Abstract
The trapping dynamics of conduction-band electrons in colloidal degenerately doped n-CdSe nanocrystals prepared by photochemical reduction (photodoping) were measured by direct optical methods. The nanocrystals show spontaneous electron trapping with distributed kinetics that extend to remarkably long timescales. Shifts in nanocrystal band-edge potentials caused by quantum confinement and surface ion stoichiometry were also measured by spectroelectrochemical techniques, and their relationship to the slow electron trapping is discussed. The very long electron-trapping timescales observed in these measurements are more consistent with atomic rearrangement than with fundamental electron-transfer processes. Such slow and broadly distributed electron-trapping dynamics are reminiscent of the well-known distributed dynamics of nanocrystal photoluminescence blinking, and potential relationships between the two phenomena are discussed.
Graphical abstract
Introduction
Surface traps on colloidal semiconductor nanocrystals (NCs) are exceedingly influential in NC photophysics, luminescence intermittency (“blinking”), photochemistry, and electron transport. Understanding and eventually controlling NC surface traps represents a major unresolved challenge confronting progress toward NC-based electronic and photonic technologies.1-5 The manifestations of carrier trapping have been studied extensively in the context of photoluminescence (PL) intermittency (“blinking”) using single-NC spectroscopies.6-17 Many models of blinking propose that “off”-state formation occurs via carrier trapping at the NC surface, and that this “off” state persists until the surface charge is eliminated.16-17 Stochastic fluctuations of relative semiconductor band-edge and trap-state energies have been proposed to explain the distributed “off”-state formation dynamics.8-10 Similarly, NC delayed luminescence is also hypothesized to involve reversible surface trapping of charge carriers,18-24 most likely electrons.20 Several experiments have demonstrated that chemical or electrochemical reduction of NC surface traps can strongly affect PL quantum yields, blinking, and related photodynamics, presumably by turning off otherwise deleterious surface electron-trapping processes.15, 25-29 Although numerous time-resolved spectroscopic studies have demonstrated sub-nanosecond carrier-trapping rates in CdSe and related semiconductor NCs, both blinking and delayed luminescence measurements routinely show distributed carrier-trapping and detrapping dynamics that extend to seconds or even longer. Rather than reflecting any elementary electron-transfer step, the relatively long blinking “on” times are suggestive of carrier trapping that is gated by another much slower process. To date, there remains little or no direct evidence of such extremely slow charge-carrier trapping in NCs. New approaches to investigating slow charge-carrier trapping in NCs may help to shed light on the processes responsible for these long timescales.
Here, we report extremely slow trapping of conduction-band (CB) electrons ( ) in freestanding colloidal n-type CdSe NCs. populations in colloidal n-type CdSe NCs generated via photochemical reduction (photodoping)30-31 are observed to decay spontaneously on timescales spanning from milliseconds to hours. We show that this slow electron decay is a unimolecular process, ruling out slow NC oxidation by adventitious O2 or other impurities, and identifying this extraordinarily slow trapping as intrinsic to the NCs themselves. The observation of such extremely slow spontaneous electron trapping in CdSe NCs provides unique opportunities for systematic investigation of the chemical factors affecting this trapping. In preliminary experiments of this type, we show here that this slow electron trapping depends on temperature, consistent with a moderate activation barrier. We also demonstrate that the maximum CdSe NC photodoping level is limited by the photostationary balance between excitation and electron-trapping rates, which in turn depends on NC size and surface stoichiometry. Using in situ NC potentiometry,32 we track the relationship between this slow carrier trapping and NC size, surface stoichiometry, electron occupancy, and Fermi level, providing additional insight into the slow trapping processes. Combined, these results suggest that this very slow electron trapping results from the stochastic appearance of new mid-gap surface traps, similar to previous proposals explaining distributed blinking dynamics. Possible relationships between this slow trapping and the phenomena of PL blinking and delayed luminescence are discussed.
Experimental Section
General Considerations
Unless stated otherwise, all measurements and synthetic manipulations were carried out using standard Schlenk techniques under a nitrogen atmosphere, or in M. Braun or Vac gloveboxes under an atmosphere of purified nitrogen. Anhydrous THF and toluene were purified through columns of activated alumina under argon. All other solvents used were reagent grade or better. Technical grade or reagent grade chemicals were purchased from Sigma-Aldrich or Strem and used without further purification.
Nanocrystal Synthesis
Zinc blende CdSe nanocrystals of various diameters were prepared by an adaptation of a previously reported heat-up synthesis method.33 As an example: CdO (0.195 g, 1.52 mmol), oleic acid (1.6 mL, 5 mmol), and octadecene (36 g) were combined in a three-necked round bottom flask equipped with a stir bar and condenser and degassed under vacuum at 115 °C for 30 min. Under nitrogen, the mixture was heated to 300 °C until it became optically clear and colorless. The mixture was cooled to 115 °C, and SeO2 (0.170 g, 1.53 mmol) was added under nitrogen flow, and the mixture was heated to 240 °C for 10 min, becoming an orange solution. The reaction mixture was cooled to room temperature, and the nanocrystals were precipitated using ethanol and then washed via redispersion/precipitation with toluene/ethanol. For nanocrystals purified under air-free, anhydrous conditions, the crude reaction mixture was distilled to remove octadecene, then dispersed in anhydrous pentane and precipitated with anhydrous methyl acetate.
Surface Treatments of CdSe NCs
The ratios of Cd and Se were changed by an adaptation of the method published by Peng and co-workers.34 A toluene solution of CdSe NCs prepared as described above was added to a three-necked round-bottom flask equipped with a stir bar and condenser and charged with octadecene (8 mL) and oleylamine (2 mL). The mixture was degassed under vacuum at 100 °C for 30 min, then heated to 140 °C under nitrogen. A dispersion of Se (0.1 mL, ca. 0.1 M in octadecene) was added via syringe, and the mixture stirred at 140 °C for 10 min. This Se treatment was repeated four times, then 0.2 mL of tributylphosphine was added and the mixture was heated to 220 °C for 5 min, then cooled to 35 °C. Cd(OAc)2•2H2O was added, and the mixture was stirred at 35 °C for 1 h, then precipitated out upon addition of ethanol. The residue was taken up in toluene, stirred with cadmium myristate for 2 h at 60 °C, then dried in vacuo. In the glovebox, the residue was dispersed in pentane and filtered to remove excess salts.
General Characterization
Nanocrystals were dried under vacuum, then dispersed in anhydrous toluene and stored in the dinitrogen glovebox. UV-vis spectra were measured using a Varian Cary 5000 spectrophotometer. Low-temperature absorption measurements were carried out using a Cary 5000 spectrophotometer equipped with a fiber-optic cable connected to a dip probe. Energy dispersive X-ray spectrometry (EDS) was measured using a Sirion XL30 scanning electron microscope at the Molecular Analysis Facility of the Molecular Engineering & Sciences Institute at the University of Washington.
Photodoping
CdSe nanocrystals were photodoped as described previously.30-31 Dispersions of NCs in anhydrous toluene with 30-100 equivalents of Li[Et3BH] (added as a 0.01 M solution in THF) were prepared anaerobically and loaded into air-free cuvettes equipped with Teflon stir bars. The nanocrystals were photodoped either by illumination with a 100 W Hg/Xe Oriel photolysis lamp (∼2 W/cm2, ∼1.5 cm illumination diameter) using a 480 nm long pass filter, or by illumination with a 405 nm diode (50 mW, ∼0.06 W/cm2). In either case, the solutions were magnetically stirred during excitation. Absorption spectra were monitored during photodoping until the band-edge absorption stopped decreasing. During absorption recovery, the NCs were kept in the dark at room temperature and their absorption spectra were measured at 1 min intervals, unless otherwise stated.
