Abstract
Fe deficiency may increase Cd accumulation in peanuts. However, the mechanisms are not yet fully understood. In the present study, two contrasting peanut cultivars, Luhua 8 (low seed-Cd cultivar) and Zhenghong 3 (high seed-Cd cultivar) were used to investigate the effect of Fe deficiency on the uptake and accumulation of cadmium (Cd) by hydroponic experiments. Under Fe-sufficient conditions, compared with Luhua 8, Zhenghong 3 had higher specific root length (SRL) and proportion of fine roots with a lower Km for Cd and showed slightly higher expression of AhIRT1 and AhNRAMP1 in the roots. These traits may be responsible for high capacity for Cd accumulation in Zhenghong 3. Under Fe deficiency, the increase of Cd accumulation was much larger in Zhenghong 3 than in Luhua 8. Kinetics studies revealed that the Vmax for Cd influx was 1.56-fold higher in Fe-deficient plants than in Fe-sufficient plants for Zhenghong 3, versus 0.48-fold higher for Luhua 8. Moreover, the increased expression levels of AhIRT1 and AhNRAMP1 induced by Fe deficiency was higher in Zhenghong 3 than in Luhua 8. Yeast complementation assays suggested that the AhIRT1 and AhNRAMP1 may function as transporters involved in Cd uptake. In conclusion, the different Cd accumulation between the two cultivars under Fe deficiency may be correlated with Vmax value for Cd uptake and the expression levels of AhIRT1 and AhNRAMP1 in the roots.
Introduction
Iron (Fe) is an important microelement for plant growth and development. As a redox-active metal, Fe is involved in many physiological processes including photosynthesis, mitochondrial respiration, nitrogen assimilation, hormone biosynthesis, production and scavenging of reactive oxygen species, osmoprotection and pathogen defence [1, 2]. Although the total Fe content in soil regularly exceeds plant requirements, it is present as oxihydrates with low bioavailability [3], particularly in calcareous soils, which represent 30% of the earth's surface [4]. Fe deficiency has become a yield-limiting factor for a variety of field crops all around the world.
Cadmium (Cd) is a highly toxic non-essential metal that is easily taken up by plant roots and transported into the aerial parts [5]. In the root, Cd is taken up by epidermal cells, radially transferred to the inner parts of the root via both apoplastic and symplastic pathway. Cd is loaded from the symplasm into the xylem by Fe transporters such as the iron-regulated transporter [6–8] and natural resistance-associated macrophage protein (NRAMP) [9–11]. Fe deficiency has been demonstrated to induce a high expression of genes of IRT and NRAMP in plant roots, leading to a considerable increase in the uptake and accumulation of Cd [7, 8, 12]. In peanuts, AhIRT1 and AhNRAMP1 have been identified as Fe transporters [13, 14]. The expression level of AhIRT1 and AhNRAMP1 were obviously induced by iron deficiency in the roots. Yeast complementation assays suggested that AhNRAMP1 and AhIRT1 encode functional iron transporter. The tobacco transgenic lines with the induced expression of AhNRAMP1 showed enhanced tolerance to iron deprivation.
Plant roots show a particularly high morphological plasticity in response to Fe deficiency. In the reference plant Arabidopsis (Arabidopsis thaliana), a mild deficiency of Fe increased root elongation; however, severe Fe deficiency caused stunting of roots [15]. Fe deficiency can enhance the formation of root hairs [2, 16], increase root diameter [16], and promote development of lateral roots [2]. Additionally, several studies have illustrated that root morphological characteristics, such as the root lengths, surface areas (SA), specific root lengths (SRL) and number of root tips, and root diameters (RD), significantly relate to the uptake and accumulation of Cd in plants [5, 17–19].
Peanut (Arachis hypogaea L.) is one of the most important oilseed and food crops worldwide. It is grown on nearly 24 million hectares of land areas globally with an annual production of 38 million tons [20]. Extensive studies have shown that peanut has particularly high capacity for accumulating Cd in both the seed and vegetative tissues, and the ability of Cd accumulation varies among cultivars [5, 21–25]. It was also demonstrated that Fe deficiency dramatically increased Cd accumulation in plant tissues of peanuts [25–27]. The accumulation of Cd in peanuts was associated with the root morphological characteristics [5, 19]. However, the mechanisms involved in Fe deficiency-induced increase of Cd accumulation in peanuts are not yet fully understood. Based on the abovementioned results, we hypothesized that Fe deficiency may induce higher expression of IRT and NRAMP and changes of root morphology in peanut as previously reported in other plant species, resulting in an increase of Cd uptake and accumulation in plants. However, to the best of our knowledge, this hypothesis has never been tested experimentally.
The present study aimed to (i) characterize the physiological aspects of Cd uptake in the two most contrasting peanut cultivars identified in our previous work; (ii) evaluate the effects of Fe deficiency on the kinetics of Cd influx, root morphology and the expression of AhIRT1 and AhNRAMP1 in the roots of peanut; and (iii) discriminate the contributions of root morphology and of Fe transporters to the increased Cd uptake induced by Fe deficiency.
Materials and methods
Plant culture
Based on previous studies [28], two peanut cultivars differing in seed Cd accumulation, Luhua 8 (low seed-Cd cultivar) and Zhenghong 3 (high seed-Cd cultivar), were selected for this study. Seeds were sterilized with 1% sodium hypochlorite for 10 min, and then they were rinsed with tap water for 24 h and germinated on well-washed sand. After 5 days, the uniform sized seedlings were selected and transferred to the nutrient solution (pH 5.8) [5]. The nutrient solution was renewed every two weeks. Plants were cultivated in a chamber at a 14-h photoperiod (average irradiance of 600 μmol m-2 s-1), with day/night temperatures 25/20°C, and a relative humidity between 50% and 60%. The pots were randomly arranged daily during the growing period.
Influence of Fe deficiency on plant growth and Cd accumulation
Seedlings were grown for 12 d in basal nutrient solution with (+Fe) or without (–Fe) 50 μM FeEDTA. Each treatment was replicated three times (pots) for each cultivar and the experiment was repeated three times. All treatments contained 0.2 μM CdCl2. The harvested seedlings were divided into roots and shoots. Roots were immersed in 20 mM Na2-EDTA for 15 min to remove metal ions adhering to the root surfaces. All plant parts were oven-dried for 30 min at 105°C, and then dried to a constant weight at 70°C. The concentrations of Cd in the dried samples were determined by flame atomic absorbance spectrometry (AAS) after digested in mixed acid [HNO3 + HClO4 (3:1, v/v)].
