government, Australia. A.S. holds a Principal Research Fellowship from the National Health and Medical Research Council of Australia.
Footnotes
Supplemental Information includes Supplemental Materials and Methods, six figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.06.017.
Contributor Information
Paul M. Howley, Email: paul.howley@sementis.com.au.
John D. Hayball, Email: john.hayball@unisa.edu.au.
Supplemental Information
References
- 1.Volz A., Sutter G. Modified vaccinia virus Ankara: history, value in basic research, and current perspectives for vaccine development. Adv. Virus Res. 2017;97:187–243. doi: 10.1016/bs.aivir.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sánchez-Sampedro L., Perdiguero B., Mejías-Pérez E., García-Arriaza J., Di Pilato M., Esteban M. The evolution of poxvirus vaccines. Viruses. 2015;7:1726–1803. doi: 10.3390/v7041726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Arriaza J., Perdiguero B., Heeney J.L., Seaman M.S., Montefiori D.C., Yates N.L., Tomaras G.D., Ferrari G., Foulds K.E., Roederer M. HIV/AIDS vaccine candidates based on replication-competent recombinant poxvirus NYVAC-C-KC expressing trimeric gp140 and Gag-derived virus-like particles or lacking the viral molecule B19 that inhibits type I interferon activate relevant HIV-1-specific B and T cell immune functions in nonhuman primates. J. Virol. 2017;91 doi: 10.1128/JVI.02182-16. e02182–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert M.F., Masson E., Artois M., Barrat J. Oral wildlife rabies vaccination field trials in Europe, with recent emphasis on France. Curr. Top. Microbiol. Immunol. 1994;187:219–243. doi: 10.1007/978-3-642-78490-3_13. [DOI] [PubMed] [Google Scholar]
- 5.Pastoret P.P., Brochier B. The development and use of a vaccinia-rabies recombinant oral vaccine for the control of wildlife rabies; a link between Jenner and Pasteur. Epidemiol. Infect. 1996;116:235–240. doi: 10.1017/s0950268800052535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith G.L., Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983;25:21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- 7.Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat. Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 8.Amanna I.J., Carlson N.E., Slifka M.K. Duration of humoral immunity to common viral and vaccine antigens. N. Engl. J. Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 9.Alcock R., Cottingham M.G., Rollier C.S., Furze J., De Costa S.D., Hanlon M., Spencer A.J., Honeycutt J.D., Wyllie D.H., Gilbert S.C. Long-term thermostabilization of live poxviral and adenoviral vaccine vectors at supraphysiological temperatures in carbohydrate glass. Sci. Transl. Med. 2010;2:19ra12. doi: 10.1126/scitranslmed.3000490. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein J.A., Neff J.M., Lane J.M., Koplan J.P. Smallpox vaccination reactions, prophylaxis, and therapy of complications. Pediatrics. 1975;55:342–347. [PubMed] [Google Scholar]
- 11.Mayr A., Hochstein-Mintzel V., Stickl H. Passage history, properties and applicability of the attenuated vaccinia virus strain MVA. Infection. 1975;3:6–14. [Google Scholar]
- 12.Tartaglia J., Cox W.I., Taylor J., Perkus M., Riviere M., Meignier B., Paoletti E. Highly attenuated poxvirus vectors. AIDS Res. Hum. Retroviruses. 1992;8:1445–1447. doi: 10.1089/aid.1992.8.1445. [DOI] [PubMed] [Google Scholar]
- 13.Blanchard T.J., Alcami A., Andrea P., Smith G.L. Modified vaccinia virus Ankara undergoes limited replication in human cells and lacks several immunomodulatory proteins: implications for use as a human vaccine. J. Gen. Virol. 1998;79:1159–1167. doi: 10.1099/0022-1317-79-5-1159. [DOI] [PubMed] [Google Scholar]
- 14.Suter M., Meisinger-Henschel C., Tzatzaris M., Hülsemann V., Lukassen S., Wulff N.H., Hausmann J., Howley P., Chaplin P. Modified vaccinia Ankara strains with identical coding sequences actually represent complex mixtures of viruses that determine the biological properties of each strain. Vaccine. 2009;27:7442–7450. doi: 10.1016/j.vaccine.2009.05.095. [DOI] [PubMed] [Google Scholar]
- 15.European Medicines Agency. (2013). IMVANEX assessment report. Committee for Medicinal Products for Human Use, 30 May 2013, EMA/369203/2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002596/WC500147898.pdf.