Potentiometry and Spectroelectrochemistry
Fermi levels and nanocrystal redox potentials were measured using the method of spectroelectrochemical potentiometry as detailed previously.32 Briefly, an air-free septum-capped quartz cuvette was modified by inserting two platinum wires (working and counter electrodes) and a 1 mm Ag/AgCl reference electrode through the septum cap to make a standard three-electrode electrochemical cell. CdSe NCs were suspended in a solution of 0.1 M [Bu4N][PF6] in THF, and Na[Et3BH] was added to the solution in the cuvette in an N2 glovebox. The samples were then irradiated with supra-band-gap light (λ = 405 nm, 50 mW). The solution's Fermi level (EF) was recorded potentiometrically during illumination with a computer-controlled Eco Chemie Autolab II potentiostat. At the same time, the transmittance was measured using a 250 W tungsten halogen lamp directed through an Oriel Cornerstone 74000 monochromator at 590 nm (the peak of the first exciton) and passed through the sample perpendicular to the photodoping excitation source. The transmitted probe light was detected with a Thorlabs PDA 55 Si diode and converted into absorbance through the equation A = –log(T/T0), where T is the transmittance through the sample and T0 is the transmittance absent the sample. T0 was collected prior to the experiment. A constant baseline measured independently was subtracted from this absorbance, and the resulting absorbance values were then used to calculate <n>. The solution was stirred continuously with a magnetic stir bar throughout the measurements. Following the potentiometric measurement, decamethylferrocene was added to the mixture and a cyclic voltammogram was collected as a reference for EF. All data are plotted against the ferrocenium/ferrocene (Fc+/Fc) redox couple.
Results and Analysis
(a) Extremely slow intrinsic electron decay
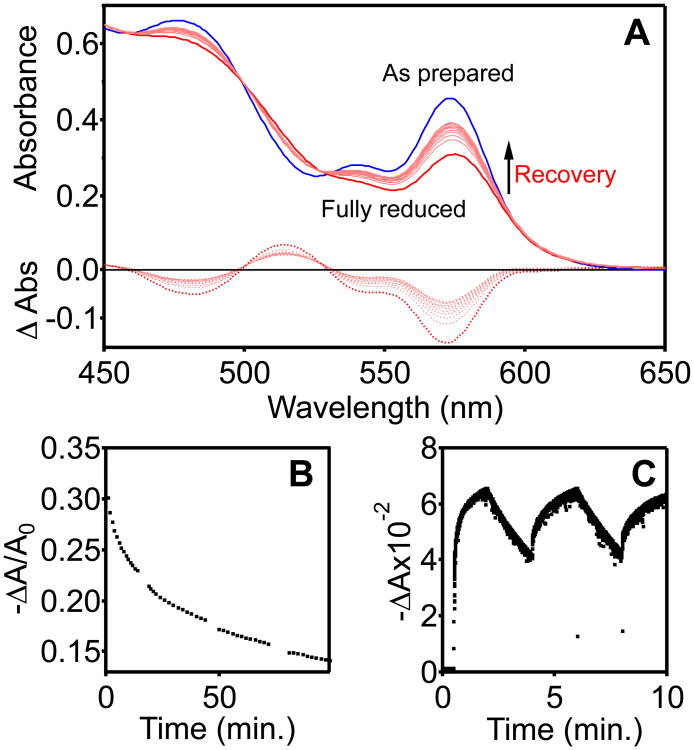
Carboxylate-capped colloidal CdSe NCs were photodoped by excitation with visible light using Li[Et3BH] as a hole quencher.30-31 Figure 1A plots the bleach of the first excitonic absorption feature caused by the introduction of delocalized . As described previously,30 this bleach is reversed within seconds upon exposure of the solution to oxidants such as O2 or decamethylferrocenium ([FeCp*2]+). This reversibility is quantitative, with the absorption spectra of the re-oxidized NCs being indistinguishable from those collected before photodoping, ruling out any significant size or compositional changes of the NCs during this process. Figures 1A and 1B show that the CdSe absorption still recovers even in the absence of added oxidant and under rigorously anaerobic conditions, but much more slowly, on the order of minutes or hours. This slow anaerobic loss is found to occur with approximately the same rate in the dark as in the light beam of the absorption instrument (see Supporting Information (SI)), indicating that it is not a light-promoted process. It is also observable as a slow brightening of the sample's PL, attributable to diminishing non-radiative Auger recombination with the loss of (see SI), although the PL is undoubtedly also impacted by excess surface-trapped electrons.30
Figure 1.
(A) Absorption spectra of photodoped CdSe NCs at room temperature measured over 90 min (NC diameter = ∼3.6 nm, concentration = 2.8 μM in toluene). (B) Normalized first-exciton differential absorbance (−ΔA/A0, λ = 573 nm) plotted vs time, where A0 represents the exciton absorbance prior to photodoping. (C) First-exciton differential absorbance (−ΔA, λ = 580 nm) of CdSe NCs (d ∼ 3.8 nm) in toluene with added Li[Et3BH]. The sample was excited using a 405 nm LED (50 mW) which was switched on and off at 2 min intervals.
A similar slow decay has been reported previously for CdSe NCs reduced by addition of ∼500-fold excess of the strong reductant sodium biphenyl.35 In this case, the decay was attributed to the presence of trace impurities, exposure to air, or incomplete dryness of the solution,35 i.e., to extrinsic factors. Extremely slow decay was also observed in PbSe NCs reduced by addition of ∼100-fold excess cobaltocene.36 Here, the slow decay was attributed to surface electron trapping. In both cases, the kinetics were not analyzable because of the presence of excess chemical reductant in redox equilibrium with the NCs. The present experiments circumvent this limitation, because the reductants used in photodoping are incapable of directly injecting into the CdSe NCs.31 This chemical difference allows decay kinetics to be monitored without complication from such additional redox equilibria.
To test the possibility of decay due to adventitious O2, moisture, or other contaminants, solutions of photodoped CdSe NCs were prepared in a nitrogen-filled glovebox (<0.5 ppm O2, <1.0 ppm H2O) in dry, deoxygenated solvents. These samples display the same slow decay whether stored in Teflon-stoppered cuvettes or in the glovebox. Additionally, mixtures of CdSe NCs and Li[Et3BH] prepared in the dark and stored under nitrogen for 1 day at room temperature still show photodoping to the same extent as freshly prepared samples, ruling out the possibility that small concentrations of adventitious oxidant or slow leaks of air or moisture are responsible for the loss observed in Figure 1. Li[Et3BH] decomposes quickly in the presence of moisture and slowly in the presence of dry air (>50% in 24 h).37 In fact, the NCs can be photodoped again after the anaerobic absorption recovery, indicating that active reducing equivalents are still present after loss (Fig. 1C). Corroborating these observations, we find that in photodoped ZnO NCs prepared under parallel conditions are stable indefinitely,38-40 ruling out many possible scenarios involving trace oxidants in the chemicals or glove-box atmosphere. Finally, and most compellingly, the decay rate is found to be independent of CdSe NC solution concentration (see SI), which implicates a unimolecular mechanism. These data provide strong evidence that this slow decay is intrinsic to the NCs themselves.
(b) Nanocrystal size dependence
Figure 2 plots the absorption spectra (solid lines) of a series of zinc blende CdSe NCs of different diameters (d = 2.6–5.6 nm), all prepared using established synthetic procedures33 and capped by carboxylate ligands. To maintain similar surface compositions, the NC samples were purified via the same method of repeated ethanol-precipitation/toluene-resuspension cycles. The dashed lines in Figure 2 correspond to the absorption spectra of these samples collected after photodoping to the maximum possible concentration by the incremental addition of Li[Et3BH] and excitation with 405 nm light, i.e., until no further exciton absorption bleach is observed. As we communicated previously,30 larger CdSe NCs display a greater maximum absorption bleach than smaller ones, reflecting a higher maximum population via photodoping. Maximum per-NC occupancies were estimated using the relationship <n> = 2(1 – A/A0), where <n> represents the average number of per NC and A and A0 represent absorbance values at the first excitonic maximum, quantified by multi-peak Gaussian fitting. The resulting values are as follows: for d = 2.6 nm, <nmax> = 0.5; for d = 3.6 nm, <nmax> = 1.3; for d = 5.6 nm, <nmax> ∼ 2 (complete bleach of the first excitonic absorption feature). These maximum average electron occupancies correspond to maximum average electron densities of <Nmax> = 5.4, 5.3, and 2.2 × 1019 cm−3, respectively. For comparison, photodoped ZnO NCs display a similar dependence of <nmax> on NC diameter, but they can accommodate many more per NC (<Nmax> ∼ 6 × 1020 cm-3 for all diameters when using Li[Et3BH]).40
Figure 2.