The translocation factors (TFs) of Cd from root to shoot and total Cd in the whole plant were calculated as follows:
| (1) |
| (2) |
Influence of Fe deficiency on Cd uptake kinetics
Five-d-old seedlings with uniform sizes were transferred to 250 ml plastic pots (one seedling per pot). Seedlings were grown in full nutrient solution for 2 d. After this period, Fe deficiency was induced in one-half of the plants replacing the full nutrient solution with a nutrient solution without Fe for 12 d. The nutrient solution was then replaced with a pretreatment solution containing 2 mM MES (pH adjusted to 6.0 with KOH) and 0.5 mM CaCl2. After 24 h pretreatment, seedlings were exposed to ten concentrations of CdCl2 (0.2–60 μM) respectively. The uptake solutions also contained 0.5 mM CaCl2 and 2 mM MES (pH 6.0). Each treatment concentration was replicated three times. After 20 min uptake, the seedlings were quickly rinsed with the pretreatment solution, and then transferred to pots containing 100 ml of ice-cold desorption solution (2 mM MES, and 5 mM CaCl2) for 30 min. After desorption, seedlings were separated into roots and shoots. All plant samples were oven-dried for 30 min at 105°C, and then dried to a constant weight at 70°C. The dried root tissues were weighed and digested with mixed acid [HNO3 + HClO4 (3:1, v/v)]. Cd was determined by flame-atomic absorption spectrometry (AAS).
Influence of Fe deficiency on leaf chlorophyll, active Fe content, and root morphology
Seedlings were grown in basal nutrient solution with (+Fe) or without (–Fe) 50 μM FeEDTA for 12 d. Each cultivar and treatment was replicated three times (pots). Mature leaves (0.2 g) from two plants in each pot were extracted in the dark at 4°C in a 5-ml mixture of acetone and ethanol (v/v = 1:1) until the color had disappeared. Light absorbance at 663 and 645 nm was determined by spectrophotometry. Chlorophyll contents (Chla+b) as the sum of chlorophyll a and b contents were calculated according to Lichtenthaler [29].
Active Fe content was determined according to the procedure of Takker and Kaur [30]. Fresh young leaves were cut into pieces and extracted with 1 M HCl (in 1:10 tissue: extractant), shaken for 5 h and filtered, and the Fe concentration in the filtrate was measured with AAS.
Detopped root systems were scanned by using a root automatism scanning apparatus (MIN Mac, STD1600+) as the method described by Lu et al. [5]. The root lengths [17], surface area (SA), root diameters (RD), and root volumes (RV) were measured from the root images using the WinRHIZOTM2000 software (Regent Instruments, QC, Canada). Specific root length (SRL, m g-1) was calculated as the ratio of RL to root dry biomass. Five root diameter classes with an interval width of 0.2 mm were defined to determine the root hierarchical architecture. According to Lu et al. [5], the roots with diameter less than 0.4 mm were defined as the fine roots, and their proportions in root system were calculated on the basis of RL in different diameter classes.
Real-time quantitative PCR
Seedlings were grown in basal nutrient solution with (+Fe) or without (–Fe) 50 μM FeEDTA for 12 d. Each cultivar and treatment was replicated three times (pots). Total RNA was extracted from the roots of Fe-deficient or Fe-sufficient seedlings by using TRIzol reagent (Takara, Japan). First-strand cDNA was synthesized from 1 μg of total RNA, using PrimeScript RT reagent kit with gDNA Eraser (Perfect Real Time) (Takara, Japan). Quantitative real time PCR (qRT-PCR) was performed on ABI 7300 system (Applied Biosystems, USA), using the SYBR Premix Ex Taq kit (Takara, Japan) according to the manufacturer’s protocol. The primers used for qRT-PCR are listed in Table 1. The peanut Actin gene was used as an internal control for normalization of gene expression. Each experiment was replicated three times.
Table 1. The primers used for qRT-PCR and yeast analysis in this study.
| Primer name | Directions | Sequence (5’–3’) |
|---|---|---|
| Ahactin-qPCR | Forward | CTGAAAGATTCCGATGCCCTGA |
| Reverse | AACCACCACTCAAGACAATGTTACCA | |
| AhNRAMP1-qPCR | Forward | TTACTCCCAAACTCAGTGGTCAAG |
| Reverse | GTGGAGGAAGAGGTTGTGCG | |
| AhIRT1-qPCR | Forward | GTTCTCTGCCTTATTCACGCTCAT |
| Reverse | GCCAACACTAACAACAACACCCAT | |
| AhNRAMP1-CDS | Forward | GGGGTACCATGGCAAGCGTTCTTAGACAGC |
| Reverse | CCGCTCGAGTTATTCCGGTAGTGGGATATCAGC | |
| AhIRT1-CDS | Forward | CGAGCTCATGGGTACTAATTCAGAAGTAAAAC |
| Reverse | GCTCTAGATTAATTCCATTTTGCCATGA |
Functional analysis of AhIRT1 and AhNRAMP1 in yeast
The full-length coding regions of AhIRT1 and AhNRAMP1 were amplified by PCR with the primers listed in Table 1 and inserted into the yeast expression vector pYES2 and transformed into the wild-type yeast strain BY4741. The transformed yeasts were selected on a SD medium without uracil (SD-Ura). Positive clones were cultured in SD-Ura liquid media with 2% glucose for growth assays, and 6 μl drops (diluted to an OD600 of 0.5) and three serial 1:10 dilutions were spotted on SD-Ura plates containing 0 or 30 μM CdCl2 in the presence of 2% galactose. The yeast was grown on the plates at 30°C for 3 d for the comparison.
Statistical analysis
Data were analyzed by One-Way ANOVA using IBM SPSS statistics 19.0 (IBM SPSS Inc., Chicago, IL). Duncan’s test was used to determine the significant differences between means (p<0.05). A Michaelis-Menten model combined with a linear component was applied to mathematically resolve the concentration-dependent kinetics of Cd using SigmaPlot 12.0 (Systat Software Inc., Chicago, IL).
