Abstract
The incorporation of an endogenous safety switch represents a rational strategy for the control of toxicities following the administration of adoptive T cell therapies. An ideal safety switch should be capable of depleting the transferred T cells with minimal injury to normal tissues. We generated a fusion receptor by engineering a cryptic 806 epitope of human epidermal growth factor receptor (EGFR) into the N terminus of the full-length human folate receptor 1 (FOLR1), designated as FR806. The expression of FR806 allows transduced T cells to be targeted with CH12, a monoclonal antibody recognizing the 806 epitope, but not wild-type EGFR in healthy tissues. FR806, therefore, constitutes a specific cell-surface marker for the elimination of transduced T cells. We demonstrate that the antibody-drug conjugate (ADC) CH12-MMAF is efficiently internalized by FR806-expressing T cells and has the potential to eliminate them. Transfected T cells could, furthermore, be efficiently detected and purified using CH12 antibodies. In immuno-compromised mice, CH12-MMAF eliminated the majority of transferred T cells expressing FR806 and anti-CD19 chimeric antigen receptor (CAR). The selectivity for the 806 epitope and internalization capacity of FOLR1 makes FR806 an efficient safety switch, which may additionally be used as a detection and purification biomarker for human T cell immunotherapies.
Keywords: gene therapy, safety switch, gene transfer to lymphocytes
Therapeutic T cells should be selectively eliminated in the event that severe adverse events are observed. Li and colleagues describe a fusion receptor (FR806) empowered with high specificity and internalization capacity. T cells transduced with FR806 can be efficiently deleted by antibody-drug conjugate with minimal damage to normal tissues.
Introduction
Cell-base therapies have clinical utility in the treatment of multiple different tumor types. Recent successes include the use of adoptively transferred T cells expressing anti-CD19 chimeric antigen receptors (CARs) for the treatment of relapsed or refractory B cell malignancies.1, 2, 3, 4, 5 However, the administration of CAR-T cells has been associated with significant adverse events, which have in some cases been fatal. Fatal on-target off-tumor toxicity and fatal cytokine release syndrome (CRS) have, for example, been reported in clinical trials of Her2-targeted and CD19-targeted CAR-T cell therapy, respectively.6, 7
To control toxicities of adoptive T cell therapy, suicide genes including inducible caspase-9 (iCasp9)8 or herpes simplex virus thymidine kinase (HSV-TK)9 have been introduced to selectively eliminate infused T cells in the event of severe toxicities. However, T cells expressing iCasp9 or HSV-TK are hard to be positively selected or detected. An alternative strategy is to express a cell-surface marker on T cells, which includes truncated epidermal growth factor receptor (EGFRt),10 truncated CD19,11 truncated nerve growth factor receptor (ΔNGFR),12 CD20,13 or RQR8.14 Although these markers facilitate positive selection, detection, and in vivo attenuation of marker-expressing T cells with corresponding antibodies, these methods have several shortcomings. First, monoclonal antibodies (mAbs) against these antigens bind to antigen-positive normal tissues and may result in adverse events such as cetuximab-induced skin exanthema15 or rituximab-induced healthy B cell depletion.16 Additionally, antibody-mediated depletion is principally dependent on complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC), which may be compromised in patients with malignancies in whom immunosuppression is common.17, 18
In contrast to mAbs, antibody-drug conjugates (ADCs), which are comprised of mAbs and conjugated cytotoxins are able to destroy target cells in an ADCC- and CDC-independent manner.19 Generally, ADCs have higher cytotoxic activity than parent mAbs. Brentuximab vedotin, for example, a CD30-targeted ADC, could induce complete responses (CRs) in 34% of refractory Hodgkin’s lymphoma (HL) patients, while no CRs or partial responses (PRs) were observed in HL patients treated with an identical naked anti-CD30 mAb.20, 21 The enhanced killing activity of the ADCs over the parent mAbs suggests that the use of T cell-targeted ADCs may represent an efficient strategy for the rapid and efficient depletion of T cells in patients experiencing significant toxicities.
In order to minimize toxicity to healthy tissues, an exogenous epitope can be introduced into CAR-T cells for the purpose of selective ADC targeting. The cryptic 806 epitope is one such candidate, as it is only exposed as a result of EGFR overexpression or extracellular domain truncations.22 FOLR1 is a glycosylphosphatidylinositol (GPI)-linked membrane glycoprotein that mediates cellular uptake of folate.23 Its capacity for efficient endocytosis has made FOLR1 an important target for the delivery of drugs to FOLR1-positive tumor cells.24 We engineered the 806 epitope and FOLR1 to generate a fusion receptor, which was named FR806 and capable of mediating the internalization of ADCs, with a view to eliminating T cells expressing FR806. Therefore, an ADC comprising an 806 epitope-specific mAb CH12 and anti-mitotic agent monomethylauristatin-F (MMAF) was developed.25, 26 Our data demonstrated that FR806-engineered T cells can be efficiently isolated and detected by mAb CH12 and eliminated by CH12-MMAF.
