Abstract
Hepatocellular carcinoma (HCC) has a high morbidity and mortality rate worldwide, with limited treatment options. Glypican-3 (GPC3) is a glycosylphosphatidylinositol-anchored glycoprotein that is overexpressed in most HCC tissues but not in normal tissues. GPC3-targeting antibody therapy shows limited response in a clinical trial due to the lack of a tumor-specific cytotoxic T lymphocyte (CTL) response. Here, in C57/B6 mice, we demonstrated that intravenous infusion of GPC3-coupled lymphocytes (LC/GPC3+) elicited robust GPC3-specific antibody and CTL responses, which effectively restricted proliferation and lysed cultured-HCC cells. Treatment with LC/GPC3+ induced durable tumor regression in HCC-bearing C57/B6 mice. Administration of LC/GPC3+ induced elevated levels of the cytotoxic T cell bioactive factors tumor necrosis factor alpha (TNF-α), interferon-γ (IFN-γ), granzyme B, and perforin, and substantially increased the number of infiltrating CD8+ T cells in tumor tissues. Moreover, immune responses elicited by LC/GPC3+ selectively suppressed GPC3+ tumors, but didn’t affect the GPC3− tumors in BALB/c mice. Our findings provide the first preclinical evidence that intravenous infusion of the LC/GPC3+ complex can induce a strong anti-HCC effect through regulating systemic and local immune responses. These results indicate that the LC/GPC3+ complex could be developed as precision therapeutics for HCC patients in the future.
Keywords: glypican-3, immunotherapy, cytotoxic T lymphocyte response, hepatocellular carcinoma
Wu et al. develop a novel immunotherapeutic vaccine for hepatocellular carcinoma (HCC) treatment through targeting the HCC-specific antigen GPC3. The antigen GPC3 is coupled on and delivered through lymphocytes. The lymphocyte-GPC3 complex vaccine triggers significantly prophylactic and therapeutic antitumor effects in the murine model through regulating systemic and local immune responses.
Introduction
Hepatocellular carcinoma (HCC) is the most common form of liver cancer and is the fifth highest cause of cancer-related mortality globally. HCC has a poor prognosis, with a 5-year survival rate below 9%.1 Unfortunately, there are limited options for treating HCC. The only Food and Drug Administration (FDA) approved systemic treatment for HCC is sorafenib, which is a multi-kinase inhibitor for several tyrosine protein kinases and Raf kinase and has been shown to prolong survival for 3 months.2
HCC is typically a chronic inflammation-associated cancer, which is mainly driven by hepatotropic viral infection or exposure to toxic compounds, such as ethanol and aflatoxin.1 Hepatomas variably express major histocompatibility complex (MHC) class I molecules and have low levels of the costimulatory molecules CD80 and CD86.3 Antigen presentation by MHC class II molecules is also attenuated in HCC tissue because liver-resident dendritic cells (DCs) appear to be less potent than their counterparts in other organs on stimulating T cells.4 Thus, the tumor microenvironment is characterized by a chronic hypo-responsive status and impaired cytotoxic response and is anergic to cancer neoantigens.5 However, there have been fragmentary clinical reports of spontaneous remission and tumor shrinkage in HCC patients.6 The results of retrospective studies suggest that despite an overall immunosuppressive environment, certain patient are able to mount a protective immunity against HCC.7 HCC-specific antigens, such as glypican-3 (GPC3), have been identified. GPC3 has been shown to play a role in the activation of a cytotoxic T lymphocyte (CTL) response against HCC.8 GPC3 is overexpressed in HCC tissues but not in normal and benign tissues.9 GPC3 appears to promote tumor cell proliferation and invasiveness, and is also a biomarker of poor prognosis.8 Thus, GPC3 could be a potential target for HCC immunotherapy because it is a cell surface glycoprotein and exhibits immunogenicity.10, 11
Strategies to restore intrinsic antitumor immunity have been applied to reverse the milieu favorable for HCC growth. For example, administration of antibody against GPC3 can block the signaling pathway to inhibit HCC growth as well as exhibit well tolerability in vivo.12, 13 However, anti-GPC3 antibody monotherapy cannot completely eliminate tumors in a mouse model, and only a partial response was observed in HCC patients in a phase II clinical trial.13, 14 These outcomes indicate that the passive immunotherapy by a GPC3 antibody may be not potent for HCC treatment and this is probably due to the lack of a tumor-specific CTL response.13, 15, 16 Supporting this notion, a therapeutic regimen that is able to elicit antitumor CTL may synergistically augment tumor rejection.17 Due to the ability of regulating systemic immunity against the tumor and reprogramming the tumor microenvironment, vaccine-mediated immunotherapeutic intervention exhibits a promising approach for clinical practice.18, 19 Several clinical studies have demonstrated that the enhanced interaction between antigen presented by MHC molecules and the T cell receptor determines CD8+ T cell response and also induces a high level of perforin and granzyme B.17, 20 One example is that pre-conditioning the vaccine site with tetanus toxoid significantly promotes antigen-pulsed DCs that migrate into lymph nodes and improves the antigen delivery efficiency to elicit immune responses against advanced glioblastoma in a mouse model and patients.21 However, DCs usually do not expand well in vitro, which results in a limited number of cells for in vivo injection.
Lymphocytes naturally possess the ability to home to lymphoid organs, where various types of immune-competent cells expressing non-clonal pattern recognition receptors could sense the antigen and initiate crosstalk and a systemic immune response.17, 22 In this study, we proved the concept of a unique approach to deliver the antigen GPC3 into lymphoid organs by intravenous administration of the GPC3-coupled lymphocyte complex (LC/GPC3+). GPC3 protein was first coupled to lymphocytes by co-incubating with heterobifunctional crosslinker sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (sulfo-SMCC). Then, we assessed the antitumor effect of LC/GPC3+ in vivo. The results showed LC/GPC3+ exhibited a robust anti-HCC effect through eliciting specific antibody and cytotoxic responses and reprogramming the tumor microenvironment. Our study provides strong preclinical evidence for developing LC/GPC3+ as a future HCC therapeutic agent.
