Abstract
Immune defense against hepatotropic viruses such as hepatitis B (HBV) and hepatitis C (HCV) poses a major challenge for therapeutic approaches. Intrahepatic cytotoxic CD8 T cells that are crucial for an immune response against these viruses often become exhausted resulting in chronic infection. We elucidated the T cell response upon therapeutic vaccination in inducible transgenic mouse models in which variable percentages of antigen-expressing hepatocytes can be adjusted, providing mosaic antigen distribution and reflecting the varying viral antigen loads observed in patients. Vaccination-induced endogenous CD8 T cells could eliminate low antigen loads in liver but were functionally impaired if confronted with elevated antigen loads. Strikingly, only by conditioning the liver environment with TLR9 ligand prior and early after peripheral vaccination, successful immunization against high intrahepatic antigen density with its elimination was achieved. Moreover, TLR9 immunomodulation was also indispensable for functional memory recall after high frequency antigen challenge. Together, the results indicate that TLR9-mediated conditioning of liver environment during therapeutic vaccination or antigen reoccurrence is crucial for an efficacious intrahepatic T cell response.
Keywords: CD8 T cells, vaccination, liver, antigen load, hepatitis, immune regulation, hepatic viral infection, HBV, HCV
Graphical Abstract
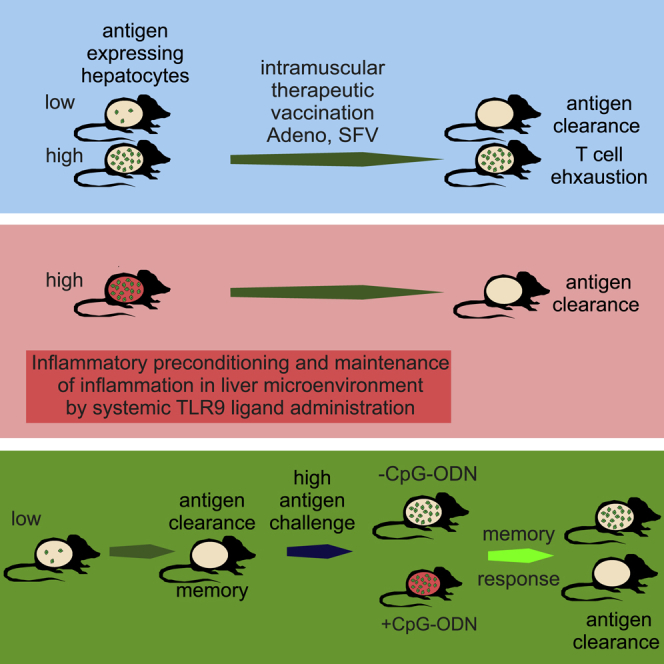
Therapeutic vaccination against HBV and HCV infections represents a challenge. Cebula et al. show that vaccination-induced T cells only eliminate low, but not elevated, antigen loads in liver. However, specific conditioning of the liver environment during vaccination improves T cell performance and antigen-presenting hepatocytes are efficiently cleared.
Introduction
The liver represents an exceptional organ, crucial not only for various metabolic and detoxifying functions in the body but also for its paradoxical immunological properties. Faced with the enormous task of balancing the immunological scale toward tolerance because of the continuous exposure to harmless dietary antigens from the gastrointestinal tract, the liver needs to be on its constant guard, ready to mount an immune response to harmful incoming pathogens.1, 2, 3, 4 Exploiting this liver tolerance effect, hepatotropic viruses such as hepatitis B (HBV) and C (HCV) have been successful in establishing persistent infection with over 500 million people worldwide showing chronic infection thereby posing a major global health concern. The intrahepatic cytotoxic CD8 T cell response that is critical for an effective immune response has been reported to be dysfunctional and exhausted in chronically infected patients.5, 6 Still, the question remains why some individuals (∼90% in case of HBV and ∼20%–40% in case of HCV) show viral control, whereas in the remaining patients, persistent infections are established.7 Our understanding of the liver immunity is still elusive. In particular, the mechanisms and key regulators that are involved in the determination of T cell fate in liver (active versus tolerized state) remain to be elucidated. Identifying these mechanisms would be fundamental for rational design of immunomodulatory strategies and therapeutic vaccination options that are currently emerging as powerful tools against chronic infections and cancer. While a preventive vaccine for HBV is available, immune therapy for HBV-infected patients is still a challenge. Further, although potent treatment options with small molecules are available for HCV, efficient immune-based interventions are missing. An ideal therapeutic vaccination should enhance immune responses, curb viral load, accelerate recovery, and develop protective memory. Such vaccinations would not only be efficacious in chronic viral infection but also in bacterial infection and cancer. Accordingly, new immunotherapeutic strategies that restore T cell responses in chronic viral infections are emerging. These rely on either blocking inhibitory receptor pathways such as PD-1,8 use of direct acting antivirals,9 and development of T cell vaccines.10, 11 However, the majority of such therapies developed so far have variable outcomes in patients.10, 11, 12
An important consideration is that patients infected with HBV or HCV show varying viral loads.13 This discrepancy in viral frequency could well play a critical role in immunotherapy outcome. To that end, recently, we and others have reported that the intrahepatic antigen load is a potent regulator determining the outcome of hepatocyte-primed CD8 T cell responses.14, 15 However, while the previous studies relied on adoptively transferred antigen-specific T cells, the impact of varying intrahepatic antigen densities on vaccine-induced endogenous T cells has not been addressed. We investigated this question in a mouse model under conditions of therapeutic vaccination. We show that irrespective of the type of vaccination, clearance of low but not of high frequencies of antigen-expressing cells was possible. Further, successful immunotherapy directed against “high antigen conditions” was achieved by conditioning the liver environment with a TLR 9 agonist. Additionally, we observed a particular threshold for effective memory recall, whereby memory responses against high antigen were functional only in presence of CpG-ODN-induced inflammatory conditions.