Results
The mAb CH12 Selectively Binds to the FR806 Fusion Receptor
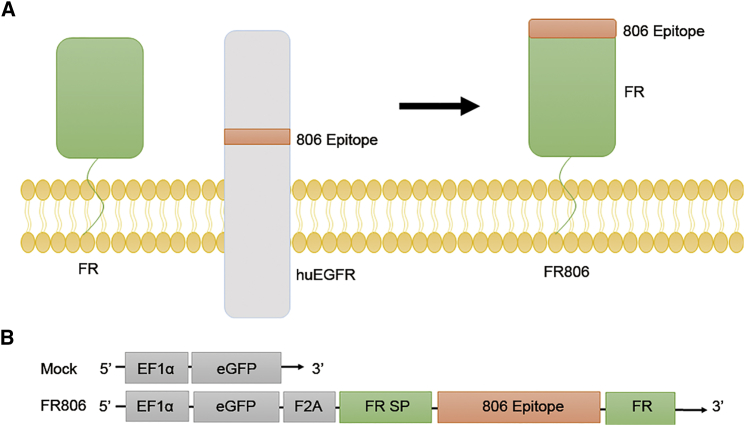
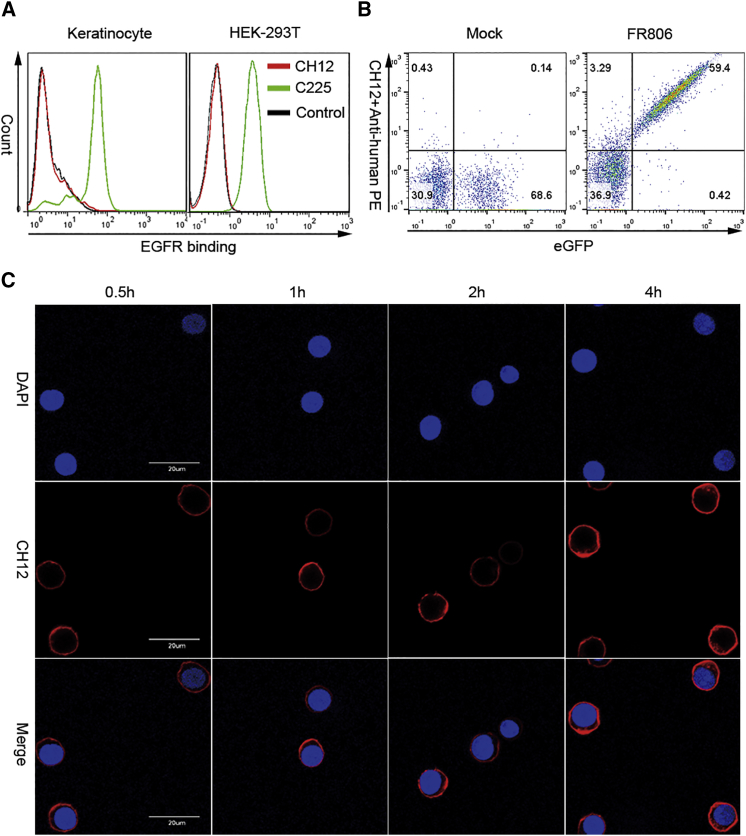
As show in Figure 1A, the 806 epitope of EGFR was directly fused to the N terminus of the whole-length human FOLR1 gene (Figure 1A). For easy detection, FR806 was co-expressed with EGFP through a self-cleaving/ribosome skip F2A peptide (Figure 1B). To demonstrate the binding specificity of CH12, fluorescence-activated cell sorting (FACS) analysis was performed on EGFR-expressing keratinocytes and HEK293T. The data in Figure 2A indicate that CH12 does not bind significantly to keratinocytes and HEK293T, whereas the anti-EGFR antibody cetuximab binds strongly to these cells. The data shown in Figure 2B demonstrate that the anti-806 mAb CH12 binds to FR806-transduced human T cells in a highly efficient manner, but not to T cells that lack FR806. In order to test the internalization capacity of CH12 mediated by FR806, FR806 transduced T cells were incubated with saturating concentrations of CH12 for 4 hr. The time course of the internalization of CH12 was studied using FR806+ T cells (Figure 2C). These results indicate that the introduction of FR806/CH12 may form the basis of a safety switch for the elimination of transduced T cells.
Figure 1.
Construction of FR806
(A) Human full-length FR and 806 epitope of human EGFR were engineered into a fusion receptor. (B) Schematic diagram of the lentiviral vectors used for the expression of FR806. EGFR-derived 21-amino acid (aa) sequence (284VRACGADSYEMEEDGVRKCKK304) including 806 epitope (287CGADSYEMEEDGVRKC302) was inserted between FR-derived signal peptide (FR SP) and the rest of human FR (FR). F2A, ribosomal skipping sequence derived from the foot and mouth disease virus; EGFP; EF1α, elongation factor 1 alpha promoter sequences. A negative control vector was designed containing EGFP only.
Figure 2.
FR806-Mediated Recognization and Endocytosis of CH12 in Human T Cells
(A) Binding capacity of CH12 on keratinocytes (left) and HEK293T cells (right). Compared to anti-EGFR mAb C225 (cetuximab), CH12 has no affinity to human keratinocytes and HEK293T with endogenous WTEGFR expression. (B) Detection of human T cells transduced with mock or FR806 by CH12 staining. CH12 binds significantly to T cells transduced with FR806. (C) Internalization of CH12 in FR806+ T cells. FR806+ T cells were treated with saturating concentration of biotinylated CH12 and collected at the indicated time points. After intracellular staining, the time-dependent internalization of CH12 in FR806+ T cells was observed using confocal microscopy. Scale bars, 20 μM.
