Abstract
T cells expressing anti-CD19 chimeric antigen receptors (CARs) can induce complete remissions (CRs) of diffuse large B cell lymphoma (DLBCL). The long-term durability of these remissions is unknown. We administered anti-CD19 CAR T cells preceded by cyclophosphamide and fludarabine conditioning chemotherapy to patients with relapsed DLBCL. Five of the seven evaluable patients obtained CRs. Four of the five CRs had long-term durability with durations of remission of 56, 51, 44, and 38 months; to date, none of these four cases of lymphomas have relapsed. Importantly, CRs continued after recovery of non-malignant polyclonal B cells in three of four patients with long-term complete remissions. In these three patients, recovery of CD19+ polyclonal B cells took place 28, 38, and 28 months prior to the last follow-up, and each of these three patients remained in CR at the last follow-up. Non-malignant CD19+ B cell recovery with continuing CRs demonstrated that remissions of DLBCL can continue after the disappearance of functionally effective anti-CD19 CAR T cell populations. Patients had a low incidence of severe infections despite long periods of B cell depletion and hypogammaglobulinemia. Only one hospitalization for an infection occurred among the four patients with long-term CRs. Anti-CD19 CAR T cells caused long-term remissions of chemotherapy-refractory DLBCL without substantial chronic toxicities.
Keywords: chimeric antigen receptors, lymphoma, adoptive T cell therapy
Five of seven patients receiving anti-CD19 chimeric antigen receptor (CAR) T cells obtained complete remissions. Four of the five CRs had long-term durability with durations of remissions ranging from 38 to 56 months. Remissions persisted despite recovery of normal B cells in three of the four patients with long-term remissions.
Introduction
Chimeric antigen receptors (CARs) are fusion proteins that have antigen recognition domains and T cell signaling domains.1, 2, 3 CAR-expressing T cells can specifically recognize malignancy-associated antigens and destroy cells expressing a targeted antigen.2, 4, 5, 6, 7 Anti-CD19 CAR T cells can induce remissions of B cell lymphoma,8, 9, 10, 11, 12, 13, 14 but the long-term durability of these remissions remains a critical unanswered question.
Diffuse large B cell lymphoma (DLBCL) is the most common type of lymphoma and can be divided into numerous subtypes.15 Relapsed DLBCL carries a grim prognosis.16, 17 Patients with DLBCL not entering at least a partial remission (PR) after second-line chemotherapy had a median overall survival of 4 months.18 The median overall survival of patients with DLBCL that progressed after autologous hematopoietic stem cell transplantation (HSCT) was less than 10 months.19, 20 When newly diagnosed DLBCL relapsed from complete remission (CR) in a large study of standard therapies, 87% of relapses occurred within 3 years of the end of therapy, which emphasized that late relapses of DLBCL are much rarer than early relapses.16
After anti-CD19 CAR T cell therapy, normal B cells are often depleted for varying lengths of time.8, 21, 22, 23, 24 Patients with B cell depletion from long-term anti-CD20 monoclonal antibody therapy have a modestly increased risk of infections.25 B cell depletion after anti-CD19 CAR T cell infusions could also increase the risk of infections, so durability of lymphoma remissions after recovery of normal B cells is preferable.
The results reported here show that anti-CD19 CAR T cells can induce long-term remissions of DLBCL that continue after recovery of normal B cells.
Results
Long-Term CRs of Relapsed DLBCL after Anti-CD19 CAR T Cell Therapy
This report covers seven patients with subtypes of DLBCL treated in a completed clinical trial cohort.10 All patients with lymphoma evaluable for response are included. Our previous report of these same patients covered toxicities and short-term lymphoma responses.10 We are now reporting long-term response durability, long-term CAR T cell persistence, and long-term B cell recovery. All patients underwent extensive lymphoma therapy prior to protocol enrollment (Table 1). Of the seven patients, five entered CR after CAR T cell infusion. Of the five CRs, four were durable, with durations of response ranging from 38 to 56 months (Figures 1A and 1B; Table 1). None of the patients with long-term CRs received any lymphoma therapy during the follow-up period after CAR T cell infusion.
Table 1.