Results
Plant growth and leaf chlorophyll content in response to Fe deficiency
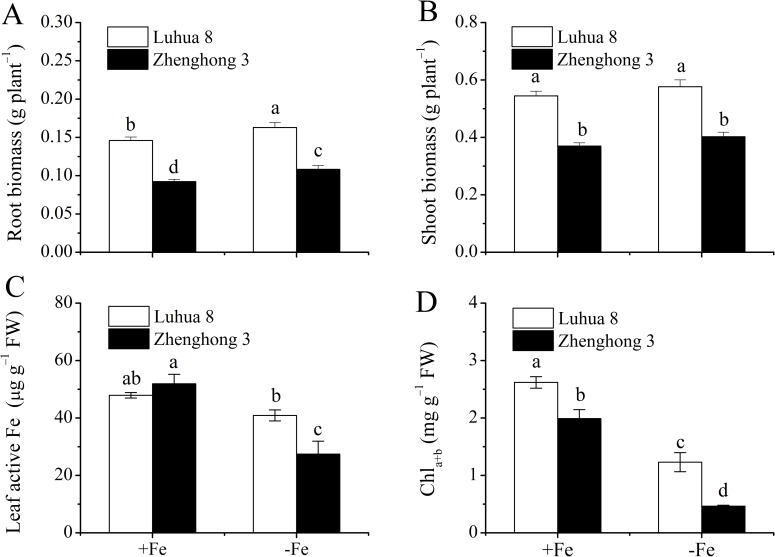
The biomasses of the roots and shoots and leaf chlorophyll content in Luhua 8 were larger than that in Zhenghong 3 under both the Fe-sufficient and -deficient conditions (Fig 1A and 1B). Fe deficiency significantly enhanced the root biomass for both cultivars (Fig 1A), while the shoot biomasses were not affected (Fig 1B).
Fig 1.
The biomasses of roots (a) and shoots (b), leaf active Fe contents (c) and chlorophyll contents (d) of Luhua 8 and Zhenghong 3 exposed to 0.2 μM CdCl2 for 12 d, under Fe-sufficient (+Fe) and -deficient (−Fe) conditions. Different letters above error bars indicate values (mean ± SE, n = 3) are significantly different between treatments at the 0.05 level.
Active Fe contents in leaves of Fe-sufficient plants were similar between the two cultivars (Fig 1C). Fe-deficient treatment decreased the active Fe content in the leaves for both cultivars, and the decrease was more pronounced in Zhenghong 3 (by 47%) than in Luhua 8 (by 15%) (Fig 1C). In the case of the chlorophyll contents (Chla+b), it was consistently higher in Luhua 8 than in Zhenghong 3. Fe-deficient treatment decreased the Chla+b by 53% and 77% for Luhua 8 and Zhenghong 3 respectively (Fig 1D).
Influence of Fe deficiency on Cd accumulations in plants
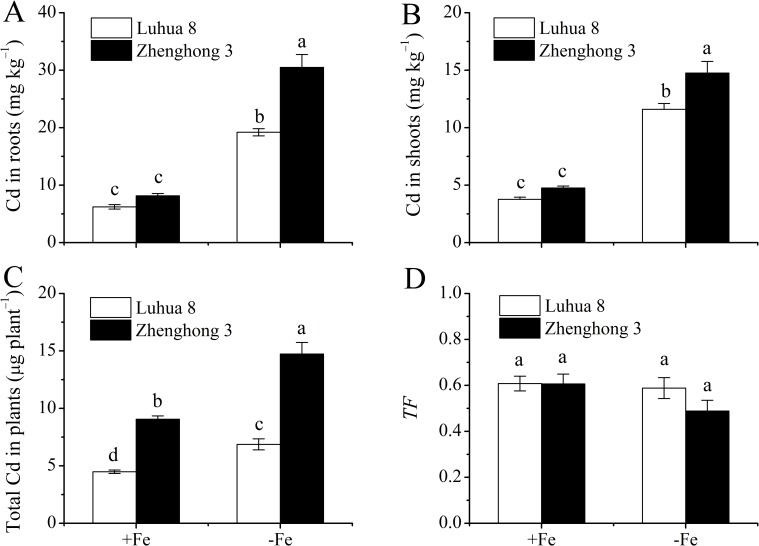
Accumulations of Cd in plants of the two cultivars followed an opposite pattern in comparison with Fe. Although Cd concentrations in the roots (Fig 2A) and shoots (Fig 2B) of Luhua 8, the low-Cd cultivar, were slightly lower than in those of Zhenghong 3, the high-Cd cultivar, the total Cd in plants was one fold higher in Zhenghong 3 than in Luhua 8 under Fe sufficient conditions (Fig 2C). Fe deficiency significantly increased Cd concentrations in the shoots and roots as well as the total Cd in plants of the two cultivars (Fig 2A–2C). The increases of Cd concentration in roots (Fig 2A) and total Cd in plants (Fig 2C) induced by Fe deficiency were higher in Zhenghong 3 (2.74- and 0.63-fold) than those in Luhua 8 (2.08- and 0.53-fold), whereas the increases of Cd concentration in shoots were similar between Luhua 8 (2.07-fold) and Zhenghong 3 (2.10-fold) (Fig 2B). The translocation factors of Cd from roots to shoots (TFs) were not significantly affected by cultivar and Fe treatments (Fig 2D).
Fig 2.
Cd concentrations in roots (a) and shoots (b), total Cd in plants (c) and TFs (d) in Luhua 8 and Zhenghong 3 exposed to 0.2 μM CdCl2 for 12 d, under Fe-sufficient (+Fe) and -deficient (−Fe) conditions. Different letters above error bars indicate values (mean ± SE, n = 3) are significantly different between treatments at the 0.05 level.