- 16.Brown S.W., Mehtali M. The avian EB66(R) cell line, application to vaccines, and therapeutic protein production. PDA J. Pharm. Sci. Technol. 2010;64:419–425. [PubMed] [Google Scholar]
- 17.Lohr V., Rath A., Genzel Y., Jordan I., Sandig V., Reichl U. New avian suspension cell lines provide production of influenza virus and MVA in serum-free media: studies on growth, metabolism and virus propagation. Vaccine. 2009;27:4975–4982. doi: 10.1016/j.vaccine.2009.05.083. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y., Moss B. Immature viral envelope formation is interrupted at the same stage by lac operator-mediated repression of the vaccinia virus D13L gene and by the drug rifampicin. Virology. 1992;187:643–653. doi: 10.1016/0042-6822(92)90467-4. [DOI] [PubMed] [Google Scholar]
- 19.Liu L., Cooper T., Howley P.M., Hayball J.D. From crescent to mature virion: vaccinia virus assembly and maturation. Viruses. 2014;6:3787–3808. doi: 10.3390/v6103787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzer G.W., Remp G., Antoine G., Pfleiderer M., Enzersberger O.M., Emsenhuber W., Hämmerle T., Gruber F., Urban C., Falkner F.G., Dorner F. Highly efficient induction of protective immunity by a vaccinia virus vector defective in late gene expression. J. Virol. 1999;73:4536–4542. doi: 10.1128/jvi.73.6.4536-4542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spehner D., Gillard S., Drillien R., Kirn A. A cowpox virus gene required for multiplication in Chinese hamster ovary cells. J. Virol. 1988;62:1297–1304. doi: 10.1128/jvi.62.4.1297-1304.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer S., Handrick R., Otte K. The art of CHO cell engineering: a comprehensive retrospect and future perspectives. Biotechnol. Adv. 2015;33:1878–1896. doi: 10.1016/j.biotechadv.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Jayapal K.P., Wlashchin K.F., Hu W.S., Yap M.G.S. Recombinant protein therapeutics from CHO cells—20 years and counting. Chem. Eng. Prog. 2007;103:40–47. [Google Scholar]
- 24.Genzel Y. Designing cell lines for viral vaccine production: where do we stand? Biotechnol. J. 2015;10:728–740. doi: 10.1002/biot.201400388. [DOI] [PubMed] [Google Scholar]
- 25.Graham B.S., Repik P.M., Yactayo S. Chikungunya in the Americas: recommendations and conclusions. J. Infect. Dis. 2016;214(Suppl 5):S510–S513. doi: 10.1093/infdis/jiw370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burt F.J., Chen W., Miner J.J., Lenschow D.J., Merits A., Schnettler E., Kohl A., Rudd P.A., Taylor A., Herrero L.J. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect. Dis. 2017;17:e107–e117. doi: 10.1016/S1473-3099(16)30385-1. [DOI] [PubMed] [Google Scholar]
- 27.Suhrbier A., Jaffar-Bandjee M.C., Gasque P. Arthritogenic alphaviruses—an overview. Nat. Rev. Rheumatol. 2012;8:420–429. doi: 10.1038/nrrheum.2012.64. [DOI] [PubMed] [Google Scholar]
- 28.Schilte C., Staikowsky F., Couderc T., Madec Y., Carpentier F., Kassab S., Albert M.L., Lecuit M., Michault A. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl. Trop. Dis. 2013;7:e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon F., Javelle E., Cabie A., Bouquillard E., Troisgros O., Gentile G., Leparc-Goffart I., Hoen B., Gandjbakhch F., Rene-Corail P., Société de pathologie infectieuse de langue francaise French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med. Mal. Infect. 2015;45:243–263. doi: 10.1016/j.medmal.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Suhrbier A., Devine G. Chikungunya virus, risks and responses for Australia. Aust. N. Z. J. Public Health. 2016;40:207–209. doi: 10.1111/1753-6405.12515. [DOI] [PubMed] [Google Scholar]
- 31.Glosser J.W. United States Department of Agriculture, Animal and Plant Health Inspection Service; 1980. Environmental assessment and preliminary finding of no significant impact. Veterinary Biologics authorized field trial of an experimental biologic: the Wistar Institute of Anatomy and Biology proposed field trial of a live experimental vaccinia vectored rabies vaccine. [Google Scholar]