Results
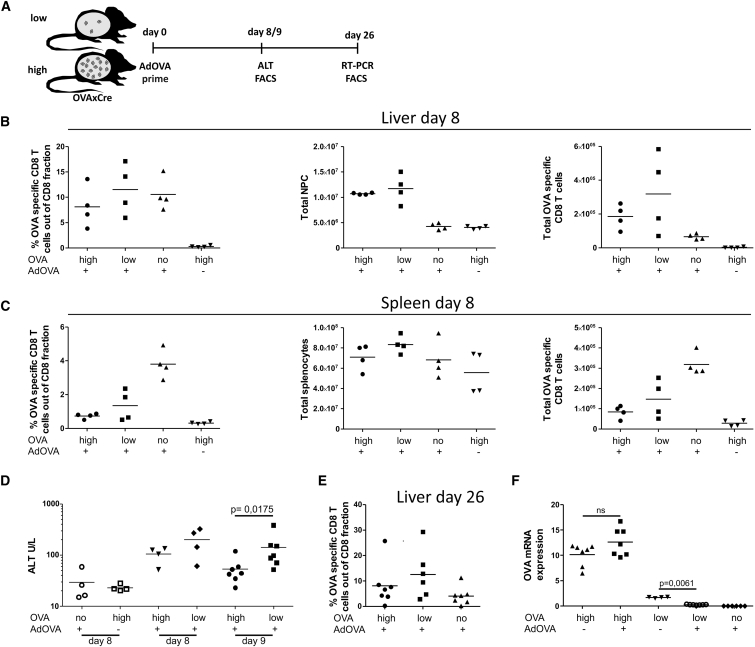
Efficacy of Standard Vaccination Inversely Correlates with Antigen Density in the Liver
We evaluated if the cytotoxic function and antigen clearance capacity of vaccine-induced, peripherally primed CD8 T cells is affected by the intrahepatic antigen density. To this end, we employed an infection-free transgenic mouse model (OVAXAlbCreERT2, short OVAxCre) expressing non-secreted ovalbumin (OVA) exclusively in hepatocytes. In the steady state, these mice display antigen expression in <10% of hepatocytes (low antigen condition) while single administration of 50 μg tamoxifen (TAM) induces OVA expression in 50% of all hepatocytes (high antigen condition).14 To elicit CD8 T cell responses, we vaccinated these mice by intramuscular injection of an adenoviral vector encoding the cognate MHC I OVA epitope (AdOVA) (Figure 1A). Eight days post vaccination, OVA-specific CD8 T cells were observed in liver (Figure 1B) and spleen (Figure 1C) in low and high antigen condition in OVAxCre mice as well as in control mice not expressing OVA in the liver. This confirmed the efficacy of immunization and qualified the OVAxCre model for vaccination purposes. Notably, vaccinated OVAxCre mice expressing OVA in the liver displayed a 2-fold increase of intrahepatic non-parenchymal cell (NPC) fractions in comparison to non-vaccinated controls or vaccinated mice without liver-specific OVA expression. This antigen-specific accumulation of NPCs and OVA-specific CD8 T cells is indicative of an inflammatory response. This was found to correlate with liver injury observed on day 8 and 9 post vaccination as measured by alanine aminotransferase (ALT) activity in blood plasma (Figure 1D). Interestingly, we observed a trend toward higher ALT values in mice expressing low antigen levels, which was statistically significant on day 9 post vaccination. Analysis on day 26 post vaccination revealed that frequencies of OVA-specific T cells were still elevated in livers of antigen-expressing mice (Figure 1E). This indicates that presence of cognate antigen in the liver may result in prolonged accumulation of OVA-specific CD8 T cells after vaccination. As ultimate outcome of the CD8 T cell response, we quantified antigen expression in the liver. To this end, qRT-PCR analysis of OVA expression was performed in the liver on day 26 post vaccination. Analysis revealed complete elimination of intrahepatic antigen expression in mice expressing low antigen levels, whereas in mice with high antigen levels, OVA expression was comparable to non-vaccinated littermates (Figure 1F). Thus, in this vaccination setting, only low antigen levels could be cleared. Insufficient response observed in mice with high antigen might have been due to limited efficacy of the immunization protocol. Therefore, we optimized the vaccination regime. First, we evaluated a protocol involving additional boosting steps. For this purpose, animals were primed by a single intramuscular injection of the OVA-encoding plasmid pCIOVA, followed by a boost with AdOVA applied on day 28 (Figure 2A). On day 14 after the boost, we found OVA-specific CD8 T cells in the liver of mice expressing both low and high antigen levels (Figure 2B). To determine the functionality of OVA-specific CD8 T cells in the liver, we analyzed their effector cytokine production capacity upon in vitro peptide stimulation. The highest cytokine production was displayed by CD8 T cells in vaccinated control mice demonstrating the actual potential of the applied vaccination regime (Figure 2C). Of note, intrahepatic CD8 T cells isolated from high antigen load mice showed impaired production of interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), which correlated with the antigen burden. Further analysis revealed that expression levels of exhaustion markers PD-1 and LAG-3 were directly proportional to intrahepatic expression of OVA antigen (Figure 2D). To estimate the efficacy of the therapeutic vaccination, we assessed the remaining antigen load in liver. Despite implementation of a boosting step, clearance of OVA-expressing hepatocytes was seen exclusively in the low antigen setting (Figure 2E).
Figure 1.
Immune Response upon AdOVA Vaccination of OVAxCre Mice
OVAxCre mice displaying low and high antigen frequencies as well as single transgenic non-OVA-expressing mice (no) were vaccinated with 109 AdOVA virions and analyzed according to the scheme (A). One group of animals was sacrificed on day 8. Liver (B) and spleen (C) were isolated and total number and frequencies of OVA-specific T cells, stained by OVA-specific pentamers, as well as liver non-parenchymal cells (NPC) and total splenocytes were determined. (D) Liver damage after vaccination was determined on days 8 and 9 by measuring ALT activity in blood plasma (A and D, data out of two independent experiments). (E) A second group of animals was sacrificed on day 26 post vaccination and the frequency of OVA-specific CD8 T cells was determined. (F) OVA mRNA expression was determined by qRT-PCR analysis in liver tissue on day 26 post vaccination. OVA mRNA levels were normalized to albumin mRNA and multiplied by constant factor of 105. The graphs represent individual mice as well as the calculated mean. The p value was calculated by Mann-Whitney test.
Figure 2.
CD8 T Cell Phenotype and Effector Functions Induced upon Various Vaccine Regimes and Hepatic Antigen Load
(A) Scheme and timescale of vaccination regime of a plasmid (pCIOVA) prime/AdOVA boost protocol in low/high OVAXCre and no antigen-expressing control mice with analysis on day 42. (B) Frequency of OVA-specific CD8 T cells in liver and spleen 42 days post vaccination. (C) IFN-γ and TNF-α effector cytokine expression profile of intrahepatic CD8 T cells was determined upon ex vivo OVA peptide restimulation. Cumulative data from two independent experiments as well as representative dot plots shows IFN-γ and TNF-α double positive CD8 T cells. (D) PD-1 and LAG-3 expression on intrahepatic OVA-specific CD8 T cells (two independent experiments) are depicted in the graphs. The representative dot plots show PD-1 and LAG-3 double-positive OVA-specific CD8 T cells. (E) Relative OVA mRNA expression was determined by qRT-PCR in liver tissue on day 42 post vaccination (data of two independent experiments). (F) The multiple boosting vaccination strategies comprise the following regimes: pCIOVA prime/pCIOVA boost, pCIOVA prime/AdOVA boost, and pCIOVA prime/ dOVA boost followed by three subsequent pCIOVA boosts. Relative OVA mRNA expression was determined by qRT-PCR in liver tissue 2 weeks after boost with pCIOVA or AdOVA, and 9 weeks after triple pCIOVA boosts (G) SFV-OVA prime/boost vaccination (5 × 106 virions each step). Blood analysis depicting OVA-specific CD8 T cell 8 days post SFV-OVA boost. Relative OVA mRNA expression was determined by RT-PCR in liver tissue on day 42 post vaccination (two independent experiments).
