Abstract
Background
People aging with HIV show variable health trajectories. Our objective was to identify longitudinal predictors of frailty severity and mortality among a group aging with HIV.
Methods
Exploratory analyses employing a multistate transition model, with data from the prospective Modena HIV Metabolic Clinic Cohort Study, based in Northern Italy, begun in 2004. Participants were followed over four years from their first available visit. We included all 963 participants (mean age 46.8±7.1; 29% female; 89% undetectable HIV viral load; median current CD4 count 549, IQR 405–720; nadir CD4 count 180, 81–280) with four-year data. Frailty was quantified using a 31-item frailty index. Outcomes were frailty index score or mortality at four-year follow-up. Candidate predictor variables were baseline frailty index score, demographic (age, sex), HIV-disease related (undetectable HIV viral load, current CD4+ T-cell count, nadir CD4 count, duration of HIV infection, and duration of antiretroviral therapy [ARV] exposure), and behavioral factors (smoking, injection drug use (IDU), and hepatitis C virus co-infection).
Results
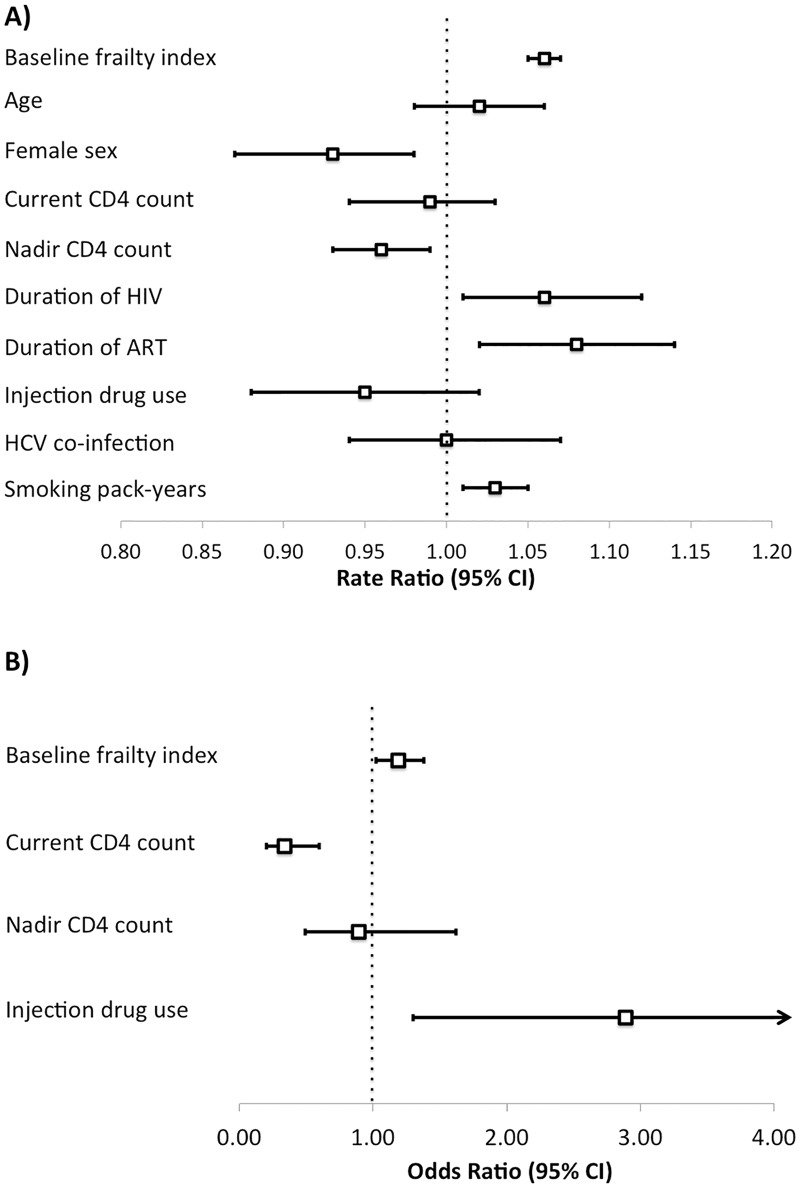
Four-year mortality was 3.0% (n = 29). In multivariable analyses, independent predictors of frailty index at follow-up were baseline frailty index (RR 1.06, 95% CI 1.05–1.07), female sex (RR 0.93, 95% CI 0.87–0.98), nadir CD4 cell count (RR 0.96, 95% CI 0.93–0.99), duration of HIV infection (RR 1.06, 95% CI 1.01–1.12), duration of ARV exposure (RR 1.08, 95% CI 1.02–1.14), and smoking pack-years (1.03, 1.01–1.05). Independent predictors of mortality were baseline frailty index (OR 1.19, 1.02–1.38), current CD4 count (0.34, 0.20–0.60), and IDU (2.89, 1.30–6.42).