CH12 ADCs Efficiently Deplete T Cells Transduced with FR806 In Vitro
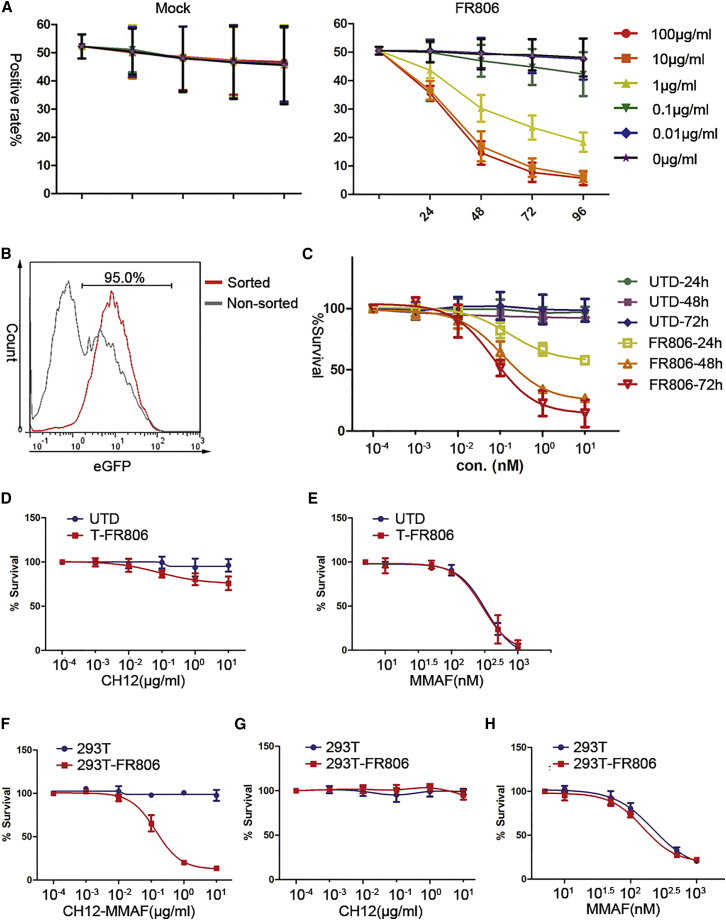
CH12 was conjugated with maleimidocaproyl monomethylauristatin F (MC-MMAF). The binding potency of the resulting antibody-drug conjugate CH12-MMAF against FR806-expressing T cells was equivalent to that of unconjugated CH12 (Figure S1), indicating that drug conjugation did not alter the binding properties of CH12. Additionally, CH12-MMAF could be internalized by FR806+ T cells, but not by T cells following mock transduction (Figure S2). To test whether FR806-expressing T cells could be eliminated in vitro, a 1:1 mix of FR806-transduced and untransduced T cells were exposed to CH12-MMAF at different concentrations. As FR806 and EGFP were coordinately expressed in human T cells, we observed the ongoing depletion of EGFP+ T cells following CH12-MMAF administration. CH12-MMAF showed effective cell cytotoxic capacity for FR806+ T cells in a time- and dose-dependent manner, but not for mock-transduced T cells (Figure 3A). 88% of FR806-expressing cells were eliminated following a single 10 μg/mL dose of CH12-MMAF for 96 hr.
Figure 3.
In Vitro Depletion of T Cells Transduced with FR806 by CH12-MMAF
(A) Time course and CH12-MMAF dose-titration assay. Mock+ or FR806+ T cells were exposed to CH12-MMAF at the indicated concentrations for 96 hr. The positive rates of GFP were monitored and 10 μg/mL CH12-MMAF was deemed sufficient for attenuation of FR806-transduced T cells. Each of the data reflect the mean ± SD of triplicates from three different donors. (B) Sorting of FR806+ T cells. FR806+ T cells (95% positive) could be sorted with biotinylated CH12 and anti-biotin microbeads. (C) Cytotoxity mediated by CH12-MMAF to sorted FR806+ T cells or untransduced T cells (UTD). UTD or sorted FR806+ T cells were exposed to CH12-MMAF at various concentrations, and cell viability assays were performed at the indicated time points. Each of the data reflect the mean ± SD of triplicates from three different donors. (D and E) Cytotoxity mediated by mAb CH12 or free MMAF to FR806+ T cells. Cell viability of UTD or sorted FR806+ T cells was shown after being treated with the indicated concentrations of mAb CH12 (D) or free MMAF (E) for 72 hr. Each of the data reflect the mean ± SD of triplicates from three different donors. (F–H) Untransduced and FR806-transduced HEK293T cells were exposed to various concentrations of CH12-MMAF (F), mAb CH12 (G), or free MMAF (H) for 72 hr followed by a cell viability assay. Each of the data points reflect the mean ± SD of triplicates.
In order to detect and purify FR806+ T cells, CH12 was biotinylated. Biotinylated CH12 is able to bind to FR806-expressing T cells in a dose-dependent manner (Figure S3). Furthermore, FR806+ T cells (95% positive) could be isolated using biotinylated CH12 and an immuno-magnetic bead cell sorting system (Figure 3B). Cell viability assays were performed on the sorted FR806+ T cells to assess the cytotoxicity of CH12-MMAF. Sorted FR806+ T cells had a time- and dose-dependent reduction in cell viability following treatment with CH12-MMAF (Figure 3C). However, mAb CH12 showed limited cytotoxicity to FR806+ T cells (Figure 3D). FR806-expressing and non-transduced T cells had the same response to free MMAF (Figure 3E). In order to investigate the potential safety of administering CH12-MMAF in the presence of cells expressing wild-type EGFR (WTEGFR), we tested the cytotoxicity of CH12-MMAF using HEK293T cells. As shown in Figure 3F, HEK293T cells were not sensitive to CH12-MMAF, as compared with FR806-expressing HEK293T cells. In contrast, FR806-expressing and untransduced HEK293T cells showed similar sensitivity to mAb CH12 (Figure 3G) or free MMAF (Figure 3H). Additionally, CH12-MMAF had less influence on the cell survival of human keratinocytes (Figure S4). These results indicate that CH12-MMAF selectively targets T cells transduced with FR806, but not T cells that lack FR806 transduction and cells with WTEGFR expression.
