Abstract
Reflux hypersensitivity, recently introduced by Rome IV as a new functional esophageal disorder, is currently considered as the presence of typical heartburn symptoms in patients with normal upper endoscopy and esophageal biopsies, normal esophageal pH test and with evidence of a close correlation between patients’ heartburn and reflux events. Reflux hypersensitivity is very common and together with functional heartburn accounts for more than 90% of the heartburn patients who failed treatment with proton pump inhibitor twice daily. In addition, reflux hypersensitivity affects primarily young to middle aged women, commonly overlaps with another functional gastrointestinal disorders, and is often associated with some type of psychological comorbidity. Diagnosis is made by using endoscopy with esophageal biopsies, pH-impedance, and high-resolution esophageal manometry. Reflux hypersensitivity is primarily treated with esophageal neuromodulators, such as tricyclic anti-depressants and selective serotonin reuptake inhibitors among others. Surgical anti-reflux management may also play an important role in the treatment of reflux hypersensitivity.
Keywords: Chest pain, Esophagus, Functional esophageal disorder, Heartburn, Reflux hypersensitivity
Introduction
The composition and definition of functional esophageal disorders have evolved over the years, primarily driven by the widely accepted Rome criteria. Presently, there are 5 functional esophageal disorders, functional heartburn, functional chest pain, reflux hypersensitivity, globus, and functional dysphagia (Table 1).1
Table 1.
Functional Esophageal Disorders (Rome IV)
| Functional chest pain |
| Functional heartburn |
| Reflux hypersensitivity |
| Globus |
| Functional dysphagia |
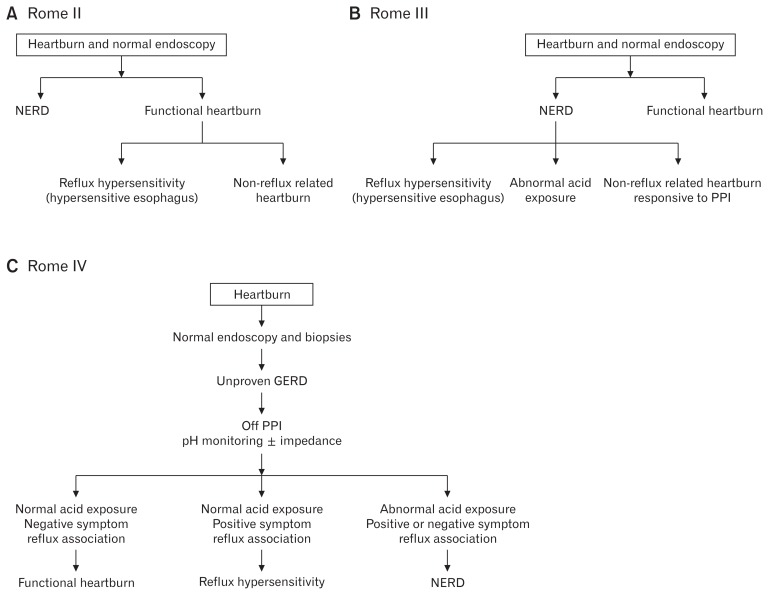
The evolution of the definitions of non-erosive reflux disease (NERD), functional heartburn, and recently reflux hypersensitivity, went in tandem with our improved capability to physiologically assess patients with heartburn who demonstrated normal esophageal mucosa on upper endoscopy. Rome II suggested that patients with heartburn and normal endoscopy are divided into patients with NERD (abnormal esophageal acid exposure) and those with functional heartburn (normal esophageal acid exposure).2 The functional heartburn group was further divided into patients with reflux related symptoms (hypersensitive esophagus) and those with heartburn unrelated to reflux symptoms. Rome III, on the other hand, proposed that patients with heartburn and normal endoscopy are divided into those with NERD and those with functional heartburn.3 However, unlike Rome II, Rome III divided NERD into 3 subgroups: patients with abnormal esophageal acid exposure, the hypersensitive esophagus group, and patients with non-reflux related symptoms who are responsive to proton pump inhibitor (PPI) treatment. In contrast, Rome IV proposed the introduction of the reflux hypersensitivity group (formerly called the hypersensitive esophagus) as a new functional esophageal disorder.1 Consequently, the group of NERD was reduced to only include patients with abnormal esophageal acid exposure with or without positive symptom association indexes. More importantly, Rome IV recognized that diagnosis of a functional esophageal disorder may take place while the patient is on anti-reflux treatment. In addition, Rome IV suggests for the first time the possibility of having functional heartburn or reflux hypersensitivity overlapping with gastroesophageal reflux disease (GERD).4 Figure 1 summarizes the evolution of reflux hypersensitivity from Rome II to Rome IV.