Attempts to provoke more potent immune responses capable of clearing high antigen loads were undertaken. More stringent regimes involving multiple boosting steps were tested, however, without any beneficial effects in terms of antigen clearance or reduction (Figure 2F). We also applied a recently developed immunization regime based on Semliki Forest virus (SFV) that provides transient and high antigen expression at the vaccination site16 and induces highly cytotoxic CD8 T cell responses.17 To this end, mice with low and high antigen load were immunized with SFV-OVA twice within a 2-week period (Figure 2G). We confirmed generation of a high frequency of OVA-specific CD8 T cells upon SFV-OVA vaccination by analyzing blood samples 6–8 days post boost (Figure 2G). Nevertheless, determination of liver-specific OVA antigen expression 42 days post vaccination showed clearance solely at low antigen conditions (Figure 2G).
In order to exclude epitope-specific dependence of the observed tolerance to high expression of intrahepatic antigen, we established an analogous mouse model expressing the VWLSVIWM epitope derived from HBV surface antigen (HBsxCre mouse model). We tested both a plasmid pCIHBs prime/boost vaccination, adenoviral boosting steps, and multiple (3×) plasmid pCIHBs boosts post adenoviral boost and evaluated the formation of intrahepatic antigen-specific T cells by tetramer staining. Notably, despite the generation of HBs-specific CD8 T cells in the liver in both high and low antigen load conditions, only mice expressing low antigen load showed significant reduction (see also Figure S1). Thus, for both OVA and HBs antigen-expressing mice, we conclude that irrespective of the type of vaccine or vaccination regime, intrahepatic antigen density plays a decisive role in controlling the outcome of the immune response.
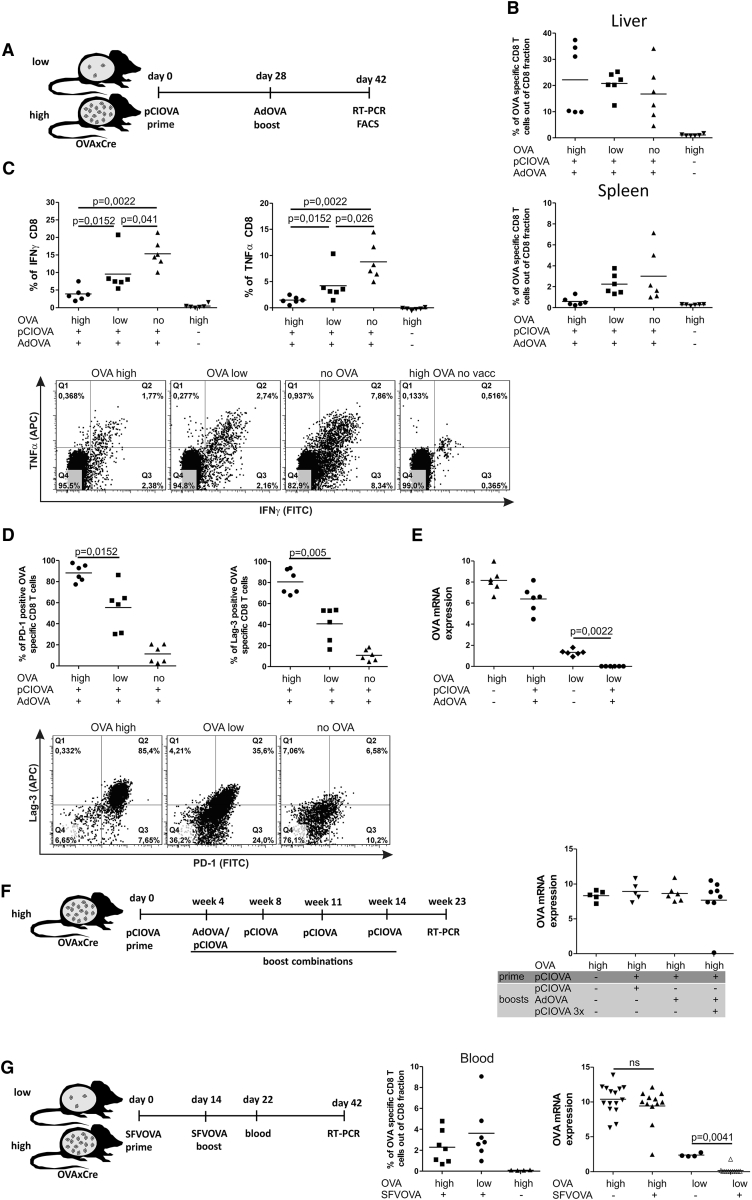
Effective Vaccination against Low Antigen Load Provides Protective Memory Response Only against Low Antigen Challenge
All vaccination regimes tested so far were exclusively mounting sterilizing immune responses that were effective only against low antigen level. We asked if these successful immune responses would lead to formation of protective memory. We took advantage of the transgenic OVAxCre model that allows for controlled TAM-dependent (re)induction of the antigen expression.18 Accordingly, we challenged the low antigen-expressing mice after successful AdOVA vaccination by administering TAM 4 weeks later (Figure 3A). To determine if the antigen load might also regulate the intrahepatic immunity at the level of memory response, we induced antigen reoccurrence at low or high level by application of different TAM doses (12.5 μg or 50 μg). Two weeks (15–18 days) after TAM application, mice were analyzed with respect to OVA antigen expression in the liver. Notably, the memory T cells were sufficient for clearing antigen reoccurring at low levels (12.5 μg TAM) (Figure 3B), but not at high levels (50 μg TAM) (Figure 3C). These results show that protective memory was established with the AdOVA vaccination regime. However, this memory is only capable of clearing subsequent challenge with low but not with high antigen levels. Thus, the primary T cell response as well as the memory response was found to be compromised by elevated intrahepatic antigen loads.
Figure 3.