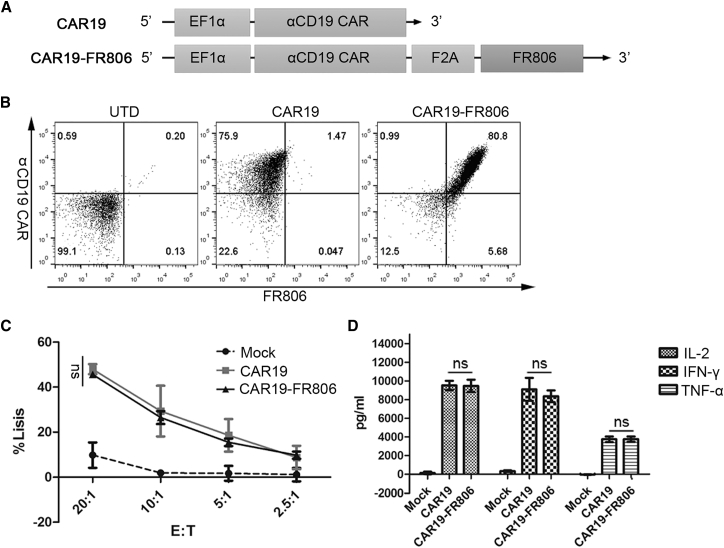
In Vitro Anti-tumor Activity and ADC-Mediated Depletion of FR806+ CAR-T Cells
Anti-CD19 CAR was successfully co-expressed with FR806 in a lentiviral vector using the self-cleaving F2A peptide (Figures 4A and 4B). T cells transduced with CAR19-FR806 and CAR19 showed comparable cytotoxicity and cytokine production against CD19-positive lymphoma cells (Figures 4C and 4D). Consequently, incorporation of FR806 has no influence on the anti-tumor activity of anti-CD19 CAR-T cells.
Figure 4.
In Vitro Anti-tumor Activities of FR806+ CAR-T Cells
(A) Lentiviral constructs used to transduce activated T cells. (B) Flow cytometry plots for anti-CD19 CAR and FR806 expression in UTD, CAR19-transduced, or CAR19-FR806-transduced T cells. (C) Similar cytotoxicity is observed with incubation of Daudi cells with CAR19 or CAR19-FR806-transduced T cells at varying effector: target (E:T) ratios for 4 hr, as determined by a standard non-radioactive cytotoxic assay. Each of the data reflect the mean ± SD of triplicates from two donors. (D) In vitro co-culture of CAR19+ or CAR19-FR806+ T cells with Daudi cells at 1:1 ratio for 24 hr have similar IFN-γ, IL-2, and TNF-α production as measured by the ELISA. Each of the data reflect the mean ± SD of triplicates from two donors.
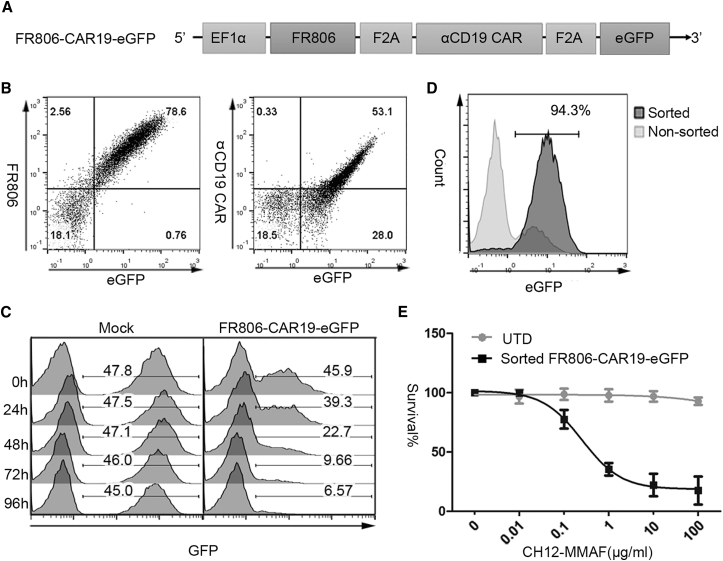
In order to determine whether the FR806/CH12-MMAF safety system may be applied to deplete CAR-modified T cells, EGFP was co-expressed with FR806 and anti-CD19 CAR through a F2A peptide to facilitate the detection of FR806+ CAR-T cells in vitro and in vivo (Figures 5A and 5B). Treatment with 10 μg/mL CH12-MMAF for 96 hr resulted in a time-dependent reduction of the FR806-expressing population (Figure 5C). FR806-CAR19-EGFP-transduced T cells were also sorted using biotinylated CH12 and anti-biotin microbeads (Figure 5D). Cell viability assays showed a drug concentration-associated toxicity of CH12-MMAF on sorted FR806+ CAR-T cells (Figure 5E).
Figure 5.
Detection, Isolation, and Elimination of Anti-CD19 CAR-T Cells with FR806 Co-expression
(A) Diagram of the lentiviral vectors used for the expression of FR806, anti-CD19 CAR, and GFP separated by F2A sequence. (B) Flow cytometry detection of FR806, anti-CD19 CAR, and GFP in FR806-CAR19-EGFP-transduced T cells. (C) 50:50 mixing assays were performed to test the depletion of FR806-CAR19-EGFP or mock-transduced T cells treated with 10 μg/mL CH12-MMAF. Survival of GFP+ cells was measured at the indicated time points. (D) T cells transduced with FR806-CAR19-EGFP (94.3% positive) could be isolated with biotinylated CH12 and anti-biotin microbeads. (E) Percentage of surviving UTD or sorted FR806-CAR19-EGFP+ T cells was measured by cell viability assay after incubation with the indicated concentrations of CH12-MMAF for 72 hr. Each of the data reflect the mean ± SD of triplicates from three different donors.