Figure 1.
The evolution of reflux hypersensitivity. NERD, non-erosive reflux disease; PPI, proton pump inhibitor; GERD, gastroesophageal reflux disease.
As a group, functional esophageal disorders are characterized by the presence of chronic symptoms attributed to the esophagus without evidence of structural, inflammatory, motor or metabolic disorder as the underlying etiology. According to the Rome IV criteria, diagnosis of a functional esophageal disorder requires having symptoms for the past 3 months with symptom onset at least 6 months before diagnosis. Non-esophageal causes for symptoms should be excluded first before esophageal etiology is entertained. GERD, major esophageal motility disorders, and eosinophilic esophagitis may be responsible for chronic heartburn symptoms. Hence, it is imperative that these conditions be ruled out before a diagnosis of any of the aforementioned functional esophageal disorders is established.
Although benign in nature, functional esophageal disorders including reflux hypersensitivity cause considerable impairment in quality of life and result in a significant economic burden on the health care system. Additionally, the limited understanding of the pathophysiologic basis of these conditions commonly results in frustration of patients as well as physicians. Moreover, therapies are mainly empiric in nature and, in many cases, of limited value.
Definition
Known in the past as the hypersensitive esophagus group, reflux hypersensitivity is a new functional esophageal disorder that was introduced for the first time by Rome IV.5
Based on the Rome IV criteria, the definition of reflux hypersensitivity includes retrosternal symptoms including heartburn or chest pain, normal endoscopy, and absence of eosinophilic esophagitis or major esophageal motor disorders (achalasia, esophagogastric junction outflow obstruction, distal esophageal spasm, jackhammer esophagus, and absent contractility) as the etiology of symptoms, and evidence of triggering of symptoms by reflux events despite normal acid exposure on pH or pH-impedance monitoring (Table 2).5 Criteria must be fulfilled for the last 3 months, with symptom onset at least 6 months prior to diagnosis with a frequency of at least twice a week. Importantly, response to anti-secretory therapy does not exclude the diagnosis. In addition, like functional heartburn, reflux hypersensitivity may overlap with GERD.5
Table 2.
Diagnostic Criteria for Reflux Hypersensitivity (Rome IV)
| Must include all of the following: |
|---|
|
Criteria fulfilled for the last 3 months with symptom onset at least 6 months prior to diagnosis with a frequency of at least twice a week.
Achalasia/esophagogastric junction outflow obstruction, diffuse esophageal spasm, jackhammer esophagus, and absent peristalsis.
Response to anti-secretory therapy dose not exclude the diagnosis.
The definition of reflux hypersensitivity emphasizes the need for positive symptom indexes to acidic or nonacidic reflux in the context of normal esophageal acid exposure, regardless if reflux assessment is done off or on therapy.
Epidemiology
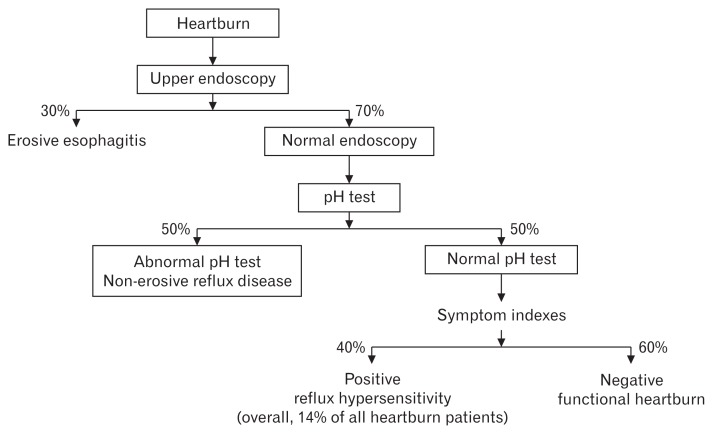
Studies have demonstrated that up to 70% of patients with heartburn have normal endoscopy.6 Of those, 50% have an abnormal pH test and thus belong to the NERD group. The other 50% are divided into functional heartburn (60%) and reflux hypersensitivity (40%). Thus, reflux hypersensitivity accounts for 14% of all patients presenting with heartburn (Fig. 2).