Patient Characteristics and Responses
| Patient Number | Age (Years) | Sex | Lymphoma Type | Prior Lymphoma Therapy before CAR T Cell Protocol Enrollment | Chemotherapy Conditioning Regimenc |
CAR T Cell Dose (CAR+ T Cells/kg)d | Best Response, Overall Duration (Months)e | |
|---|---|---|---|---|---|---|---|---|
| Cy (mg/kg) | Flu (mg/m2) | |||||||
| 2 | 43 | F | PMBCLa | R-CHOP, R-ICE, methotrexate + Ara-C, X-ray therapy | 60 | 125 | 5 × 106 | CR, 56f |
| 7 | 42 | M | DLBCL NOSa | R-CHOP, R-ICE + lenalidomide, phase I clinical trial, X-ray therapy, rituximab + cytarabine + etoposide | 60 | 75 | 2.5 × 106 | CR, 51f |
| 8 | 44 | F | PMBCLa | R-CHOP, R-ICE, brentuximab, R-high-dose Ara-C, lenalidomide + rituximab, R-GDP, X-ray therapy, three different phase I clinical trials | 60 | 125 | 2.5 × 106 | CR, 38g |
| 9 | 38 | M | PMBCLa | R-EPOCH, R-ICE, gemcitabine + oxaliplatin + rituximab | 120 | 125 | 2.5 × 106 | SD, 1 |
| 11 | 58 | F | DLBCLa from CLL (Richter’s transformation) | fludarabine, fludarabine + R, bendamustine + R (two different times), hyper-CVAD (two different times), R-ICE, X-ray therapy, R + methylprednisolone, R + cisplatin + gemcitabine + dexamethasone, methotrexate + Ara-C, R + Ara-C | 60 | 125 | 1 × 106 | PR, 1 |
| 14 | 43 | M | DLBCL NOSa | R-CHOP, R-ESHAP | 60 | 125 | 1 × 106 | CR, 6 |
| 15 | 64 | F | DLBCL NOSb | R-CHOP, R-ICE, high-dose chemotherapy followed by autologous stem cell transplantation | 60 | 125 | 1 × 106 | CR, 42f |
Ara-C, cytarabine; CLL, chronic lymphocytic leukemia; CVAD, cyclophosphamide, vincristine, doxorubicin, dexamethasone; Cy, cyclophosphamide; DLBCL, diffuse large B cell lymphoma; F, female; Flu, fludarabine; M, male; NOS, not otherwise specified; PMBCL, primary mediastinal B cell lymphoma; PR, partial response; R, rituximab; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-EPOCH, rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; R-ESHAP, rituximab, etoposide, methylprednisolone, high-dose Ara-C, cisplatin R-GDP, rituximab, gemcitabine, dexamethasone, cisplatin; R-ICE, rituximab, ifosfamide, carboplatin, and etoposide; SD, stable disease.
Lymphoma was chemotherapy refractory. Chemotherapy refractory was defined as failure of the lymphoma to be in partial remission or complete remission 1 month after the end of the regimen.
Lymphoma relapsed after autologous stem cell transplantation.
Cy was administered on days −7 and −6. Flu was administered on days −5 to −1, except it was administered on days −5 to −3 for patient 7. Doses listed are the total doses of each agent given to each patient, not the daily doses.
CAR T cells were infused on day 0.
The best response obtained is given. For all patients with ongoing CRs, the best response and current responses are both CR. Duration of response is defined as the time that response was first documented, which was usually 1 month after infusion, until the last follow-up or progression of lymphoma.
Ongoing response.
Patient 8 became not evaluable for lymphoma response 39 months after CAR T cell infusion because she developed myelodysplastic syndrome and underwent allogeneic hematopoietic stem cell transplantation while her lymphoma remained in CR.
Figure 1.
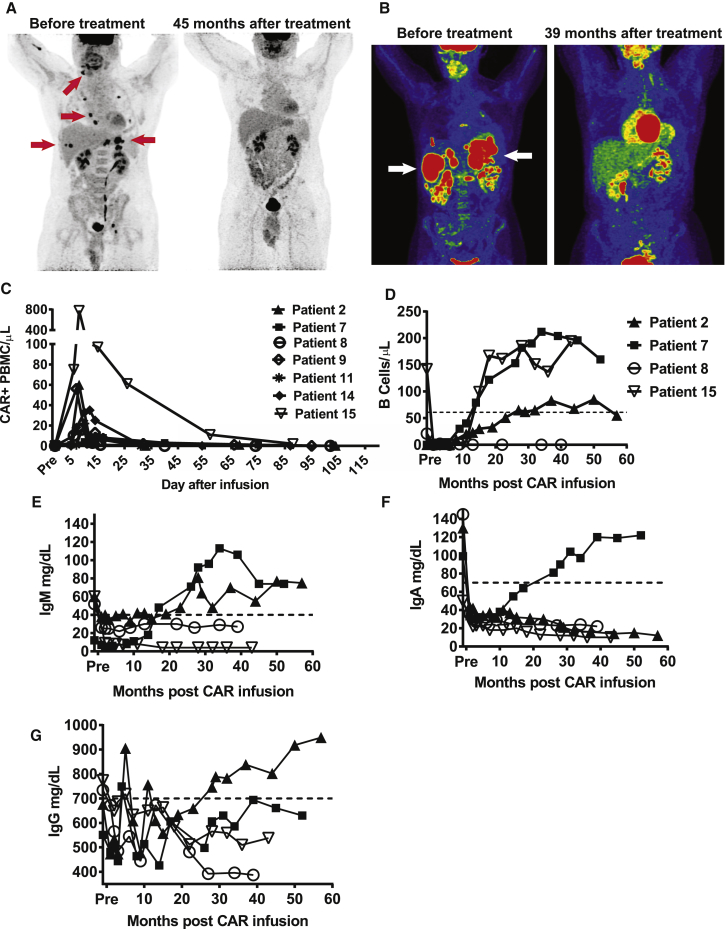
Complete Remissions of Long Duration and Assessment of B Cell and Immunoglobulin Recovery in Patients Receiving Anti-CD19 CAR T Cells
(A) Patient 7 had chemotherapy-refractory DLBCL NOS. Patient 7’s lymphoma went into a CR that is ongoing (at the time of this report) after CAR T cell infusion as shown on positron emission tomography (PET) imaging. Examples of sites of lymphoma in this patient are indicated by the red arrows pointing to the black lesions. Note that the brain, heart, kidneys, and bladder are normally dark on these images and do not represent lymphoma in the after- treatment images. (B) Patient 8 had chemotherapy-refractory PMBCL that had undergone 10 previous lines of therapy. At the time of enrollment in the anti-CD19 CAR trial, she had extensive abdominal lymphoma, as shown by PET. The patient entered a complete remission that was ongoing 39 months after CAR T cell infusion, at which time she was diagnosed with myelodysplastic syndrome. Lymphoma is indicated by the white arrows pointing to red areas. Note that the brain, heart, kidneys, and bladder are normally red on these images and do not represent lymphoma in the after-treatment images. (C) Blood CAR+ cell levels were determined by qPCR. CAR+ cell numbers peaked in the first 2 weeks after infusion for all patients and then decreased. (D) Blood B cell levels were determined by flow cytometry