In Vivo Depletion of T Cells Transduced with FR806-CAR19-EGFP
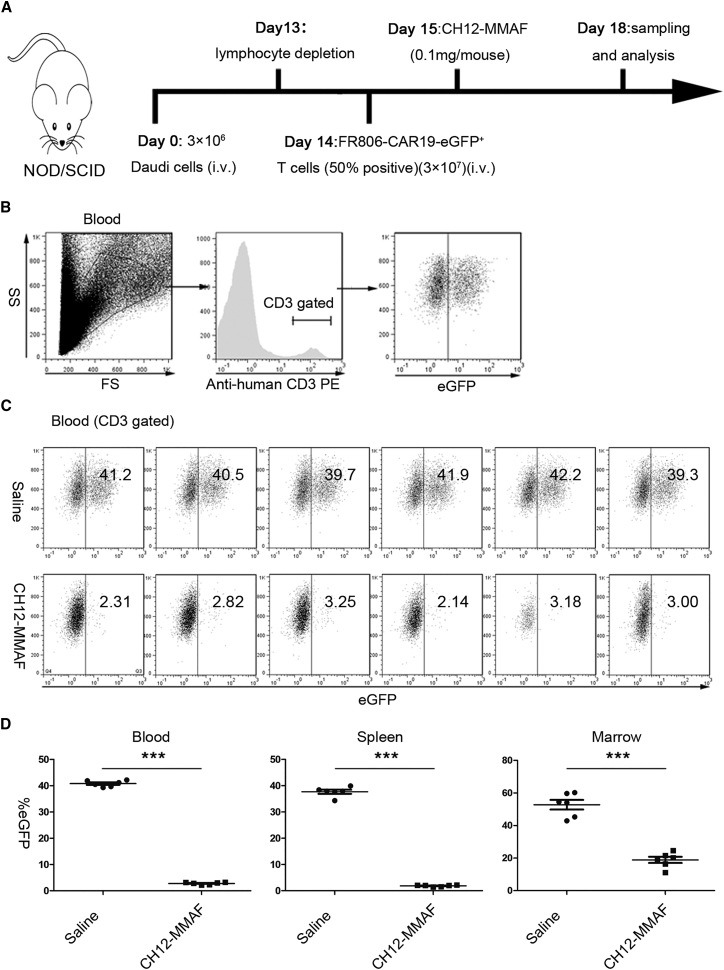
In order to demonstrate that FR806-CAR19-EGFP-transduced T cells could be eliminated in vivo, non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice were engrafted intravenously (i.v.) with Daudi tumor cells as described in Figure 6A. After 14 days, FR806-CAR19-EGFP-transduced T cells (50% efficiency) were i.v. infused into NOD/SCID mice. On day 15, a single dose of 100 μg CH12-MMAF or saline was administrated by tail-vein infusion. Since CH12 is a chimeric mAb containing a human immunoglobulin G (IgG) Fc fragment, the CDC and ADCC effects are not generated in NOD/SCID mice following CH12-MMAF administration. On day 18, mice were sacrificed, and peripheral blood, spleen, and bone marrow were collected to analyze the EGFP positivity rate in human T cells gated on human CD3 (Figure 6B). In mice that received CH12-MMAF, human CD3+/EGFP+ cells were reduced by 93% in blood, 94% in spleen, and 64% in bone marrow. In contrast, CD3+/EGFP+ cells were observed in the blood (mean 40.8%), spleen (mean 37.7%), and bone marrow (mean 52.8%) of mice in the saline treated group (Figures 6C and 6D).
Figure 6.
CH12-MMAF-Mediated Depletion of FR806+ CAR-T Cells In Vivo
(A) Experimental scheme of in vivo depletion experiments. NOD/SCID mice were injected intravenously (i.v.) with 3 × 106 Daudi cells. On day 13, cyclophosphamide was administrated to deplete host lymphocytes. At 24 hr later, 3 × 107 FR806-CAR19-EGFP-transduced T cells (50% positive) were intravenously infused into NOD/SCID mice. CH12-MMAF treatment commenced with 0.1 mg doses at day 15 by i.v. injection or saline carrier for the control cohort. Mice were sacrificed at day 18. Peripheral blood, spleen, and bone marrow were sampled and the presence of human CD3+/EGFP+ T cells was analyzed. (B) The human CD3+ population was gated to determine the rate of EGFP positivity. (C) Flow cytometry analysis of the peripheral blood samples from mice treated with CH12-MMAF or saline. (D) Percentage of CD3+/EGFP+ cells in peripheral blood, spleen, and bone marrow from mice treated with CH12-MMAF or saline. n = 6. Data represent mean ± SEM of six mice per group.
Discussion
The selective elimination of engineered immune cells, while simultaneously causing only minimal damage to healthy tissues, is an important adjunct to adoptive immunotherapy so as to limit the adverse and potentially life-threatening toxicities that are observed in an unpredictable manner in some individuals. Here, we demonstrate that the fusion receptor FR806 co-expressed with an anti-CD19 CAR can successfully be applied to immune detection, selection, and in vivo elimination of engineered T cells. The 806 epitope is masked in EGFR derived from healthy individuals; therefore, the targeting of this epitope significantly reduces the potential for non-specific binding to healthy tissues.
