Abstract
Birt-Hogg-Dubé syndrome (BHDS) is a rare disease with autosomal dominant inheritance that manifests through skin tumors, pulmonary cystic lesions, and renal tumors. A mutation of FLCN located on chromosome 17p11.2, which encodes a tumor-suppressor protein (folliculin), is responsible for the development of BHDS. We report the case of a patient presenting with spontaneous pneumothorax, in whom a familial genetic study revealed a novel nonsense mutation: p.(Arg379*) in FLCN.
Keywords: Birt-Hogg-Dubé syndrome, Pneumothorax, FLCN, Thoracoscopy, Video-assisted thoracic surgery
Case report
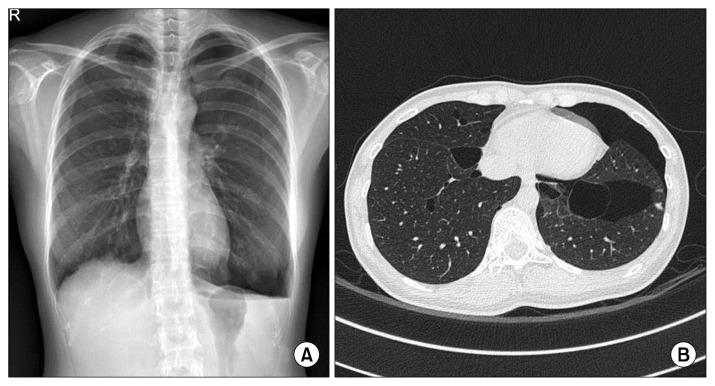
A 32-year-old female presented with an incidentally found pneumothorax during a workup for a vocal cord mass, after having experienced the symptom of voice change for several months. A chest X-ray and high-resolution chest tomography (HRCT) scan showed a left-side pneumothorax with a small amount of pleural effusion. In addition, several variably-sized thin-walled air cysts were found, mostly located on the basal or medial side of the bilateral lungs (Fig. 1). However, she had neither a history of smoking nor of pneumothorax. She had no history of underlying disease, such as an immunologic or collagen vascular disorder. Of note, her maternal relatives had a family history of pneumothorax. Because the multiple cysts in the bilateral lungs could increase the risk of recurrence, surgical repair was planned.
Fig. 1.
(A) A chest X-ray showing a left-sided pneumothorax. (B) A chest computed tomography scan reveals left-sided pneumothorax with the lower lung predominant, and several variably-sized thin-walled air cysts in both lungs.
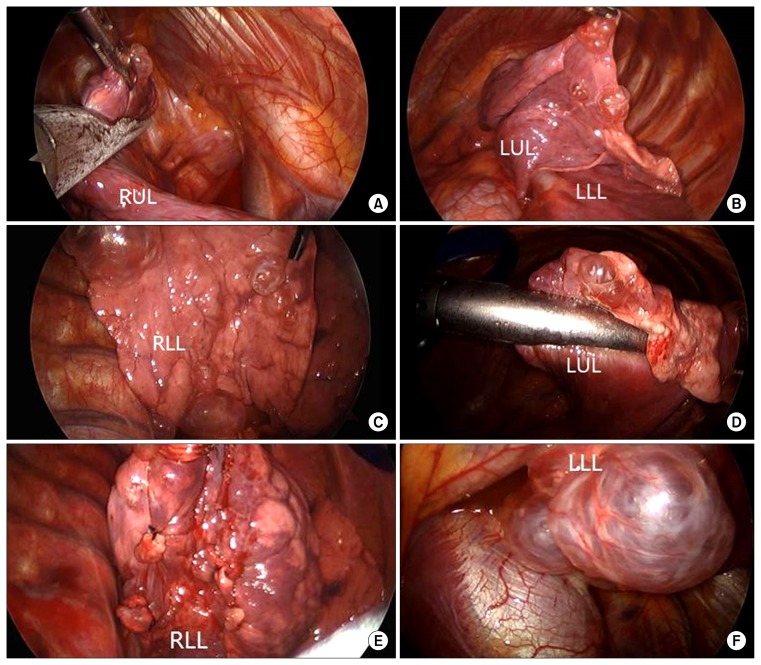
Video-assisted thoracoscopic surgery (VATS) was done for the single-stage resection of bilateral multiple cystic lesions. A double-lumen endotracheal tube was placed for bilateral sequential one-lung ventilation under general anaesthesia. The patient was placed in the lateral decubitus position, and 2-ports VATS exploration was performed using a 30° thoracoscope (Karl Storz endoscope; Karl Storz, Tuttlingen, Germany) for multiple wedge resections using endostaplers. Both bulla plication and ligation were also performed to remove multiple discrete small lesions, and sequential VATS for the contralateral