Results
Functional Antibody Elicited by LC/GPC3+ Vaccination
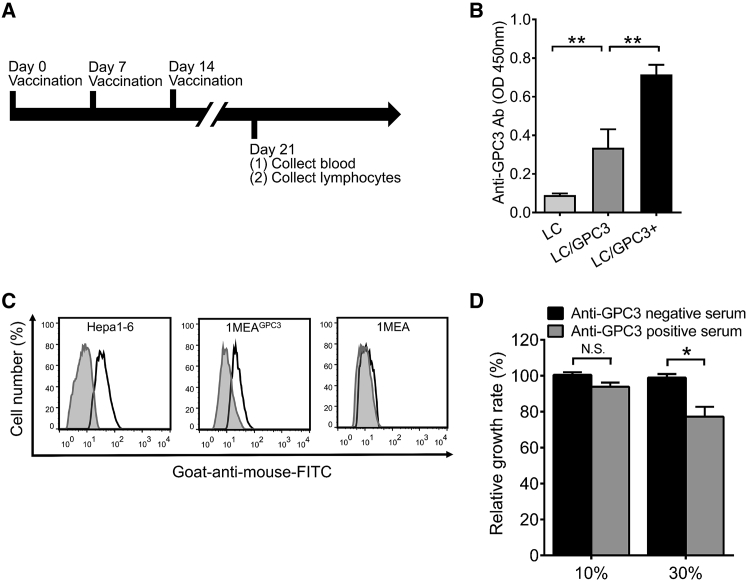
We determined the immunogenicity of the LC/GPC3+ complex by evaluating the antibody response in C57/B6 mice. LC/GPC3+ induced a stronger antibody response than did LC/GPC3 (Figures 1A and 1B). Immunization of LC generated only a background spectrometric signal comparable to that of native mice sera. It has been previously reported that the anti-GPC3 antibody could suppress HCC cell growth in vitro.13, 23 Thus, we first examined the binding of anti-GPC3 sera to the native GPC3 on the HCC cell surface. The flow cytometry data showed that anti-GPC3 positive sera could specifically bind to the surface of Hepa1-6 and 1MEAGPC3 cells, rather than GPC3− 1MEA cells, compared with the binding activity of anti-GPC3 negative sera (Figure 1C). The anti-GPC3 serum samples also exhibited binding ability to the human HCC cell HepG2 (Figure S4A). The human GPC3 protein shares 94% identity, within 579 aa, with murine GPC3.24 This suggests that polyclonal anti-GPC3 antibody from vaccinated mice targets multi-epitopes of the GPC3 protein and potentially recognizes antigenic epitopes of human GPC3. To determine whether anti-GPC3 antibody restricted HCC cell growth, we performed an HCC cell proliferation assay by incubating HCC cells with anti-GPC3 sera, as indicated. It showed that anti-GPC3 sera exhibited a dose-dependent inhibition of HCC cell proliferation (Figure 1D). At a concentration of 30% (v/v), anti-GPC3+ sera inhibited Hepa1-6 cells growth up to 24% compared with anti-GPC3− sera. In addition, the maximum inhibition efficiency of anti-GPC3+ sera reached 45% in HepG2 cells and 23% in 1MEAGPC3 cells (Figure S4B). However, anti-GPC3+ sera did not have a significant inhibitory effect on the growth of GPC3− 1MEA cells.
Figure 1.
Functional Antibody Response Elicited by Vaccination
(A) Schematic diagram of vaccination strategy in C57/B6 mice. (B) GPC3-specific antibody response. GPC3-specific antibody response was examined by ELISA (n = 10). Sera were diluted at 1:2,000. (C) The binding of anti-GPC3 sera to the HCC cell surface. HCC cells were incubated with anti-GPC3− (shaded area) and GPC3+ (heavy solid line) sera from immunized mice and then probed with goat-anti-mouse Dylight 488-labeled IgG. The stained cells were analyzed with flow cytometry. Representative histograms show the binding of sera to HCC cells. (D) Cell proliferation in the presence of anti-GPC3 sera. Hepa1-6 cells were cultured with 10% and 30% of anti-GPC3− and -GPC3+ mice sera. Cell growth rate was measured by the CCK-8 kit and normalized to treatment with GPC3− serum post 72 hr culture (n = 3).
Cellular Immunity Induced by LC/GPC3+ Vaccination
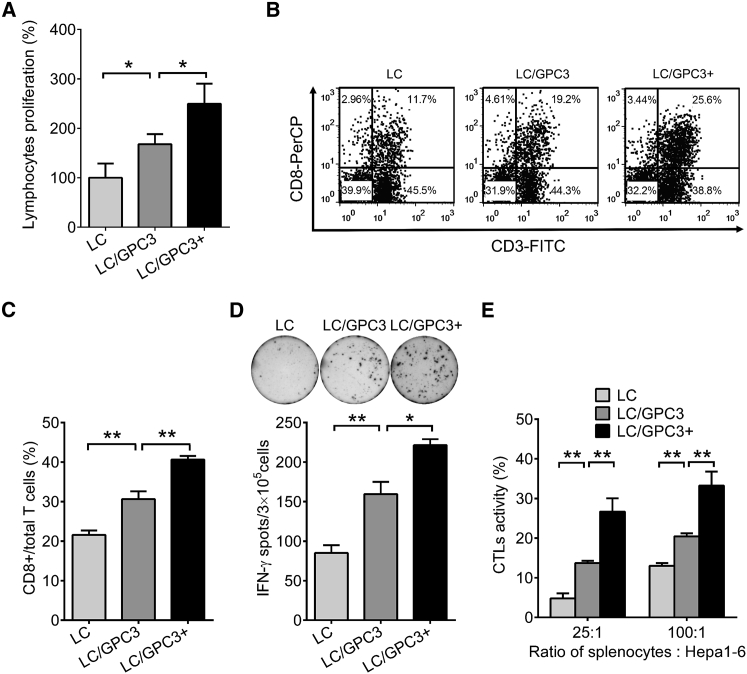
To evaluate the cellular immune response after intravenous administration of LC/GPC3+, we performed the lymphocyte proliferation assay in vitro in the presence of GPC3 protein. LC/GPC3+ elicited substantially higher lymphocyte proliferation than the control groups (Figure 2A). We also analyzed the T lymphocyte subtypes by flow cytometry and ELISpot assay. It showed that LC/GPC3+ significantly expanded antigen-specific CD8+ T cells (Figure 2B). Compared with LC/GPC3, LC/GPC3+ induced a much more robust CD8+ T cell response because it augmented the percentage of CD8+ T cells by as much as 40% (Figure 2C). In addition, the frequency of GPC3-specific interferon-γ (IFN-γ) secreting cells in the LC/GPC3+ group was significantly higher than that in the other groups (Figure 2D). There were approximately 740 spots/106 lymphocytes in the LC/GPC3+ groups, which was three times more than that in the vehicle control group. Moreover, in comparison with irrelevant ovalbumin (OVA) peptide OVA-I, incubation of H2-Kd- and H2-Db-restricted peptides GPC3395–404 and GPC3418–427 with lymphocytes from vaccinated mice triggered a much stronger IFN-γ response (Figure S5). These results demonstrated that GPC3-specific T cell response was induced by vaccination of LC/GPC3+.
Figure 2.
Cellular Response Induced by Vaccination
(A) Lymphocyte proliferation in vitro. Lymphocytes were re-stimulated with GPC3 protein. The cell number was determined by the CCK-8 assay after 3 days’ incubation (n = 10). (B) Analysis of CD8+ T lymphocyte response in mice by flow cytometry. Lymphocytes were stained with anti-CD3 and anti-CD8a antibody. Representative dot plots showing CD8+ T cell recall response to GPC3 protein. (C) The percentage of CD8+ T cells in total T cells (n = 5). (D) ELISpot analysis of IFN-γ secreting cells in lymphocytes from immunized mice (n = 5). (E) Evaluation of cytolytic rate of lymphocytes on HCC cells. Lymphocytes from vaccinated mice were co-cultured with Hepa1-6 cells at E:T ratios of 25:1 and 100:1 for 48 hr. Cytolysis efficiency was evaluated by measuring the release of LDH (n = 10).
To investigate CTL-mediated cytolysis of Hepa1-6 cells, we performed a cytotoxic killing assay in vitro by co-culturing Hepa1-6 cells and lymphocytes from immunized mice. An obvious dose-dependent antitumor cytolysis was detected (Figure 2E). The lytic efficiency of lymphocytes from the LC/GPC3+ group was found to be up to 35% at an effector to target cell (E:T) ratio of 100:1 and was about 28% at E:T ratio of 25:1. Markedly less cytolysis were elicited in the control groups LC/GPC3 and LC. It is notable that control LC/GPC3 induced significantly higher antibody and cellular responses than did the vehicle control LC (Figures 1 and 2). However, the immune responses to LC/GPC3 were much weaker than those evoked by LC/GPC3+. It may be attributable to the GPC3 proteins captured by DCs and other antigen-presenting cells during preparation of the LC/GPC3 complex. The data indicated that LC/GPC3+ efficiently delivered antigens in vivo and elicited a robust systemic immune response in the mouse model.