Efficacy of Vaccination-Induced Memory Response upon OVA Antigen Challenge
(A) Scheme and timescale of vaccination regime (AdOVA prime), antigen challenge, and analysis. OVAXCre mice expressing low antigen frequency as well as non-OVA-expressing control mice were vaccinated with a single AdOVA application. This protocol clears the low antigen levels within 26 days (see Figure 1F). On day 27, mice were treated with 12.5 μg or 50 μg TAM to (re)induce OVA expression in low or high numbers of hepatocytes, respectively. (B and C) Relative OVA mRNA expression determined by qRT-PCR in liver tissue on day 15 (B) or 17 (C) post OVA antigen challenge (two independent experiments)
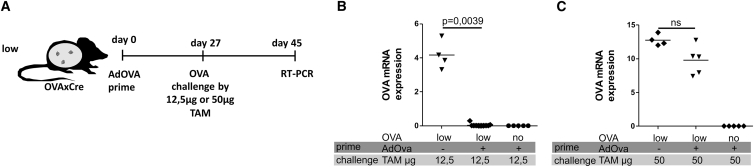
Functional Memory Recall against High Antigen Challenge Depends on Simultaneous Conditioning of the Liver Environment by a TLR9 Agonist
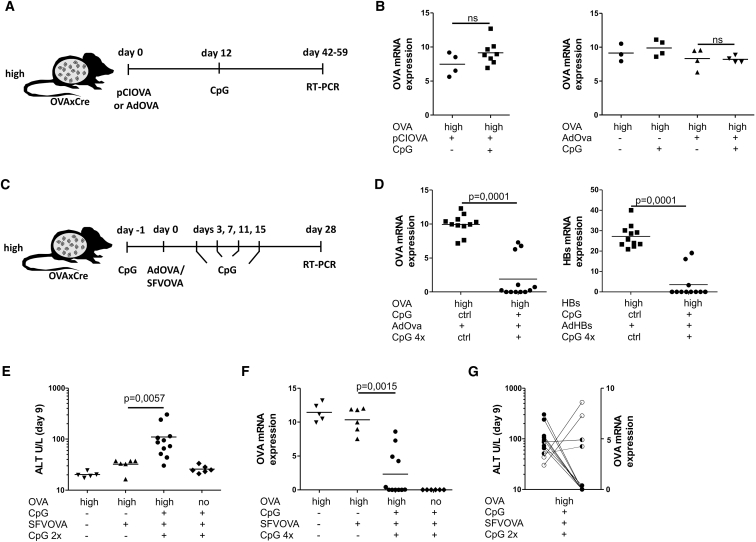
The limitation in memory recall against high level antigen conditions let us test the hypothesis that additional immunomodulatory signals could improve the efficacy of liver-specific memory responses. Huang et al.19 showed that systemic injections of the TLR9 ligand CpG-ODN at day 12 after vaccination can support intrahepatic CD8 T cell expansion by induction of intrahepatic inflammatory cell clusters to provide a costimulatory environment within the liver parenchyma. We investigated if this protocol could improve the memory quality with respect to reoccurring high levels of antigen. Accordingly, mice expressing OVA antigen at low levels were vaccinated with AdOVA and subjected to TLR9 ligand treatment at day 12 (see Figure 4A part I for the vaccination regime). To evaluate the memory response, we challenged these mice on day 39 post vaccination with 50 μg TAM to reestablish antigen expression in 50% of hepatocytes. Seven weeks post challenge, the antigen level remained in the range of the non-vaccinated controls indicating that the TLR9 treatment on day 12 did not improve the quality of memory (Figure 4B, part I).
Figure 4.
Efficacy of Vaccination-Induced Memory Response upon OVA Antigen Challenge in the Presence of CpG-Based Immunomodulation
(A) Scheme and timescale of vaccination regime (AdOVA prime, CpG-ODN), antigen challenge, and analysis. OVAxCre mice expressing low antigen frequency were vaccinated with a single dose of AdOVA followed on day 12 by i.v. injection of 20 μg CpG-ODN TLR9 ligand. On day 39, mice were treated with 50 μg TAM leading to the induction of OVA expression in high numbers of hepatocytes either in absence (I) or presence (II) of CpG-ODN treatment on day 40. (B) Relative OVA mRNA expression determined by qRT-PCR in liver tissue on days 14–49 post OVA antigen challenge. Data of two independent experiments are shown. (C) HBsxCre mice with low antigen levels were vaccinated and challenged with high antigen either in absence (I) or presence (II) of CpG-ODN treatment according to the experimental procedure depicted in (A). On day 17 after TAM induced antigen (re)induction, animals were sacrificed and analyzed for relative HBs antigen levels.
We then investigated if inflammatory conditions at the time of antigen challenge would improve the recall of memory response against high intrahepatic antigen loads. To this end, we first vaccinated OVAxCre mice at low antigen expression conditions with AdOVA and stimulated them on day 12 with TLR9 ligand. Four weeks after vaccination, we co-administered 50 μg TAM and CpG-ODN TLR9 ligand (see Figure 4A, part II). Strikingly, analysis of intrahepatic OVA expression revealed a significant reduction of antigen in 3/14 mice while 11/14 showed complete clearance (Figure 4B, part II). To validate this interesting finding, we translated this vaccine regime to the HBsxCre mouse model. All mice subjected to high antigen challenge with subsequent TLR9 stimulation achieved a significant reduction or complete clearance of HBs antigen levels (Figure 4C) confirming the results from the OVA model. This proves that a functional liver-specific memory response against high frequencies of antigen-expressing hepatocytes essentially depends on a conditioned liver environment.