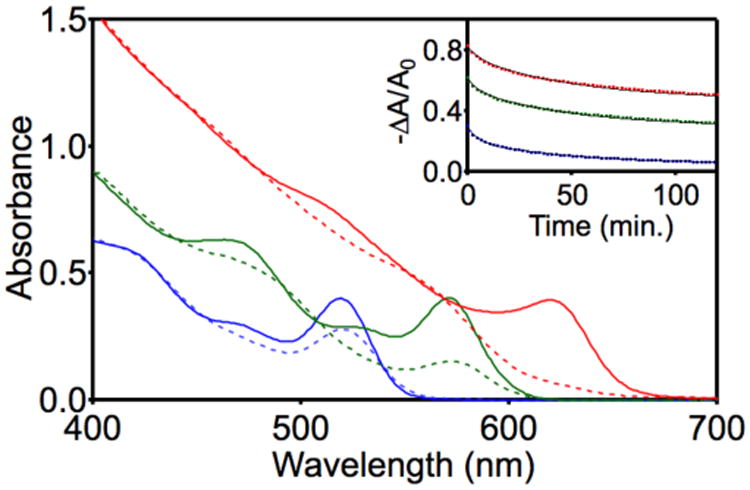
Absorption spectra of CdSe NCs of various diameters, measured before (solid lines) and after photodoping (dashed lines) using Li[Et3BH] as the reductant and 405 nm irradiation for excitation. d = 5.6 nm (red), 3.6 nm (green), and 2.6 nm (blue). Inset: Normalized first-exciton differential absorbance (−ΔA/A0) of these photodoped CdSe nanocrystals plotted vs time over the first 2 h after 405 nm irradiation is terminated (at t = 0). The black curves in the inset depict double-exponential fits to the data using τ1 ∼ 7 min and τ2 ∼ 80 min, and different asymptotes (see text).
The data suggest that <nmax> in these CdSe NCs is determined by a steady-state balance between photodoping and trapping rates under cw photoexcitation, i.e., a photostationary state. We find that the value of <nmax> achieved under illumination depends on the specific photoexcitation conditions, and that after photoexcitation is terminated, the population decays slowly and asymptotically to a value of <n> that is independent of the excitation rate, i.e., a quasi-equilibrium value (<nequil>). Further decay of this quasi-equilibrated population is immeasurably slow over several days of observation.
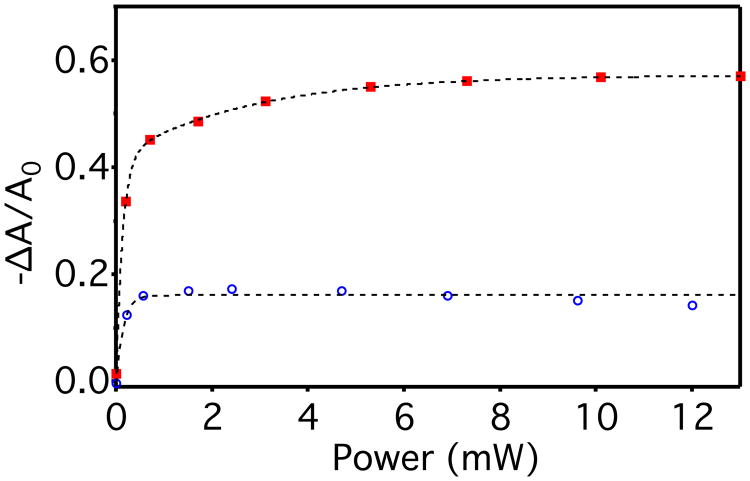
The dependence of −ΔA/A0 on photoexcitation rate confirms that <nmax> is limited by the rate of decay. Figure 3 plots steady-state values of −ΔA/A0 for d ∼ 4.0 nm and d ∼ 2.4 nm CdSe NCs measured under different 405 nm irradiation powers (proportional to excitation rates). For both NC samples, −ΔA/A0 (and hence <nmax>) is smaller at small excitation rates than at large excitation rates. Moreover, the saturation of −ΔA/A0 at large excitation rates implies that the rate of decay increases sharply with increasing <n> under those conditions. This increase in trapping rates could suggest additional depletion pathways of the conduction-band electrons or of trapped electrons to regenerate empty trap states. Possible pathways might include Shockley-Read-Hall or Auger recombination processes. We note, however, that the observation of <nmax> ∼ 0.32 in the d = 2.4 nm CdSe NCs of Figure 3 eliminates the hypothesis41 that NC photodoping is limited to one electron per NC by rapid Auger recombination because this nonradiative process does not turn on until an has been deposited, so photodoping should continue until at least <n> = 1 in this scenario. The conditions used for Figure 3 correspond to NC excitation rates up to ∼150 excitations•s-1 (see SI for calculations), which implies trapping on timescales as short as milliseconds at excitation powers where saturation is observed. This trapping rate is far greater than observed in Figure 2, indicating an extremely broad distribution of electron-trapping kinetics.
Figure 3.
Normalized differential first-exciton absorbance (−ΔA/A0, λ = 586 or 518 nm) of CdSe NCs of two sizes (d ∼ 4.0 nm, red squares, or d ∼ 2.4 nm, blue circles) in toluene with added Li[Et3BH] (60 equiv/NC or 7 equiv/NC, respectively) under 405 nm illumination at various CW excitation powers. The dashed lines are guides to the eye.
After maximum photodoping, solutions of n-CdSe NCs of different sizes were stored in the dark under anaerobic conditions, and their absorption spectra were measured periodically over several hours. The inset to Figure 2 shows the dark recovery of the band-edge absorption measured at room temperature over the first 2 h for each sample, plotted as −ΔA/A0 vs time. The kinetics of loss are similar for all three NCs, but such similar decay kinetics are not always observed (see SI), and they appear to depend on NC processing and handling prior to photodoping in a complex way. The decay data for all three NC samples of Figure 2 are fit reasonably well to the same double-exponential function with time constants of τ1 ∼ 7 min and τ2 ∼ 80 min, and with similar amplitudes (see SI). Their difference comes from their apparent asymptotes, which increase from <nequil> = 0.05 to 0.53 to 0.88 across this series (from small to large diameter). This result shows that the highest-energy (most negative potential) CB electrons added to each of these NCs trap at comparable rates, but also that the lowest-energy CB electrons in large CdSe NCs trap far more slowly than the lowest-energy CB electrons in small CdSe NCs do.
(c) Nanocrystal surface chemistry
Monitoring decay via absorption spectroscopy provides a relatively simple and direct tool for studying the effects of NC surface perturbations on <nmax>, <nequil>, and the trapping kinetics of the colloidal ensemble. For example, we find that growth of a ZnS shell over the CdSe NCs significantly increases <nmax> and <nequil>, and slows decay at short times (see SI), consistent with elimination of surface electron traps. This result is similar to the effect of CdSe shell growth over PbSe NCs.36
Beyond demonstrating suppressed trapping, we hypothesized that such measurements might provide a unique way to investigate the microscopic nature of native CdSe surface traps. For example, we find that CdSe NCs purified under nitrogen atmosphere display the same recovery characteristics as those purified under ambient conditions, ruling out any influence of surface oxidation on this electron trapping. Importantly, the addition of typical hydrophobic ligands such as trioctylphosphine, octylamine, or trioctylphosphine oxide (technical grade, 90%, containing phosphinic acids) to the NC solutions also does not have any noticeable effect on the trapping kinetics (see SI). These data, along with the NC-concentration independence of the electron-trapping rates, suggest that this trapping is not impacted by ligand association/dissociation equilibria.