Durable Tumor Regression Induced by Administration of LC/GPC3+ in the C57/B6 Mouse HCC Model
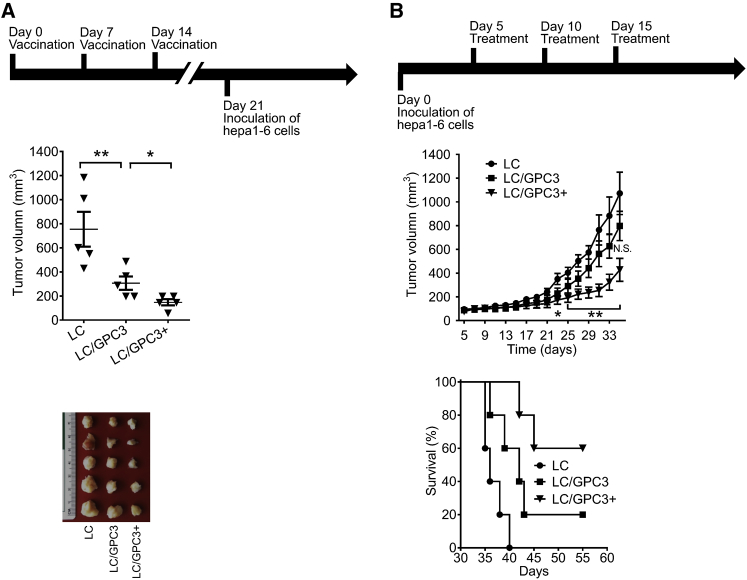
The ability of the antibody and lymphocytes elicited by vaccination to suppress HCC cells in vitro encouraged us to evaluate its in vivo anti-HCC efficacy. Considering that patients with chronic hepatitis have a high risk of HCC and they may benefit from vaccination, we first investigated the prophylactic ability of LC/GPC3+ by immunizing mice prior to inoculating tumor cells. As shown in Figure 3A, infusion of LC/GPC3+ induced substantial tumor rejection following Hepa1-6 cell challenge. LC/GPC3+ restricted tumor size to 147.2 mm3, compared with 306.1 mm3 in the LC/GPC3 group and 753.9 mm3 in the LC group. It indicated that LC/GPC3+ conferred a preventive effect on suppressing the HCC tumor in a prophylactic setting.
Figure 3.
Antitumor Effect of LC/GPC3+ in C57/B6 Mice
(A) Prophylactic antitumor effect. C57/B6 mice were challenged with Hepa1-6 cells after immunization with different formulations (top panel). The tumor size was monitored for 35 days (n = 6, middle panel). The tumors were collected at the end of the experiment (bottom panel). (B) Therapeutic effects of vaccination on the advanced, aggressively growing HCC tumors. C57/B6 mice were inoculated with Hepa1-6 cells. After tumors are palpable, mice were treated by intravenous infusion with LC/GPC3+, LC/GPC3, and LC (top panel). The tumor size was measured every 2 days for a total of 30 days (n = 6, middle panel). The overall survival of tumor-bearing mice treated with the indicated vaccine formulations (LC versus LC/GPC3, p < 0.05; LC/GPC3 versus LC/GPC3+, p < 0.05) (bottom panel).
Next, we investigated the therapeutic effects of vaccination on established and poorly immunogenic liver tumors. Mice were inoculated with Hepa1-6 cells first. After the tumors reached approximately 100 mm3, the mice were treated with the different vaccine formulations. Tumors in the LC/GPC3+ group showed slower growth, with significantly reduced tumor size at the end of experiment compared with the other treatment groups (Figure 3B). The average tumor size was 427.3 mm3 in the LC/GPC3+ group, 797.4 mm3 in the LC/GPC3 group, and 1,072.1 mm3 in the LC group. A significant antitumor effect was achieved in the LC/GPC3+ treatment group compared with the other treatments on days 27–35. Importantly, 60% of mice treated with LC/GPC3+ survived for longer than 55 days, whereas the median survival durations of the mice treated with LC and LC/GPC3 were 36 and 42 days, respectively (Figure 3B).
Activation of Tumor-Specific T Cell Response by LC/GPC3+ Infusion
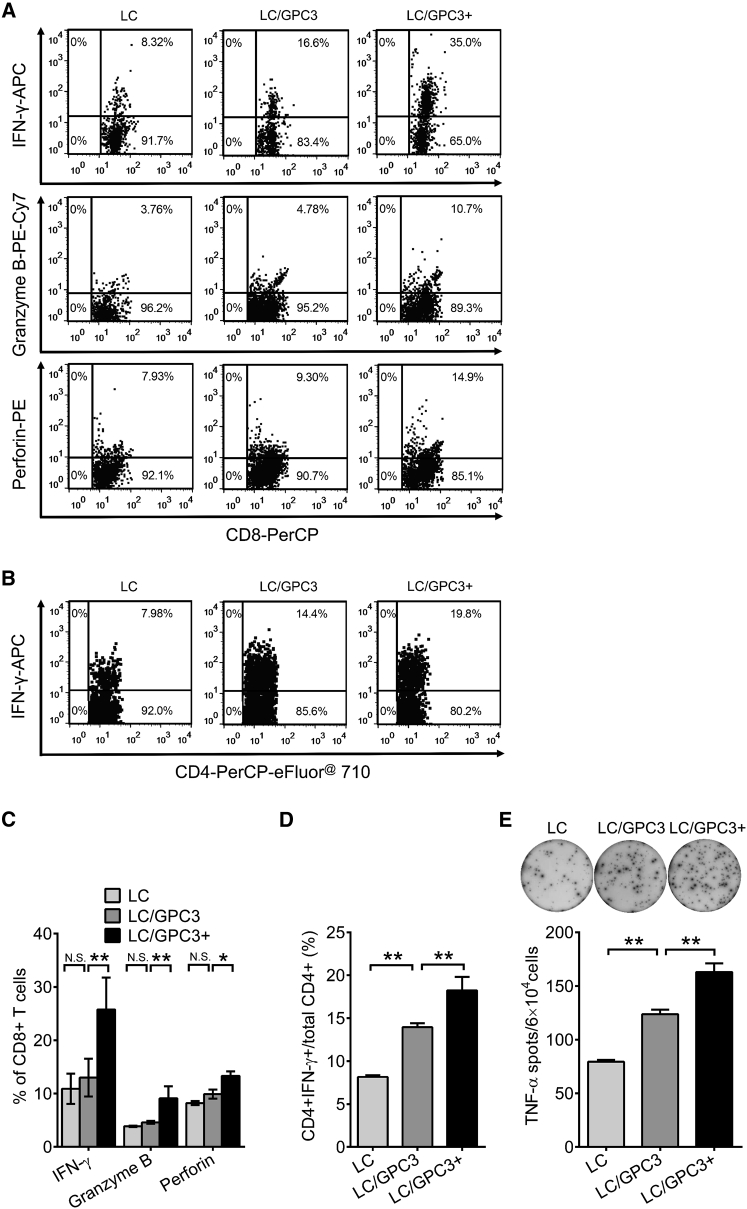
To further explore the antitumor cellular immunity in the therapeutic setting, we harvested the lymphocytes from vaccine-treated tumor-bearing mice and analyzed the expression of IFN-γ, granzyme B, and perforin. As shown in Figure 4A, IFN-γ expression was significantly increased in mice treated with LC/GPC3+ compared with the other treatment groups, with the ratio of CD8+IFN-γ+ cells reaching an average of 26% (Figure 4C). In the LC/GPC3+ group, similar increases of granzyme B and perforin expression in the CD8+ T cell subset were observed (Figures 4A and 4C). In addition, treatment with LC/GPC3+ significantly increased the number of IFN-γ-secreting cells in the CD4+ T population (Figures 4B and 4D). Meanwhile, tumor necrosis factor alpha (TNF-α) was also substantially activated by vaccine treatment (Figure 4E). The activated spots reached 2,800 per 106 lymphocytes in the LC/GPC3+ group. These data suggest that the LC/GPC3+ therapeutic vaccine primarily triggers tumor-specific CTLs, leading to the regression of a poorly immunogenic HCC tumor.