Concentration-dependent kinetics of Cd uptake in roots
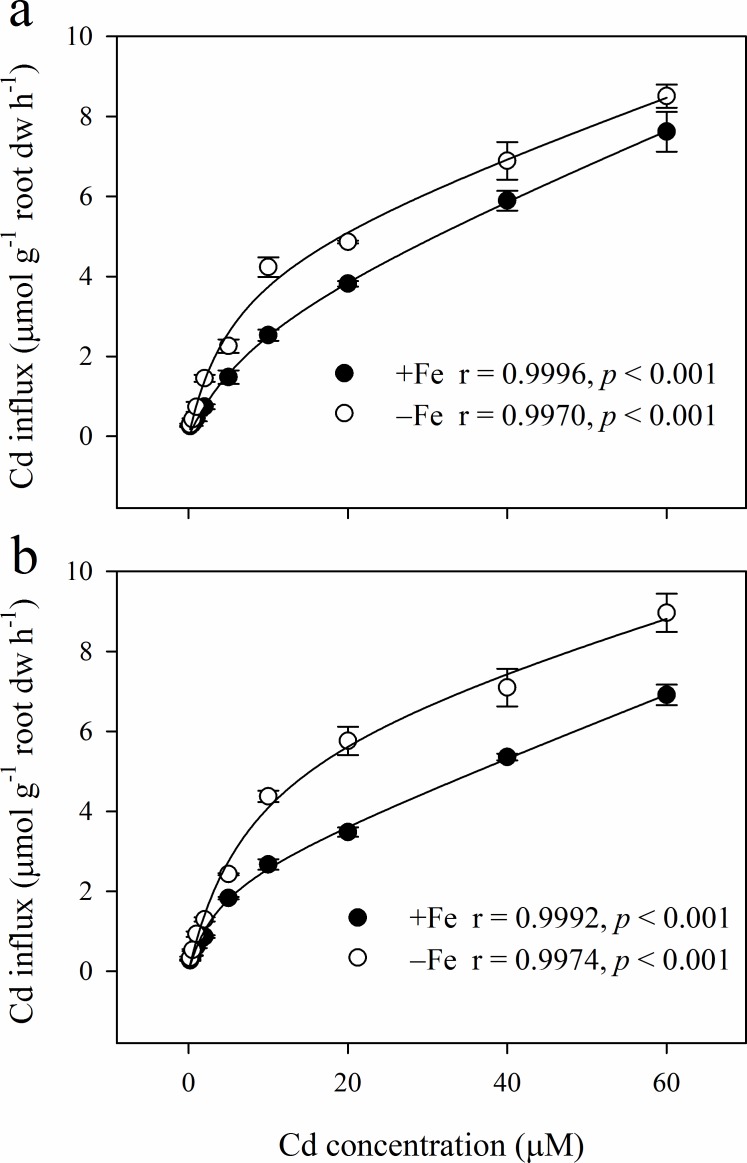
The concentration-dependent kinetics of Cd influx showed a saturable (hyperbolic) component and a linear component for both cultivars (Fig 3A and 3B). In all cases, the model fitted closely the experimental data as demonstrated by R values of between 0.9970 and 0.9996 (Fig 3A and 3B).
Fig 3.
Concentration-dependent kinetics of Cd uptake in roots of Luhua 8 (a) and Zhenghong 3 (b) grown in full nutrient solution (+Fe) or without Fe (−Fe) for 12 d. Data points represent mean values ± SE, n = 3. The lines represent the best fit of the data using a Michaelis-Menten plus linear model.
Plants grown for 12 d in hydroponics without Fe showed obvious symptoms of chlorosis, indicating Fe deficiency; however, no decrease in the biomass of roots or shoots was observed (Fig 1). The Cd influx was similar between the two cultivars in Fe-sufficient conditions (Fig 3A and 3B). Fe deficiency considerably enhanced the rate of Cd influx in both cultivars, showing a cultivar-dependent relationship. A 1.56-fold increase in the maximal Cd influx (Vmax) was observed for Zhenghong 3 when the plants were Fe deficient compared with the treatment where Fe was supplied (Table 2). In the case of Luhua 8, only a 0.48-fold increase in Vmax was induced by Fe deficiency. The Vmax for Cd was similar between the two cultivars when the plants were grown in the presence of Fe. However, Zhenghong 3 showed a larger Vmax than Luhua 8 under the conditions of Fe deficiency. The saturable component of the Cd influx was characterized by similar Km values and significant differences were observed between cultivars and between Fe treatments. Compared with Luhua 8, Zhenghong 3 showed a higher Km under Fe-sufficient conditions. Fe deficiency affected the Km values in a cultivar dependent manner. Fe deficiency considerably enhanced the Km in Zhenghong 3, whereas that in Luhua 8 was decreased. The angular coefficients characterizing the linear component of the Cd influx curves were slightly higher in Luhua 8 than in Zhenghong 3. Fe deficiency slightly but not significantly decreased the angular coefficients for both cultivars (Table 2).
Table 2. Parameters of the Michaelis-Menten plus linear model used to resolve the kinetic of influx curves in Fig 3.
Data represent mean values ± SE.
| Treatments |
Vmax (μmol g−1 root dw h−1) |
Km (μM) |
Angular coefficient (μmol g−1 root dw h−1 μM−1) |
|---|---|---|---|
| Luhua 8 | |||
| +Fe | 3.23±0.48 | 9.01±2.12 | 0.081±0.006 |
| –Fe | 4.78±0.90 | 5.64±2.03 | 0.068±0.014 |
| Zhenghong 3 | |||
| +Fe | 2.44±0.26 | 3.67±0.87 | 0.077±0.005 |
| –Fe | 6.25±1.19 | 7.62±2.46 | 0.055±0.017 |
Expression of AhIRT1 and AhNRAMP1 in response to Fe deficiency
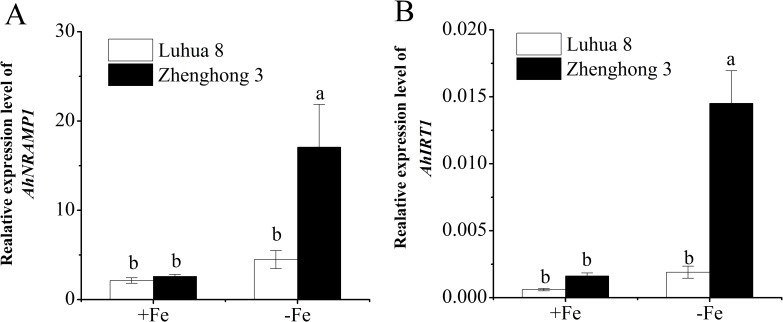
Fig 4 shows the expression of AhIRT1 and AhNRAMP1 in the roots of Luhua 8 and Zhenghong 3 under Fe-sufficient and -deficient conditions. The two cultivars showed a similar expression of AhNRAMP1 in the roots, while the expression of AhIRT1 was slightly higher in Zhenghong 3 than in Luhua 8. Fe deficiency induced expression of AhNRAMP1 and AhIRT1 in the roots by 2.1- and 2.9-fold for Luhua 8, and by 6.6- and 9.0-fold for Zhenghong 3 respectively (Fig 4).