Conclusions
Demographic, HIV-disease related, and social and behavioral factors appear to confer risk for changes in frailty severity and mortality among people aging with HIV.
Introduction
In the era of effective antiretroviral therapy, the number of people aging with HIV is growing. A product of this success, aging with HIV presents new challenges. Treated HIV infection is associated with an increased risk of many non-communicable health problems, including cardiovascular disease, osteoporosis, kidney disease, chronic obstructive pulmonary disease, and cognitive and mobility impairment [1–3]. These conditions span different physiological systems but are all strongly associated with advanced age, leading to the controversial idea that the aging process itself might be accelerated among people with HIV [4]. Potential contributing factors have been proposed, including chronic inflammation, long-term antiretroviral drug toxicity, and associated social and behavioral risk factors [2,5,6].
Understanding aging, and factors that might contribute to differences in the aging process, warrants looking beyond individual age-related diseases to the health of the whole person. Though health generally worsens and diseases accumulate with age, these processes are stochastic, inevitably leading to people of the same chronological age exhibiting differences in overall vulnerability [7,8] including some people who will improve over given, shorter-term intervals. People who are more vulnerable than others of the same age are said to be frailer [9,10]. In this way, frailty can be considered a model of biological (as opposed to chronological) aging [11,12]. Some factors associated with frailty at the same time-point have been identified among people aging with HIV, but few studies have assessed potential predictors of longitudinal changes in frailty severity [6,13,14].
Here, we applied a multistate transition model of changes in frailty severity over a fixed time interval, based on the frailty index, among a cohort people aging with HIV. Using longitudinal data over a four-year interval we sought to identify potential predictors of transitions in frailty severity, including demographic, HIV and treatment-related, and social and behavioral factors.
Methods
Setting & sample
This is an exploratory analysis of longitudinal data from the ongoing Modena HIV Metabolic Clinic (MHMC) cohort study, which includes data from patients at the multidisciplinary HIV metabolic clinic at the University of Modena and Reggio Emilia School of Medicine in Modena, Italy. The MHMC cohort was initiated in 2004 to assess metabolic changes among people with HIV aged 18 years and older [15]. Participants undergo an annual study assessment. As this is a clinical study, participants’ data are included in the clinical record, and vital status is updated regularly. Of 2272 enrolled in the longitudinal study, all MHMC participants with four-year follow-up data from their first visit through July, 2014 were included.
Frailty index
Frailty severity was measured using a frailty index, following the deficit accumulation approach [16–20]. A person’s frailty index score is calculated as the proportion of deficits present out of all health variables considered. (For example, if 40 health variables were available in a database, and a person had 8 health deficits present, their frailty index score would be 8/40 = 0.20). Health variables (including signs, symptoms, laboratory abnormalities, or self-reported health measures) can be included in a frailty index if they meet some basic criteria: variables should characterize acquired health deficits that are generally age-associated, and as a group, should number at least around 30 and cover a range of physiologic systems [9,16,20,21]. The frailty index is robust to the items included, so that in different settings it often incorporates different health variables, and does not require the use of the same variables in each index that is constructed. If the above criteria are met, frailty indices exhibit consistent and reliable characteristics regardless of the individual health variables included–showing typical stochastic dynamics that arise from how deficits interact [7,8]. Frailty index values theoretically range from 0 to 1, although mortality consistently approaches 100% at values around 0.7 (i.e. 2/3 of possible deficits) [18,21]. The frailty index approach has been applied among people as young as 15 years old [22], and in multiple clinical populations including among people living with HIV [23]. The frailty index approach has been compared favorably to other approaches for measuring frailty [24,25], including among people living with HIV [26].
In the present study, thirty-one health variables were selected from the MHMC electronic medical record and re-coded so that they represented the presence or absence of a health deficit (Table 1).
Table 1. Health variables included in the frailty index and descriptions of health deficit criteria.