for CD19 at multiple time points after CAR T cell infusion in patients who obtained long-term CRs. Blood B cell numbers recovered in three of four patients in long-term CRs. B cell and immunoglobulin recovery of patients not obtaining long-term CRs is not included in this figure because these patients developed progressive lymphoma, which was a criteria for patients to be removed from the study, so the patients were removed from the study. (D–G) The dashed line represents the lower limit of the normal range. The patient symbols used in (D) were used to represent the same patients in (E)–(G). (E) Serum IgM levels were determined for patients in long-term CRs. (F) Serum IgA levels were determined for patients in long-term CRs. (G) Serum IgG levels are shown. Note that IgG levels fluctuate because patients 2, 7, and 8 received infusions of intravenous IgG.
Three of the four patients with long-term remissions had ongoing CRs at the time of this report. Patient 2 had chemotherapy-refractory primary mediastinal B cell lymphoma (PMBCL) that is currently in an ongoing CR. Patient 7 had chemotherapy-refractory DLBCL not otherwise specified (NOS) that is currently in an ongoing CR. Patient 15 had DLBCL NOS that relapsed 17 months after autologous HSCT; the lymphoma of patient 15 is also in an ongoing CR. Patient 8 had chemotherapy-refractory PMBCL. Patient 8 obtained a CR after anti-CD19 CAR T cell infusion. Because of concern over new cytopenias 39 months after CAR T cell infusion, patient 8 underwent a bone marrow biopsy that revealed myelodysplastic syndrome (MDS) with excess blasts. Standard bone marrow cytogenetics analysis showed a normal karyotype. MDS is common in patients with heavily treated lymphoma because of extensive chemotherapy exposure,26, 27 and patient 8 had received 10 prior lines of therapy before anti-CD19 CAR therapy (Table 1). No T cell abnormalities were detected in the bone marrow of patient 8 by flow cytometry or immunohistochemistry, and only 0.04% of total bone marrow cells contained the CAR gene as assessed by qPCR. At the time of the MDS diagnosis, patient 8’s lymphoma was in CR, and she underwent allogeneic HSCT to treat her MDS.
Blood CAR+ Cell Quantification
The number of CAR T cells peaked during the first 14 days after infusion in all patients and then dropped to very low or undetectable levels (Figure 1C). Very low levels of CAR T cells persisted long term in two of four patients with long-duration CRs. At the last follow-up 39 months after infusion, 0.08% of patient 8’s peripheral blood mononuclear cells (PBMCs) were CAR+. At the last follow-up 43 months after infusion, 0.02% of patient 15’s PBMC were CAR+. B cells did not recover in patient 8, but B cells did recover in patient 15 despite the low level of persisting CAR T cells at the time of and after B cell recovery (Figure 1D). In contrast, blood CAR T cells were last detected by qPCR 5 months after infusion in patient 2 and 4 months after infusion in patient 7. These results show that CRs can continue after blood CAR T cells are undetectable. A summary of the duration of persistence of CAR T cells in all seven patients is provided in Table 2.
Table 2.
Summary of Low-Level CAR+ Cell Persistence and Time Points When CAR+ Cells Were Last Detected
| Patient | Last Time Point after Infusion That Blood CAR+ Cells Were Detectable by qPCR | Limit of Detection in Each Patient (%) |
|---|---|---|
| 2 | CAR+ cells last detected 5 months after infusion | 0.004 |
| 7 | CAR+ cells last detected 4 months after infusion | 0.005 |
| 8 | CAR+ cells still detectable at a level of 0.08% 39 months after CAR T cell infusion prior to being removed from the study to receive allogeneic stem cell transplantation for myelodysplastic syndrome | 0.017 |
| 9 | CAR+ cells still detectable at a level of 0.03% prior to going off-study to receive standard lymphoma therapy 3 months after infusion | 0.005 |
| 11 | CAR+ cells still detectable at a level of 0.06% prior to going off-study to receive standard lymphoma therapy 2 months after CAR T cell infusion | 0.020 |
| 14 | CAR+ cells last detected 3 months after infusion | 0.005 |
| 15 | CAR+ cells still detectable at a level of 0.02% 43 months after CAR T cell infusion | 0.004 |
CAR+ cell levels are given as a percentage of total PBMCs that contained the CAR gene. CAR gene levels were determined by qPCR. The limit of detection is different in each patient because an individual PCR standard curve was made for each patient by diluting DNA from each patient’s infused CAR T cells in the patient’s pretreatment peripheral blood mononuclear cell DNA.