Figure 4.
Antigen-Specific T Cell Responses in Therapeutically Treated C57/B6 Mice
Lymphocytes were harvested from vaccine-treated tumor-bearing mice and re-stimulated with GPC3 protein. (A) Flow cytometry analysis of expression of IFN-γ, granzyme B, and perforin in CD8+ T cells. The data were shown as representative dot plots. (B) Flow cytometry analysis of IFN-γ expression in gated CD4+ T cells. (C) The percentage of IFN-γ, granzyme B, and perforin+ cells in total CD8+ T cells (n = 5). (D) The percentage of IFN-γ+ cells in total CD4+ T cells (n = 5). (E) ELISpot analysis of TNF-α secreting lymphocytes from tumor-bearing mice after different treatments (n = 5).
Enhanced Intratumoral Lymphocyte Infiltration by Treatment with LC/GPC3+
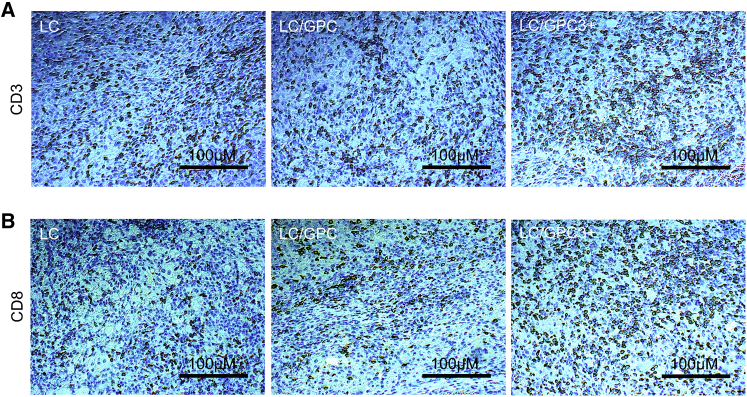
Cytotoxic CD8+ T lymphocytes are present at a low frequency within or surrounding HCC tissue and exhibit functional impairment in HCC patients, with the hallmarks of T cell exhaustion.25 These alterations result from an immunosuppressive tumor microenvironment and adversely affect clinical outcomes, suggesting that the reversal of T cell exhaustion may have therapeutic benefits. To assess whether activated T lymphocytes were recruited to the tumor site after treatment, we examined the frequency of tumor infiltrating lymphocytes (TILs). As shown in Figure 5, CD3 immunohistochemical staining revealed a substantial increase in the number of mature T lymphocytes in tumor tissues in the LC/GPC3+ group compared with that in the LC/GPC3 and LC groups. Further CD8a staining showed a significantly elevated number of CD8+ T cells in tumors from the LC/GPC3+ treated mice, whereas fewer CD8+ T cells were observed in the LC/GPC3 group, especially in the LC group. Analysis of the percentage of CD8+ T cells in total of the CD3+ lymphocytes (positive staining per square cm2) revealed that CD8+ lymphocytes accounted for the majority of TILs. This finding suggests that LC/GPC3+ treatment could promote the migration of cytotoxic T lymphocytes to the tumor and proliferation in situ. Supporting this, with a lower microscope magnification and compared with the control treatments, it was remarkable that some areas had high density of aggregated T cells in the tumor tissue from the LC/GPC3+ group, forming a possible T cell zone (Figure S6).
Figure 5.
Massive Infiltration of T Cells within the Tumor Site
(A and B) The tumor tissues were collected following by immunohistochemical staining with anti-mouse CD3 (A) and CD8a (B) mAbs (eBioscience). The representative images were captured using Olympus X73 fluorescence microscopy at 200 × magnifications.
Antitumor Effect of LC/GPC3+ on HCC Is Antigen Specific
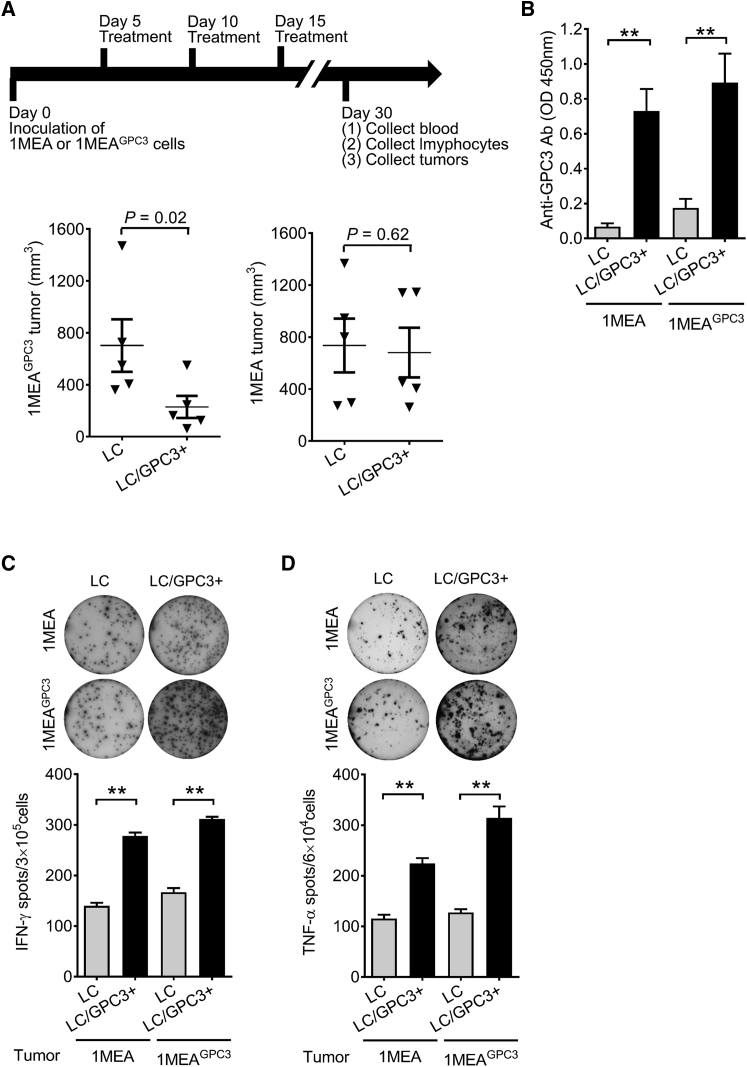
LC/GPC3+ exhibited significantly prophylactic and therapeutic antitumor effects in the C57/B6 murine HCC model. We next examined whether LC/GPC3+ selectively mount the antitumor immunity against the GPC3+ HCC tumor. We developed both GPC3− and GPC3+ HCC tumors in a BALB/c murine model by subcutaneous injection of 1MEA and 1MEAGPC3 cells, respectively (Figure 6). When the tumors became palpable, the mice were treated with LC/GPC3+ and LC following the same protocol used in the therapeutic experiments on C57/B6 mice. LC/GPC3+ treatment did not have a significant inhibitory effect on the 1MEA tumor, but did significantly suppress 1MEAGPC3 tumor growth, which was similar to its effect on the C57/B6 HCC model (Figure 6A). A substantial GPC3-specific antibody response was induced by LC/GPC3+ in both 1MEA and 1MEAGPC3 tumor-bearing BALB/c mice (Figure 6B). In addition, significantly augmented IFN-γ and TNF-α responses were triggered by LC/GPC3+ treatment in these mice (Figures 6C and 6D). These data indicate that antitumor immunity triggered by LC/GPC3+ treatment selectively targets the GPC3+ tumor and is anergic to the GPC3− tumor. In line with the above finding, staining of CD3 and CD8a demonstrated that many more CD3+ and CD8+ T cells were observed in the 1MEAGPC3 tumor than in the 1MEA tumor (Figure S7).