| Variable | Description of health deficit |
|---|---|
| Chronic kidney disease |
|
| NAFLD |
|
| Osteoporosis |
|
| Menopause or male hypogonadism |
|
| High or low body mass index |
|
| High waist circumference |
|
| High visceral adipose tissue (VAT) |
|
| Sarcopenia or presarcopenia |
|
| Unemployment |
|
| Insulin resistance (HOMA) |
|
| High total cholesterol |
|
| High low density lipoprotein |
|
| Low high density lipoprotein |
|
| High triglycerides |
|
| Abnormal leukocyte counts |
|
| Anemia |
|
| Cirrhosis |
|
| Abnormal potassium |
|
| Abnormal phosphorus |
|
| Abnormal thyroid stimulating hormone |
|
| Proteinuria or albuminuria |
|
| Elevated aspartate transaminase (AST) |
|
| Elevated alanine transaminase (ALT) |
|
| Elevated gamma-glutamyl transphosphatase (GGT) |
|
| Thrombocytopenia |
|
| Elevated total bilirubin |
|
| Abnormal parathyroid hormone |
|
| Elevated C-reactive protein |
|
| Vitamin D deficiency |
|
| Lipoatrophy |
|
| Lipohypertrophy |
|
Covariates
We evaluated the effects on transitions in frailty severity of baseline frailty index, demographic, HIV-related, and social/behavioral factors using generalized linear models [27]. Demographic factors were age and sex. HIV-related factors were current CD4+ T-cell count (indicating the degree of immune recovery with antiretroviral [ARV] treatment), nadir CD4 cell count (indicating the severity of immune depletion before initiation of antiretroviral treatment; low nadir CD4 count is associated with late presentation for care). viral load, duration of HIV infection, and duration of ARV exposure. Social/behavioral factors were pack-years smoking history, history of injection drug use, and hepatitis C virus (HCV) co-infection. Age was categorized by decade. Current and nadir CD4 cell counts were categorized into four clinically relevant groups (<100, 101–250, 251–500, and >500 cells/mm3). Viral load was categorized as detectable or undetectable (≤40 copies/mL). Duration of HIV and ARV were categorized by decade. Smoking history was categorized by decades of pack-years.
Transition model
Probabilities of transitions in frailty severity with age can be summarized using a simple stochastic transition model based on the frailty index [21,27–30]. Although health generally worsens with age, the relationship between aging and health is dynamic and periodic improvement and stability in health are common [9,21,31]. Useful models of biological ageing allow for changes in health that include improvement, maintenance, deterioration, and death [32]. Covariate estimation allows quantification of other factors that might modify such transitions [27].
While individual trajectories of health transitions can appear chaotic, they typically behave in an orderly fashion, and depend on the starting health state–here the number of deficits (i.e. frailty index score) that the person has at baseline. Specifically, as detailed previously [28], the chance of going from any health state (frailty index score at baseline) to a future health state (frailty index score at follow-up) depends first on survival. This is estimated as a logistic function among those who died. The ambient risk of death is estimated by the chance of dying for the people with the fewest deficits at baseline, and the chance of dying increases with the more things people have wrong with them (i.e. the higher their frailty index score). For survivors, the probability of the follow-up health state (frailty index score at follow-up) follows a Poisson distribution and therefore the contribution of multiple covariates can be estimated with a Poission regression, based on the generalized linear model [27,28].
Analysis
The primary outcomes were transitions in frailty severity and in mortality at four years. The probability of transitions from any frailty index value (number of health deficits out of 31) at baseline to any other value (or death) were assessed using a multistate transition model, described above [27–29]. Each covariate was first evaluated in univariate models, and covariates significantly associated with the outcomes were added into multivariable models. Significance was set at p<0.05. Analyses were performed using SPSS 21·0 (IBM Corp., Armonk, NY). All relevant data are within the paper and its Supporting Information file (S1 Table).
Ethics committee approval
The research ethics board of the University of Modena and Reggio Emilia provided approval for the MHMC cohort study and all participants provided written consent.
Results
Of the 2722 participants in the MHMC cohort, 963 had available frailty index scores at baseline and either died or were followed for four years and were included in this study. Participants were generally middle-aged, and just under a third were female (Table 2). Participants generally had high current CD4 cell counts (median 549, interquartile range [IQR] 405–720) and undetectable HIV viral load. Median nadir CD4 was 180 (IQR 81–280). Fifty three percent of participants had a lower frailty index score after four years than at first study visit, while 18.5% maintained the same frailty index score and 28.1% worsened. Mortality was 3·0% (n = 29 deaths).
Table 2. Characteristics of study participants.
| Sample size (n) | 963 |
| Age, years (mean ± SD) | 46·8 ± 7·1 |
| Female (%) | 29 |
| Current CD4, cells/mm3 (mean ± SD) | 591 ± 276 |
| Nadir CD4, cells/mm3 (mean ± SD) | 193 ± 150 |
| Undetectable viral load (%) | 89 |
| Duration of HIV infection, years (mean ± SD) | 15.0 ± 5.8 |
| Duration of ART, years (mean ± SD) | 13.0 ± 9.1 |
| People who inject drugs (%) | 27 |
| Hepatitis C virus co-infection (%) | 30 |
| Smoking, pack-years (mean ± SD) | 16.0 ±15.9 |
Baseline frailty index scores strongly influenced transition probabilities and survival. All covariates except for detectable viral load were associated with frailty index scores at follow-up in univariate analyses (Table 3). In multivariable analyses, baseline frailty index (RR 1.06, 95% CI 1.05–1.07), female sex (RR 0.93, 95% CI 0.87–0.98), nadir CD4 cell count (RR 0.96, 95% CI 0.93–0.99), duration of HIV infection (RR 1.06, 95% CI 1.01–1.12), duration of ARV exposure (RR 1.08, 95% CI 1.02–1.14), and smoking pack-years (1.03, 1.01–1.05) were independent predictors of frailty index at follow-up (Fig 1A).