- 32.Joklik W.K., Becker Y. The replication and coating of vaccinia DNA. J. Mol. Biol. 1964;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- 33.Salzman N.P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- 34.Hsiao J.C., Chao C.C., Young M.J., Chang Y.T., Cho E.C., Chang W. A poxvirus host range protein, CP77, binds to a cellular protein, HMG20A, and regulates its dissociation from the vaccinia virus genome in CHO-K1 cells. J. Virol. 2006;80:7714–7728. doi: 10.1128/JVI.00207-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner J.D., Tscharke D.C., Reading P.C., Smith G.L. Vaccinia virus semaphorin A39R is a 50–55 kDa secreted glycoprotein that affects the outcome of infection in a murine intradermal model. J. Gen. Virol. 2001;82:2083–2093. doi: 10.1099/0022-1317-82-9-2083. [DOI] [PubMed] [Google Scholar]
- 36.Thomas S., Rai J., John L., Günther S., Drosten C., Pützer B.M., Schaefer S. Functional dissection of the alphavirus capsid protease: sequence requirements for activity. Virol. J. 2010;7:327. doi: 10.1186/1743-422X-7-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liljeström P., Garoff H. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J. Virol. 1991;65:147–154. doi: 10.1128/jvi.65.1.147-154.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Fugère M., Day R., Kielian M. Furin processing and proteolytic activation of Semliki Forest virus. J. Virol. 2003;77:2981–2989. doi: 10.1128/JVI.77.5.2981-2989.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elick T.A., Bauser C.A., Fraser M.J. Excision of the piggyBac transposable element in vitro is a precise event that is enhanced by the expression of its encoded transposase. Genetica. 1996;98:33–41. doi: 10.1007/BF00120216. [DOI] [PubMed] [Google Scholar]
- 40.Fisher R.W., Reed J.L., Snoy P.J., Mikolajczyk M.G., Bray M., Scott D.E., Kennedy M.C. Postexposure prevention of progressive vaccinia in SCID mice treated with vaccinia immune globulin. Clin. Vaccine Immunol. 2011;18:67–74. doi: 10.1128/CVI.00280-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeZure A.D., Berkowitz N.M., Graham B.S., Ledgerwood J.E. Whole-inactivated and virus-like particle vaccine strategies for chikungunya virus. J. Infect. Dis. 2016;214(Suppl 5):S497–S499. doi: 10.1093/infdis/jiw352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erasmus J.H., Rossi S.L., Weaver S.C. Development of vaccines for chikungunya fever. J. Infect. Dis. 2016;214(Suppl 5):S488–S496. doi: 10.1093/infdis/jiw271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramsauer K., Tangy F. Chikungunya virus vaccines: viral vector-based approaches. J. Infect. Dis. 2016;214(Suppl 5):S500–S505. doi: 10.1093/infdis/jiw369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lum F.M., Teo T.H., Lee W.W., Kam Y.W., Rénia L., Ng L.F. An essential role of antibodies in the control of chikungunya virus infection. J. Immunol. 2013;190:6295–6302. doi: 10.4049/jimmunol.1300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal P., Dowd K.A., Brien J.D., Edeling M.A., Gorlatov S., Johnson S., Lee I., Akahata W., Nabel G.J., Richter M.K. Development of a highly protective combination monoclonal antibody therapy against chikungunya virus. PLoS Pathog. 2013;9:e1003312. doi: 10.1371/journal.ppat.1003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kam Y.W., Lum F.M., Teo T.H., Lee W.W., Simarmata D., Harjanto S., Chua C.L., Chan Y.F., Wee J.K., Chow A. Early neutralizing IgG response to chikungunya virus in infected patients targets a dominant linear epitope on the E2 glycoprotein. EMBO Mol. Med. 2012;4:330–343. doi: 10.1002/emmm.201200213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner J., Anraku I., Le T.T., Larcher T., Major L., Roques P., Schroder W.A., Higgs S., Suhrbier A. Chikungunya virus arthritis in adult wild-type mice. J. Virol. 2010;84:8021–8032. doi: 10.1128/JVI.02603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson J.A., Prow N.A., Schroder W.A., Ellis J.J., Cumming H.E., Gearing L.J., Poo Y.S., Taylor A., Hertzog P.J., Di Giallonardo F. RNA-seq analysis of chikungunya virus infection and identification of granzyme A as a major promoter of arthritic inflammation. PLoS Pathog. 2017;13:e1006155. doi: 10.1371/journal.ppat.1006155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoarau J.J., Jaffar Bandjee M.C., Krejbich Trotot P., Das T., Li-Pat-Yuen G., Dassa B., Denizot M., Guichard E., Ribera A., Henni T. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J. Immunol. 2010;184:5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 50.Poo Y.S., Rudd P.A., Gardner J., Wilson J.A., Larcher T., Colle M.A., Le T.T., Nakaya H.I., Warrilow D., Allcock R. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl. Trop. Dis. 2014;8:e3354. doi: 10.1371/journal.pntd.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayr A., Stickl H., Müller H.K., Danner K., Singer H. [The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism (author’s transl)] Zentralbl. Bakteriol. B. 1978;167:375–390. [PubMed] [Google Scholar]