Fig 4.
Expression pattern of AhNRAMP1 (a) and AhIRT1 (b) in the roots of Luhua 8 and Zhenghong 3 grown in full nutrient solution (+Fe) or without Fe (−Fe) for 12 d. The expression levels of AhNRAMP1 and AhIRT1 were normalized to that of Ahactin gene. Different letters above error bars indicate values (mean ± SE, n = 3) are significantly different between treatments at the 0.05 level.
Expression of AhIRT1 and AhNRAMP1 in yeast
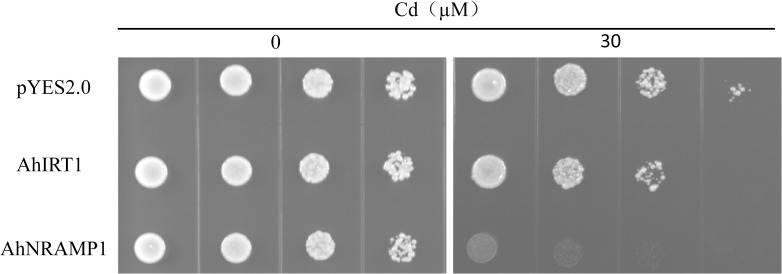
To investigate whether AhIRT1 and AhNRAMP1 transport Cd, we carried out a yeast functional complementation assay using the wild type strain BY4741. In the presence of galactose, the yeast strains expressing AhIRT1 or AhNRAMP1 showed more sensitivity to Cd than the vector control (Fig 5), suggesting that AhIRT1 and AhNRAMP1 may function as transporters involved in Cd uptake.
Fig 5. Growth of wild-type yeast cells transformed with empty vector pYES2, AhIRT1 and AhNRAMP1 in the presence of galactose.
Serial dilutions of the transformed yeast cells with OD600nm 0.5 to 0.0005 were spotted on SD-Ura plates containing 0 or 30 μM CdCl2 in the presence of galactose. The yeast was grown on the plates at 30°C for 3 d for the comparison.
Root morphology of the two peanut cultivars in response to Fe deficiency
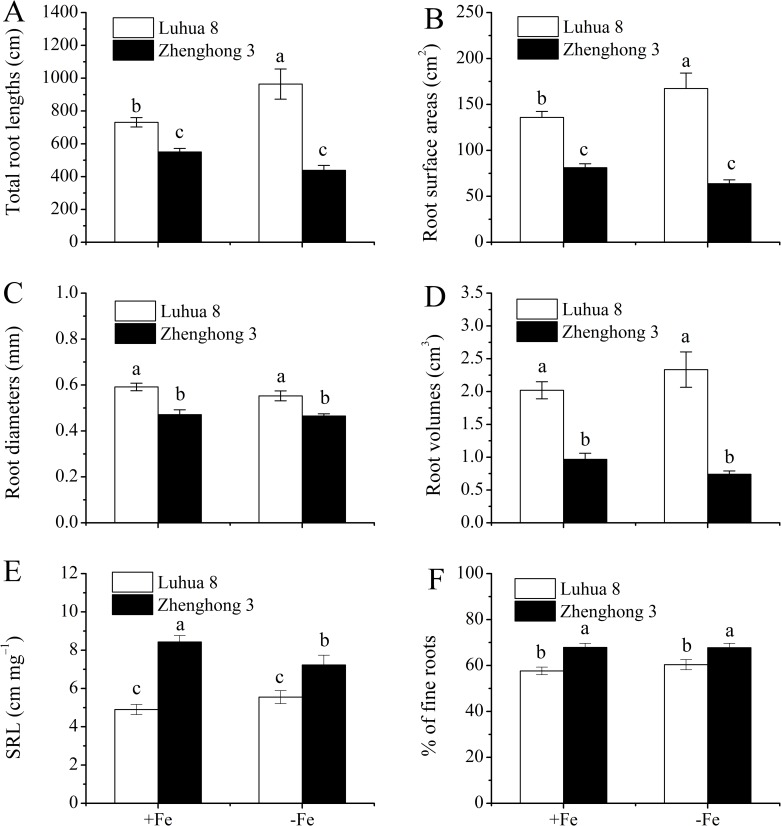
The two cultivars differed in root morphology in terms of RL, SA, RD and RV, and these parameters were generally higher in Luhua 8 than in Zhenghong 3 (Fig 6A–6D). Fe deficiency significantly increased the RL and SA in Luhua 8, while those in Zhenghong 3 remained unaffected (Fig 6A and 6B). The RD and RV in both cultivars were not affected by Fe deficiency.
Fig 6. Root morphological traits of Luhua 8 and Zhenghong 3 grown in full nutrient solution (+Fe) or without Fe (−Fe) for 12 d.
Different letters above error bars indicate values (mean ± SE, n = 3) are significantly different between treatments at the 0.05 level.
The specific root length (SRL) was consistently higher in Zhenghong 3 than in Luhua 8. Fe deficiency caused a slight but not significant increase in the SRL of Luhua 8, while those in Zhenghong 3 significantly decreased (Fig 6E). In the case of the proportion of fine roots (0–0.4 mm diameter classes), it was consistently higher in Zhenghong 3 than in Luhua 8, and was not affected by Fe treatments (Fig 6F).