Figure 2.
Percentage of functional heartburn patients among patients with normal endoscopy.
Several recent studies, using pH-impedance monitoring evaluated the prevalence of reflux hypersensitivity. Savarino et al7 assessed 329 endoscopy negative patients with a pH-impedance monitoring off PPI treatment. The authors demonstrated that 40% of the patients had NERD, 24% functional heartburn, and 36% reflux hypersensitivity. The higher prevalence of reflux hypersensitivity in non-treated heartburn patients in this study likely reflects the incorporation of non-acidic reflux into the symptom indexes.
The prevalence of reflux hypersensitivity in patients with heartburn who failed PPI twice a day varies greatly. Savarino et al8 demonstrated in a retrospective study that 28% of the patients had reflux hypersensitivity and 39% functional heartburn. Patel et al9 reported that 29% of 266 refractory heartburn patients were found to have positive symptom association probability (SAP) with acidic reflux, weakly acidic reflux or both. Importantly, 6.50% had positive SAP only for acid reflux, 50.65% for weakly acidic reflux, and 42.86% for both. In another study, the authors demonstrated that 35.90% of 78 refractory heartburn patients who failed PPI twice-daily had reflux hypersensitivity.10 The last 2 trials suggest that both functional heartburn and reflux hypersensitivity account for more than 90% of the heartburn patients who failed twice-daily PPI. Thus, the introduction of Rome IV criteria for functional esophageal disorders clarified the main underlying mechanisms for failure of PPI treatment and consequently allowed new therapeutic options for these challenging patients.
Pathophysiology
Assessments of the underlying mechanisms for reflux hypersensitivity have been relatively scarce. As with the other functional esophageal disorders, esophageal hypersensitivity due to peripheral and/or central sensitization appears to be the main underlying mechanism for symptom generation. Esophageal hypersensitivity is defined as the perception of non-painful esophageal stimuli as being painful and the perception of painful esophageal stimuli as being more painful.11 In one study, the authors demonstrated that the reflux hypersensitivity group has the highest percentage of patients demonstrating increased chemo- and mechano-receptor sensitivity to acid perfusion and balloon distension, respectively, as compared with healthy subjects, patients with NERD, and those with functional heartburn.12
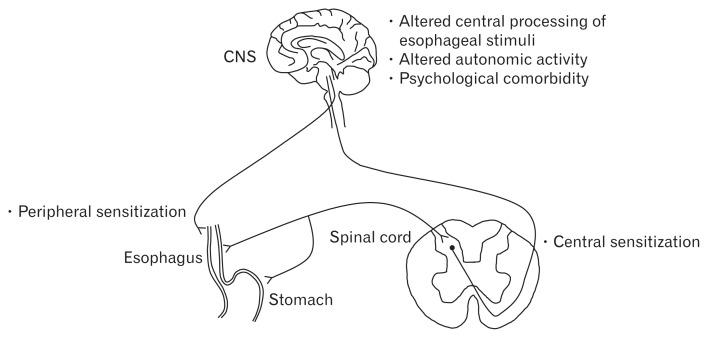
Other proposed underlying mechanisms for reflux hypersensitivity include altered central processing of esophageal stimuli, hypervigilance, altered autonomic activity, and psychological commodity (Fig. 3).13,14
Figure 3.
Underlying mechanisms for esophageal hypersensitivity in reflux hypersensitivity. Adapted from Miwa et al.14
What is unique about patients with reflux hypersensitivity as compared to those with functional heartburn is their sensitivity to physiological amounts of gastroesophageal reflux. Originally, it was noted that the reflux hypersensitivity group is sensitive to physiologic levels of acidic reflux. The introduction of the pH-impedance test revealed esophageal sensitivity to non-acid reflux as well in the context of normal esophageal acid exposure. In one study, the authors demonstrated that 29% of the patients (normal endoscopy and pH test) had a positive SAP with acid reflux using pH testing alone.15 However, when using pH-impedance, the percentage increased by an additional 19%, and thus 48% of the patients with normal esophageal acid exposure had positive SAP with acidic and/or non-acidic reflux.