- 52.Overton E.T., Stapleton J., Frank I., Hassler S., Goepfert P.A., Barker D., Wagner E., von Krempelhuber A., Virgin G., Meyer T.P. Safety and immunogenicity of modified vaccinia Ankara-Bavarian Nordic smallpox vaccine in vaccinia-naive and experienced human immunodeficiency virus-infected individuals: an open-label, controlled clinical phase II trial. Open Forum Infect. Dis. 2015;2:ofv040. doi: 10.1093/ofid/ofv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greenberg R.N., Hurley M.Y., Dinh D.V., Mraz S., Vera J.G., von Bredow D., von Krempelhuber A., Roesch S., Virgin G., Arndtz-Wiedemann N. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18–40 year old subjects with diagnosed atopic dermatitis. PLoS ONE. 2015;10:e0138348. doi: 10.1371/journal.pone.0138348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.García-Arriaza J., Cepeda V., Hallengärd D., Sorzano C.O., Kümmerer B.M., Liljeström P., Esteban M. A novel poxvirus-based vaccine, MVA-CHIKV, is highly immunogenic and protects mice against chikungunya infection. J. Virol. 2014;88:3527–3547. doi: 10.1128/JVI.03418-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weger-Lucarelli J., Chu H., Aliota M.T., Partidos C.D., Osorio J.E. A novel MVA vectored chikungunya virus vaccine elicits protective immunity in mice. PLoS Negl. Trop. Dis. 2014;8:e2970. doi: 10.1371/journal.pntd.0002970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shchelkunov S.N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013;9:e1003756. doi: 10.1371/journal.ppat.1003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frey S.E., Couch R.B., Tacket C.O., Treanor J.J., Wolff M., Newman F.K., Atmar R.L., Edelman R., Nolan C.M., Belshe R.B., National Institute of Allergy and Infectious Diseases Smallpox Vaccine Study Group Clinical responses to undiluted and diluted smallpox vaccine. N. Engl. J. Med. 2002;346:1265–1274. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- 58.Chakrabarti S., Sisler J.R., Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- 59.Smith G.L. Expression of genes by vaccinia virus vectors. In: Davison A.J., Elliotand R.M., editors. Molecular Virology a Practical Approach. IRL Press at Oxford University Press; 1993. pp. 257–283. [Google Scholar]
- 60.Falkner F.G., Moss B. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J. Virol. 1988;62:1849–1854. doi: 10.1128/jvi.62.6.1849-1854.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyle D.B., Coupar B.E. A dominant selectable marker for the construction of recombinant poxviruses. Gene. 1988;65:123–128. doi: 10.1016/0378-1119(88)90424-6. [DOI] [PubMed] [Google Scholar]
- 62.Isaacs S.N., Kotwal G.J., Moss B. Reverse guanine phosphoribosyltransferase selection of recombinant vaccinia viruses. Virology. 1990;178:626–630. doi: 10.1016/0042-6822(90)90367-z. [DOI] [PubMed] [Google Scholar]
- 63.Kotwal G.J., Abrahams M.-R. Poxviruses and determining virus titer. In: Issacs S.N., editor. Vaccinia Virus and Poxvirology Methods and Protocols. Humana Press; 2004. pp. 101–112. [DOI] [PubMed] [Google Scholar]
- 64.Kremer M., Volz A., Kreijtz J.H.C.M., Fux R., Lehmann M.H., Sutter G. Easy and efficient protocols for working with recombinant vaccinia virus MVA. In: Isaacs S.N., editor. Vaccinia Virus and Poxvirology Methods and Protocols. Humana Press; 2012. pp. 59–92. [DOI] [PubMed] [Google Scholar]
- 65.Newman F.K., Frey S.E., Blevins T.P., Mandava M., Bonifacio A., Jr., Yan L., Belshe R.B. Improved assay to detect neutralizing antibody following vaccination with diluted or undiluted vaccinia (Dryvax) vaccine. J. Clin. Microbiol. 2003;41:3154–3157. doi: 10.1128/JCM.41.7.3154-3157.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang D., Suhrbier A., Penn-Nicholson A., Woraratanadharm J., Gardner J., Luo M., Le T.T., Anraku I., Sakalian M., Einfeld D., Dong J.Y. A complex adenovirus vaccine against chikungunya virus provides complete protection against viraemia and arthritis. Vaccine. 2011;29:2803–2809. doi: 10.1016/j.vaccine.2011.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.