Therapeutic Vaccination against High Antigen Requires Conditioning of Liver Environment by CpG-ODN during Priming
The potent activity of memory in inflamed liver conditions let us ask if the primary response upon vaccination could also be improved by TLR9 stimulation. Because CpG-ODN treatment on day 12 application after vaccination did not give rise to any reduction of hepatocytes at high level antigen-expression conditions (Figure 5B), we argued that activation of T cell responses would require an earlier stimulation. Thus, we aimed to ensure inflammatory intrahepatic conditions before antigen-specific CD8 T cells entered the liver (i.e., day 8 post vaccination, cf. Figure 1). For this purpose, OVAxCre or HBsXCre mice with high antigen load were injected intravenously (i.v.) with CpG-ODN prior to vaccination in order to precondition the liver. Subsequently, mice were vaccinated with AdOVA or AdHBs vectors, respectively. To maintain the altered liver milieu, CpG-ODN was administered four times after vaccination. This covers the period of T cell priming, liver entry, and effector response (Figure 5C). Antigen load was then determined on day 28 post vaccination (Figure 5D). In comparison to groups preconditioned with a control TLR9 ligand, the group receiving CpG-ODN achieved significant reduction or even clearance of high antigen load in 67% and 81%, respectively. To show whether this therapeutic conditionalized liver immunization strategy can be adapted to a clinically relevant immunizing vector, we tested the SFV vector-encoding OVA antigen. Assuming that liver damage during vaccination could serve as a predictive marker of a successful immune response, we monitored the ALT activity. An increase of ALT on day 9 post vaccination was observed in CpG-ODN-treated and SFV-OVA-vaccinated group of OVA-expressing mice, indicating antigen-specific cytotoxic activity of vaccine-induced CD8 T cells (Figure 5E). These data correlated with complete OVA antigen clearance observed in 7 of 11 mice (Figures 5F and 5G). In conclusion, these results indicate the crucial role of intrahepatic antigen levels as one of the key regulatory factors influencing the outcome of intrahepatic CD8 T cell responses. By providing adequate intervention, e.g., by using a TLR9 agonist, we could define the conditions to overcome the tolerizing effects of high intrahepatic antigen expression as well as induce functional memory responses against reoccurring high antigen in the liver.
Figure 5.
Effects of CPG-ODN-Based Immunomodulation of the Liver during Therapeutic Vaccination in High Antigen Mice
(A) Scheme and timescale of CpG ODN containing vaccination regime. OVAxCre mice expressing high antigen frequency were vaccinated with a single dose of pCIOVA or AdOVA followed by i.v. injection of 20 μg CpG-ODN on day 12. (B) Relative OVA mRNA expression levels were determined by qRT-PCR in liver tissues at least 6 weeks post vaccination. (C) OVAxCre or HBsxCre mice expressing high antigen frequencies were conditioned by i.v. injection of 20 μg CpG-ODN or control non-CpG-ODN 1 day before vaccination with a single dose of AdOVA or AdHBs, respectively. Subsequently, four doses of CpG-ODN or control non-CpG-ODN were administered i.v. every fourth day. (D) Relative OVA (left) or HBs (right) mRNA expression determined by qRT-PCR in liver tissue after a minimum of 4 weeks post vaccination in OVA model (left) and HBs (right). Data of two independent experiments are shown. (E and F) Viral particles (5 × 107) of the SFV-OVA vector was used to immunize OVAxCre mice with high antigen load according to (C). (E) ALT activity in blood plasma was determined on day 9 after SFV-OVA vaccination. (F) Relative OVA mRNA expression determined by qRT-PCR in liver tissue after minimum of 4 weeks post SFV-OVA vaccination. (G) Correlation between elevated ALT measured in therapeutically vaccinated mice and observed antigen clearance (two independent experiments).
Discussion
The dichotomous properties of liver have challenged the development of immune-based strategies against liver pathogens. While the liver is the only non-lymphoid organ capable of inducing immune responses and directly prime CD8 T cells,20 current literature shows contradicting results concerning the outcome of such immune responses; tolerance21, 22 versus functional activation.23, 24 The differential results concerning the fate of intrahepatically primed CD8 T cells were addressed by models that show antigen expression induced either by viral vector titration15 or in a sterile, infection-free setting.14 Studies using varying antigen loads in the liver revealed that high antigen loads impair the cytotoxic potential of adoptively transferred cells. Overcoming this impairment would be of particular relevance in the context of therapeutic vaccination that relies on reviving T cell function to combat hepatic viral infection or transformed tumor cells. Thus, the question was raised if endogenous T cell responses evoked by priming in the periphery could obliterate the tolerance in high antigen density conditions.
In this study, we investigated if the cytotoxic capacity of vaccine-induced CD8 T cells is affected by the intrahepatic antigen load. To this end, we employed an infection-free mouse model in which the number of antigen-expressing cells can be adjusted in a range of 10%–50% of cells by feeding with TAM.14 This model reflects the range of infected cells in patients infected with HCV, where 1%–54% of infected hepatocytes have been found.13 Further, it mimics the condition of antigen presentation in chronic HBV infections, which are hardly accompanied by activation of innate immune responses.25, 26 We could provoke the establishment of a population of antigen-specific CD8 T cells upon vaccination, irrespective of intrahepatic expression of the cognate antigen. However, the cytotoxic activity of these cells was found to be impaired proportionally to the level of antigen expression in liver. The inability to clear high antigen loads was also observed when employing vaccination protocols that have been described as highly potent in extrahepatic compartments. These protocols include adenoviral vaccinations, multiple boosting regimes,27, 28 and SFV-based vaccinations that recently entered clinical trials for cervical papilloma immunotherapy (http://www.vicinivax.com).17, 29 These observations are supported by a recent study in an HBV transgenic model where vaccination-induced T cells could only combat low viremia.30 Although the mechanism that underlies the dampening of T cell activation remains to be elucidated, evidence is accumulating that this observation is not restricted to the liver but also plays a role for other tissues as demonstrated in chronic lymphocytic choriomeningitis virus (LCMV) infection models31 or certain lymphoma.32
A key objective of therapeutic vaccinations is to induce (and/or invigorate) cytotoxic T cell responses against chronically infected cells or tumors that eventually differentiate into long-lasting protective memory. A recent study shows the existence and essential role of liver-resident memory T cells that confer protection against malaria.33 Such tissue-resident memory T cells have been recognized as a subset of the memory population that have a crucial role in ensuring protection against pathogens.34
In our model, we challenged the memory response established by AdOVA vaccination by de novo induction of different densities of OVA-expressing hepatocytes. We observed protective immunity against reoccurring low antigen densities but not at high antigen densities. This suggests that memory can be established below a certain threshold of antigen-expressing cells. Beyond this threshold, intrahepatic regulatory cues limit memory recall.
While memory has been thought to depend on antigen recognition (i.e., T cell receptor signaling) recent studies shed new light on the details of memory recall. They indicate that the response of memory cells is shaped by inflammatory cytokines. Particularly, interleukin (IL)-12, IL-15, IL-18, and type I IFN are released by sentinel cells upon reinfection and support memory recall35, 36, 37. Currently, it is not understood how inflammatory factors determine the quality of memory recall, in particular in recently identified tissue-resident memory T cells.38 Similarly, standard experimental conditions did not allow dissecting the role of antigen-induced TCR signaling versus the impact of inflammation.39
The sterile OVAxCre model allows us to address the requirements for memory recall within the liver environment. While antigenic stimulation was sufficient when antigen reoccurred at low levels, the recall of functional memory against high antigen challenge requires the simultaneous activation of TLR9 signaling pathway. This finding provides a first important clue toward the (potential) role of earlier-described TLR9-induced intrahepatic myeloid structures19 for the establishment of a functional memory response in the liver.