Studies have produced conflicting results regarding the role of non-acidic reflux or proximal esophageal migration of gastroesophageal reflux in reflux hypersensitivity. Savarino et al7 demonstrated that an increased number of weakly acidic reflux events and a high rate of proximal reflux are the main causes of symptoms in reflux hypersensitivity patients who were evaluated with multichannel impedance (MII)-pH monitoring. In contrast, Tamura et al16 reported that the total and proximal acid reflux events were significantly higher in NERD patients with abnormal esophageal acid exposure as compared with reflux hypersensitivity patients. In addition, another study compared high-resolution esophageal manometry (HREM) and MII-pH monitoring values between NERD and reflux hypersensitivity patients. The authors demonstrated that NERD and reflux hypersensitivity patients showed similar values on HREM. However, NERD patients had greater acid exposure time, bolus exposure time, proximal and distal acid reflux events, and increased impairment of chemical clearance and mucosal integrity than reflux hypersensitivity patients. The authors also showed that distal non-acid reflux events were significantly more common in reflux hypersensitivity patients as compared with NERD patients.17
Frazzoni et al18 developed pH-impedance related parameters, post-reflux swallow-induced peristaltic wave index, and mean nocturnal baseline impedance. The former parameter may assess esophageal chemical clearance and the latter mucosal integrity. While these parameters have not achieved wide use in clinical practice and their clinical value remains to be determined, the authors have demonstrated that both parameters were significantly lower in NERD patients as compared to those with reflux hypersensitivity, and in both NERD and reflux hypersensitivity as compared to those with functional heartburn.18 However, the definition of reflux hypersensitivity used in this study included PPI responsiveness, which is not required by the Rome IV criteria. A recent study suggested that reflux hypersensitivity patients demonstrate a hypercontractile response of the distal esophagus due to acid swallowing as compared with functional heartburn patients.19
Regarding acid sensitive receptors, it was shown by immunostaining that transient receptor potential vanilloid-1 (TRPV1)-positive nerve fibers are increased in erosive esophagitis mucosa. In addition, an increased expression of TRPV1 has been demonstrated in NERD patients as well.20 Recently, Yoshida et al21 reported that esophageal hypersensitivity in NERD patients is related to neurogenic inflammation with an increase in both substance P release and neurokinin-1 receptor expression, which may be associated with the activation of TRPV1 and protease-activated receptor 2. These acid-sensitive receptors are likely involved in esophageal hypersensitivity of patients with reflux hypersensitivity.
Studies have also demonstrated that central factors, such as stress, hypervigilance psychological disorders, and poor sleep, play an important role in enhancing perception of intraesophageal stimuli.22 Psychological factors have been shown to be an important factor in the generation and exacerbation of the overlap syndrome in functional gastrointestinal disorders.23 Acute psychological stress has been shown to increase sensitivity to intraesophageal acid perfusion in patients with GERD.24 The increase in perceptual responses to acid was associated with greater emotional response to the stressor and was not related to the presence or absence of esophageal mucosal breaks. Recent daily stressful life events have been associated with symptom onset or exacerbation and may alter perception thresholds for pain.5 Acute stress by itself can lead to increase in esophageal mucosal permeability and the development of dilated intraepithelial spaces.25,26 These mechanisms suggest a complex relationship among stress, acid exposure, and esophageal hypersensitivity in generating reflux symptoms.
Clinical Presentation
The clinical presentation of reflux hypersensitivity is not different from the clinical presentation of functional heartburn patients. Many of the original studies, using the Rome II criteria for functional heartburn, did not distinguish between the 2 groups of patients. However, like functional heartburn, one cannot differentiate among any of the phenotypic presentations of GERD and heartburn-related functional esophageal disorders, using patients’ severity, frequency, or duration of heartburn symptoms.27 Overlap symptoms with other functional bowel disorders, GERD and psychological comorbidity are not uncommon.5,28 A recent study suggested that anxiety may be more common in reflux hypersensitivity patients as compared with functional heartburn patients.29
In one study, the authors demonstrated that 66.5% of reflux hypersensitivity patients were woman, 15.1% smokers, 39.0% alcohol consumers, 47.7% with hiatal hernia, 4.1% Helicobacter pylori-positive, 48.2% with irritable bowel syndrome diagnosis, 35.8% with anxiety, 6.0% with depression, and the mean body mass index was 24.1 kg/m2.30 Using multivariate logistic regression analysis as compared with GERD, the authors found that female gender, irritable bowel syndrome diagnosis, hiatal hernia, H. pylori status, and anxiety are associated with reflux hypersensitivity. However, more studies were needed to better describe the typical demographics of reflux hypersensitivity patients.