Figure 6.
GPC3-Specific Antitumor Effect in the BALB/c HCC Model
(A) Treatment effect of LC/GPC3+ on GPC3− and GPC3+ HCC tumors. BALB/c mice were inoculated 1MEA and 1MEAGPC3 cells. After tumors became visible, mice were treated by intravenous administration of LC/GPC3+ and LC. The tumor size was monitored for 30 days (n = 10). (B) GPC3-specific antibody response. GPC3-specific antibody was determined at the end of the experiment by ELISA (n = 10). Sera were diluted at 1:2,000. (C and D) ELISpot analysis of IFN-γ (C) and TNF-α (D) secreting lymphocytes from tumor-bearing BLAB/c mice after treatment (n = 10).
Discussion
Numerous preclinical and clinical studies of the therapeutic cancer vaccines suggest that the critical step of vaccination is the efficient presentation of cancer neoantigens to the host immune system, especially to the T lymphocytes.22, 26 Our study is the first to provide preclinical evidence that intravenous delivery of GPC3-coupled lymphocyte complex LC/GPC3+ effectively presents the antigen and induces substantial antitumor immunity in murine HCC models.
Tumor-specific functional antibody plays a crucial role in defending against the progression of malignant tumors.27 They have been widely applied in clinical practice for non-solid and solid tumors, including lymphoma, breast cancer, and prostate carcinoma.27 The clinical trial shows that administration of anti-GPC3 antibody brings clinical benefit in HCC patients. In this study, the antibody elicited by LC/GPC3+ vaccination was able to bind to the HCC cell surface via interacting with GPC3 protein and exhibited cell-arresting activity. Anti-GPC3 antibody may interfere with Wnt and Yes-associated protein signaling pathways and activate antibody-dependent cell-mediated cytotoxicity, which inhibits cell proliferation and induces apoptosis.13, 27, 28, 29 In addition, booster vaccination of LC/GPC3+ augmented the antibody level (Figure S4C). Our previous study has shown that the immune response induced by the OVA-sulfo-SMCC-lymphocyte complex (harboring approximate 7.5 ng of OVA) is two-to-six times more robust than the aluminum-adjuvanted 100 μg of OVA does.30 This enhanced immune response may contribute to the characteristics of antigen-lymphocyte complex homing to lymph nodes throughout and to the spleen, thereby efficiently presenting antigens to the entire immune system.31
Although the mechanisms of the adaptive immune response-mediated HCC regression have not been fully elucidated, it is widely accepted that both functional antibody and the induction of cell-mediated antitumor immunity bring clinical benefits.32, 33, 34, 35 In a phase I clinical trial, the frequency of CTL induced by administration of GPC3298–306 peptide correlates with overall survival in advanced HCC patients.10, 11 In this study, substitution of GPC3 peptide by GPC3 protein guarantees many more antigen-specific T cells to be primed, including CD4+ and CD8+ T cell clones.17 It has been demonstrated that proteins and long peptides induce broader CD4+ and CD8+ T cell responses against multiple epitopes compared with short peptides.36 In two different HCC murine models, GPC3 protein-based treatment significantly induced CD8+ T cell expansion and increased intratumoral lymphocyte infiltration. Our previous data suggest that this treatment endows the proliferation potential of T cells and the increased number of memory T cells.30, 37 Although LC/GPC3+ exhibited comparable effectiveness in inhibiting the HCC tumor in both BALB/c and C57/C mice, this antitumor immunity selectively targeted the antigen-specific tumors, suggesting that LC/GPC3+ might be further studied as a precision treatment for GPC3+ HCC patients.38, 39 In addition, the high density area of TILs in LC/GPC3+-treated tumors indicates a possible intratumoral tertiary lymphoid structure (TLS), which reflects an active inflammatory tumor microenvironment and correlates with a favorable clinical outcome.40 Comparative analysis of systemic and intratumoral T cell responses implies that CTL may be primed in lymphoid tissues upon homing of the LC/GPC3+ complex, subsequently migrates to the tumor sites, and is possibly further expanded in situ by tumor-associated DCs and TLS.41, 42 In addition, the cytotoxic recall response to lyse tumor cells in vitro indicates that the generation of long-term memory T cells by LC/GPC3+ would act to prevent tumor metastasis and relapse.30, 37
Compared with LC/GPC3+, it was noted that infusion of LC/GPC3 also induced a moderate anti-HCC effect. It suggests that fewer antigen loaded to the lymphocytes through MHC-I molecules may not be sufficient for eliciting strong antibody and CTL responses against the tumor. In addition, sulfo-SMCC-mediated antigen-coupled LC/GPC3+ induced a higher level of IFN-γ, TNF-α, granzyme B, and perforin, indicating that CD8+ CTLs may execute an anti-HCC function through both the perforin-granzyme cytolytic pathway and cytokine-mediated pathway.43 TNF-α induces hyper-permeability of tumor vasculature by binding to the TNF-α receptor 1.44 The leaky vascular linings throughout the tumor facilitate the distribution and accumulation of tumor-specific antibody and infiltration of activated lymphocytes into the tumor tissue, resulting in better exposure of tumor cells to cytostatic agents.44 Furthermore, IFN-γ has immuno-editing activity within HCC tissue that correlates with prognosis following curative treatment.45 IFN-γ produced by activated TILs has been shown to restore proteasome function and augment tumor antigen presentation.46 IFN-γ also induces cell cycle regulator p21-dependent tumor cell cycle arrest at the G1 stage.47 In clinical reports, IFN-γ treatment was found to induce HCC cell apoptosis either by activation of caspase-9 or mitochondria.47 The level of IFN-γ is positively associated with the frequency of activated T cells and natural killer cells in the circulating system and local HCC tissue as well as clinical outcomes in HCC patients.45
Although significant tumor suppression was observed in murine HCC models after LC/GPC3+ treatment, the tumor was not completely eradicated at the endpoint of studies. Several strategies have been developed to achieve a more potent antitumor effect. For example, immunization with tumor-derived exosomes, which carry miscellaneous neoantigens, was reported to substantially suppress HCC and improve the tumor microenvironment in a mouse model.48 Thus, coupling multiple antigens to lymphocytes would be an attractive option because manipulation of the immunogenic complex is feasible for timely intervention in HCC patients. On the other hand, co-administration of vaccine with immune checkpoint inhibitors or chemotherapeutic agents might also augment antitumor activity. We have found that pre-treating HCC cells with sorafenib greatly increased the killing effects of GPC3-specific T cells on HCC by downregulating programmed death-ligand 1 (PD-L1) (data for another manuscript). It suggests an obvious rationale to combine checkpoint inhibitors, such as antibodies against PD-1 and PD-L1, to augment the antitumor effect of LC/GPC3+.49, 50 Further studies to understand the mechanism of how the coupled antigen interacts with immune cells in lymphoid organs and how infusion of antigen-coupled lymphocytes systemically and locally regulate the immune response will definitely benefit the improvement of the effect of this therapeutic vaccine.