While in the past, TLR9 ligands were mainly employed as adjuvants at the site of vaccination, the impact of systemic CpG-ODN administration on intrahepatic CD8 T cell expansion has been recently highlighted. As shown by Huang et al.,19 systemic application of CpG-ODN-induced intrahepatic myeloid structures consisting of inflammatory monocytes. These express co-stimulatory molecules like CD80, CD86, and OX40L that are relevant for expansion of vaccine-induced T cell responses. By challenging the suggested vaccination protocol (that includes CpG-ODN treatment on day 12) with elevated antigen load, we failed, however, to induce immune responses capable of reducing the antigen load. Strikingly, only when we pre-treated the mice with TLR9 before vaccination and extended the time of TLR9-induced inflammation could we significantly improve the intrahepatic T cell response. Under these conditions, the T cells were capable of reducing high antigen loads in all vaccinated animals, and in the majority of the animals, we could even achieve complete clearance. Thus, we conclude that it is crucial to condition the tolerogenic liver environment by CpG-ODN before and during vaccine-induced T cell responses, thereby ensuring inflammatory conditions in the liver throughout the complete immune reaction, including antigen recognition and cytotoxic effector phase. It remains to be shown if extra-hepatic effects of systemic CpG-ODN administration can support T cells in other organs.40
It would be tempting to translate this finding to patients chronically infected with HBV and HCV. To this end, peripherally applied vaccines directed against virus-specific epitopes would need to be combined with repeated doses of CpG-ODN. In this regard, it is of note that maintaining inflammation in liver over 2 weeks during vaccination is not expected to cause adverse autoimmune effects because TLR9 induction has been shown to be completely reversible in mice.41 While CpG has been considered as an adjuvants for B cell vaccines,42 more studies would be required to validate this regimen in the context of T cell-based therapeutic strategies.
Our results show that immunotherapeutic intervention, even in an extremely immune-privileged organ/site like the liver, can be achieved by providing appropriate immunomodulatory conditions. We identified the requirements for efficacious liver memory responses as well as the conditions supporting therapeutic vaccinations directly against high antigen burden. These findings may contribute to more rational design of immunotherapeutic interventions.
Materials and Methods
Transgenic Mice and Experimental Procedures
The transgenic OVAXAlbCreERT2, (OVAxCre) mice used in the study have C57BL6/J genetic background and have been described before.43 In brief, the OVAxCre mice carry a silent synthetic OVA gene cassette flanked by inversely oriented LoxP sites integrated in the ubiquitously expressed ROSA26 locus. Expression was induced by tamoxifen (TAM) administration, leading to liver-specific Cre-mediated inversion of the cassette. Similarly, HBsAgXAlbCreERT2 (HBsxCre) mice carry a gene cassette in a similar configuration encoding the 86 amino acid residues (aa 140–226) of hepatitis B virus surface antigen (SIII) possessing well-defined MHC class I Kb, Kd, and Dd binding epitopes.44 All of the experiments were performed with mice 8–20 weeks of age that were maintained and bred in individually ventilated cages under specific pathogen-free conditions. The TAM (Ratiopharm, Ulm, Aluid Pharma) application procedure (50 μg or 12.5 μg) is described elsewhere.18 All animal experiments were done in accordance with the German Animal Welfare Law and have been approved by the local government of Lower Saxony.
Vaccination Vectors and Immunization of Mice
Plasmids pCIOVA and pCIHBs, described in Schirmbeck et al.,45 were produced by Plasmid Factory. Used AdOVA and AdHBs vectors were based on Ad5 E1-deleted first-generation vectors as described in Schirmbeck et al.45 and Wortmann et al.46 SFV-OVA-replicon particles were produced as previously described in Daemen et al.16 Immunization of mice was performed by intramuscular bilateral injections into tibialis anterior muscle. Per injection (25–50 μL), mice received 50 μg plasmid DNA, 109 Ad virions, 5 × 106 SFV virions (Figure 1G), or 5 × 107 (Figure 5F) SFV virions, resuspended in PBS. To induce an inflammatory liver environment, mice were additionally treated with 20 μg of TLR9 ligand, CpG-ODN1668 (5′-S-TCCATGACGTTCCTGATGCT-3′) or control non-CpG-ODN 1720 (5′-S-TCCATGAGCTTCCTGATGCT-3′) (TIB Molbiol) injected i.v. (100 μL of 0.9% NaCl).
ALT Measurement
Retro-orbital bleeding technique was used to collect blood samples that were mixed 1:4 with heparin 1.25 I.E., (Ratiopharm). ALT activity measurement in blood plasma was carried out using a Reflovet Plus reader (Roche Diagnostics).
Immune Cell Isolation and Flow Cytometry
Isolation of liver non-parenchymal cells (NPC) and splenocytes was performed as described in Cebula et al.18 The staining procedure using antiCD8-PerCPCy5.5 antibody, antiPD-1-FITC, antiLAG-3-PE, antiTNFα-APC (eBioscience), and antiIFNγ-FITC (BD) was described in Ochel et al.14 For identification of OVA- or HBs-specific T cells, H-2Kb-SIINFEKL pentamer-PE or H-2Kb-VWLSVIWM pentamer-PE (HBs) were used, respectively (Proimmune). Alternatively, to identify OVA-specific CD8 T cell dextramers, JD2163-PE were used (Immudex). Specific effector cytokine expression upon SIINFEKL peptide stimulation of CD8 cells was analyzed by subtracting minimal signal of peptide unstimulated controls. Acquisition was performed using the LSR II (BD). Analysis was done with FlowJo (TriStar).
RNA Isolation and qRT-PCR
RNA isolation was performed as described previously.18 Total RNA (2 μg) was reverse-transcribed using the RevertAid First Strand cDNA synthesis kit (ThermoFisher Scientific) or Ready-To-Go You-Prime First-Strand Beads kit (GE Healthcare Life Science). qRT-PCR was performed as described previously18 using primer pairs 1a (5′-CAGGCACTCCTTTCAAGACC-3′) and 4a (5′-GCGGTTGAGGACAAACTCTT-3′) for quantification of OVA/HBsAg and normalized to albumin expression (forward primer: 5′-GACAAGGAAAGCTGCCTGAC-3′/reverse primer: 5′-TTCTGCAAAGTCAGCATTGG-3′).
Statistical Analysis
Data are represented as a mean of biological replicates and additionally depict individual mice as specified in the respective figure legends. PRISM software (GraphPad) was used for graph generation and statistical analysis. Mann-Whitney U test was used for all comparison of respective datasets, and p values are given above compared datasets.