Table 3. Univariate predictors of frailty index scores over four years.
| Predictors | Rate Ratio | 95% CI | p |
|---|---|---|---|
| Baseline frailty index (each deficit) | 1.08 | 1.08–1.09 | <0.001 |
| Age (per 10 years) | 1.12 | 1.08–1.15 | <0.001 |
| Sex (female) | 0·89 | 0·85–0·94 | <0·001 |
| Current CD4 (4 groups) | 0·90 | 0·87–0·93 | <0·001 |
| <100 cells/mm3 | 0.91 | 0.55–1.51 | 0.7 |
| 101–250 cells/mm3 | 1.27 | 1.16–1.40 | <0.001 |
| 251–500 cells/mm3 | 1.11 | 1.06–1.17 | <0.001 |
| >500 cells/mm3 | 1.0 | - | - |
| Nadir CD4 (4 groups) | 0.88 | 0.86–0.91 | <0.001 |
| <100 cells/mm3 | 1.14 | 1.00–1.32 | 0.05 |
| 101–250 cells/mm3 | 1.02 | 0.89–1.17 | 0.8 |
| 251–500 cells/mm3 | 0.86 | 0.75–0.99 | 0.03 |
| >500 cells/mm3 | 1 | - | - |
| Viral load (undetectable) | 1.02 | 0.95–1.09 | 0.7 |
| Duration of HIV infection (per 10 years) | 1.16 | 1.13–1.20 | <0·001 |
| Duration of ARV exposure (per 10 years) | 1·20 | 1·15–1·25 | <0·001 |
| Injection drug use | 1·12 | 1·07–1·17 | <0·001 |
| Hepatitis C virus co-infection | 1·11 | 1·06–1·17 | <0·001 |
| Smoking (per 10 pack-years) | 1·05 | 1·04–1·07 | <0·001 |
CD4: CD4+ T-cell count. ARV: antiretroviral. CI: confidence interval.
For predictors of frailty index scores at follow-up, Poisson distributions were used to generate Rate Ratios.
Fig 1. Demographic, HIV-related, and behavioral factors predict changes in frailty and mortality among people aging with HIV.
Multivariable analysis of factors associated with (A) frailty index score (per deficit) and with (B) mortality at four-year follow-up in the Modena HIV Metabolic Clinic cohort study.
Baseline frailty index, current and nadir CD4 cell count, injection drug use, and HCV co-infection were associated with mortality in univariate analyses (Table 4). In multivariable analyses, independent predictors were baseline frailty index (OR 1.19, 95% CI 1.02–1.38), current CD4 cell count (OR 0.34, 95% CI 0.20–0.60), and injection drug use (2.89, 95% CI 1.30–6.42) (Fig 1B).
Table 4. Univariate predictors of mortality over four years.
| Predictors | Odds Ratio | 95% CI | p |
|---|---|---|---|
| Baseline frailty index (each deficit) | 1.33 | 1.17–1.53 | <0.001 |
| Age (per 10 years) | 1.43 | 0.89–2.31 | 0.1 |
| Sex (female) | 0.63 | 0.25–1.55 | 0.3 |
| Current CD4 (4 groups) | 0.27 | 0.17–0.44 | <0.001 |
| <100 cells/mm3 | 90.17 | 10.84–750.20 | <0.001 |
| 101–250 cells/mm3 | 11.27 | 3.50–36.30 | <0.001 |
| 251–500 cells/mm3 | 4.03 | 1.53–10.60 | 0.005 |
| >500 cells/mm3 | 1.00 | - | - |
| Nadir CD4 (4 groups)* | 0.51 | 0.31–0.85 | 0.01 |
| Viral load (undetectable) | 0.56 | 0.21–1.49 | 0.2 |
| Duration of HIV infection (per 10 years) | 1.30 | 0.74–2.30 | 0.4 |
| Duration of ARV exposure (per 10 years) | 0·86 | 0·42–1·77 | 0·7 |
| Injection drug use | 4·05 | 1·91–8·61 | <0·001 |
| Hepatitis C virus co-infection | 2·78 | 1·27–6·08 | 0·01 |
| Smoking (per 10 pack-years) | 0·67 | 0·35–1·29 | 0·2 |
CD4: CD4+ T-cell count. ARV: antiretroviral. CI: confidence interval.