Taken together, our findings demonstrate a novel approach for efficient HCC immunotherapy by infusion of the LC/GPC3+ complex. This approach poses a great potential for clinical translation.
Materials and Methods
Cell Lines and Mice
The Hepa1-6 murine HCC cell line, which expresses GPC3 and is derived from the BW7756 tumor in C57/B6 mice, was purchased from ATCC (Manassas, VA) and used for in vitro and in vivo procedures. The 1MEA murine cell line (ATCC) derived from the BNL cell by transformation with methylcholanthrene epoxid is GPC3 negative. 1MEAGPC3 cells stably express GPC3 by transducing 1MEA cells with a recombinant lentivirus expressing murine GPC3 (Supplemental Materials and Methods; Figure S2). Human HepG2 hepatoma cells were purchased from ATCC, and human LH86 hepatoma cells were established in our laboratory.51 All cell lines were cultured in DMEM containing 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 10 μg/mL streptomycin sulfate at 37°C in a humidified 5% CO2 incubator. All cell lines were confirmed to be mycoplasma free with the LookOut mycoplasma qPCR detection kit (Sigma, St. Louis, MO). Lymphocytes were harvested from mice spleens using HISTOPAQUE 1083 (Sigma) and cultured in RPMI Medium 1640 plus 10% FBS. The cell density was determined prior to each experiment using a Countess II FL automated cell counter (Life Technologies, Waltham, MA).
Preparation of GPC3-Coupled Vaccine Formulations
Lymphocytes are rich with free thiols on the cell surface.52 We previously demonstrated that the heterobifunctional crosslinker sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (sulfo-SMCC, an FDA-approved chemical for drug delivery) could efficiently couple OVA onto the lymphocyte surface by forming stable amide bonds with lysine residues of OVA and reacting with cell surface sulfhydryl.37 In this study, 10 × 106 lymphocytes from naive BALB/c or C57/B6 mice were incubated with 4 μM GPC3 protein (Supplemental Materials and Methods; Figure S3) and a 500-fold molar excess sulfo-SMCC (2 mM, Sigma) in 1 mL of PBS for 1 hr at room temperature to form an LC/GPC3+ complex.37 Lymphocytes incubated with GPC3 without sulfo-SMCC were referred to LC/GPC3 and lymphocytes incubated with sulfo-SMCC were referred to LC as vehicle control (Figure S1). The cells were washed three times with PBS to remove un-coupled sulfo-SMCC and GPC3 protein. The viability of these three cell-based vaccine formulations was confirmed by trypan blue staining prior to infusion to mice.
Immunization and Murine HCC Models
4- to 6-week old BALB/c and C57/B6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and housed at the animal facility of the University of Florida. The protocol was approved by the Institutional Animal Care and Use Committee, University of Florida. Vaccine formulations were administrated intravenously for 5 × 106 cells/injection at the indicated time point. The murine HCC model was developed by subcutaneously injecting 5 × 106 HCC cells in 100 μL of PBS into the right flank. At the endpoint, the mice were euthanatized with CO2. Tumor size was calculated as (width2 × length)/2.
ELISA
GPC3-specific antibody was detected by ELISA using GPC3 protein as the capture antigen in a similarly described method.53 Briefly, the 96-well ELISA plates were coated with 10 μg/mL GPC3 protein in 100 μL of carbonate-bicarbonate buffer (Sigma) at 4°C overnight. After incubation with blocking reagent (Sigma), the serum dilutions were added and probed with horseradish peroxidase (HRP) conjugated goat-anti-mouse secondary antibody. The final signal was developed by substrate 3,3′,5,5′-tetramethylbenzidine and read at OD450 nM using a microplate reader (BioTech BioTek, Winooski, VT).
Flow Cytometry Analysis
Lymphocytes were harvested from immunized mice at the end of the experiments and cultured in the presence of 5 μg/mL GPC3 protein for 48 hr. The cells were then stained with mouse monoclonal antibodies (mAbs) against CD3 (fluorescein isothiocyanate [FITC] labeled, BD Bioscience, San Diego, CA), CD4 (peridinin-chlorophyll-protein [PerCP]-eFluor710 labeled, eBioscience), CD8a (PerCP labeled, eBioscience), IFN-γ (allophycocyanin [APC] labeled, eBioscience), granzyme B (PE-Cyanine7 labeled, eBioscience), perforin (PE labeled, eBioscience), and anti-mouse isotype control immunoglobulin G (IgG) (eBioscience). For adherent HCC cells, the cells were detached with 5 mM EDTA and incubated with 30% pooled anti-GPC3− and -GPC3+ mice sera and then probed with goat-anti-mouse Dylight 488-labeled IgG (Life Technologies). The stained cells were analyzed by the FACSCanto II instrument (BD Bioscience, San Jose, CA) and FlowJo software.
Cell Proliferation Assay
Cell growth was examined by a CCK-8 assay (Dojindo, Rockville, MA). For the effect of anti-GPC3 sera on tumor cell growth, 1 × 104 HCC cells were seeded in 96-well plates. The freshly isolated anti-GPC3− and GPC3+ serum was pooled and then added to the cell culture medium at a concentration of 10% or 30%. After incubation at 37°C for 3 days, cell viability was performed by adding CCK-8 and measuring the OD450 nM. For the lymphocyte proliferation assay, 5 × 105 lymphocytes were harvested from immunized mice and seeded in 96-well plates in the presence of 5 μg/mL GPC3 protein. The CCK-8 reagent was added after 72 hr incubation.
Lactate Dehydrogenase Cytotoxicity Assay
Cytotoxic function of activated lymphocytes from immunized mice was determined by a lactate dehydrogenase (LDH) cytotoxicity assay (Pierce, Waltham, MA).48 Briefly, lymphocytes were regarded as effector cells and HCC cells were used as target cells. In 96-well plates, the effector cells (E) were co-cultured with 1 × 104 target cells (T) at E:T ratios of 25:1 or 100:1. After 48 hr, the supernatant was transferred into another 96-well plate in triplicated wells and an equal volume of reaction mixture was added. Absorbance was read at 490 and 680 nM.
ELISpot Assay
Antigen-specific IFN-γ and TNF-α secreting cells were assessed by the ELISpot assay (R&D Systems, Minneapolis, MN). Briefly, in the 96-well plates coated with anti-mouse IFN-γ or TNF-α mAbs, lymphocytes were cultured at two different densities (6 × 105 cells/mL and 3 × 106 cells/mL) in 100 μL of RPMI-1640 medium. GPC3 protein (5 μg/mL) and peptides GPC3395–404 KSFINFYSAL (H2-Kb restricted) and GPC3418–427 DTLCWNGQEL (H2-Db restricted) (10 μg/mL, synthesized by GenScript, Piscataway, NJ) were used as stimulators.54 The irrelevant peptide OVA-I SIINFEKL (InvivoGen) was used as the negative control.55 After 48 hr incubation at 37°C, the plates were washed and incubated with biotinylated anti-IFN-γ or anti-TNF-α antibody overnight at 4°C. On the next day, the plates were incubated with streptavidin-AP, and the spots were developed by adding BCIP/NBT chromogen. The spots were read and automatically counted using an AID EliSpot reader.