Author Contributions
Concept & Design, M.C. and D.W.; Experiments & Procedures, M.C., M.R., and U.H.; Materials: R.F.K., F.K., G.K., and T.D.; Critical Discussion of Data: M.C., M.R., T.D., H.H., U.H., and D.W.; Writing, M.C., U.H., H.H., and D.W. All authors read and approved the manuscript.
Acknowledgments
We thank the Central Animal Facility (TEE) at HZI for excellent support. The work was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) via the SFB900 (Chronic Infection) and the Cluster of Excellence REBIRTH (From Regenerative Biology to Reconstructive Therapy). Further, we acknowledge support by a grant from the Helmholtz cross-program topic “Metabolic Dysfunction.”
Footnotes
Supplemental Information includes one figure and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.06.018.
Supplemental Information
References
- 1.Heymann F., Tacke F. Immunology in the liver--from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 2.Jenne C.N., Kubes P. Immune surveillance by the liver. Nat. Immunol. 2013;14:996–1006. doi: 10.1038/ni.2691. [DOI] [PubMed] [Google Scholar]
- 3.Wong Y.C., Tay S.S., McCaughan G.W., Bowen D.G., Bertolino P. Immune outcomes in the liver: Is CD8 T cell fate determined by the environment? J. Hepatol. 2015;63:1005–1014. doi: 10.1016/j.jhep.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 4.Adams D.H., Eksteen B., Curbishley S.M. Immunology of the gut and liver: a love/hate relationship. Gut. 2008;57:838–848. doi: 10.1136/gut.2007.122168. [DOI] [PubMed] [Google Scholar]
- 5.Radziewicz H., Ibegbu C.C., Fernandez M.L., Workowski K.A., Obideen K., Wehbi M., Hanson H.L., Steinberg J.P., Masopust D., Wherry E.J. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boni C., Fisicaro P., Valdatta C., Amadei B., Di Vincenzo P., Giuberti T., Laccabue D., Zerbini A., Cavalli A., Missale G. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin E.C., Sung P.S., Park S.H. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 2016;16:509–523. doi: 10.1038/nri.2016.69. [DOI] [PubMed] [Google Scholar]
- 8.Gardiner D., Lalezari J., Lawitz E., DiMicco M., Ghalib R., Reddy K.R., Chang K.M., Sulkowski M., Marro S.O., Anderson J. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS ONE. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin B., Hennecke N., Lohmann V., Kayser A., Neumann-Haefelin C., Kukolj G., Böcher W.O., Thimme R. Restoration of HCV-specific CD8+ T cell function by interferon-free therapy. J. Hepatol. 2014;61:538–543. doi: 10.1016/j.jhep.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 10.Kelly C., Swadling L., Capone S., Brown A., Richardson R., Halliday J., von Delft A., Oo Y., Mutimer D., Kurioka A. Chronic hepatitis C viral infection subverts vaccine-induced T-cell immunity in humans. Hepatology. 2016;63:1455–1470. doi: 10.1002/hep.28294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ip P.P., Nijman H.W., Wilschut J., Daemen T. Therapeutic vaccination against chronic hepatitis C virus infection. Antiviral Res. 2012;96:36–50. doi: 10.1016/j.antiviral.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Zabaleta A., D’Avola D., Echeverria I., Llopiz D., Silva L., Villanueva L., Riezu-Boj J.I., Larrea E., Pereboev A., Lasarte J.J. Clinical testing of a dendritic cell targeted therapeutic vaccine in patients with chronic hepatitis C virus infection. Mol. Ther. Methods Clin. Dev. 2015;2:15006. doi: 10.1038/mtm.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wieland S., Makowska Z., Campana B., Calabrese D., Dill M.T., Chung J., Chisari F.V., Heim M.H. Simultaneous detection of hepatitis C virus and interferon stimulated gene expression in infected human liver. Hepatology. 2014;59:2121–2130. doi: 10.1002/hep.26770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochel A., Cebula M., Riehn M., Hillebrand U., Lipps C., Schirmbeck R., Hauser H., Wirth D. Effective intrahepatic CD8+ T-cell immune responses are induced by low but not high numbers of antigen-expressing hepatocytes. Cell. Mol. Immunol. 2016;13:805–815. doi: 10.1038/cmi.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tay S.S., Wong Y.C., McDonald D.M., Wood N.A., Roediger B., Sierro F., Mcguffog C., Alexander I.E., Bishop G.A., Gamble J.R. Antigen expression level threshold tunes the fate of CD8 T cells during primary hepatic immune responses. Proc. Natl. Acad. Sci. USA. 2014;111:E2540–E2549. doi: 10.1073/pnas.1406674111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daemen T., Regts J., Holtrop M., Wilschut J. Immunization strategy against cervical cancer involving an alphavirus vector expressing high levels of a stable fusion protein of human papillomavirus 16 E6 and E7. Gene Ther. 2002;9:85–94. doi: 10.1038/sj.gt.3301627. [DOI] [PubMed] [Google Scholar]
- 17.Riezebos-Brilman A., Walczak M., Regts J., Rots M.G., Kamps G., Dontje B., Haisma H.Y., Wilschut J., Daemen T. A comparative study on the immunotherapeutic efficacy of recombinant Semliki Forest virus and adenovirus vector systems in a murine model for cervical cancer. Gene Ther. 2007;14:1695–1704. doi: 10.1038/sj.gt.3303036. [DOI] [PubMed] [Google Scholar]
- 18.Cebula M., Ochel A., Hillebrand U., Pils M.C., Schirmbeck R., Hauser H., Wirth D. An inducible transgenic mouse model for immune mediated hepatitis showing clearance of antigen expressing hepatocytes by CD8+ T cells. PLoS ONE. 2013;8:e68720. doi: 10.1371/journal.pone.0068720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang L.R., Wohlleber D., Reisinger F., Jenne C.N., Cheng R.L., Abdullah Z., Schildberg F.A., Odenthal M., Dienes H.P., van Rooijen N. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat. Immunol. 2013;14:574–583. doi: 10.1038/ni.2573. [DOI] [PubMed] [Google Scholar]
- 20.Bertolino P., Bowen D.G., McCaughan G.W., Fazekas de St Groth B. Antigen-specific primary activation of CD8+ T cells within the liver. J. Immunol. 2001;166:5430–5438. doi: 10.4049/jimmunol.166.9.5430. [DOI] [PubMed] [Google Scholar]