Discussion
In previous papers, we have found that Luhua 8 and Zhenghong 3 differ from each other in Cd accumulation in both the seeds of mature plants and the shoots of seedlings [5, 25, 27, 28]. Differential responses in Cd accumulation to Fe deficiency were also observed between the two cultivars [25, 27]. The present results showed that, although the two cultivars were similar in the responses of plant growth to Fe deficiency (Fig 1A and 1B), the decreases in the active Fe content and chlorophyll contents in the leaves as a consequence of Fe deficiency were larger in Zhenghong 3 than in Luhua 8 (Fig 1C and 1D), indicating Zhenghong 3 is more sensitive to Fe deficiency compared with Luhua 8.
The results of the long-term accumulation experiment indicated that Zhenghong 3, the high seed-Cd cultivar, shows a high capacity for Cd uptake and accumulation in plants than Luhua 8, the low seed-Cd cultivar (Fig 2A–2C). The observation was in agreement with our previous results [5, 25, 27, 28]. Fe deficiency greatly enhanced Cd accumulation in plants for both cultivars (Fig 2A–2C), while the TFs were not affected (Fig 2D). The increases of Cd concentration in the roots and shoots were larger in Zhenghong 3 than in Luhua 8. The results indicate that increased Cd uptake by roots induced by Fe deficiency may account for higher Cd accumulation observed in Zhenghong 3. The increases in Cd accumulation as a consequence of Fe deficiency have been reported by other authors in various plants including peanut [6, 12, 25, 27, 31, 32].
The curves of the concentration-dependent kinetics of Cd influx were characterized by a saturable and a linear component in both cultivars (Fig 3). Similar results have been reported in various plants including wheat (Triticum aestivum) [33, 34], alpine pennycress (Thlaspi caerulescens) [35–37], maize [36, 38], pea (Pisum sativum) [6], Arabidopsis halleri [39] and Sedum alfredii [40]. According to previous studies [6, 31], the saturable component is generally considered as the symplastic absorption of Cd across the plasma membrane. Although the two cultivars showed a similar Vmax of Cd influx under Fe-sufficient conditions, the Km values were much lower in Zhenghong 3 than in Luhua 8 (Table 2). These results suggest that the higher Cd accumulation in Zhenghong 3 is a direct consequence of a higher affinity for Cd influx.
The two cultivars exhibited different responses of Vmax and Km values of Cd influx to Fe deficiency. In Zhenghong 3, Fe deficiency markedly increased both the Vmax and Km values of Cd influx. In Luhua 8, however, Fe deficiency slightly increased the Vmax but greatly decreased the Km (Table 2). These results indicate that a high Vmax is probably more important than a low Km for the increase of Cd influx induced by Fe deficiency in Zhenghong 3, while in Luhua 8, a higher affinity (lower Km) may be involved. The increase of the Vmax for Cd influx induced by Fe deficiency in peanut seedlings were 0.48- and 1.56-fold for Luhua 8 and Zhenghong 3 respectively, the values were lower than that in pea (6.94-fold) [6] and T. caerulescens (9-fold) [31].
The angular coefficients characterizing the linear component of the influx curves that is generally considered to reflect Cd that remain bound to cell walls after desorption [6, 34, 37, 38, 40, 41]. In the present study, we found that the angular coefficients were similar between the two cultivars and shows a slight but nonsignificant reduction in response to Fe deficiency in both cultivars (Table 2). The results, consistent with the previous findings [6, 31], indicate that Fe deficiency had relatively little effect on the adsorption of Cd in the cell walls.
The root system is the main organ through which crops absorb water and mineral elements. Previous studies have demonstrated that Fe deficiency can alter the root morphological characteristics [2, 16], and the alterations were also proven to closely relate to the uptake and accumulation of Cd in plants [5, 17–19]. To examine the hypothesis that the changes in root morphology induced by Fe deficiency may be related to Cd uptake and accumulation in plants, root morphological responses to Fe deficiency were evaluated. The results obtained from the present study do not support the abovementioned hypothesis. For instance, although Fe deficiency caused greater increases in the Vmax for Cd influx (Table 2) and Cd accumulation in the roots and shoots (Fig 2) in Zhenghong 3, the root morphological characteristics of this cultivar remained unaffected (Fig 6).
Additionally, we found that, although Luhua 8 shows higher RL, SA, RD and RV than Zhenghong 3 under Fe-sufficient conditions, the SRL and proportion of fine roots were greatly higher in Zhenghong 3 than in Luhua 8 (Fig 6E and 6F). The SRL represents the trade-offs between producing longer and thinner roots for resource acquisition (benefit) and partitioning more biomass for construction and maintenance (cost) [42], and it was shown to positively correlate with the uptake and accumulation of Cd in peanuts [5]. The fine roots have been demonstrated to play an important role in Cd uptake and translocation [5, 43–45]. Therefore, higher Cd accumulation in Fe-sufficient plants of Zhenghong 3 may, at least partially, result from their higher SRL and proportion of fine roots.
The molecular study provides a possible explanation to the physiological data presented. Compared with Luhua 8, Zhenghong 3 showed a slightly higher expression of AhIRT1 and AhNRAMP1 in the roots (Fig 4). The ability of IRT [6–8, 12, 31] and NRAMP [9–11] to transport Cd has been well established. In the present study, the heterologous assay in yeast also indicated that AhIRT1 and AhNRAMP1 may function as transporters of Cd in peanut (Fig 5). Thus, the initial expression of AhIRT1 and AhNRAMP1 in the roots may be responsible for high capacity for Cd uptake and accumulation in Zhenghong 3.
Fe deficiency increased the transcript abundance of AhIRT1 and AhNRAMP1 in the roots for both cultivars (Fig 4). Similar results have been reported in several plants [6, 8, 10, 12, 31, 46]. Induction of AhIRT1 and AhNRAMP1 by Fe deficiency was greater in Zhenghong 3 than in Luhua 8 (Fig 4). The findings correspond to the greatly increased Vmax for Cd influx (Table 2) and Cd accumulation in roots and shoots (Fig 2). The lower induction of AhIRT1 and AhNRAMP1 is also consistent with the small increase in the Vmax for Cd influx in Luhua 8. These results suggested that the higher expression of AhIRT1 and AhNRAMP1 may be involved in Cd uptake and accumulation in the two peanut cultivars in response to Fe deficiency.