Immunohistochemical Staining Assay
Immunostaining of lymphocytes in tumor tissues was performed as previously described.56 The sections were probed with primary anti-CD3 and anti-CD8a mAb (eBioscience). The signal was developed with a diaminobenzidine substrate for 5 min.
Statistical Analysis
Data were expressed as mean ± SD. One-way ANOVA or Student’s t test was used to evaluate the significance of differences among the multiple experimental groups. The overall survival statistics were calculated using the log-rank test. Differences were considered significant at *p < 0.05 and highly significant at **p < 0.01 and not significant (N.S.) at p ≥ 0.05.
Author Contributions
Conceptualization, Q.W., T.L.T., D.R.N., P.R., C.Q.X., and C.L.; Methodology, Q.W., T.L.T., L.P., K.P., C.Z., M.X., Z.H., W.P., and P.R.; Investigation, Q.W., L.P., T.L.T., C.Z., Y.J., S.J., and W.P.; Writing – Original Draft, Q.W., L.P., T.L.T., C.Q.X. and C.L.; Writing – Review and Editing, Q.W., C.Q.X., D.O., and C.L.; Funding Acquisition, K.R., C.L. and L.P.; Supervision, C.Q.X. and C.L.
Conflicts of Interest
The authors declare there is no conflict of interest.
Acknowledgments
We thank Cathy Sun (Ph.D., Molecular Pathology Core at the University of Florida) for the immunohistochemical staining assay. This work was funded by National Institute on Alcohol Abuse and Alcoholism K01AA024174 and Children Miracle Network grants to L.P.
Footnotes
Supplemental Information includes Supplemental Materials and Methods and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.ymthe.2017.08.005.
Contributor Information
Chang Qing Xia, Email: xia@pathology.ufl.edu.
Chen Liu, Email: chen.liu@rutgers.edu.
Supplemental Information
References
- 1.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin. Liver Dis. 2005;25:143–154. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 2.Palmer D.H. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:2498. [PubMed] [Google Scholar]
- 3.Tatsumi T., Takehara T., Katayama K., Mochizuki K., Yamamoto M., Kanto T., Sasaki Y., Kasahara A., Hayashi N. Expression of costimulatory molecules B7-1 (CD80) and B7-2 (CD86) on human hepatocellular carcinoma. Hepatology. 1997;25:1108–1114. doi: 10.1002/hep.510250511. [DOI] [PubMed] [Google Scholar]
- 4.Pillarisetty V.G., Shah A.B., Miller G., Bleier J.I., DeMatteo R.P. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J. Immunol. 2004;172:1009–1017. doi: 10.4049/jimmunol.172.2.1009. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y., Li Y., Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015;6:e1792. doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhardwaj N., Li M., Price T., Maddern G.J. Spontaneous regression of a biopsy confirmed hepatocellular carcinoma. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-204897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizukoshi E., Nakamoto Y., Arai K., Yamashita T., Sakai A., Sakai Y., Kagaya T., Yamashita T., Honda M., Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–1216. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 8.Komori H., Nakatsura T., Senju S., Yoshitake Y., Motomura Y., Ikuta Y., Fukuma D., Yokomine K., Harao M., Beppu T. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin. Cancer Res. 2006;12:2689–2697. doi: 10.1158/1078-0432.CCR-05-2267. [DOI] [PubMed] [Google Scholar]
- 9.Capurro M., Wanless I.R., Sherman M., Deboer G., Shi W., Miyoshi E., Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology. 2003;125:89–97. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 10.Sayem M.A., Tomita Y., Yuno A., Hirayama M., Irie A., Tsukamoto H., Senju S., Yuba E., Yoshikawa T., Kono K. Identification of glypican-3-derived long peptides activating both CD8(+) and CD4(+) T cells; prolonged overall survival in cancer patients with Th cell response. OncoImmunology. 2015;5:e1062209. doi: 10.1080/2162402X.2015.1062209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawada Y., Yoshikawa T., Nobuoka D., Shirakawa H., Kuronuma T., Motomura Y., Mizuno S., Ishii H., Nakachi K., Konishi M. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin. Cancer Res. 2012;18:3686–3696. doi: 10.1158/1078-0432.CCR-11-3044. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda M., Ohkawa S., Okusaka T., Mitsunaga S., Kobayashi S., Morizane C., Suzuki I., Yamamoto S., Furuse J. Japanese phase I study of GC33, a humanized antibody against glypican-3 for advanced hepatocellular carcinoma. Cancer Sci. 2014;105:455–462. doi: 10.1111/cas.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng M., Gao W., Wang R., Chen W., Man Y.G., Figg W.D., Wang X.W., Dimitrov D.S., Ho M. Therapeutically targeting glypican-3 via a conformation-specific single-domain antibody in hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA. 2013;110:E1083–E1091. doi: 10.1073/pnas.1217868110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu A.X., Gold P.J., El-Khoueiry A.B., Abrams T.A., Morikawa H., Ohishi N., Ohtomo T., Philip P.A. First-in-man phase I study of GC33, a novel recombinant humanized antibody against glypican-3, in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2013;19:920–928. doi: 10.1158/1078-0432.CCR-12-2616. [DOI] [PubMed] [Google Scholar]
- 15.Abou-Alfa G.K., Puig O., Daniele B., Kudo M., Merle P., Park J.W., Ross P., Peron J.M., Ebert O., Chan S. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2016;65:289–295. doi: 10.1016/j.jhep.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt N., Neumann-Haefelin C., Thimme R. Cellular immune responses to hepatocellular carcinoma: lessons for immunotherapy. Dig. Dis. 2012;30:483–491. doi: 10.1159/000341697. [DOI] [PubMed] [Google Scholar]
- 17.Palucka K., Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambricht L., Vanvarenberg K., De Beuckelaer A., Van Hoecke L., Grooten J., Ucakar B., Lipnik P., Sanders N.N., Lienenklaus S., Préat V. Coadministration of a plasmid encoding HIV-1 gag enhances the efficacy of cancer DNA vaccines. Mol. Ther. 2016;24:1686–1696. doi: 10.1038/mt.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin C.C., Chou C.W., Shiau A.L., Tu C.F., Ko T.M., Chen Y.L., Yang B.C., Tao M.H., Lai M.D. Therapeutic HER2/Neu DNA vaccine inhibits mouse tumor naturally overexpressing endogenous neu. Mol. Ther. 2004;10:290–301. doi: 10.1016/j.ymthe.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Appay V., Douek D.C., Price D.A. CD8+ T cell efficacy in vaccination and disease. Nat. Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell D.A., Batich K.A., Gunn M.D., Huang M.N., Sanchez-Perez L., Nair S.K., Congdon K.L., Reap E.A., Archer G.E., Desjardins A. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–369. doi: 10.1038/nature14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 23.Nakano K., Orita T., Nezu J., Yoshino T., Ohizumi I., Sugimoto M., Furugaki K., Kinoshita Y., Ishiguro T., Hamakubo T. Anti-glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2009;378:279–284. doi: 10.1016/j.bbrc.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 24.Ishiguro T., Sugimoto M., Kinoshita Y., Miyazaki Y., Nakano K., Tsunoda H., Sugo I., Ohizumi I., Aburatani H., Hamakubo T. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 2008;68:9832–9838. doi: 10.1158/0008-5472.CAN-08-1973. [DOI] [PubMed] [Google Scholar]