B Cell Recovery and Immunoglobulin Recovery
Among patients with long-term CRs, blood B cell counts recovered in patients 2, 7, and 15 but not patient 8 (Figure 1D). Recovery of CD19+ polyclonal B cells in patients 2, 7, and 15 took place 28, 38, and 28 months prior to their last follow-up, respectively, and these three patients all remained in CR at their last follow-up appointments. The recovery of polyclonal CD19+ B cells demonstrated that remissions of DLBCL can continue after functionally effective CAR T cells capable of eliminating B cells were no longer present. The lack of B cell recovery in patient 8 may or may not have been due to the low level of persisting CAR T cells in this patient. Immunoglobulin M (IgM) recovered to normal levels in two of three patients in long-term CRs with B cell recovery (Figure 1E). For unknown reasons, immunoglobulin A (IgA) serum levels returned to normal in only one of three patients in long-term CRs with B cell recovery (Figure 1F). Patients 2, 7, and 8 received prophylactic intravenous immunoglobulin G (IgG) to prevent infections; serum IgG levels are shown in Figure 1G. We administered intravenous IgG when the serum IgG level dropped below 500 mg/dL. Only patient 2 had a serum IgG level in the normal range at the last follow-up, but both patients 7 and 15 had serum IgG levels of greater than 500 mg/dL without intravenous IgG support at the last follow-up.
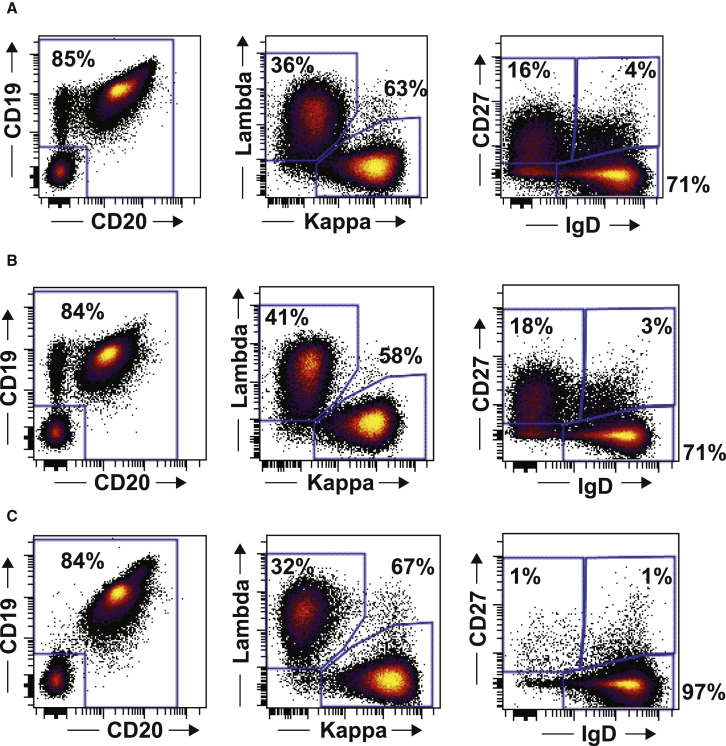
We analyzed the phenotypes of blood B cells after recovery of B cells in patients 2, 7, and 15. The B cell phenotypes are shown in Figure 2. The B cells that recovered after anti-CD19 CAR T cell therapy expressed both CD19 and CD20. The recovering B cells were polyclonal, as shown by a normal ratio of kappa to lambda light chains on these B cells (Figure 2). After recovery, most B cells expressed immunoglobulin D (IgD) but not CD27, which is a phenotype consistent with naive B cells.28 Naive B cells usually make up 60%–70% of blood B cells; patient 15’s B cells contained a higher than normal percentage of naive B cells with 97% IgD+CD27− B cells.28
Figure 2.
Phenotype of Recovering B Cells after Anti-CD19 CAR T Cell Therapy
The phenotypes of recovering B cells in the three patients with recovery of B cells during long-term complete remissions are shown. (A) is patient 2, (B) is patient 7, and (C) is patient 15. The time from CAR T cell infusion until B cell analysis was 50 months for patient 2, 45 months for patient 7, and 43 months for patient 15. The first column of plots shows the CD19 and CD20 phenotype of B cells. The number on the plot is the percentage of cells outside the box in the left lower quadrant. This number also represents the sum of the percentages of cells that are either CD19+ or CD20+. The plot is gated on CD3−, CD56−, CD16−, and CD14− lymphocytes. The second column of plots shows that the recovering B cells were polyclonal as shown by flow cytometry for immunoglobulin kappa and lambda. Plots are gated on all CD19+ and/or CD20+ lymphocytes. The third column of plots shows the IgD and CD27 phenotype of recovering B cells after CAR T cell infusion. Plots are gated on CD20+ lymphocytes. CD27 is a marker of memory B cells, and IgD expression is found on B cells that have not undergone isotype switching. Most cells had a CD27−, IgD+ phenotype, which is consistent with naive B cells.