Diagnosis
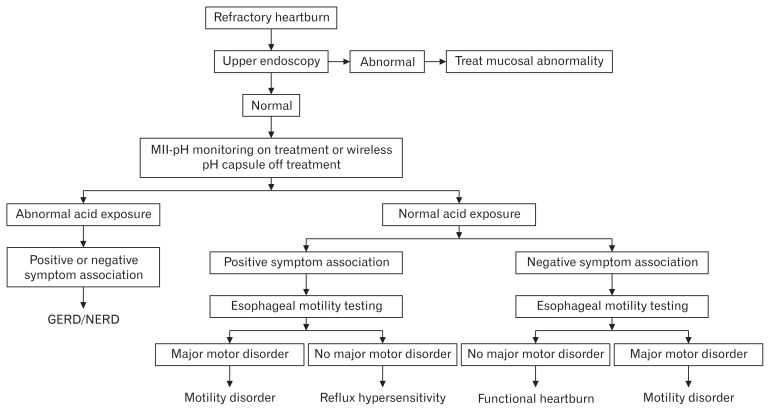
The process of diagnosing reflux hypersensitivity is similar to the algorithm required for diagnosing functional heartburn. Rome IV proposed 2 diagnostic pathways for reflux hypersensitivity: one in patients on anti-reflux treatment and the other in patients off anti-reflux treatment.5 In patients on PPI treatment, assessment should start with an upper endoscopy and biopsies to rule out eosinophilic esophagitis. If the test is normal, then the GERD history of the patient will determine the next step. If the patient has a positive history of GERD (abnormal endoscopy and/or pH testing), then pH-impedance on PPI treatment should be performed. If the patient has no history of GERD, then a wireless pH capsule should be done off PPI treatment. In case any of the aforementioned tests is normal, symptom indexes should be assessed and, if positive, then the diagnosis of reflux hypersensitivity is established. All patients should undergo HREM to exclude major esophageal motor disorders. In patients with a history of GERD (abnormal upper endoscopy and/or abnormal pH test), the diagnosis would be reflux hypersensitivity that is overlapping with GERD (Fig. 4). Table 3 demonstrates a typical wireless pH capsule result.
Figure 4.
Diagnostic algorithm of reflux hypersensitivity in refractory heartburn patients (failed proton pump inhibitor twice daily). MII, multichannel impedance; GERD, gastroesophageal reflux disease; NERD, non-erosive reflux disease.
Table 3.
A Typical Wireless pH Capsule of a 52 Year Old Female With Reflux Hypersensitivity, Who Failed Proton Pump Inhibitor Twice Daily. The Test Was Done Off Treatment
| Days | Fraction time pH < 4 (%) | ||
|---|---|---|---|
|
| |||
| Upright | Supine | Total | |
| Day #1 | 2.6 | 0.0 | 1.4 |
| Day #2 | 1.3 | 0.0 | 0.8 |
| Combined | 1.9 | 0.0 | 1.1 |
|
| |||
| Heartburn (%) | Chest pain (%) | ||
|
| |||
| Symptom index | 22.2 | 50.0 | |
| Symptom association probability | 99.9 | 99.6 | |
In patients with burning retrosternal discomfort or pain who are not on anti-reflux medication, then the diagnostic algorithm is basically similar to those on anti-reflux treatment, except the patients should undergo pH testing using the wireless pH capsule.
Treatment
Patients with reflux hypersensitivity will likely benefit from assurance about the nature of their disorder. However, many patients may require further medical intervention, some with a more comprehensive approach. The latter may include psychologists or psychiatrists, alternative/complimentary medicine therapists, acupuncturists as well as other experts in functional medicine.