For predictors of mortality, logistic distributions were used to generate Odds Ratios.
*No deaths occurred in the subgroup with nadir CD4 cell count >500 cells/mm3, and so Odds Ratios for each subgroup were unreliable.
Discussion
We assessed four-year transitions in frailty severity and mortality among Italians aging with HIV using a multistate modeling approach based on a frailty index, and identified predictors of frailty severity and of mortality. Many predictors were important, with baseline frailty index, sex, current and nadir CD4 cell count, duration of HIV infection, duration of HIV exposure, injection drug use, HCV co-infection and smoking history all associated with outcomes after four years. Frailty is a model of biological aging, and our findings suggest that biological aging is a multifactorial process among people living with HIV. Notably, many of these factors are not specific to HIV infection. Over half of the sample improved in frailty status over four years, and protective factors included female sex, higher current and nadir CD4 cell counts, and fewer smoking pack years. Some factors contributing to frailty might be meaningfully modifiable for individuals and for populations, including baseline frailty, immune status, and behavioral factors.
Our findings should be interpreted with caution. The MHMC cohort includes men and women living in northern Italy who are generally middle-aged, and around a quarter of whom inject drugs, and our findings may not be generalizable to other settings or comparable to some previously published research. For example, frailty has previously been assessed among people living with HIV within cohorts of men who have sex with men [33,34] and within cohorts of people who inject drugs [35,36]. Also, our analysis of morality predictors identified fewer and different factors compared to our analysis of frailty predictors, and included wider confidence intervals. This may be due in part to the relatively few deaths over the follow-up period (which is in turn reflective of the greatly increased life expectancy among people living with treated HIV today), and thereby likely reflects a lack of statistical power. Over longer follow-up further predictors of long-term mortality may be identified.
Recent reviews have summarized what is known about frailty and age-related health conditions in people living with HIV [4–6,13,14,37–39]. Most studies use modified versions of the Fried frailty phenotype instrument [13,40,41], which classifies people as “frail”, “pre-frail”, or “robust”/”not frail”. Few previous studies have applied the frailty index approach in an HIV setting [23,26,42]. As it grades degrees of frailty, the frailty index approach is suitable for investigating what can be subtle changes in how health changes as people age, a benefit that we have aimed to exploit here by situating aging with HIV in the context of aging as deficit accumulation. In cross-sectional analyses, frailty has been associated with demographic, biological, and social factors, including older age, immune system dysfunction, duration of ARV, medical comorbidities, lower education, and lower income [13].
Few longitudinal studies have assessed the progression of frailty among people with HIV [6,13,14]. Escota and colleagues assessed frailty status over time in the SUN study using a modified version of Fried frailty phenotype instrument [43]. Over a median follow-up of 12 months, the majority of participants who were “not frail” at baseline remained “not frail” at follow-up, while the majority of participants who were “frail” at baseline transitioned to “not frail”. Participants who remained “prefrail” or “frail” from baseline to follow-up more often had HCV co-infection, lower median hemoglobin, and higher Veterans Aging Cohort Study index scores [43]. Althoff and colleagues evaluated factors associated with transitioning from not frail to frail between study visits among HIV-positive men who have sex with men in the MACS study [33]. Predictors of transitions included less than college education, having a history of AIDS, and having depressive symptoms, diabetes, or kidney disease. Our findings are consistent with this in that we found the progression of frailty to be multifactorial, including having a low nadir CD4 cell count (or history of AIDS) and HCV co-infection.
Other measures of overall age-related health, associated with frailty, have been assessed in longitudinal studies which identify predictors of biological aging. In the ANRS CO3 Aquitaine Cohort, Richert and colleagues found that middle-aged adults with HIV experienced greater-than-expected declines in six-minute walk test time and five times sit-to-stand (5TSTS) test time [44]. Predictors of decline in 5TSTS test time were history of IDU, cerebral complications of HIV disease, and diabetes. Molsberry and colleagues assessed longitudinal trajectories of cognition in men with and without HIV in the MACS study [45]. They identified heterogeneity in cognitive aging, with a history of AIDS, hepatitis C virus infection, depression, and race all associated with premature cognitive aging.
Our finding that duration of HIV disease and duration of ARV exposure were independently associated with worse health transitions conflicts with a recent report finding no association between these factors and multiple age-related diseases [1]. It is also complicated by the notion that earlier start of ARV, often at higher CD4 counts, is associated with better health outcomes. This is an important area for future research, if ongoing ARV exposure could actually accelerate the aging process in some way. This question of the independent contributions of nadir CD4 cell count and duration of ARV is motivating further inquiry by our group.