Despite prolonged B cell depletion and incomplete immunoglobulin recovery, severe infections were rare. Among the patients with long-term CRs, the only hospitalization for infection was a 2-day hospitalization that was required for treatment of pneumonia in patient 2. Patient 8 had a mild respiratory infection from respiratory syncytial virus and was treated as an outpatient. Patient 15 had herpes zoster that resolved with outpatient treatment. Aside from minor upper respiratory infections, patients with long-term remissions did not have any other infections.
Discussion
The most important finding of this work is that multi-year CRs of DLBCL occur after anti-CD19 CAR T cell infusions. These results raise the possibility that anti-CD19 CAR T cells can be curative for chemotherapy-refractory lymphoma. Patients had substantial acute toxicity that was previously reported,10 but they did not have chronic toxicity directly attributable to CAR T cells except B cell depletion and hypogammaglobulinemia.
It is unclear what degree of CAR T cell persistence is necessary to obtain prolonged CRs of lymphoma. If CAR T cells kill all malignant cells early after infusion, only short-term persistence of the CAR T cells is necessary; however, if all malignant cells are never eliminated, CAR T cells persisting long term might be able to maintain remissions by continuously detecting and killing malignant cells to prevent clinically evident relapse. In some of the currently reported patients, remissions continued after the disappearance of CAR T cells from the blood. This is consistent with our finding that remissions can continue after recovery of non-malignant CD19+ B cells. Continued remissions with recovery of non-malignant B cells provided clear evidence that lymphoma remissions can continue after the disappearance of an effective anti-CD19 T cell response. One limitation of our work is that CAR T cells were quantified in blood but not in other tissues except in patient 8, where CAR T cells were assessed in the bone marrow. It is possible that CAR T cells persisted longer in tissues such as lymph nodes but again, the recovery of non-malignant CD19+ B cells demonstrated a lack of functional anti-CD19 CAR T cells in patients 2, 7, and 15 while the lymphomas of these patients remained in remission.
Different B cell malignancies might have varying susceptibility to complete elimination by anti-CD19 CAR T cells. Varying susceptibilities to complete elimination might determine the importance of CAR T cell persistence in maintaining remissions of different malignancies. Acute lymphoblastic leukemia (ALL) has a CR rate of 70%–94% with anti-CD19 CAR T cell treatment but a substantial number of patients with ALL relapse within 1 year after CAR T cell therapy, with some patients relapsing because of loss of CD19 expression on the ALL blasts.3, 23, 29 Some patients with ALL enter sustained remissions after anti-CD19 CAR T cell therapy.3 It is possible that these patients with ALL stayed in remission because their leukemia cells were completely eliminated by a powerful CAR T cell response early after infusion.
We have not identified characteristics that differentiate the four of seven patients with DLBCL in the currently reported cohort who obtained long-term CRs from the other three patients in the cohort. There was not an association between peak blood CAR+ cell levels and long-term CR. Patients 7 and 8 had low peak CAR+ cell levels of 8/μL and 16/μL, respectively, while patients 2 and 15 had high peak CAR+ cell levels of 60/μL and 777/μL, respectively (Figure 2C). The phenotypes of the infused CAR T cells were previously determined by flow cytometry and reported; the T cell phenotypes were not consistent among patients obtaining long-term CRs.10 Of course, given the small number of patients in this report, T cell characteristics associated with response could have been missed.
The patients with long-term remissions of DLBCL reported here were treated on a protocol that had certain characteristics that were different than some current anti-CD19 CAR T cell protocols. One important difference was the very limited use of immunosuppressive drugs such as tocilizumab and corticosteroids despite the significant cytokine-release syndrome and neurologic toxicity experienced by the patients soon after CAR T cell infusion.10 Aside from a single 4-mg/kg dose of tocilizumab that was administered to patient 15, the four patients with long-term remissions did not receive any immunosuppressive drugs. We were willing to tolerate toxicity to avoid any chance of abrogating the efficacy of the CAR T cells by administering immunosuppressive agents. Whether the durability of the responses was related to this lack of administration of immunosuppressive drugs is not known. No immunosuppressive drugs were administered to the three patients in this cohort who did not obtain long-term remissions. Another factor to consider is that all seven reported patients received a chemotherapy conditioning regimen of cyclophosphamide and fludarabine. The chemotherapy given prior to CAR T cell infusions is intended to enhance the activity of the CAR T cells. We have previously reported that chemotherapy regimens of cyclophosphamide and fludarabine lead to increases in serum interleukin (IL)-15 and IL-7, among other cytokines.13, 30 Conditioning regimens of cyclophosphamide and fludarabine have been utilized in multiple anti-CD19 CAR studies that have reported high remission rates of lymphoma.10, 11, 12, 13 We also treated other patients in this cohort in addition to the patients with DLBCL reported here, including three patients with chronic lymphocytic leukemia, one patient with an indolent B cell lymphoma NOS, and one patient with splenic marginal zone lymphoma. Aside from the patient with splenic marginal zone lymphoma, all of these patients are in ongoing CRs.