Conclusions
This study has clearly established that, compared with Luhua 8, Zhenghong 3 shows lower Km, higher SRL and proportion of fine roots, and slightly higher expression of AhIRT1 and AhNRAMP1 in the roots. These traits may be responsible for high capacity for Cd accumulation in Zhenghong 3. Fe deficiency induces considerable increase in the uptake and accumulation of Cd in plants for both cultivars. The increase of Cd accumulation as a consequence of Fe deficiency was greatly larger in Zhenghong 3 than in Luhua 8, in which a greater increase of Vmax for Cd influx and higher expression of AhIRT1 and AhNRAMP1 are involved.
Supporting information
(OPJ)
(OPJ)
(JNB)
(OPJ)
(OPJ)
Acknowledgments
We thank Dr. Ronghua Xu (Anhui Science and Technology University) for kindly providing the vector pYES2 and the yeast strain BY4741. Financial support from the National Natural Science Foundation of China (No. 31671599, 31370515) is gratefully acknowledged.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Financial support from the National Natural Science Foundation of China (No. 31671599, 31370515) is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hansch R, Mendel RR. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr Opin Plant Biol. 2009;12(3):259–266. doi: 10.1016/j.pbi.2009.05.006 . [DOI] [PubMed] [Google Scholar]
- 2.Zamboni A, Zanin L, Tomasi N, Pezzotti M, Pinton R, Varanini Z, et al. Genome-wide microarray analysis of tomato roots showed defined responses to iron deficiency. BMC Genomics. 2012;13(1):101 doi: 10.1186/1471-2164-13-101 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiol. 1994;104(3):815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imsande J. Iron, sulfur, and chlorophyll deficiencies: a need for an integrative approach in plant physiology. Physiol Plant. 1998;103(1):139–144. doi: 10.1034/j.1399-3054.1998.1030117.x [Google Scholar]
- 5.Lu Z, Zhang Z, Su Y, Liu C, Shi G. Cultivar variation in morphological response of peanut roots to cadmium stress and its relation to cadmium accumulation. Ecotoxicol Environ Saf. 2013;91:147–155. doi: 10.1016/j.ecoenv.2013.01.017 . [DOI] [PubMed] [Google Scholar]
- 6.Cohen CK, Fox TC, Garvin DF, Kochian LV. The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol. 1998;116(3):1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connolly EL, Fett JP, Guerinot ML. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell. 2002;14(6):1347–1357. doi: 10.1105/tpc.001263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr. 2006;52(4):464–469. doi: 10.1111/j.1747-0765.2006.00055.x [Google Scholar]
- 9.Ishimaru Y, Takahashi R, Bashir K, Shimo H, Senoura T, Sugimoto K, et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep. 2012;2:286 doi: 10.1038/srep00286 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi R, Ishimaru Y, Nakanishi H, Nishizawa NK. Role of the iron transporter OsNRAMP1 in cadmium uptake and accumulation in rice. Plant Signal Behav. 2011;6(11):1813–1816. doi: 10.4161/psb.6.11.17587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI. Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. PNAS. 2000;97(9):4991–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astolfi S, Zuchi S, Neumann G, Cesco S, Sanita di Toppi L, Pinton R. Response of barley plants to Fe deficiency and Cd contamination as affected by S starvation. J Exp Bot. 2012;63(3):1241–1250. doi: 10.1093/jxb/err344 . [DOI] [PubMed] [Google Scholar]
- 13.Ding H, Duan L, Li J, Yan H, Zhao M, Zhang F, et al. Cloning and functional analysis of the peanut iron transporter AhIRT1 during iron deficiency stress and intercropping with maize. J Plant Physiol. 2010;167(12):996–1002. doi: 10.1016/j.jplph.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 14.Xiong H, Kobayashi T, Kakei Y, Senoura T, Nakazono M, Takahashi H, et al. AhNRAMP1 iron transporter is involved in iron acquisition in peanut. J Exp Bot. 2012;63(12):4437–4446. doi: 10.1093/jxb/ers117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber BD, Giehl RF, Friedel S, von Wirén N. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013;163(1):161–179. doi: 10.1104/pp.113.218453 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Römheld V, Marschner H. Iron deficiency stress induced morphological and physiological changes in root tips of sunflower. Physiol Plant. 1981;53(3):354–360. [Google Scholar]
- 17.Berkelaar E, Hale B. The relationship between root morphology and cadmium accumulation in seedlings of two durum wheat cultivars. Can J Bot. 2000;78(3):381–387. doi: 10.1139/cjb-78-3-381 [Google Scholar]
- 18.Li T, Yang X, Jin X, He Z, Stoffella P, Hu Q. Root responses and metal accumulation in two contrasting ecotypes of Sedum alfredii Hance under lead and zinc toxic stress. J Environ Sci Health, Pt A. 2005;40(5):1081–1096. doi: 10.1081/ESE-200056163 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Liu C, Wang X, Shi G. Cadmium-induced alterations in morpho-physiology of two peanut cultivars differing in cadmium accumulation. Acta Physiol Plant. 2013;35(7):2105–2112. doi: 10.1007/s11738-013-1247-4 [Google Scholar]
- 20.Kumari V, Gowda M, Tasiwal V, Pandey MK, Bhat RS, Mallikarjuna N, et al. Diversification of primary gene pool through introgression of resistance to foliar diseases from synthetic amphidiploids to cultivated groundnut (Arachis hypogaea L.). Crop J. 2014;2(2):110–119. doi: 10.1016/j.cj.2014.03.002 [Google Scholar]
- 21.Bell M, McLaughlin MJ, Wright G, Cruickshank A. Inter-and intra-specific variation in accumulation of cadmium by peanut, soybean, and navybean. Aust J Agric Res. 1997;48:1151–1160. [Google Scholar]