Prior studies have demonstrated that the expression of a cell-surface-target antigen on T cells renders engineered cells recognizable by a cognate antibody. One example of this is the cell-surface expression of EGFRt.10 Targeting EGFRt with cetuximab results in the elimination of EGFRt+ T cells in vivo. The use of FR806 as the target antigen, however, confers several advantages as compared with EGFRt. First, as a result of non-specific binding to EGFR expressed on healthy cells, follicular skin exanthema has been observed in 80% of patients treated with cetuximab. Severe exanthema (grade 3/4) develops in about 9% to 19% of patients, necessitating dose reduction or cessation.15 The 806 epitope is not exposed in the EGFR present in healthy tissues. Treatment of EGFR-positive tumors with ch806 (a mAb targeting the 806 epitope in a manner similar to CH12) was well tolerated, with no significant toxicities observed at doses of up to 40 mg/m2.27 Our preclinical study on cynomolgus monkeys, furthermore, indicates that even a 20 mg/kg dose of mAb CH12 does not damage healthy EGFR-expressing tissues (data not shown). Second, the cytotoxic activity of cetuximab mediated by CDC and antibody-dependent cellular cytotoxicity (ADCC) is likely to be compromised in individuals with suppressed immunity. However, in addition to being recognized by CH12, FR806 also mediates endocytosis. The depletion of engineered T cells mediated by the antibody-drug conjugate CH12-MMAF is consequently independent of both CDC and ADCC.
Other approaches have included the introduction of the HSV-TK9 or iCasp98 for conditional depletion of infused T cells. Indeed, clinical studies have shown that HSV-TK and iCasp9 suicide genes were effective in controlling graft-versus-host disease (GVHD) resulting from allogeneic T cell infusion.28, 29 However, therapeutic cells expressing virus-derived HSV-TK protein may be rejected by the host immune system.30 In contrast, FR806 is less likely to be immunogenic, as the 806 epitope and folate receptor are human-derived molecules and were fused together without the formation of junctional sequences. Additionally, unlike FR806, HSV-TK and iCasp9 are intracellular proteins, which should be co-expressed with a recognizable marker such as truncated ΔNGFR12 or truncated CD1911 for convenient detection or selection of engineered T cells. In this study, CH12 was biotinylated and shown to successfully detect FR806-expressing T cells with streptavidin-PE. Biotinylated CH12 was also shown to facilitate the selection of FR806+ T cells with anti-biotin microbeads.
The characteristics of the CAR-T cells are expected to be unaffected following the introduction of a cell-surface marker or suicide gene. We have, additionally, demonstrated that the expression of FR806 has no effect on the cytotoxicity and cytokine production of CAR-T cells. One potential concern relates to whether the binding of FR806 to folate will impact other CAR-T cell functions. Additional studies will be necessary to address this issue. Engineering FOLR1 into a mutant devoid of the folate-binding site, but which remains competent for endocytosis would be one possible approach.
In summary, we have generated a fusion receptor that is efficiently expressed on the surface of T cells following lentiviral transduction that can be used to efficiently eliminate engineered T cells in conjunction with a selective antibody-drug conjugate. FR806 can be safely targeted with CH12, a mAb that targets a masked epitope of EGFR with minimal non-specific binding to healthy tissues. In addition, the capacity of FR806 for internalization renders T cells sensitive to CH12-MMAF-mediated depletion. Together, our data indicate that the use of the fusion receptor FR806 represents a rational strategy for the detection and purification of T cell immunotherapeutic agents, as well as for their safe and rapid depletion in the event that their administration is associated with significant clinical toxicities.
Materials and Methods
Construction of Lentiviral Vectors
As shown in Figure 1A and Table S1, the FR806 receptor was built by interposing the sequence including the 806 site (Val284 to Lys304) of EGFR (GenBank: X00588.1) between the signal peptide (Met1 to Thr24) and residues (Arg25 to Ser257) of human FOLR1 (GenBank: NM_016729.2). The sequence encoding the anti-human CD19 antigen ligand binding single-chain fragment variable (scFv) was based on the sequence of a bispecific anti-CD19/anti-CD3 single-chain antibody.31 This anti-CD19 scfv was subcloned into a lentiviral vector containing the human CD8α signal peptide, the human CD8α hinge and transmembrane domain, and the human 4-1BB co-stimulation and CD3ζ immunoreceptor tyrosine-based activation motif (ITAM) signaling chains, which were obtained from the anti-GPC3 CARs in our laboratory.32 A ribosomal skipping sequence (F2A) derived from the foot and mouth disease virus was used to co-express the fusion protein FR806, EGFP, and/or anti-CD19 CAR. Finally, standard molecular cloning techniques were applied to construct FR806 expression plasmids using a third-generation non-self-inactivating EF-1a promoter-based lentiviral expression vector pWPT-EGFP.
Cell Lines
Daudi cells (CD19+, Burkitt lymphoma cell lines) and HEK293T cells were purchased from American Type Culture Collection (ATCC). Daudi cells were cultured in RPMI-1640 (GE Healthcare, catalog # SH30809.01), supplemented with 10% fetal bovine serum (FBS) (Gibco, catalog # 10099-141). HEK293T were cultured in DMEM (Gibco, catalog # C11995500), supplemented with 10% FBS. Peripheral blood mononuclear cells (PBMCs) were obtained from the Shanghai Blood Center. PBMCs and T cells were maintained in culture in AIM-V (Gibco, catalog # 0870112), supplemented with 10% human AB serum (ABS) (Gemini, catalog # 100-512) and 500 U/mL recombinant human interleukin-2 (IL-2) (Shanghai Huaxin High Biotechnology). Human epidermal keratinocytes from a healthy donor were cultured in EpiLife Medium (Gibco, catalog # MEPI500CA) with the addition of Human Keratinocyte Growth Supplement (Gibco, catalog # S0015).
Lentivirus Production
Lentiviruses were generated using a polyethylenimine-based DNA transfection reagent. Viruses were harvested from conditioned medium at 72 hr post-transfection, filtering through a 0.45 μm filter unit to remove cell debris, followed by concentration and purification with polyethylene glycol.