- 25.Kalathil S., Lugade A.A., Miller A., Iyer R., Thanavala Y. Higher frequencies of GARP(+)CTLA-4(+)Foxp3(+) T regulatory cells and myeloid-derived suppressor cells in hepatocellular carcinoma patients are associated with impaired T-cell functionality. Cancer Res. 2013;73:2435–2444. doi: 10.1158/0008-5472.CAN-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finn O.J. Cancer immunology. N. Engl. J. Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 27.Scott A.M., Wolchok J.D., Old L.J. Antibody therapy of cancer. Nat. Rev. Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 28.Gao W., Kim H., Feng M., Phung Y., Xavier C.P., Rubin J.S., Ho M. Inactivation of Wnt signaling by a human antibody that recognizes the heparan sulfate chains of glypican-3 for liver cancer therapy. Hepatology. 2014;60:576–587. doi: 10.1002/hep.26996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y.F., Ho M. Humanization of high-affinity antibodies targeting glypican-3 in hepatocellular carcinoma. Sci. Rep. 2016;6:33878. doi: 10.1038/srep33878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang X., Xia C.Q. Administration of sulfosuccinimidyl-4-[N-maleimidomethyl] cyclohexane-1-carboxylate conjugated GP100(25-33) peptide-coupled spleen cells effectively mounts antigen-specific immune response against mouse melanoma. Biochem. Biophys. Res. Commun. 2015;468:46–52. doi: 10.1016/j.bbrc.2015.10.168. [DOI] [PubMed] [Google Scholar]
- 31.Ohlfest J.R., Andersen B.M., Litterman A.J., Xia J., Pennell C.A., Swier L.E., Salazar A.M., Olin M.R. Vaccine injection site matters: qualitative and quantitative defects in CD8 T cells primed as a function of proximity to the tumor in a murine glioma model. J. Immunol. 2013;190:613–620. doi: 10.4049/jimmunol.1201557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat. Rev. Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eager R., Nemunaitis J. GM-CSF gene-transduced tumor vaccines. Mol. Ther. 2005;12:18–27. doi: 10.1016/j.ymthe.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Ye G.W., Park J.B., Park Y.J., Choi Y.S., Sin J.I. Increased sensitivity of radiated murine cervical cancer tumors to E7 subunit vaccine-driven CTL-mediated killing induces synergistic anti-tumor activity. Mol. Ther. 2007;15:1564–1570. doi: 10.1038/sj.mt.6300149. [DOI] [PubMed] [Google Scholar]
- 36.Quakkelaar E.D., Melief C.J. Experience with synthetic vaccines for cancer and persistent virus infections in nonhuman primates and patients. Adv. Immunol. 2012;114:77–106. doi: 10.1016/B978-0-12-396548-6.00004-4. [DOI] [PubMed] [Google Scholar]
- 37.Guo Y., Werbel T., Wan S., Wu H., Li Y., Clare-Salzler M., Xia C.Q. Potent antigen-specific immune response induced by infusion of spleen cells coupled with succinimidyl-4-(N-maleimidomethyl cyclohexane)-1-carboxylate (SMCC) conjugated antigens. Int. Immunopharmacol. 2016;31:158–168. doi: 10.1016/j.intimp.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 38.Chen R., Snyder M. Promise of personalized omics to precision medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013;5:73–82. doi: 10.1002/wsbm.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collins F.S., Varmus H. A new initiative on precision medicine. N. Engl. J. Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dieu-Nosjean M.C., Giraldo N.A., Kaplon H., Germain C., Fridman W.H., Sautès-Fridman C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 2016;271:260–275. doi: 10.1111/imr.12405. [DOI] [PubMed] [Google Scholar]
- 41.Allan R.S., Waithman J., Bedoui S., Jones C.M., Villadangos J.A., Zhan Y., Lew A.M., Shortman K., Heath W.R., Carbone F.R. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Joyce J.A., Fearon D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 43.Voskoboinik I., Whisstock J.C., Trapani J.A. Perforin and granzymes: function, dysfunction and human pathology. Nat. Rev. Immunol. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 44.Mocellin S., Rossi C.R., Pilati P., Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Lee I.C., Huang Y.H., Chau G.Y., Huo T.I., Su C.W., Wu J.C., Lin H.C. Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int. J. Cancer. 2013;133:2895–2902. doi: 10.1002/ijc.28311. [DOI] [PubMed] [Google Scholar]
- 46.Matsui M., Machida S., Itani-Yohda T., Akatsuka T. Downregulation of the proteasome subunits, transporter, and antigen presentation in hepatocellular carcinoma, and their restoration by interferon-gamma. J. Gastroenterol. Hepatol. 2002;17:897–907. doi: 10.1046/j.1440-1746.2002.02837.x. [DOI] [PubMed] [Google Scholar]
- 47.Detjen K.M., Murphy D., Welzel M., Farwig K., Wiedenmann B., Rosewicz S. Downregulation of p21(waf/cip-1) mediates apoptosis of human hepatocellular carcinoma cells in response to interferon-gamma. Exp. Cell Res. 2003;282:78–89. doi: 10.1016/s0014-4827(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 48.Rao Q., Zuo B., Lu Z., Gao X., You A., Wu C., Du Z., Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64:456–472. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- 49.Calderaro J., Rousseau B., Amaddeo G., Mercey M., Charpy C., Costentin C., Luciani A., Zafrani E.S., Laurent A., Azoulay D. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology. 2016;64:2038–2046. doi: 10.1002/hep.28710. [DOI] [PubMed] [Google Scholar]
- 50.Hato T., Goyal L., Greten T.F., Duda D.G., Zhu A.X. Immune checkpoint blockade in hepatocellular carcinoma: current progress and future directions. Hepatology. 2014;60:1776–1782. doi: 10.1002/hep.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu H., Dong H., Eksioglu E., Hemming A., Cao M., Crawford J.M., Nelson D.R., Liu C. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology. 2007;133:1649–1659. doi: 10.1053/j.gastro.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Sahaf B., Heydari K., Herzenberg L.A., Herzenberg L.A. Lymphocyte surface thiol levels. Proc. Natl. Acad. Sci. USA. 2003;100:4001–4005. doi: 10.1073/pnas.2628032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu Q., Xiao S., Fan H., Li Y., Xu J., Li Z., Lu W., Su X., Zou W., Jin M. Protective immunity elicited by a pseudotyped baculovirus-mediated bivalent H5N1 influenza vaccine. Antiviral Res. 2011;92:493–496. doi: 10.1016/j.antiviral.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Iwama T., Horie K., Yoshikawa T., Nobuoka D., Shimomura M., Sawada Y., Nakatsura T. Identification of an H2-Kb or H2-Db restricted and glypican-3-derived cytotoxic T-lymphocyte epitope peptide. Int. J. Oncol. 2013;42:831–838. doi: 10.3892/ijo.2013.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Evel-Kabler K., Song X.T., Aldrich M., Huang X.F., Chen S.Y. SOCS1 restricts dendritic cells’ ability to break self tolerance and induce antitumor immunity by regulating IL-12 production and signaling. J. Clin. Invest. 2006;116:90–100. doi: 10.1172/JCI26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weng H.L., Liu Y., Chen J.L., Huang T., Xu L.J., Godoy P., Hu J.H., Zhou C., Stickel F., Marx A. The etiology of liver damage imparts cytokines transforming growth factor beta1 or interleukin-13 as driving forces in fibrogenesis. Hepatology. 2009;50:230–243. doi: 10.1002/hep.22934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.