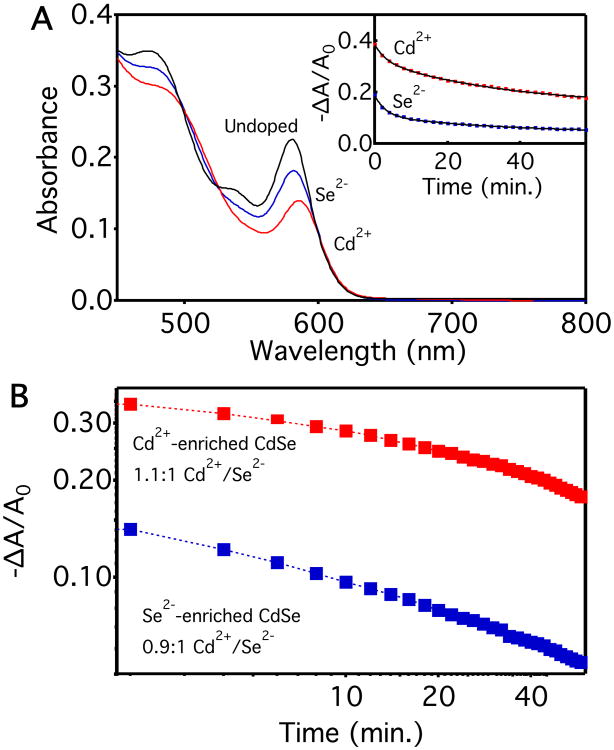
In contrast, we find that changing the surface Cd2+:Se2− ratio has a large impact. Surface stoichiometry has previously been shown to significantly affect the PL quantum yields of CdSe NCs, primarily attributed to changes in the densities of selenide-derived hole traps.34, 42-44 Recently, we reported that surface stoichiometry also dramatically affects CdSe NC band-edge potentials, with tunability exceeding ∼0.4 V,45 but electron trapping was not examined. Comparably large effects have also been observed elsewhere following various surface modifications.46-48 Here, in one set of experiments, we treated CdSe NCs with elemental selenium and oleylamine in octadecene to prepare selenium-enriched CdSe NCs (PLQY ∼ 0%).34, 42 We anticipate that surface selenium exists as Se2− during the photodoping experiments, because Li[Et3BH] can reduce elemental selenium to Li2Se.49 This Se2−-enriched sample was then treated with cadmium acetate to passivate the surface Se2− sites and form Cd2+-enriched CdSe NCs (PLQY ∼ 7%). Figure 4A plots absorption spectra of the as-prepared CdSe NCs before photodoping, the photodoped Se2−-enriched CdSe NCs from the same batch, and the photodoped Cd2+-enriched NCs prepared as described above. At the same NC concentration, a smaller steady-state absorption bleach is observed for the Se2−-enriched NCs than for the Cd2+-enriched NCs, indicating a smaller <nmax>, consistent with our previous report.45 The inset of Figure 4A plots the recovery of excitonic absorption in these Se2−- and Cd2+-enriched NCs over the first hour after photodoping, measured anaerobically at room temperature. These curves again show fairly similar kinetics but differ in their y-asymptotes, which correspond to <nequil> = 0.08 and 0.30 for the Se2−- and Cd2+-enriched NCs, respectively. In Figure 4B, the excitonic absorption recovery data from Figure 4A are plotted on log-log axes. As discussed above, the trapping kinetics also extend to faster timescales than those depicted here, reaching at least milliseconds. Clearly, CdSe NCs exhibit extraordinarily broadly distributed -trapping dynamics.
Figure 4.
(A) Absorption spectra of photodoped CdSe NCs (cores d ∼ 3.9 nm) after Se2- and Cd2+ enrichment. Inset: Normalized first-exciton differential absorbance (−ΔA/A0, λ = 581, and 585 nm for Se2−-enriched, and Cd2+-enriched NC samples respectively) measured over 60 min at room temperature for CdSe NCs with the different surface compositions. The black lines depict double exponential fits as described in the text (τ1 ∼ 3 min, τ2 ∼ 35 min). (B) The excitonic absorption recovery data from the inset of panel (A), plotted vs time in a log-log representation. Cd2+/Se2− ratios measured using energy-dispersive X-ray spectroscopy (EDS) are listed for each sample.
(d) Potentiometry
To understand the role of surface-ion enrichment in this electron trapping, we performed spectroelectrochemical potentiometric measurements on the freestanding colloidal CdSe NCs.32 These measurements allow the changes in NC Fermi level to be quantified during photodoping and subsequent carrier trapping, as a function of the surface modifications described in Figure 4.
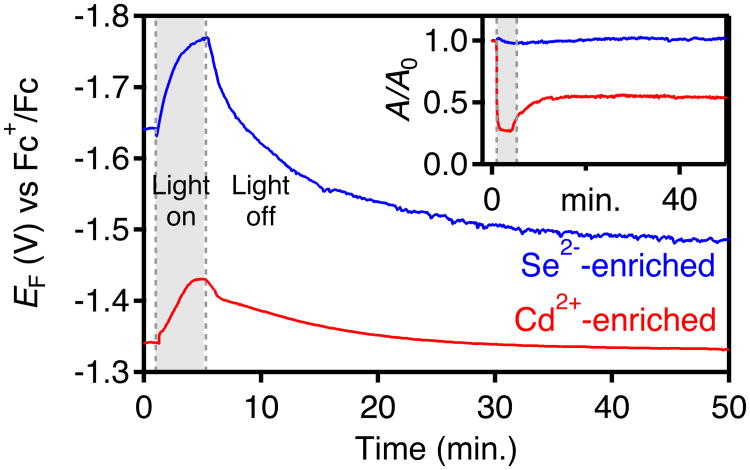
Figure 5 compares transient potentiometric data for Se2−- and Cd2+-enriched CdSe NCs collected before, during, and after photodoping in the presence of Na[Et3BH]. Prior to irradiation (t = 0 min), the Se2−-enriched NCs already display a resting potential >280 mV more negative than that of the Cd2+-enriched NCs. Upon irradiation (t = 1 min), EF for both samples shifts ∼120 mV more negative before reaching a maximum. At this maximum, <nmax> < 0.1 for the Se2--enriched NCs and <nmax> ∼ 1.5 for the Cd2+-enriched NCs. When irradiation is terminated (t = 5 min), EF slowly returns more positive for both NC samples until nearly constant potentials are reached at <nequil>. Overall, these plots are thus roughly parallel for the two NC samples, but EF is nearly 300 mV more negative for the Se2−-enriched NCs than for the Cd2+-enriched NCs at all stages of photodoping and recovery. Importantly, these data also demonstrate that electron trapping after termination of the photoexcitation is accompanied by a positive shift of EF. We further note that the Cd2+-enriched NCs return to approximately their initial EF after recovery, but the Se2−-enriched NCs plateau at a value of EF that is >100 mV more positive than it was before photodoping. This observation suggests that the cycle of photodoping followed by electron trapping alters the surfaces of the Se2--enriched NCs significantly.
Figure 5.
Transient potentiometric data for photodoping d = 3.8 nm Cd2+-enriched (red) and Se2--enriched (blue) CdSe NCs with Na[Et3BH] as the hole quencher in a THF solution with 0.1 M TBAPF6 as the supporting electrolyte. For each sample, the NCs were photodoped using a 405 nm diode (50 mW) between t = 1 and 5 min. At t = 5 min, photoexcitation was stopped but EF was monitored further. Inset: A/A0 collected in concert with EF.