- 22.McLaughlin MJ, Bell M, Wright G, Cozens G. Uptake and partitioning of cadmium by cultivars of peanut (Arachis hypogaea L.). Plant Soil. 2000;222(1–2):51–58. doi: 10.1023/A:1004771712840 [Google Scholar]
- 23.Su G, Li F, Lin J, Liu C, Shi G. Peanut as a potential crop for bioenergy production via Cd-phytoextraction: a life-cycle pot experiment. Plant Soil. 2013;365(1–2):337–345. doi: 10.1007/s11104-012-1394-1 [Google Scholar]
- 24.Wang S, Li G. Assessment of cadmium bioaccumulation and distribution in the kernels of peanut widely cultivated in China. Ecotoxicol Environ Saf. 2014;108:23–28. doi: 10.1016/j.ecoenv.2014.06.029 . [DOI] [PubMed] [Google Scholar]
- 25.Su Y, Wang X, Liu C, Shi G. Variation in cadmium accumulation and translocation among peanut cultivars as affected by iron deficiency. Plant Soil. 2013;363(1–2):201–213. doi: 10.1007/s11104-012-1310-8 [Google Scholar]
- 26.Shi G, Sun L, Wang X, Liu C. Leaf responses to iron nutrition and low cadmium in peanut: anatomical properties in relation to gas exchange. Plant Soil. 2014;375(1–2):99–111. doi: 10.1007/s11104-013-1953-0 [Google Scholar]
- 27.Su Y, Liu J, Lu Z, Wang X, Zhang Z, Shi G. Effects of iron deficiency on subcellular distribution and chemical forms of cadmium in peanut roots in relation to its translocation. Environ Exp Bot. 2014;97:40–48. doi: 10.1016/j.envexpbot.2013.10.001 [Google Scholar]
- 28.Shi G, Su G, Lu Z, Liu C, Wang X. Relationship between biomass, seed components and seed Cd concentration in various peanut (Arachis hypogaea L.) cultivars grown on Cd-contaminated soils. Ecotoxicol Environ Saf. 2014;110:174–181. doi: 10.1016/j.ecoenv.2014.09.003 . [DOI] [PubMed] [Google Scholar]
- 29.Lichtenthaler H. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1 [Google Scholar]
- 30.Takkar P, Kaur N. HCl method for Fe2+ estimation to resolve iron chlorosis in plants. J Plant Nutr. 1984;7(1–5):81–90. [Google Scholar]
- 31.Lombi E, Tearall KL, Howarth JR, Zhao FJ, Hawkesford MJ, McGrath SP. Influence of iron status on cadmium and zinc uptake by different ecotypes of the hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2002;128(4):1359–1367. doi: 10.1104/pp.010731 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siedlecka A, Krupa Z. Interaction between cadmium and iron and its effects on photosynthetic capacity of primary leaves of Phaseolus vulgaris. Plant Physiol Biochem. 1996;34(6):833–841. [Google Scholar]
- 33.Harris NS, Taylor GJ. Cadmium uptake and translocation in seedlings of near isogenic lines of durum wheat that differ in grain cadmium accumulation. BMC Plant Biol. 2004;4(1):4 doi: 10.1186/1471-2229-4-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart JJ, Welch RM, Norvell WA, Sullivan LA, Kochian LV. Characterization of cadmium binding, uptake, and translocation in intact seedlings of bread and durum wheat cultivars. Plant Physiol. 1998;116(4):1413–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lombi E, Zhao F, McGrath S, Young S, Sacchi G. Physiological evidence for a high-affinity cadmium transporter highly expressed in a Thlaspi caerulescens ecotype. New Phytol. 2001;149(1):53–60. doi: 10.1046/j.1469-8137.2001.00003.x [DOI] [PubMed] [Google Scholar]
- 36.Redjala T, Sterckeman T, Morel JL. Cadmium uptake by roots: contribution of apoplast and of high-and low-affinity membrane transport systems. Environ Exp Bot. 2009;67(1):235–242. doi: 10.1016/j.envexpbot.2009.05.012 [Google Scholar]
- 37.Zhao FJ, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP. Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J Exp Bot. 2002;53(368):535–543. doi: 10.1093/jexbot/53.368.535 . [DOI] [PubMed] [Google Scholar]
- 38.Han F, Shan X, Zhang S, Wen B, Owens G. Enhanced cadmium accumulation in maize roots-the impact of organic acids. Plant Soil. 2006;289(1–2):355–368. doi: 10.1007/s11104-006-9145-9 [Google Scholar]
- 39.Zhao F, Jiang R, Dunham S, McGrath S. Cadmium uptake, translocation and tolerance in the hyperaccumulator Arabidopsis halleri. New Phytol. 2006;172(4):646–654. doi: 10.1111/j.1469-8137.2006.01867.x . [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Tian S, Yang X, Wang X, Brown P, Li T, et al. Enhanced root-to-shoot translocation of cadmium in the hyperaccumulating ecotype of Sedum alfredii. J Exp Bot. 2008;59(11):3203–3213. doi: 10.1093/jxb/ern174 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He J, Zhu C, Ren Y, Jiang D, Sun Z. Root morphology and cadmium uptake kinetics of the cadmium-sensitive rice mutant. Biol Plant. 2007;51(4):791–794. doi: 10.1007/s10535-007-0162-1 [Google Scholar]
- 42.Eissenstat D, Yanai R. The ecology of root lifespan. Adv Ecol Res. 1997;27(1):60 doi: 10.1016/S0065-2504(08)60005-7 [Google Scholar]
- 43.Li T, Di Z, Han X, Yang X. Elevated CO2 improves root growth and cadmium accumulation in the hyperaccumulator Sedum alfredii. Plant Soil. 2012;354(1–2):325–334. doi: 10.1007/s11104-011-1068-4 [Google Scholar]
- 44.Shi G, Xia S, Ye J, Huang Y, Liu C, Zhang Z. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environ Exp Bot. 2015;111:127–134. doi: 10.1016/j.envexpbot.2014.11.008 [Google Scholar]
- 45.Wang R, Dai S, Tang S, Tian S, Song Z, Deng X, et al. Growth, gas exchange, root morphology and cadmium uptake responses of poplars and willows grown on cadmium-contaminated soil to elevated CO2. Environ Earth Sci. 2012;67(1):1–13. doi: 10.1007/s12665-011-1475-0 [Google Scholar]
- 46.Curie C, Alonso JM, Marie L, Ecker JR, Briat J-F. Involvement of Nramp1 from Arabidopsis thaliana in iron transport. Biochem J. 2000;347(3):749–755. doi: 10.1042/bj3470749 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(OPJ)
(OPJ)
(JNB)
(OPJ)
(OPJ)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.