A growing body of data from general population and non-HIV clinical settings suggests that the rate of aging is influenced by both extrinsic factors, which determine environmental insults, and intrinsic factors, which determine an organism’s ability to repair damage [27,29]. This was confirmed among people with HIV in the present study, where we found that frailty severity, demographic factors, immune status, duration of HIV infection, duration of ARV exposure, and behavioral factors were all predictive of transitions in health status and/or mortality. The fact that health state transitions, measured by changes in frailty index over time, are multifactorial supports previous cross-sectional data [6,13]. Some of these factors are modifiable, suggesting future opportunities for interventions to prevent or delay the progression of frailty. Other factors may help illustrate the pathophysiology of aging and frailty in people living with HIV and aging in general. For example, nadir CD4+ T-cell count is a predictor of health transitions, suggesting that lasting effects on the immune system from CD4 cell depletion may influence the rate of aging. Clinically, early detection of HIV and initiation of ARV may prevent the lasting effects of immune depletion.
As the HIV-positive population continues to age, understanding of the aging process, risk for age-related diseases, geriatric syndromes, and frailty among people with HIV becomes even more important [39]. Higher rates of many different age-related comorbidities have been identified among people aging with HIV compared to general population rates [1–3,5]. The available evidence suggests that the aging process might differ in some ways among people living with HIV, and might be modestly accelerated in general. People aging with HIV represent a highly heterogeneous group in terms of health status and risk factors [39]. Exploration of the present model of biological aging in other datasets is needed, as well as further longitudinal studies of ageing and health among people with HIV. Further research is needed on the intersection between frailty, HIV, and related, acquired age-associated risk states, such as diabetes [46], COPD [47], social isolation [48], and food insecurity [49,50]. We identified factors across multiple domains that were predictive of health transitions, and not all were specific to HIV infection. Trials to test interventions aimed at recognized risk factors are also needed.
Supporting information
This table contains the data used in this analysis.
(XLSX)
Acknowledgments
The authors would like to acknowledge the invaluable contributions of all MHMC study participants.
TDB was supported by a Canadian Institutes of Health Research Summer Research Studentship and an award from the Dalhousie University Internal Medicine Research Foundation. GG was partially supported by 'Co-morbidity in relation to AIDS' grant agreement (305522), European Union Seventh Framework. KR is supported by the Dalhousie Medical Research Foundation through the Kathryn Allen Weldon Chair in Alzheimer Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
TDB was supported by a Canadian Institutes of Health Research Summer Research Studentship and an award from the Dalhousie University Internal Medicine Research Foundation. GG was partially supported by 'Co-morbidity in relation to AIDS' grant agreement (305522), European Union Seventh Framework. KR is supported by the Dalhousie Medical Research Foundation through the Kathryn Allen Weldon Chain in Alzheimer Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rasmussen LD, May MT, Kronborg G, Larsen CS, Pedersen C, Gerstoft J, et al. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: a nationwide population-based cohort study. Lancet HIV. 2015;2: e288–e298. doi: 10.1016/S2352-3018(15)00077-6 [DOI] [PubMed] [Google Scholar]
- 2.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. The Lancet. 2013;382: 1525–1533. doi: 10.1016/S0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53: 1120–1126. doi: 10.1093/cid/cir627 [DOI] [PubMed] [Google Scholar]
- 4.Pathai S, Bajillan H, Landay AL, High KP. Is HIV a model of accelerated or accentuated aging? J Gerontol A Biol Sci Med Sci. 2014;69: 833–842. doi: 10.1093/gerona/glt168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brothers TD, Rockwood K. Biologic aging, frailty, and age-related disease in chronic HIV infection: Curr Opin HIV AIDS. 2014;9: 412–418. doi: 10.1097/COH.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 6.Levett TJ, Cresswell FV, Malik MA, Fisher M, Wright J. Systematic review of prevalence and predictors of frailty in individuals with Human Immunodeficiency Virus. J Am Geriatr Soc. 2016;64: 1006–1014. doi: 10.1111/jgs.14101 [DOI] [PubMed] [Google Scholar]