- 21.Holz L.E., Benseler V., Bowen D.G., Bouillet P., Strasser A., O’Reilly L., d’Avigdor W.M., Bishop A.G., McCaughan G.W., Bertolino P. Intrahepatic murine CD8 T-cell activation associates with a distinct phenotype leading to Bim-dependent death. Gastroenterology. 2008;135:989–997. doi: 10.1053/j.gastro.2008.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morimoto J., Tan X., Teague R.M., Ohlén C., Greenberg P.D. Induction of tolerance in CD8+ T cells to a transgenic autoantigen expressed in the liver does not require cross-presentation. J. Immunol. 2007;178:6849–6860. doi: 10.4049/jimmunol.178.11.6849. [DOI] [PubMed] [Google Scholar]
- 23.Wuensch S.A., Pierce R.H., Crispe I.N. Local intrahepatic CD8+ T cell activation by a non-self-antigen results in full functional differentiation. J. Immunol. 2006;177:1689–1697. doi: 10.4049/jimmunol.177.3.1689. [DOI] [PubMed] [Google Scholar]
- 24.Wuensch S.A., Spahn J., Crispe I.N. Direct, help-independent priming of CD8+ T cells by adeno-associated virus-transduced hepatocytes. Hepatology. 2010;52:1068–1077. doi: 10.1002/hep.23745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wieland S.F., Chisari F.V. Stealth and cunning: hepatitis B and hepatitis C viruses. J. Virol. 2005;79:9369–9380. doi: 10.1128/JVI.79.15.9369-9380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertoletti A., Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754–1764. doi: 10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 27.Fiedler M., Kosinska A., Schumann A., Brovko O., Walker A., Lu M., Johrden L., Mayer A., Wildner O., Roggendorf M. Prime/boost immunization with DNA and adenoviral vectors protects from hepatitis D virus (HDV) infection after simultaneous infection with HDV and woodchuck hepatitis virus. J. Virol. 2013;87:7708–7716. doi: 10.1128/JVI.00645-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosinska A.D., Zhang E., Johrden L., Liu J., Seiz P.L., Zhang X., Ma Z., Kemper T., Fiedler M., Glebe D. Combination of DNA prime--adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog. 2013;9:e1003391. doi: 10.1371/journal.ppat.1003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ip P.P., Boerma A., Regts J., Meijerhof T., Wilschut J., Nijman H.W., Daemen T. Alphavirus-based vaccines encoding nonstructural proteins of hepatitis C virus induce robust and protective T-cell responses. Mol. Ther. 2014;22:881–890. doi: 10.1038/mt.2013.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Backes S., Jäger C., Dembek C.J., Kosinska A.D., Bauer T., Stephan A.S., Dišlers A., Mutwiri G., Busch D.H., Babiuk L.A. Protein-prime/modified vaccinia virus Ankara vector-boost vaccination overcomes tolerance in high-antigenemic HBV-transgenic mice. Vaccine. 2016;34:923–932. doi: 10.1016/j.vaccine.2015.12.060. [DOI] [PubMed] [Google Scholar]
- 31.Wherry E.J., Blattman J.N., Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J. Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Segal G., Prato S., Zehn D., Mintern J.D., Villadangos J.A. Target density, not affinity or avidity of antigen recognition, determines adoptive T cell therapy outcomes in a mouse lymphoma model. J. Immunol. 2016;196:3935–3942. doi: 10.4049/jimmunol.1502187. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Ruiz D., Ng W.Y., Holz L.E., Ma J.Z., Zaid A., Wong Y.C., Lau L.S., Mollard V., Cozijnsen A., Collins N. Liver-resident memory CD8(+) T cells form a front-line defense against malaria liver-stage infection. Immunity. 2016;45:889–902. doi: 10.1016/j.immuni.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Park C.O., Kupper T.S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 2015;21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexandre Y.O., Ghilas S., Sanchez C., Le Bon A., Crozat K., Dalod M. XCR1+ dendritic cells promote memory CD8+ T cell recall upon secondary infections with Listeria monocytogenes or certain viruses. J. Exp. Med. 2016;213:75–92. doi: 10.1084/jem.20142350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohlmeier J.E., Cookenham T., Roberts A.D., Miller S.C., Woodland D.L. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soudja S.M., Ruiz A.L., Marie J.C., Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauvau G., Boutet M., Williams T.M., Chin S.S., Chorro L. Memory CD8(+) T cells: innate-like sensors and orchestrators of protection. Trends Immunol. 2016;37:375–385. doi: 10.1016/j.it.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wirth T.C., Martin M.D., Starbeck-Miller G., Harty J.T., Badovinac V.P. Secondary CD8+ T-cell responses are controlled by systemic inflammation. Eur. J. Immunol. 2011;41:1321–1333. doi: 10.1002/eji.201040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crispe I.N., Pierce R.H. Killer T cells find meaningful encounters through iMATEs. Nat. Immunol. 2013;14:533–534. doi: 10.1038/ni.2620. [DOI] [PubMed] [Google Scholar]
- 41.Sacher T., Knolle P., Nichterlein T., Arnold B., Hämmerling G.J., Limmer A. CpG-ODN-induced inflammation is sufficient to cause T-cell-mediated autoaggression against hepatocytes. Eur. J. Immunol. 2002;32:3628–3637. doi: 10.1002/1521-4141(200212)32:12<3628::AID-IMMU3628>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 42.Scheiermann J., Klinman D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014;32:6377–6389. doi: 10.1016/j.vaccine.2014.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandhu U., Cebula M., Behme S., Riemer P., Wodarczyk C., Metzger D., Reimann J., Schirmbeck R., Hauser H., Wirth D. Strict control of transgene expression in a mouse model for sensitive biological applications based on RMCE compatible ES cells. Nucleic Acids Res. 2011;39:e1. doi: 10.1093/nar/gkq868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schirmbeck R., Stober D., El-Kholy S., Riedl P., Reimann J. The immunodominant, Ld-restricted T cell response to hepatitis B surface antigen (HBsAg) efficiently suppresses T cell priming to multiple Dd-, Kd-, and Kb-restricted HBsAg epitopes. J. Immunol. 2002;168:6253–6262. doi: 10.4049/jimmunol.168.12.6253. [DOI] [PubMed] [Google Scholar]
- 45.Schirmbeck R., Reimann J., Kochanek S., Kreppel F. The immunogenicity of adenovirus vectors limits the multispecificity of CD8 T-cell responses to vector-encoded transgenic antigens. Mol. Ther. 2008;16:1609–1616. doi: 10.1038/mt.2008.141. [DOI] [PubMed] [Google Scholar]
- 46.Wortmann A., Vöhringer S., Engler T., Corjon S., Schirmbeck R., Reimann J., Kochanek S., Kreppel F. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol. Ther. 2008;16:154–162. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.