The fact that only one hospitalization for infection occurred in the four patients with long-term remissions of lymphoma suggests that infection risk is not a limiting problem for anti-CD19 CAR T cell therapies, but studies in a larger number of patients are needed to further address the risk of infection. Our results show that remissions can continue for long periods of time after B cell recovery, so any increased infection risk that might occur after anti-CD19 CAR T cell infusions is potentially temporary, but remissions are potentially permanent in some patients.
In patients 2 and 7, there was a substantial lag time between the disappearance of CAR T cells from the blood and full recovery of blood B cell counts. We hypothesize that the lag time between the disappearance of CAR T cells from the blood until full recovery of B cell counts is attributable to one of two reasons. First, an undetected persisting bone marrow CAR T cell population could have been present. We did not regularly assess for bone marrow CAR T cells, since we infrequently did post-treatment bone marrow biopsies on these patients. Another possible explanation for this lag time between the disappearance of CAR T cells from the blood until recovery of blood B cells is that the anti-CD19 CAR T cells cause profound depletion of primitive B cell precursors, so a substantial amount of time is required for recovery of B-lineage cells.
These results show that anti-CD19 CAR T cells can cause long CRs of chemotherapy-refractory DLBCL and that anti-CD19 CAR T cells are possibly a curative therapy for lymphoma. Importantly, patients in long-term remissions did not suffer substantial chronic toxicity. Not all patients with lymphoma who receive anti-CD19 CAR T cells obtain sustained CRs, and CAR T cells cause substantial short-term toxicity early after infusion; therefore, more research is needed to improve anti-CD19 CAR T cell therapies.
Materials and Methods
Clinical Trial
This clinical trial (ClinicalTrials.gov NCT00924326) tested an anti-CD19 CAR designated FMC63-28Z.31 This CAR is encoded by a gamma-retrovirus, and it includes a murine single-chain variable fragment and a CD28 costimulatory domain.31 The same CAR is used in axicabtagene ciloleucel.12 All patients gave informed consent. This research was approved by the National Cancer Institute (NCI) Institutional Review Board. Patients treated in a previously reported cohort of this trial were included in this report if they had any subtype of DLBCL and were evaluable for lymphoma response (Table 1).10 Nine patients with DLBCL were treated in this previously reported cohort. Note that two patients from the previously reported cohort were not evaluable for long-term response: one patient refused to return for follow-up, and the other patient died 16 days after CAR T cell infusion.10 Patient 15 was previously reported as having a PR, but this patient converted from PR to CR 8 months after treatment when subtle positron emission tomography abnormalities resolved. Patient numbers used in this report are the same numbers used in the first report of these patients.10 Patients received a conditioning chemotherapy regimen of cyclophosphamide and fludarabine followed 1 day later by a single infusion of anti-CD19 CAR T cells (Table 1). Chemotherapy was administered to enhance CAR T cell activity.32, 33, 34 No further lymphoma therapy was administered after anti-CD19 CAR T cell infusions. Lymphoma was staged by standard methods.35
Anti-CD19-CAR T Cell Preparation
PBMCs obtained from each patient by apheresis were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 IU/mL IL-2 (Novartis). T cell proliferation was initiated by adding 50 ng/mL of the anti-CD3 monoclonal antibody OKT3 (Ortho Biotech). Six-well plates were coated with RetroNectin (Takara Bio), and gamma-retroviruses encoding the anti-CD19 CAR were coated onto the plates as previously described.36 Two days after initiation of the PBMC cultures, 2 × 106 stimulated PBMCs were added to each well of the virus-coated plates, and the plates were cultured overnight. The next day, the cells were returned to culture in AIM V medium with 5% human AB serum and 300 IU/mL IL-2. All cell products were assessed for potency by interferon (IFN)-γ release and for CAR expression by anti-Fab antibody staining. Release criteria for clinical T cell products were at least 200 pg/mL IFNγ release against CD19+ targets in a standard ELISA and at least 30% CAR expression on T cells as measured by anti-Fab flow cytometry as previously described.10 Cells were tested for sterility by bacteria culture, fungal culture, and mycoplasma PCR testing. Endotoxin testing was performed with the limulus amoebocyte lysis assay, and replication competent retrovirus testing was performed with PCR. Cells were harvested and washed on day 10 of culture and were infused intravenously over 20–30 min.
Quantification of Serum Immunoglobulins
IgG, IgA, and IgM levels were determined by a standard clinical immunoturbidimetric assay with a Roche Cobas 6000 analyzer.