- 7.Mitnitski AB, Rutenberg AD, Farrell S, Rockwood K. Aging, frailty and complex networks. Biogerontology. 2017; 1–14. [DOI] [PubMed] [Google Scholar]
- 8.Mitnitski A, Bao L, Rockwood K. Going from bad to worse: A stochastic model of transitions in deficit accumulation, in relation to mortality. Mech Ageing Dev. 2006;127: 490–493. doi: 10.1016/j.mad.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 9.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet. 2013;381: 752–762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Mañas L, Fried LP. Frailty in the clinical scenario. The Lancet. 2015;385: e7–e9. doi: 10.1016/S0140-6736(14)61595-6 [DOI] [PubMed] [Google Scholar]
- 11.Mitnitski A, Rockwood K. Biological Age Revisited. J Gerontol A Biol Sci Med Sci. 2014;69A: 295–296. doi: 10.1093/gerona/glt137 [DOI] [PubMed] [Google Scholar]
- 12.Mitnitski A, Howlett SE, Rockwood K. Heterogeneity of human aging and its assessment. J Gerontol A Biol Sci Med Sci. 2017;72: 877–884. doi: 10.1093/gerona/glw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis. 2014;210: 1170–1179. doi: 10.1093/infdis/jiu258 [DOI] [PubMed] [Google Scholar]
- 14.Piggott DA, Erlandson KM, Yarasheski KE. Frailty in HIV: Epidemiology, biology, measurement, interventions, and research needs. Curr HIV/AIDS Rep. 2016;13: 340–348. doi: 10.1007/s11904-016-0334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guaraldi G, Orlando G, Squillace N, De Santis G, Pedone A, Spaggiari A, et al. Multidisciplinary approach to the treatment of metabolic and morphologic alterations of HIV-related lipodystrophy. HIV Clin Trials. 2006;7: 97–106. doi: 10.1310/EYWJ-8B5K-X7VQ-9CPE [DOI] [PubMed] [Google Scholar]
- 16.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001;1: 323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saum K-U, Dieffenbach AK, Müller H, Holleczek B, Hauer K, Brenner H. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol. 2014;29: 171–179. doi: 10.1007/s10654-014-9891-6 [DOI] [PubMed] [Google Scholar]
- 18.Clegg A, Bates C, Young J, Ryan R, Nichols L, Teale EA, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45: 353–60. doi: 10.1093/ageing/afw039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pajewski NM, Williamson JD, Applegate WB, Berlowitz DR, Bolin LP, Chertow GM, et al. Characterizing frailty status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci. 2016;71: 649–655. doi: 10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8: 24 doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27: 17–26. doi: 10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Can Med Assoc J. 2011;183: E487–494. doi: 10.1503/cmaj.101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guaraldi G, Brothers TD, Zona S, Stentarelli C, Carli F, Malagoli A, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS. 2015;29: 1633–1641. doi: 10.1097/QAD.0000000000000753 [DOI] [PubMed] [Google Scholar]
- 24.Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61: 1537–1551. doi: 10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- 25.Theou O, Brothers TD, Peña FG, Mitnitski A, Rockwood K. Identifying common characteristics of frailty across seven scales. J Am Geriatr Soc. 2014;62: 901–906. doi: 10.1111/jgs.12773 [DOI] [PubMed] [Google Scholar]
- 26.Guaraldi G, Malagoli A, Theou O, Brothers TD, Wallace LM, Torelli R, et al. Correlates of frailty phenotype and frailty index with clinical outcomes in people aging with HIV. HIV Med. 2017;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Song X, Mitnitski A, Fang X, Tang Z, Yu P, et al. Effect of health protective factors on health deficit accumulation and mortality risk in older adults in the Beijing Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62: 821–828. doi: 10.1111/jgs.12792 [DOI] [PubMed] [Google Scholar]
- 28.Mitnitski AB, Fallah N, Dean CB, Rockwood K. A multi-state model for the analysis of changes in cognitive scores over a fixed time interval. Stat Methods Med Res. 2014;23: 244–256. doi: 10.1177/0962280211406470 [DOI] [PubMed] [Google Scholar]
- 29.Mitnitski A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology. 2013;14: 709–717. doi: 10.1007/s10522-013-9446-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitnitski A, Rockwood K. Aging as a Process of Deficit Accumulation: Its Utility and Origin. Aging Health–Syst Biol Perspect Interdiscipl Top Gerontol. 2015;50: 85–98. [DOI] [PubMed] [Google Scholar]
- 31.Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166: 418–423. doi: 10.1001/archinte.166.4.418 [DOI] [PubMed] [Google Scholar]