side was conducted. There were multiple large cysts, especially at the base of the lower lobes, and some cysts were assessed as being at the stage of impending rupture (Fig. 2). After a air-leak test using saline solution, 20-Fr chest tubes were placed in the bilateral pleural space through the scope ports. Mechanical pleurodesis was not performed. The examination of the gross specimen revealed multiple, variably-sized subpleural cysts on both lungs, and the largest one measured up to 6.5×4.6 cm.
Fig. 2.
(A–F) Thoracoscopic findings showing wedge resection and ligation of multiple cystic lesions. RUL, right upper lobe; LUL, left upper lobe; LLL, left lower lobe; RLL, right lower lobe.
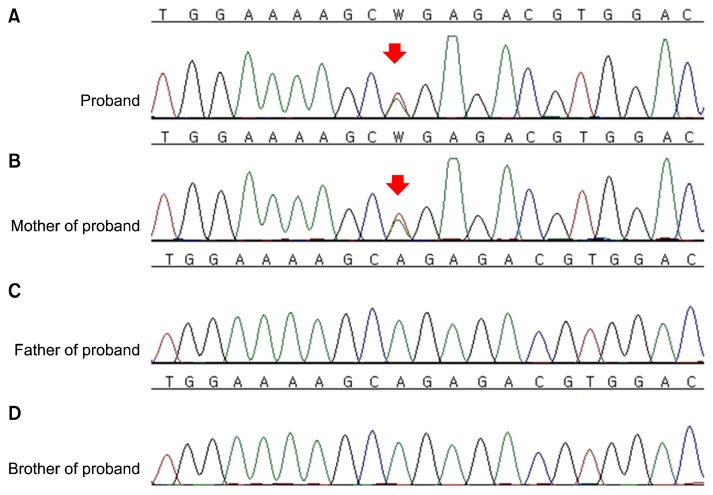
The HRCT and operative findings were uncommon; it is rare for young females to present with numerous thin-walled cysts in the bilateral lungs, especially in the lower lung fields. Furthermore, her grandmother had a history of pneumothorax at a relatively young age. After obtaining an informed consent for a genetic analysis with the suspicion of Birt-Hogg-Dubé syndrome (BHDS), genomic DNA was isolated from peripheral blood leukocytes using the QIAmp DNA Mini Kit (Qiagen, Hamburg, Germany). Polymerase chain reaction (PCR) was carried out using gene-specific primers for all coding exons and splice junctions of FLCN as previously described [1]. PCR amplicons were bidirectionally sequenced using a BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, USA) on an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). The chromatograms were analyzed using Sequencher ver. 5.0 (Gene Codes, Ann Arbor, MI, USA) and all mutations were described according to the Human Genome Variation Society nomenclature [2]. The RefSeq ID NM_144997.5 was used for alignment. Direct sequencing of the PCR products revealed a heterozygous, novel nonsense mutation, c.1135A>T; p.Arg379* in exon 10 of FLCN. This mutation has not been previously reported and is absent from locus-specific databases [3,4] as well as public databases (HGMD; ExAC; 1000 Genomes; dbSNP; and the ethnically specific Korean Reference Genome database, KRGDB). A subsequent familial genetic study was performed in both parents and an older brother of the proband, revealing an identical heterozygous mutation in the proband’s mother (Fig. 3).