(e) Temperature dependence
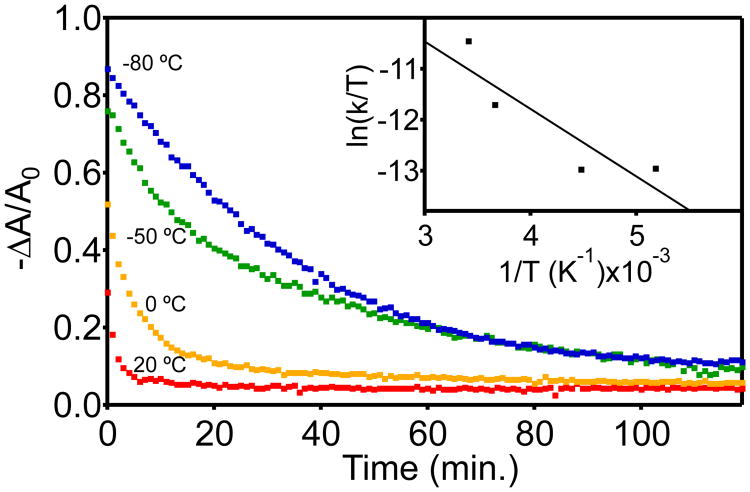
The temperature dependence of this slow electron trapping was also measured. Reducing the temperature to 20 K has already been reported to stabilize dramatically in chemically reduced CdSe NCs,35 again in the presence of excess reductant. To explore the effect of temperature here, we performed photodoping and recovery measurements at a series of temperatures at which these NC solutions remain in the liquid phase. Toluene solutions of d ∼ 3.6 nm CdSe NCs were cooled below room temperature (−80 to 20 °C), photodoped to <nmax> by exciting with 405 nm light after treatment with Li[Et3BH], and the recovery of the band-edge absorbance was measured. Figure 6 plots −ΔA/A0 vs time for measurements performed at four different temperatures. At t = 0 min, a significant increase in the steady-state value of −ΔA/A0 is observed as the temperature is lowered (see SI), indicating a shift in the photostationary state consistent with slower trapping at lower temperatures. Slower trapping is confirmed directly by the slower recovery in −ΔA/A0 with decreasing temperature after irradiation is terminated. To quantify this temperature dependence, these decay data were fitted to single-exponential functions to extract approximate decay rate constants (see SI), although the true dynamics are obviously more complicated, e.g., Figure 4B. Figure 6 (inset) plots these effective decay rate constants in an Eyring plot. Fitting these data (solid line) yields a low transition-state enthalpy of ΔH‡ ∼ 3 kcal/mol (∼130 meV) and a small, negative entropic parameter. These parameters indicate that this trapping is an activated process with a barrier of ∼5 kBT at room temperature.
Figure 6.
Normalized excitonic bleach (−ΔA/A0) recovery data for photodoped d = ∼3.6 nm CdSe NCs measured at 20, 0, −50, and −80 °C. Inset: Eyring plot of the rate constants estimated from single-exponential fits of the absorption recovery data. R2 = 0.81. Fitting yields ΔH‡ ∼ 3 kcal/mol and a small, negative entropic parameter. These parameters correspond to an activation barrier of ∼5 kBT at room temperature.
Discussion
(a) Slow surface fluctuations gate electron trapping
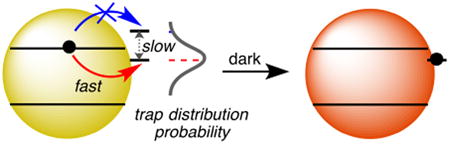
Numerous photophysical investigations have found electron transfer from the CB of a semiconductor NC to an available surface trap occurs within pico- to nanoseconds.50-52 Despite the feasibility of such rapid electron transfer, the electron trapping observed here is remarkably slow, with components extending to minutes and even hours. Clearly, the electron-trapping kinetics monitored here do not reflect the rates of the actual elementary electron-transfer steps themselves. Instead, we propose that they report on the availability of suitable electron traps. In this scenario, the data suggest that electron traps can spontaneously appear within the CdSe NC band gap, but this appearance is extremely slow. Prior to trap appearance within the band gap, an is stable, but upon appearance of a mid-gap trap, that disappears rapidly. Within this interpretation, the data show that the spontaneous formation of mid-gap electron traps is thermally activated. It is noteworthy that in all cases, <nmax> is close to only one electron per NC, meaning that the decay kinetics observed here reflect the appearance of approximately just one new mid-gap electron trap per NC. These deductions are the major qualitative conclusions of the present work.
Given these conclusions, we propose that the extremely slow trapping described here is consistent with the hypothesis of dynamic fluctuations in relative surface-trap and CB-edge potentials deduced8-10 from blinking studies. Because of the small numbers of traps involved in the present observations (one per NC), we consider the relevant trap potentials in terms of probability distributions rather than a static mid-gap density of states. For example, a given trap may dynamically sample many different relative potentials, thereby defining its probability distribution. Figure 7 illustrates one such probability distribution, depicted arbitrarily as a Gaussian function that extends both above and below the NC CB edge. The existence of multiple traps fluctuating simultaneously would scale and broaden this probability distribution curve. The actual distributions are unknown, of course, but nonetheless, within this picture, photodoping raises the Fermi level to above the CB edge, filling any traps that exist below that Fermi level at that time. This photodoping thus generates a standing population. After photoexcitation is terminated, trapping can only occur when a new trap appears with a favorable potential for spontaneous electron transfer, but the formation of a new mid-gap trap is very slow because of a ∼5 kBT activation barrier at room temperature. When no new mid-gap traps appear on the experimental time scale (hours to days), the remaining population is taken as <nequil>. Although qualitative, this interpretation is consistent with all of the experimental results described above.
Figure 7.
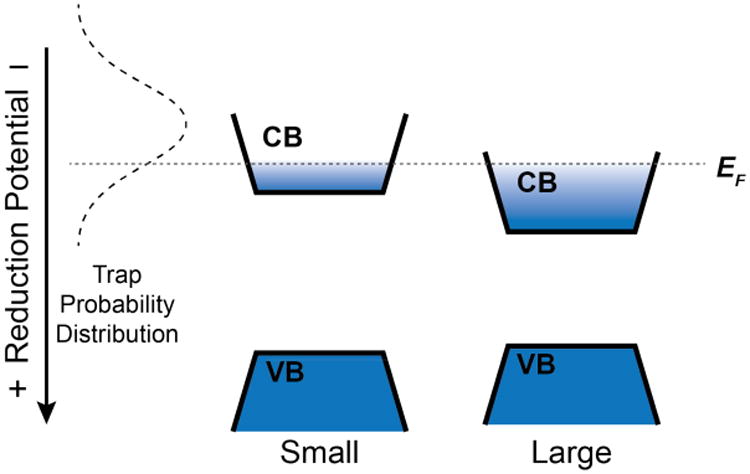
Schematic depiction of the proposed relationship between a trap potential's probability distribution and the photodoping characteristics of colloidal CdSe NCs. The dashed Gaussian curve depicts the probability distribution of a single trap's potential, which fluctuates stochastically relative to the CB edge. Within this distribution, a trap has a discrete relative potential for a period of time before hopping to another relative potential. Sampling of the full potential distribution is slow and thermally activated. The dotted horizontal line indicates the Fermi level achieved via photodoping. When the trap hops to a potential below the Fermi level, electron transfer from the CB to the trap is favorable. Under steady-state excitation conditions, the photodoping rate equals the electron-trapping rate. Increasing quantum confinement shifts the CB edge to more negative potentials, increasing the probability of thermodynamically favorable electron trapping at a given value of <n>, yielding a smaller value of <nmax>. A similar schematic applies to nanocrystal redox-potential shifting via surface non-stoichiometries. Surface perturbations likely also affect the total number of fluctuating trap states per NC, which in turn affects photodoping and electron decay, but these considerations are not depicted here.
(b) Size and surface-stoichiometry dependence
The data presented here indicate that trapping can be perturbed by shifts in the CB-edge potential induced by quantum confinement or NC surface chemistry. The effect of quantum confinement on electron trapping is also illustrated schematically in Figure 7. Increasing NC confinement shifts the CB-edge potential more negative but does not affect the localized trap-state potentials. Under illumination, steady state is reached at a given EF dictated by the balance between excitation and trapping rates, which in turn reflects the trap-potential probability distribution. At this EF, the smaller NCs have a lower <nmax>. Turning the excitation source off, CB electrons decay from both NCs at similar rates, but the larger NCs retain a larger <nequil>. A similar picture can be used to rationalize the effect of surface Se2- or Cd2+ enrichment: Se2- enrichment shifts the NC CB-edge potential more negative by 300 to 400 mV (Figure 5 and ref. 45), increasing the trap probability density at the band edge. This shift increases electron-trapping rates and thereby decreases <nmax> and <nequil>.