Flow Cytometry
Flow cytometry to determine B cell phenotypes was performed by using standard methods. The B cell counts in Figure 1D were determined by quantifying CD19+ lymphocytes.
Real-Time qPCR to Quantitate Blood CAR+ Cells
For each patient, DNA was extracted from PBMCs collected before treatment and at multiple time points after treatment. DNA was extracted by using a QIAGEN DNeasy blood and tissue kit. DNA from each time point was amplified in duplicate with a primer and probe set (Applied Biosystems) that was specific for the anti-CD19 CAR. Real-time PCR was carried out with a Roche Light Cycler 480 real-time PCR system. Similar to an approach used previously by other investigators and as published previously in our own work, we made serial 1:5 dilutions of DNA from the infused T cells of each patient into pretreatment DNA from the same patient, and we made standard curves by performing qPCR on this DNA.9, 37, 38, 39, 40 We determined the percentage of the infused T cells that expressed the anti-CD19 CAR by flow cytometry with anti-Fab antibody staining.10 The standard curve was in units of the percentage of CAR+ cells. The highest point of the standard curve was the point derived from undiluted infused T cell DNA. We assumed that only infused T cells with surface CAR expression detected by flow cytometry contained the CAR gene. This assumption probably underestimates the actual number of cells containing the CAR gene because all cells containing the CAR gene might not express the CAR protein on the cell surface. The percentage of PBMC that contained the CAR gene at each time point was determined by comparing the qPCR results obtained with DNA of PBMCs from each time point to the qPCR results obtained from each patient’s infused T cell standard curve. All samples were normalized to β-actin with an Applied Biosystems β-actin control reagent kit. After the percentage of CAR+ PBMCs was determined by PCR, the absolute number of CAR+ PBMCs was calculated by multiplying the percentage of CAR+ PBMCs by the sum of the absolute number of blood lymphocytes and monocytes.
Author Contributions
J.N.K. conceived of the study, provided patient care, analyzed the data, and wrote the paper. R.P.T.S. and T.L. produced clinical cell products, analyzed the data, and reviewed the manuscript. N.L. conducted the experiments, analyzed the data, and reviewed the manuscript. A.B. and J.R. conceived of the experiments, analyzed the data, and reviewed the manuscript. J.C.Y., R.M.S., and L.M. provided patient care, analyzed the data, and extensively reviewed the manuscript. S.A.F. produced the clinical gene therapy vector, analyzed the data, and reviewed the manuscript. S.A.R. conceived of the study, participated in clinical care, supervised the research, and extensively reviewed and edited the manuscript.
Conflicts of Interest
J.N.K. is the principal investigator of collaborative research and development agreements between the National Cancer Institute and both Kite Pharma, Inc. and bluebird bio. J.N.K. also has multiple patents on CAR T cell technology. S.A.R. is principal investigator of a collaborative research and development agreement between the National Cancer Institute and Kite Pharma Inc. A.B. and J.R. are employees of Kite Pharma, Inc.
Acknowledgments
We thank the NIH Clinical Center 3 Northwest Nursing Unit nurses and our patients. We also thank Tyler Willett for help in organizing patient specimen shipments. This work was supported in part by intramural funding from the NIH National Cancer Institute Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The work was also supported in part by Kite Pharma, Inc. through a cooperative research and development agreement with the NCI Surgery Branch.
References
- 1.Sadelain M., Brentjens R., Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochenderfer J.N., Rosenberg S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenderian S.S., Porter D.L., Gill S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: how not to put the CART before the horse. Biol. Blood Marrow Transplant. 2017;23:235–246. doi: 10.1016/j.bbmt.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos C.A., Heslop H.E., Brenner M.K. CAR-T cell therapy for lymphoma. Annu. Rev. Med. 2016;67:165–183. doi: 10.1146/annurev-med-051914-021702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadelain M. CAR therapy: the CD19 paradigm. J. Clin. Invest. 2015;125:3392–3400. doi: 10.1172/JCI80010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen M.C., Riddell S.R. Designing chimeric antigen receptors to effectively and safely target tumors. Curr. Opin. Immunol. 2015;33:9–15. doi: 10.1016/j.coi.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson H.J., Rafiq S., Brentjens R.J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 2016;13:370–383. doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A., Maric I., Raffeld M., Nathan D.A., Lanier B.J. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke F.L., Neelapu S.S., Bartlett N.L., Siddiqi T., Chavez J.C., Hosing C.M., Ghobadi A., Budde L.E., Bot A., Rossi J.M., Jiang Y. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol. Ther. 2017;25:285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kochenderfer J.N., Somerville R.P.T., Lu T., Shi V., Bot A., Rossi J., Xue A., Goff S.L., Yang J.C., Sherry R.M. Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J. Clin. Oncol. 2017;35:1803–1813. doi: 10.1200/JCO.2016.71.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos C.A., Savoldo B., Torrano V., Ballard B., Zhang H., Dakhova O., Liu E., Carrum G., Kamble R.T., Gee A.P. Clinical responses with T lymphocytes targeting malignancy-associated κ light chains. J. Clin. Invest. 2016;126:2588–2596. doi: 10.1172/JCI86000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martelli M., Ferreri A.J.M., Agostinelli C., Di Rocco A., Pfreundschuh M., Pileri S.A. Diffuse large B-cell lymphoma. Crit. Rev. Oncol. Hematol. 2013;87:146–171. doi: 10.1016/j.critrevonc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Coiffier B., Thieblemont C., Van Den Neste E., Lepeu G., Plantier I., Castaigne S., Lefort S., Marit G., Macro M., Sebban C. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedberg J.W. Relapsed/refractory diffuse large B-cell lymphoma. ASH Education Book. 2011;2011:498–505. doi: 10.1182/asheducation-2011.1.498. [DOI] [PubMed] [Google Scholar]
- 18.Elstrom R.L., Martin P., Ostrow K., Barrientos J., Chadburn A., Furman R., Ruan J., Shore T., Schuster M., Cerchietti L. Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: implications for the development of novel therapeutic strategies. Clin. Lymphoma Myeloma Leuk. 2010;10:192–196. doi: 10.3816/CLML.2010.n.030. [DOI] [PubMed] [Google Scholar]
- 19.Nagle S.J., Woo K., Schuster S.J., Nasta S.D., Stadtmauer E., Mick R., Svoboda J. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am. J. Hematol. 2013;88:890–894. doi: 10.1002/ajh.23524. [DOI] [PubMed] [Google Scholar]
- 20.Vose J.M., Bierman P.J., Anderson J.R., Kessinger A., Pierson J., Nelson J., Frappier B., Schmit-Pokorny K., Weisenburger D.D., Armitage J.O. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: clinical course and patient follow-up. Blood. 1992;80:2142–2148. [PubMed] [Google Scholar]
- 21.Brudno J.N., Somerville R.P.T., Shi V., Rose J.J., Halverson D.C., Fowler D.H., Gea-Banacloche J.C., Pavletic S.Z., Hickstein D.D., Lu T.L. Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J. Clin. Oncol. 2016;34:1112–1121. doi: 10.1200/JCO.2015.64.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vidal L., Gafter-Gvili A., Salles G., Bousseta S., Oberman B., Rubin C., van Oers M.H., Fortpied C., Ghielmini M., Pettengell R. Rituximab maintenance improves overall survival of patients with follicular lymphoma-Individual patient data meta-analysis. Eur. J. Cancer. 2017;76:216–225. doi: 10.1016/j.ejca.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 26.Leone G., Mele L., Pulsoni A., Equitani F., Pagano L. The incidence of secondary leukemias. Haematologica. 1999;84:937–945. [PubMed] [Google Scholar]
- 27.Bhatia S. Therapy-related myelodysplasia and acute myeloid leukemia. Semin. Oncol. 2013;40:666–675. doi: 10.1053/j.seminoncol.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Andres M., Paiva B., Nieto W.G., Caraux A., Schmitz A., Almeida J., Vogt R.F., Jr., Marti G.E., Rawstron A.C., Van Zelm M.C., Primary Health Care Group of Salamanca for the Study of MBL Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin. Cytom. 2010;78(Suppl 1):S47–S60. doi: 10.1002/cyto.b.20547. [DOI] [PubMed] [Google Scholar]
- 29.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudley M.E., Yang J.C., Sherry R., Hughes M.S., Royal R., Kammula U., Robbins P.F., Huang J., Citrin D.E., Leitman S.F. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kochenderfer J.N., Feldman S.A., Zhao Y., Xu H., Black M.A., Morgan R.A., Wilson W.H., Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochenderfer J.N., Yu Z., Frasheri D., Restifo N.P., Rosenberg S.A. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gattinoni L., Finkelstein S.E., Klebanoff C.A., Antony P.A., Palmer D.C., Spiess P.J., Hwang L.N., Yu Z., Wrzesinski C., Heimann D.M. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klebanoff C.A., Khong H.T., Antony P.A., Palmer D.C., Restifo N.P. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheson B.D., Pfistner B., Juweid M.E., Gascoyne R.D., Specht L., Horning S.J., Coiffier B., Fisher R.I., Hagenbeek A., Zucca E., International Harmonization Project on Lymphoma Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 36.Johnson L.A., Morgan R.A., Dudley M.E., Cassard L., Yang J.C., Hughes M.S., Kammula U.S., Royal R.E., Sherry R.M., Wunderlich J.R. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren E.H., Fujii N., Akatsuka Y., Chaney C.N., Mito J.K., Loeb K.R., Gooley T.A., Brown M.L., Koo K.K., Rosinski K.V. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115:3869–3878. doi: 10.1182/blood-2009-10-248997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kochenderfer J.N., Dudley M.E., Carpenter R.O., Kassim S.H., Rose J.J., Telford W.G., Hakim F.T., Halverson D.C., Fowler D.H., Hardy N.M. Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:4129–4139. doi: 10.1182/blood-2013-08-519413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ali S.A., Shi V., Maric I., Wang M., Stroncek D.F., Rose J.J., Brudno J.N., Stetler-Stevenson M., Feldman S.A., Hansen B.G. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]