Fig. 3.
Direct sequencing analysis of FLCN. (A) The proband was heterozygous for a nonsense mutation, c.1135A>T, p.Arg379*. (B) The same base change was also identified in her mother, but not in the proband’s father (C) or brother (D). The base substitution mutation is indicated by the red arrows.
The bilateral chest tubes were removed on postoperative day 1, and the patient was discharged on postoperative day 2 with complete resolution of the pneumothorax. The vocal cord mass was excised a month later, and was confirmed as a glomus tumor, measuring 2.0×1.2 cm. The patient has been followed for 12 months after the operation, and neither recurrence of pneumothorax nor other symptoms have been noted.
Discussion
BHDS is a rare inherited autosomal dominant systemic disorder, manifesting through skin lesions, pulmonary cystic lesions, and renal tumors. The European BHD consortium reported BHD patients should fulfill one major or two minor criteria (major criteria: (1) at least five fibrofolliculomas or trichodiscomas, at least one histologically confirmed, of adult onset; (2) pathogenic FLCN germline mutation; minor criteria: (1) bilateral basally located multiple lung cysts with no other apparent cause, with or without spontaneous primary pneumothorax; (2) early-onset [<50 years] or multifocal or bilateral renal cancer, or renal cancer of mixed chromophobe and oncocytic histology; (3) first-degree relative with BHD). The precise prevalence of BHDS remains unknown due to its considerable heterogeneity in terms of clinical symptoms, even within a family entity, and many patients with BHDS are underdiagnosed [5]. The skin-related findings, a triad of fibrofolliculomas, trichodiscomas, and acrochordons, usually develop in the third to fourth decades of life. Generally, the scalp, face, forehead, or neck is involved, with the manifestations growing in number and size, but the skin lesions alone are not diagnostic [6]. Toro et al. [7] first described the relationship between cystic lesions and pneumothorax, presenting an incidence rate of 33%–38%. Pulmonary cystic lesions have been reported in 77%–89% of BHDS patients, and usually manifest as multiple mostly well-defined, air-filled cysts varying in size and shape, from round to oval, lentiform, and often with a multiseptated appearance in the largest cyst. The large cysts tend to be located in the lower lobes of the bilateral lungs, and are asymptomatic, but pose a risk of spontaneous pneumothorax. The differential diagnoses include an unusual manifestation of bullous emphysema, lymphangioleiomyomatosis (LAM), pulmonary Langerhans cell histiocytosis (LCH), and lymphocytic interstitial pneumonia. LAM shows small to medium cysts in comparison with BHDS, and LCH demonstrates irregularly shaped cysts, including nodules, predominant in the upper lung [8]. The risk of kidney tumors is high, and approximately 15%–25% of patients develop multiple, bilateral, slowly-growing tumors that are diagnosed at the median age of 48 years old. In some family pedigrees, either kidney tumors or spontaneous pneumothorax can be found without skin manifestations [9].