The extremely long electron-trapping timescales observed here are consistent with rapid electron transfer that is gated by slow atomic rearrangement. Microscopically, these processes may relate to the formation or rearrangement of partially oxidized surface selenium species (formed during photodoping by the photogenerated holes) having redox levels within the gap. If selenium-based, then Cd2+ enrichment may decrease the total number of such surface electron traps per NC in addition to shifting band-edge potentials relative to trap potentials, which would further increase both <nmax> and <nequil>. Other potential surface electron traps could include surface Cd2+ ions53 or anion vacancies.54 It is interesting to note that the thermodynamic parameters calculated55 for cation and anion vacancy migration on the surfaces of CdSe NCs are in reasonable agreement with (but somewhat larger than) those estimated from the data in Figure 6, considering that the experimental kinetics are not truly mono-exponential and the calculations were performed using a pre-defined surface ligand coverage and without solvent. The data here do not allow more specific identification of the speciation of the surface electron traps, but they do suggest future opportunities for using slow electron trapping measurements to help identify such traps.
(c) Electron trapping and recycling during photodoping
The excitation power dependence shown in Figure 3 demonstrates that <nmax> is limited by the kinetic competition between photodoping and electron trapping. This conclusion would seemingly imply a continuous consumption of the reductant (Li[Et3BH]) at steady state, but <nmax> does not decrease after hours of photoexcitation, even though only tens of equivalents of Li[Et3BH] per NC are included during photodoping and the nanocrystals are excited many times per second. Moreover, photodoping is still effective even after most from a previous round of photodoping have decayed (Figure 1C). Combined, these observations suggest that the reducing equivalents required for photodoping are not removed from the reaction cycle over time, but can be reintroduced to the CB and then trapped again many times. It is reasonable to conclude that surface-trapped electrons can recombine with subsequent photogenerated valence-band (VB) holes in a mechanism such as illustrated in Figure 8,56 thereby recycling these electrons.
Figure 8.
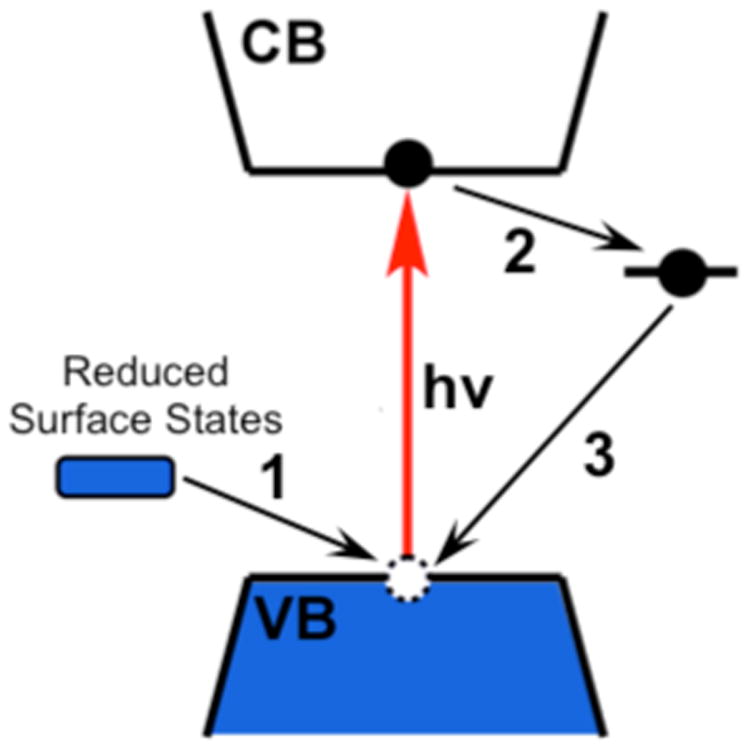
Diagram of electron-transfer processes observed during CdSe NC photodoping and subsequent electron trapping. (1) Under irradiation, the valence-band holes of CdSe NCs are initially quenched via electron transfer from reduced surface states derived from the triethylborohydride reductant (ref. 31). (2) Conduction-band electrons transfer to mid-gap surface traps. (3) Electrons transfer from reduced surface traps to the valence band to quench subsequent photogenerated holes.
Figure 8 diagrams the various electron-transfer processes proposed to occur here, including (1) initial hole-quenching by the reducing equivalents from the reductant (shown31 to proceed via a dark surface pre-reduction by [Et3BH]-) after photoexcitation, (2) surface electron trapping, and (3) VB-hole quenching from reduced surface traps after another photoexcitation event. Apparently, therefore, hole transfer to the reduced trap is orders of magnitude faster than electron transfer to the oxidized trap. Such kinetic disparity is a strong indication of significant microscopic structural rearrangement upon trap-state oxidation and reduction, consistent with the thermal activation barrier of ∼5 kBT at room temperature seen from the temperature-dependent electron-trapping kinetics.
(d) Possible relationship to blinking and delayed luminescence
It is interesting to consider the possibility that the distributed electron-trapping dynamics observed here may be related to the well-known distributed kinetics of photoluminescence intermittency (blinking) in single colloidal semiconductor NCs,6-8, 11, 16-17 and to the distributed kinetics in NC delayed luminescence.18, 20-24, 57 If related, the electron trapping studied here would be analogous to formation of a luminescence “off” state, and hence its dynamics would be analogous to the “on”-state dynamics of blinking measurements. Distributed “on”-state kinetics extending out to seconds or minutes have been documented in blinking measurements,6-8, 11, 16-17 and various observations may suggest that both the blinking and the delayed luminescence dynamics in CdSe NCs reflect specifically the dynamics of electron trapping and de-trapping. For example, application of a reducing potential to raise the Fermi level close to but below the CB can suppress blinking in CdSe/CdS nanocrystals, interpreted as reducing surface electron traps so that they can no longer accept CB electrons,15 i.e., blocking pathway 2 in Figure 8. Similarly, introduction of a Cu+ dopant as a designer hole trap within a CdSe NC shifts “on”-state probabilities toward shorter times, interpreted as enhancing the probability of surface electron trapping.20 Likewise, greater delayed luminescence is observed in samples with longer lifetimes in their luminescent core excited states.20, 24 We note that the chemical instability of delocalized holes in CdSe NCs makes it likely that photogenerated holes are also localized during blinking “off” periods and while in the metastable excited states that feed delayed luminescence, but this hole localization does not preclude the blinking or delayed luminescence dynamics from being governed by the electron's trapping/detrapping dynamics.
Other aspects of the two measurements are also similar. For example, the “on”-state blinking dynamics are temperature dependent, as observed here for the slow electron trapping, whereas the “off”-state dynamics are not.8 Some models of blinking invoke slow fluctuations of trap-state potentials relative to the NC band edge to reproduce their characteristic distributed dynamics,9-10 and a similar picture may apply here as well. Although inconclusive with respect to mechanistic specifics, the many parallels described above appear to support the hypothesis of a general fundamental analogy between the electron trapping observed here and the phenomena of intermittent and delayed luminescence in colloidal semiconductor NCs. Further experiments are necessary to test this hypothesis and explore such potential links in greater detail. At the very least, these data demonstrate conclusively that spontaneous electron trapping can occur on the same extremely long timescales as blinking and delayed luminescence.
Conclusion
Extraordinarily slow intrinsic trapping is observed in photodoped colloidal n-type CdSe NCs. This trapping displays broadly distributed kinetics spanning from milliseconds to hours. Trapping measurements have been performed under various experimental conditions, with different NC sizes, and with different NC surface stoichiometries, and the results point to an interpretation in which these slow electron-trapping dynamics reflect the thermally activated stochastic appearance of individual mid-gap electron traps, much like has been proposed to explain the distributed dynamics of photoluminescence blinking. Microscopically, the results support a description of this slow electron trapping in which intrinsically fast elementary electron-transfer steps are gated by much slower atomic rearrangements at the NC surfaces.
Supplementary Material
Acknowledgments
This research was supported by the NSF (CHE-1506014 to DRG), NIH (Postdoctoral Fellowship F32GM110876 to EYT), and the Swiss National Science Foundation (Early Postdoctoral Mobility Fellowship to AM).
Footnotes
Supporting Information. Experimental details and additional experimental results (11 figures, 3 tables).