- 32.Steinsaltz D, Mohan G, Kolb M. Markov models of aging: Theory and practice. Exp Gerontol. 2012;47: 792–802. doi: 10.1016/j.exger.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 33.Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci. 2014;69A: 189–198. doi: 10.1093/gerona/glt148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Nilles TL, Johnson JR, Margolick JB. Regulatory T cells, frailty, and immune activation in men who have sex with men in the Multicenter AIDS Cohort Study. J Gerontol A Biol Sci Med Sci. 2015; glv132. doi: 10.1093/gerona/glv132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, et al. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci. 2015;70: 1542–7. doi: 10.1093/gerona/glv107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piggott DA, Muzaale AD, Varadhan R, Mehta SH, Westergaard RP, Brown TT, et al. Frailty and cause-specific hospitalization among persons aging with HIV infection and injection drug use. J Gerontol Ser A. 2017;72: 389–394. doi: 10.1093/gerona/glw142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levett T, Wright J. How to assess and manage frailty in patients with HIV. Sex Transm Infect. 2017; sextrans–2016–052663. doi: 10.1136/sextrans-2016-052663 [DOI] [PubMed] [Google Scholar]
- 38.Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV-infection. Curr HIV/AIDS Rep. 2014;11: 279–290. doi: 10.1007/s11904-014-0215-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sangarlangkarn A, Appelbaum JS. Caring for older adults with the Human Immunodeficiency Virus. J Am Geriatr Soc. 2016; n/a–n/a. doi: 10.1111/jgs.14584 [DOI] [PubMed] [Google Scholar]
- 40.Desquilbet L, Margolick JB, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. Relationship between a frailty-related phenotype and progressive deterioration of the immune system in HIV-infected men. JAIDS J Acquir Immune Defic Syndr. 2009;50: 299–306. doi: 10.1097/QAI.0b013e3181945eb0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. A Frailty-Related Phenotype Before HAART Initiation as an Independent Risk Factor for AIDS or Death After HAART Among HIV-Infected Men. J Gerontol Ser A. 2011;66A: 1030–1038. doi: 10.1093/gerona/glr097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace LMK, Ferrara M, Brothers TD, Garlassi S, Kirkland SA, Theou O, et al. Lower frailty is associated with successful cognitive aging among older adults with HIV. AIDS Res Hum Retroviruses. 2017;33: 157–163. doi: 10.1089/AID.2016.0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Escota GV, Patel P, Brooks JT, Bush T, Conley L, Baker J, et al. The Veterans Aging Cohort Study Index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses. 2015;31: 313–7. doi: 10.1089/AID.2014.0225 [DOI] [PubMed] [Google Scholar]
- 44.Richert L, Brault M, Mercié P, Dauchy F-A, Bruyand M, Greib C, et al. Decline in locomotor functions over time in HIV-infected patients: AIDS. 2014;28: 1441–1449. doi: 10.1097/QAD.0000000000000246 [DOI] [PubMed] [Google Scholar]
- 45.Molsberry SA, Lecci F, Kingsley L, Junker B, Reynolds S, Goodkin K, et al. Mixed membership trajectory models of cognitive impairment in the multicenter AIDS cohort study: AIDS. 2015;29: 713–21. doi: 10.1097/QAD.0000000000000561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cacciatore F, Testa G, Galizia G, Della-Morte D, Mazzella F, Langellotto A, et al. Clinical frailty and long-term mortality in elderly subjects with diabetes. Acta Diabetol. 2013;50: 251–260. doi: 10.1007/s00592-012-0413-2 [DOI] [PubMed] [Google Scholar]
- 47.Galizia G, Cacciatore F, Testa G, Della-Morte D, Mazzella F, Langellotto A, et al. Role of clinical frailty on long-term mortality of elderly subjects with and without chronic obstructive pulmonary disease. Aging Clin Exp Res. 2011;23: 118–125. [DOI] [PubMed] [Google Scholar]
- 48.Mazzella F, Cacciatore F, Galizia G, Della-Morte D, Rossetti M, Abbruzzese R, et al. Social support and long-term mortality in the elderly: Role of comorbidity. Arch Gerontol Geriatr. 2010;51: 323–328. doi: 10.1016/j.archger.2010.01.011 [DOI] [PubMed] [Google Scholar]
- 49.Hessol NA, Zepf R, Zobell E, Weiser SD, John MD. Food insecurity and aging outcomes in older adults living with HIV. AIDS Behav. 2017; 1–9. doi: 10.1007/s10461-017-1838-y [DOI] [PubMed] [Google Scholar]
- 50.Smit E, Wanke C, Dong K, Grotheer A, Hansen S, Skinner S, et al. Frailty, food insecurity, and nutritional status in people living with HIV. J Frailty Aging. 2015;4: 191–197. doi: 10.14283/jfa.2015.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28: 412–419. [DOI] [PubMed] [Google Scholar]
- 52.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43: 1317–1325. doi: 10.1002/hep.21178 [DOI] [PubMed] [Google Scholar]
- 53.Palella FJ, Cole SR, Chmiel JS, Riddler SA, Visscher B, Dobs A, et al. Anthropometrics and examiner-reported body habitus abnormalities in the multicenter AIDS cohort study. Clin Infect Dis. 2004;38: 903–907. doi: 10.1086/381684 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This table contains the data used in this analysis.
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.