The mutation of the folliculin gene (FLCN), which encodes a tumor suppressor protein and is located on the short arm of chromosome 17 (17p11.2), is responsible for BHDS with an autosomal dominant pattern, and was first identified in 2001. It is known that this gene may play a key role in pulmonary cystic lesions, and that it is expressed in stromal cells and pneumocytes. The mutation is detected in about 88% of patients using direct sequencing of the whole coding exon. However, cases have been reported with genetic deletions or duplications that were not detected by direct sequencing chromosome analysis in approximately 3%–5% of patients [10]. Numerous mutations have been described, and dal Sasso et al. [6] reported that up to 142 unique DNA mutations have been implicated. The 2 most common pathogenic variants are c.1285dupC and c.1285delC, and all pathogenic variants predict protein truncation [11], as is the case in this report of a novel nonsense mutation. In accordance with the tumor suppressor role of folliculin, the fact that all germline pathogenetic variants lead to protein truncation suggests that the loss of the folliculin protein is the basis of tumor formation in BHDS [12]. In Korea, few reports have been published about the mutations found in pneumothorax patients with BHDS [10,13,14]. In our case, we found a novel nonsense mutation, p. (Arg379*) in exon 10, as the cause of BHDS in a young female patient with multiple, bilateral pulmonary cystic lesions, and the mother of the proband showed the same mutation. In order to detect the recurrence of pneumothorax and renal malignancies, radiologic surveillance of the chest and abdomen should be performed in family members, as well as in the patient with BHDS. In conclusion, we report a surgically managed, rare case of BHDS that presented with incidentally found pneumothorax, and a familial genetic study that revealed a novel nonsense mutation.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Van Steensel MA, Verstraeten VL, Frank J, et al. Novel mutations in the BHD gene and absence of loss of heterozygosity in fibrofolliculomas of Birt-Hogg-Dube patients. J Invest Dermatol. 2007;127:588–93. doi: 10.1038/sj.jid.5700592. [DOI] [PubMed] [Google Scholar]
- 2.Den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 3.Lim DH, Rehal PK, Nahorski MS, et al. A new locus-specific database (LSDB) for mutations in the folliculin (FLCN) gene. Hum Mutat. 2010;31:E1043–51. doi: 10.1002/humu.21130. [DOI] [PubMed] [Google Scholar]
- 4.Wei MH, Blake PW, Shevchenko J, Toro JR. The folliculin mutation database: an online database of mutations associated with Birt-Hogg-Dube syndrome. Hum Mutat. 2009;30:E880–90. doi: 10.1002/humu.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menko FH, van Steensel MA, Giraud S, et al. Birt-Hogg-Dube syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 6.Dal Sasso AA, Belem LC, Zanetti G, et al. Birt-Hogg-Dube syndrome: state-of-the-art review with emphasis on pulmonary involvement. Respir Med. 2015;109:289–96. doi: 10.1016/j.rmed.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Toro JR, Pautler SE, Stewart L, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dube syndrome. Am J Respir Crit Care Med. 2007;175:1044–53. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal PP, Gross BH, Holloway BJ, Seely J, Stark P, Kazerooni EA. Thoracic CT findings in Birt-Hogg-Dube syndrome. AJR Am J Roentgenol. 2011;196:349–52. doi: 10.2214/AJR.10.4757. [DOI] [PubMed] [Google Scholar]
- 9.Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med. 2005;172:39–44. doi: 10.1164/rccm.200501-143OC. [DOI] [PubMed] [Google Scholar]
- 10.Kim J, Yoo JH, Kang DY, Cho NJ, Lee KA. Novel in-frame deletion mutation in FLCN gene in a Korean family with recurrent primary spontaneous pneumothorax. Gene. 2012;499:339–42. doi: 10.1016/j.gene.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt LS, Nickerson ML, Warren MB, et al. Germline BHD-mutation spectrum and phenotype analysis of a large cohort of families with Birt-Hogg-Dube syndrome. Am J Hum Genet. 2005;76:1023–33. doi: 10.1086/430842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vocke CD, Yang Y, Pavlovich CP, et al. High frequency of somatic frameshift BHD gene mutations in Birt-Hogg-Dube-associated renal tumors. J Natl Cancer Inst. 2005;97:931–5. doi: 10.1093/jnci/dji154. [DOI] [PubMed] [Google Scholar]
- 13.Kim EH, Jeong SY, Kim HJ, Kim YC. A case of Birt-Hogg-Dube syndrome. J Korean Med Sci. 2008;23:332–5. doi: 10.3346/jkms.2008.23.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park G, Seo HJ, Jang SJ, Shin BS, Hong R, Lee SK. A case of primary spontaneous pneumothorax with a three nucleotide deletion mutation of the FLCN gene. Korean J Thorac Cardiovasc Surg. 2010;43:824–8. doi: 10.5090/kjtcs.2010.43.6.824. [DOI] [Google Scholar]