References
- 1.Hines MA, Guyot-Sionnest P. Synthesis and Characterization of Strongly Luminescing ZnS-Capped CdSe Nanocrystals. J Phys Chem. 1996;100:468–471. [Google Scholar]
- 2.Chen Y, Vela J, Htoon H, Casson JL, Werder DJ, Bussian DA, Klimov VI, Hollingsworth JA. “Giant” Multishell CdSe Nanocrystal Quantum Dots with Suppressed Blinking. J Am Chem Soc. 2008;130:5026–5027. doi: 10.1021/ja711379k. [DOI] [PubMed] [Google Scholar]
- 3.Mahler B, Spinicelli P, Buil S, Quelin X, Hermier JP, Dubertret B. Towards non-blinking colloidal quantum dots. Nat Mater. 2008;7:659–664. doi: 10.1038/nmat2222. [DOI] [PubMed] [Google Scholar]
- 4.van Embden J, Jasieniak J, Gómez DE, Mulvaney P, Giersig M. Review of the Synthetic Chemistry Involved in the Production of Core/Shell Semiconductor Nanocrystals. Aust J Chem. 2007;60:457–471. [Google Scholar]
- 5.Reiss P, Protière M, Li L. Core/Shell Semiconductor Nanocrystals. Small. 2009;5:154–168. doi: 10.1002/smll.200800841. [DOI] [PubMed] [Google Scholar]
- 6.Nirmal M, Dabbousi BO, Bawendi MG, Macklin JJ, Trautman JK, Harris TD, Brus LE. Fluorescence intermittency in single cadmium selenide nanocrystals. Nature. 1996;383:802–804. [Google Scholar]
- 7.Kuno M, Fromm DP, Hamann HF, Gallagher A, Nesbitt DJ. Nonexponential “blinking” kinetics of single CdSe quantum dots: A universal power law behavior. J Chem Phys. 2000;112:3117–3120. [Google Scholar]
- 8.Shimizu KT, Neuhauser RG, Leatherdale CA, Empedocles SA, Woo WK, Bawendi MG. Blinking statistics in single semiconductor nanocrystal quantum dots. Phys Rev B. 2001;63:205316. [Google Scholar]
- 9.Tang J, Marcus RA. Mechanisms of fluorescence blinking in semiconductor nanocrystal quantum dots. J Chem Phys. 2005;123:054704. doi: 10.1063/1.1993567. [DOI] [PubMed] [Google Scholar]
- 10.Pelton M, Smith G, Scherer NF, Marcus RA. Evidence for a diffusion-controlled mechanism for fluorescence blinking of colloidal quantum dots. Proc Natl Acad Sci USA. 2007;104:14249–14254. doi: 10.1073/pnas.0706164104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frantsuzov P, Kuno M, Jánko B, Marcus RA. Universal emission intermittency in quantum dots, nanorods, and nanowires. Nat Phys. 2008;4:519–522. [Google Scholar]
- 12.Zhao J, Nair G, Fisher BR, Bawendi MG. Challenge to the Charging Model of Semiconductor-Nanocrystal Fluorescence Intermittency from Off-State Quantum Yields and Multiexciton Blinking. Phys Rev Lett. 2010;104:157403. doi: 10.1103/PhysRevLett.104.157403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen S, Schwartz O, Oron D. Transient Fluorescence of the Off State in Blinking CdSe/CdS/ZnS Semiconductor Nanocrystals Is Not Governed by Auger Recombination. Phys Rev Lett. 2010;104:157404. doi: 10.1103/PhysRevLett.104.157404. [DOI] [PubMed] [Google Scholar]
- 14.Califano M. Off-State Quantum Yields in the Presence of Surface Trap States in CdSe Nanocrystals: The Inadequacy of the Charging Model To Explain Blinking. J Phys Chem C. 2011;115:18051–18054. [Google Scholar]
- 15.Galland C, Ghosh Y, Steinbrück A, Sykora M, Hollingsworth JA, Klimov VI, Htoon H. Two types of luminescence blinking revealed by spectroelectrochemistry of single quantum dots. Nature. 2011;479:203–207. doi: 10.1038/nature10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordones AA, Leone SR. Mechanisms for charge trapping in single semiconductor nanocrystals probed by fluorescence blinking. Chem Soc Rev. 2013;42:3209–3221. doi: 10.1039/c2cs35452g. [DOI] [PubMed] [Google Scholar]
- 17.Efros AL, Nesbitt DJ. Origin and control of blinking in quantum dots. Nat Nanotech. 2016;11:661–671. doi: 10.1038/nnano.2016.140. [DOI] [PubMed] [Google Scholar]
- 18.Sher PH, Smith JM, Dalgarno PA, Warburton RJ, Chen X, Dobson PJ, Daniels SM, Pickett NL, O'Brien P. Power law carrier dynamics in semiconductor nanocrystals at nanosecond timescales. Appl Phys Lett. 2008;92:101111. [Google Scholar]
- 19.Jones M, Lo SS, Scholes GD. Quantitative modeling of the role of surface traps in CdSe/CdS/ZnS nanocrystal photoluminescence decay dynamics. Proc Natl Acad Sci USA. 2009;106:3011–3016. doi: 10.1073/pnas.0809316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitham PJ, Knowles KE, Reid PJ, Gamelin DR. Photoluminescence Blinking and Reversible Electron Trapping in Copper-Doped CdSe Nanocrystals. Nano Lett. 2015;15:4045–4051. doi: 10.1021/acs.nanolett.5b01046. [DOI] [PubMed] [Google Scholar]
- 21.Rabouw FT, Kamp M, van Dijk-Moes RJA, Gamelin DR, Koenderink AF, Meijerink A, Vanmaekelbergh D. Delayed Exciton Emission and Its Relation to Blinking in CdSe Quantum Dots. Nano Lett. 2015;15:7718–7725. doi: 10.1021/acs.nanolett.5b03818. [DOI] [PubMed] [Google Scholar]
- 22.Rabouw FT, van der Bok JC, Spinicelli P, Mahler B, Nasilowski M, Pedetti S, Dubertret B, Vanmaekelbergh D. Temporary Charge Carrier Separation Dominates the Photoluminescence Decay Dynamics of Colloidal CdSe Nanoplatelets. Nano Lett. 2016;16:2047–2053. doi: 10.1021/acs.nanolett.6b00053. [DOI] [PubMed] [Google Scholar]
- 23.Whitham PJ, Marchioro A, Knowles KE, Kilburn TB, Reid PJ, Gamelin DR. Single-Particle Photoluminescence Spectra, Blinking, and Delayed Luminescence of Colloidal CuInS2 Nanocrystals. J Phys Chem C. 2016;120:17136–17142. [Google Scholar]
- 24.Marchioro A, Whitham PJ, Knowles KE, Kilburn TB, Reid PJ, Gamelin DR. Tunneling in the Delayed Luminescence of Colloidal Semiconductor Nanocrystals, and Relationship to Blinking. J Phys Chem C. 2016;120:27040–27049. [Google Scholar]
- 25.Jha PP, Guyot-Sionnest P. Electrochemical Switching of the Photoluminescence of Single Quantum Dots. J Phys Chem C. 2010;114:21138–21141. [Google Scholar]
- 26.Qin W, Shah RA, Guyot-Sionnest P. CdSeS/ZnS Alloyed Nanocrystal Lifetime and Blinking Studies under Electrochemical Control. ACS Nano. 2012;6:912–918. doi: 10.1021/nn2044388. [DOI] [PubMed] [Google Scholar]
- 27.Weaver AL, Gamelin DR. Photoluminescence Brightening via Electrochemical Trap Passivation in ZnSe and Mn2+-Doped ZnSe Quantum Dots. J Am Chem Soc. 2012;134:6819–6825. doi: 10.1021/ja301102h. [DOI] [PubMed] [Google Scholar]
- 28.Rinehart JD, Weaver AL, Gamelin DR. Redox Brightening of Colloidal Semiconductor Nanocrystals using Molecular Reductants. J Am Chem Soc. 2012;134:16175–16177. doi: 10.1021/ja307996b. [DOI] [PubMed] [Google Scholar]
- 29.Boehme SC, Azpiroz JM, Aulin YV, Grozema FC, Vanmaekelbergh D, Siebbeles LDA, Infante I, Houtepen AJ. Density of Trap States and Auger-mediated Electron Trapping in CdTe Quantum-Dot Solids. Nano Lett. 2015;15:3056–3066. doi: 10.1021/acs.nanolett.5b00050. [DOI] [PubMed] [Google Scholar]