Transduction of Human T Cells
On day 0, PBMCs were stimulated with anti-CD3/CD28 magnetic beads (Invitrogen, catalog # 21013) at a beads:cell ratio of 1:1 for 48 hr. T cells were then transduced with lentiviruses on RetroNectin (Takara, catalog # T100A) coated plates. The transduced T cells were cultured at a concentration of 5 × 105 cells/mL. Magnetic beads were removed on day 4, and the cell culture medium supplemented with 500 U/mL recombinant human IL-2 was replaced every other day.
Cell Proliferation Assay In Vitro
The effect of CH12-MMAF on cell viability was assessed with the CCK-8 assay. Untransduced or sorted FR806+ T cells (30,000 per well) and HEK293T cells (5,000 per well) were seeded and exposed to various concentrations of CH12-MMAF for 72 hr. After 72 hr, cell viability was measured using a CCK-8 kit (Dojindo Laboratories, catalog # CK04) according to the manufacturer’s instructions.
Cytotoxicity Assays In Vitro
The specific cytotoxicity of anti-CD19 CAR-T cells toward Daudi cells was evaluated by a 4 hr lactate dehydrogenase (LDH) release assay using the CytoTox 96 Non-Radioactive Cytotoxicity Kit (Promega, catalog # G1780) according to the manufacturer’s instructions.
Cytokine Release Assays
The IL-2, interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α) cytokines secreted by the varying genetically modified T cells were measured using an ELISA kit purchased from MultiSciences Biotech (catalog # EK1822; EK1802; and EK1022).
Antibodies and ADCs
The chimeric mAb CH12 (IgG1) was produced in dihydrofolate reductase-deficient CHO DG44 cells as described previously.25 Goat anti-mouse IgG-phycoerythrin (PE) and goat anti-human IgG-PE were purchased from Santa Cruz (catalog # GAM0041; sc-3738). Streptavidin-PE and anti-human CD3-PE were purchased from eBioscience (catalog # 12-4317-87 and 12-0039-42). For generation of the biotinylated CH12, CH12 (2.5 mg/mL) was dissolved in PBS (pH 7.4) and modified with sulfo-NHS-LC-biotin (30:1) in 4°C overnight. Excess biotin was removed using PD-10 desalting columns (GE Healthcare, catalog # 17-5175-01). The biotinylated CH12 was then buffer exchanged to PBS, glycerol was added to a final concentration of 5%. Maleimidocaproyl (mc) MMAF was synthesized and conjugated to antibodies as reported previously.26 The ADCs were determined to have more than 98% monomeric mAbs containing 3.5 to 4.2 drugs per mAb using previously published methods.26
In Vivo Engraftment Model
On day 0, 4- to 6-week-old NOD/SCID mice were injected i.v. with 3 × 106 Daudi cells. On day 13, mice were injected intraperitoneally with 100 mg/kg of cyclophosphamide to deplete host lymphocytes. FR806+ CAR-T cells (50% positive) were then infused i.v. on day 14. A single dose of 0.1 mg CH12-MMAF was administered by tail-vein infusion on day 15. Mice were sacrificed on day 18. Peripheral blood, spleen, and bone marrow were taken and analyzed by flow cytometry. All animal experiments were treated under specific pathogen-free conditions at the Experimental Animal Center of Shanghai Cancer Institute (Shanghai, China) in accordance with the protocols approved by the Shanghai Medical Experimental Animal Care Commission.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 5.0. Student’s t test was performed to assess the differences between the CAR19 and CAR19-FR806 group in the in vitro assays. Differences in the presence of human CD3+/EGFP+ T cells between the CH12-MMAF-treated and saline-treated group were also evaluated by Student’s t test. In the figures, significance of findings was defined as: ns, not significant, p > 0.05; *p < 0.05; **p < 0.01; and ***p < 0.001.
Author Contributions
Z.L. conceived the idea, designed the research, and revised the manuscript; X.W. designed subsequent experiments, performed most of the in vitro and in vivo work, and wrote the manuscript; H.J. assisted with the interpretation of data and helped to perform the in vitro and in vivo work; B.S. performed the molecular cloning work; J.Z. helped to analyze the data; Z.S., S.D., M.F., and H.G. assisted with the in vitro work; H.W. and J.G. assisted with the revision of the manuscript.
Conflicts of Interest
Z.L. and X.W. are named co-inventors in patent applications relating to this work. Z.L. is a stockholder of CARsgen Therapeutics, Inc.
Acknowledgments
This work was supported by the project of Shanghai Municipal Science and Technology Commission (14431903500 and 16DZ1910700), the Program of Shanghai Subject Chief Scientist (16XD1402600), the National Natural Science Foundation of China (NSFC grant 81402546), and the Research Fund of the State Key Laboratory of Oncogenes and Related Genes (91-17-04).
Footnotes
Supplemental Information includes four figures and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.06.026.
Contributor Information
Hua Jiang, Email: jianghuapy@163.com.
Zonghai Li, Email: zonghaili@shsmu.edu.cn.