- 30.Rinehart JD, Schimpf AM, Weaver AL, Cohn AW, Gamelin DR. Photochemical Electronic Doping of Colloidal CdSe Nanocrystals. J Am Chem Soc. 2013;135:18782–18785. doi: 10.1021/ja410825c. [DOI] [PubMed] [Google Scholar]
- 31.Tsui EY, Hartstein KH, Gamelin DR. Selenium Redox Reactivity on Colloidal CdSe Quantum Dot Surfaces. J Am Chem Soc. 2016;135:11105–11108. doi: 10.1021/jacs.6b06548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll GM, Brozek CK, Hartstein KH, Tsui EY, Gamelin DR. Potentiometric Measurements of Semiconductor Nanocrystal Redox Potentials. J Am Chem Soc. 2016;138:4310–4313. doi: 10.1021/jacs.6b00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen O, Chen X, Yang Y, Lynch J, Wu H, Zhuang J, Cao YC. Synthesis of Metal–Selenide Nanocrystals Using Selenium Dioxide as the Selenium Precursor. Angew Chem Int Ed. 2008;47:8638–8641. doi: 10.1002/anie.200804266. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Peng X. Photogenerated Excitons in Plain Core CdSe Nanocrystals with Unity Radiative Decay in Single Channel: The Effects of Surface and Ligands. J Am Chem Soc. 2015;137:4230–4235. doi: 10.1021/jacs.5b01314. [DOI] [PubMed] [Google Scholar]
- 35.Shim M, Guyot-Sionnest P. N-type colloidal semiconductor nanocrystals. Nature. 2000;407:981–983. doi: 10.1038/35039577. [DOI] [PubMed] [Google Scholar]
- 36.Koh WK, Koposov AY, Stewart JT, Pal BN, Robel I, Pietryga JM, Klimov VI. Heavily doped n-type PbSe and PbS nanocrystals using ground-state charge transfer from cobaltocene. Sci Rep. 2013;3:2004. doi: 10.1038/srep02004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown HC, Krishnamurthy S, Hubbard JL. Addition compounds of alkali metal hydrides. 15. Steric effects in the reaction of representative trialkylboranes with lithium and sodium hydrides to form the corresponding trialkylborohydrides. J Am Chem Soc. 1978;100:3343–3349. [Google Scholar]
- 38.Liu WK, Whitaker KM, Kittilstved KR, Gamelin DR. Stable Photogenerated Carriers in Magnetic Semiconductor Nanocrystals. J Am Chem Soc. 2006;128:3910–3911. doi: 10.1021/ja060488p. [DOI] [PubMed] [Google Scholar]
- 39.Liu WK, Whitaker KM, Smith AL, Kittilstved KR, Robinson BH, Gamelin DR. Room-Temperature Electron Spin Dynamics in Free-Standing ZnO Quantum Dots. Phys Rev Lett. 2007;98:186804. doi: 10.1103/PhysRevLett.98.186804. [DOI] [PubMed] [Google Scholar]
- 40.Schimpf AM, Gunthardt CE, Rinehart JD, Mayer JM, Gamelin DR. Controlling Carrier Densities in Photochemically Reduced Colloidal ZnO Nanocrystals: Size Dependence and Role of the Hole Quencher. J Am Chem Soc. 2013;135:16569–16577. doi: 10.1021/ja408030u. [DOI] [PubMed] [Google Scholar]
- 41.Shim M, Guyot-Sionnest P. Organic-Capped ZnO Nanocrystals: Synthesis and n-Type Character. J Am Chem Soc. 2001;123:11651–11654. doi: 10.1021/ja0163321. [DOI] [PubMed] [Google Scholar]
- 42.Jasieniak J, Mulvaney P. From Cd-Rich to Se-Rich - the Manipulation of CdSe Nanocrystal Surface Stoichiometry. J Am Chem Soc. 2007;129:2841–2848. doi: 10.1021/ja066205a. [DOI] [PubMed] [Google Scholar]
- 43.Anderson NC, Hendricks MP, Choi JJ, Owen JS. Ligand Exchange and the Stoichiometry of Metal Chalcogenide Nanocrystals: Spectroscopic Observation of Facile Metal-Carboxylate Displacement and Binding. J Am Chem Soc. 2013;135:18536–18548. doi: 10.1021/ja4086758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busby E, Anderson NC, Owen JS, Sfeir MY. Effect of Surface Stoichiometry on Blinking and Hole Trapping Dynamics in CdSe Nanocrystals. J Phys Chem C. 2015;119:27797–27803. [Google Scholar]
- 45.Carroll GM, Tsui EY, Brozek CK, Gamelin DR. Spectroelectrochemical Measurement of Surface Electrostatic Contributions to Colloidal CdSe Nanocrystal Redox Potentials. Chem Mater. 2016;28:7912–7918. [Google Scholar]
- 46.Brown PR, Kim D, Lunt RR, Zhao N, Bawendi MG, Grossman JC, Bulovic V. Energy Level Modification in Lead Sulfide Quantum Dot Thin Films through Ligand Exchange. ACS Nano. 2014;8:5863–5872. doi: 10.1021/nn500897c. [DOI] [PubMed] [Google Scholar]
- 47.Jeong KS, Deng Z, Keuleyan S, Liu H, Guyot-Sionnest P. Air-Stable n-Doped Colloidal HgS Quantum Dots. J Phys Chem Lett. 2014;5:1139–1143. doi: 10.1021/jz500436x. [DOI] [PubMed] [Google Scholar]
- 48.Schimpf AM, Knowles KE, Carroll GM, Gamelin DR. Electronic Doping and Redox Potential Tuning in Colloidal Semiconductor Nanocrystals. Acc Chem Res. 2015;48:1929–1937. doi: 10.1021/acs.accounts.5b00181. [DOI] [PubMed] [Google Scholar]
- 49.Gladysz JA, Hornby JL, Garbe JE. Convenient one-flask synthesis of dialkyl selenides and diselenides via lithium triethylborohydride reduction of Sex. J Org Chem. 1978;43:1204–1208. [Google Scholar]
- 50.Knowles KE, McArthur EA, Weiss EA. A Multi-Timescale Map of Radiative and Nonradiative Decay Pathways for Excitons in CdSe Quantum Dots. ACS Nano. 2011;5:2026–2035. doi: 10.1021/nn2002689. [DOI] [PubMed] [Google Scholar]
- 51.Kern SJ, Sahu K, Berg MA. Heterogeneity of the Electron-Trapping Kinetics in CdSe Nanoparticles. Nano Lett. 2011;11:3493–3498. doi: 10.1021/nl202086b. [DOI] [PubMed] [Google Scholar]
- 52.Morris-Cohen AJ, Frederick MT, Cass LC, Weiss EA. Simultaneous Determination of the Adsorption Constant and the Photoinduced Electron Transfer Rate for a Cds Quantum Dot–Viologen Complex. J Am Chem Soc. 2011;133:10146–10154. doi: 10.1021/ja2010237. [DOI] [PubMed] [Google Scholar]
- 53.Zhao J, Holmes MA, Osterloh FE. Quantum Confinement Controls Photocatalysis: A Free Energy Analysis for Photocatalytic Proton Reduction at CdSe Nanocrystals. ACS Nano. 2013;7:4316–4325. doi: 10.1021/nn400826h. [DOI] [PubMed] [Google Scholar]
- 54.Chestnoy N, Harris TD, Hull R, Brus LE. Luminescence and photophysics of cadmium sulfide semiconductor clusters: the nature of the emitting electronic state. J Phys Chem. 1986;90:3393–3399. [Google Scholar]
- 55.Voznyy O, Sargent EH. Atomistic Model of Fluorescence Intermittency of Colloidal Quantum Dots. Phys Rev Lett. 2014;112:157401. doi: 10.1103/PhysRevLett.112.157401. [DOI] [PubMed] [Google Scholar]
- 56.Shockley W, Read WT. Statistics of the Recombinations of Holes and Electrons. Phys Rev. 1952;87:835–842. [Google Scholar]
- 57.Tachiya M, Seki K. Unified explanation of the fluorescence decay and blinking characteristics of semiconductor nanocrystals. Appl Phys Lett. 2009;94:081104. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.