Supplemental Information
References
- 1.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright J.F. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A., Maric I., Raffeld M., Nathan D.A., Lanier B.J. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teachey D.T., Lacey S.F., Shaw P.A., Melenhorst J.J., Maude S.L., Frey N., Pequignot E., Gonzalez V.E., Chen F., Finklestein J. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Straathof K.C., Pulè M.A., Yotnda P., Dotti G., Vanin E.F., Brenner M.K., Heslop H.E., Spencer D.M., Rooney C.M. An inducible caspase 9 safety switch for T-cell therapy. Blood. 2005;105:4247–4254. doi: 10.1182/blood-2004-11-4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciceri F., Bonini C., Stanghellini M.T.L., Bondanza A., Traversari C., Salomoni M., Turchetto L., Colombi S., Bernardi M., Peccatori J. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489–500. doi: 10.1016/S1470-2045(09)70074-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Chang W.C., Wong C.W., Colcher D., Sherman M., Ostberg J.R., Forman S.J., Riddell S.R., Jensen M.C. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tey S.K., Dotti G., Rooney C.M., Heslop H.E., Brenner M.K. Inducible caspase 9 suicide gene to improve the safety of allodepleted T cells after haploidentical stem cell transplantation. Biol. Blood Marrow Transplant. 2007;13:913–924. doi: 10.1016/j.bbmt.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deola S., Scaramuzza S., Birolo R.S., Carballido-Perrig N., Ficara F., Mocchetti C., Dando J., Carballido J.M., Bordignon C., Roncarolo M.G. Mobilized blood CD34+ cells transduced and selected with a clinically applicable protocol reconstitute lymphopoiesis in SCID-Hu mice. Hum. Gene Ther. 2004;15:305–311. doi: 10.1089/104303404322886156. [DOI] [PubMed] [Google Scholar]
- 13.Introna M., Barbui A.M., Bambacioni F., Casati C., Gaipa G., Borleri G., Bernasconi S., Barbui T., Golay J., Biondi A., Rambaldi A. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum. Gene Ther. 2000;11:611–620. doi: 10.1089/10430340050015798. [DOI] [PubMed] [Google Scholar]
- 14.Philip B., Kokalaki E., Mekkaoui L., Thomas S., Straathof K., Flutter B., Marin V., Marafioti T., Chakraverty R., Linch D. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood. 2014;124:1277–1287. doi: 10.1182/blood-2014-01-545020. [DOI] [PubMed] [Google Scholar]
- 15.Wehler T.C., Graf C., Möhler M., Herzog J., Berger M.R., Gockel I., Lang H., Theobald M., Galle P.R., Schimanski C.C. Cetuximab-induced skin exanthema: prophylactic and reactive skin therapy are equally effective. J. Cancer Res. Clin. Oncol. 2013;139:1667–1672. doi: 10.1007/s00432-013-1483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimby E. Tolerability and safety of rituximab (MabThera) Cancer Treat. Rev. 2005;31:456–473. doi: 10.1016/j.ctrv.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Jie H.B., Schuler P.J., Lee S.C., Srivastava R.M., Argiris A., Ferrone S., Whiteside T.L., Ferris R.L. CTLA-4+ regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 2015;75:2200–2210. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy E.M., Sycz G., Arriaga J.M., Barrio M.M., von Euw E.M., Morales S.B., González M., Mordoh J., Bianchini M. Cetuximab-mediated cellular cytotoxicity is inhibited by HLA-E membrane expression in colon cancer cells. Innate Immun. 2009;15:91–100. doi: 10.1177/1753425908101404. [DOI] [PubMed] [Google Scholar]
- 19.Garnett M.C. Targeted drug conjugates: principles and progress. Adv. Drug Deliv. Rev. 2001;53:171–216. doi: 10.1016/s0169-409x(01)00227-7. [DOI] [PubMed] [Google Scholar]
- 20.Chen R., Gopal A.K., Smith S.E., Ansell S.M., Rosenblatt J.D., Savage K.J., Connors J.M., Engert A., Larsen E.K., Huebner D. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128:1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forero-Torres A., Leonard J.P., Younes A., Rosenblatt J.D., Brice P., Bartlett N.L., Bosly A., Pinter-Brown L., Kennedy D., Sievers E.L., Gopal A.K. A phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br. J. Haematol. 2009;146:171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 22.Gan H.K., Burgess A.W., Clayton A.H.A., Scott A.M. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–2930. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 23.Kamen B.A., Wang M.T., Streckfuss A.J., Peryea X., Anderson R.G. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J. Biol. Chem. 1988;263:13602–13609. [PubMed] [Google Scholar]
- 24.Sudimack J., Lee R.J. Targeted drug delivery via the folate receptor. Adv. Drug Deliv. Rev. 2000;41:147–162. doi: 10.1016/s0169-409x(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H., Wang H., Tan Z., Hu S., Wang H., Shi B., Yang L., Li P., Gu J., Wang H., Li Z. Growth suppression of human hepatocellular carcinoma xenografts by a monoclonal antibody CH12 directed to epidermal growth factor receptor variant III. J. Biol. Chem. 2011;286:5913–5920. doi: 10.1074/jbc.M110.192252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doronina S.O., Mendelsohn B.A., Bovee T.D., Cerveny C.G., Alley S.C., Meyer D.L., Oflazoglu E., Toki B.E., Sanderson R.J., Zabinski R.F. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug. Chem. 2006;17:114–124. doi: 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- 27.Scott A.M., Lee F., Tebbutt N., Herbertson R., Gill S.S., Liu Z., Skrinos E., Murone C., Saunder T.H., Chappell B. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc. Natl. Acad. Sci. USA. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., Straathof K., Liu E., Durett A.G., Grilley B. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonini C., Ferrari G., Verzeletti S., Servida P., Zappone E., Ruggieri L., Ponzoni M., Rossini S., Mavilio F., Traversari C., Bordignon C. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science. 1997;276:1719–1724. doi: 10.1126/science.276.5319.1719. [DOI] [PubMed] [Google Scholar]
- 30.Berger C., Flowers M.E., Warren E.H., Riddell S.R. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood. 2006;107:2294–2302. doi: 10.1182/blood-2005-08-3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Löffler A., Kufer P., Lutterbüse R., Zettl F., Daniel P.T., Schwenkenbecher J.M., Riethmüller G., Dörken B., Bargou R.C. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000;95:2098–2103. [PubMed] [Google Scholar]
- 32.Gao H., Li K., Tu H., Pan X., Jiang H., Shi B., Kong J., Wang H., Yang S., Gu J., Li Z. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin. Cancer Res. 2014;20:6418–6428. doi: 10.1158/1078-0432.CCR-14-1170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.