Because patients with reflux hypersensitivity have symptoms that are triggered by reflux events, anti-reflux therapeutic modalities have been considered as first line therapy. They include medical, endoscopic, and surgical interventions that are also used to treat GERD. The role of diet and lifestyle modification related to GERD in patients with reflux hypersensitivity remains unknown.31 Histamine-2 receptor antagonists (H2RA) have been shown to reduce esophageal chemoreceptor sensitivity to acid.32 Ranitidine (single 150 mg dose) significantly decreased esophageal sensitivity to acid infusion as compared with placebo in patients with Rome II-defined functional heartburn.33 These studies suggest that patients with reflux hypersensitivity may benefit from a trial of H2RA. When patients with Rome II-defined functional heartburn received PPI twice daily, only those with positive symptoms index (the reflux hypersensitivity group) responded to treatment.34 This study suggests that further suppression of gastric acid and minimization of esophageal acid exposure may improve symptoms in patients with reflux hypersensitivity. It is unclear if standard dose twice-daily PPI is a “ceiling dose,” like in GERD patients, or, in those with reflux hypersensitivity, an even higher dose may still have a therapeutic effect.
It has been reported that patients with a positive symptom index and SAP who are not responsive to PPI therapy, but demonstrate evidence of persistent non-acid or acid reflux using MII-pH monitoring can be treated successfully with laparoscopic Nissen fundoplication.35 Surgical anti-reflux management can provide reflux control for carefully selected reflux hypersensitivity patients.9 Similarly, Broeders et al36 reported that laparoscopic Nissen fundoplication drastically reduced the incidence of acid and weakly acidic reflux, as well as liquid and mixed reflux episodes. However, there are still very few studies that have assessed the value of surgical fundoplication in reflux hypersensitivity patients and thus more data are needed. The current clinical approach is to avoid surgery in reflux hypersensitivity patients and to consider it only in a small number of very carefully selected patients.
As with all functional esophageal disorders, neuromodulators are considered to be the cornerstone of therapy of reflux hypersensitivity. However, there are almost no studies assessing their value in this patient population.
Tricyclic anti-depressants (TCA) have been shown to be efficacious in controlling esophageal pain in patients with functional esophageal disorders (functional chest pain, globus, and non-cardiac chest pain).11 Presently, there are no TCA studies in reflux hypersensitivity patients. The range of initial therapeutic dose is 10–50 mg/day, and the range of maximal therapeutic dose is 25–150 mg/day. Dosing changes of TCAs should depend on symptom improvement and development of side effects. Generally, patients are started on 5–10 mg once a day. Due to their anti-cholinergic and sedative side effects, TCAs are commonly administered before bedtime.
Selective serotonin reuptake inhibitors (SSRIs) have also been shown to be efficacious in various patients with functional esophageal disorders including, functional chest pain, esophageal hypersensitivity, NERD, and refractory heartburn.37–41 It is the only neuromodulator that was tested in patients with reflux hypersensitivity. In a randomized, double-blind, placebo-controlled study, 75 patients with reflux hypersensitivity were randomized to receive citalopram 20 mg or placebo. At the end of the follow-up period which lasted 6 months, 38.5% of the patients receiving citalopram and 66.7% of those receiving placebo continued to report heartburn symptoms (P = 0.021).42 The study suggested that citalopram was effective in controlling heartburn in patients with reflux hypersensitivity.
SSRIs have only 5-hydroxytryptamine activity and thus have less side effects as compared with TCAs. In addition, this class of drugs is better tolerated than TCAs. Dosing (initial and maximal dose) of SSRIs in functional disorders differ from one medication to another, fluoxetine, 10–80 mg/day, paroxetine, 10–60 mg/day, citalopram, 10–40 mg/day, and sertraline, 25–200 mg/day, respectively.
Another neuromodulator is Trazodone, which was solely evaluated for the treatment of non-cardiac chest pain. Its value in reflux hypersensitivity remained to be elucidated.
Of all serotonin-norepinephrine reuptake inhibitors, only venlafaxine has been studied in a functional esophageal disorder. While considered to be the most efficacious anti-depressant in reducing esophageal pain and improving global health assessment, it has been associated with agitation and inability to fall asleep.
Other esophageal neuromodulators include adenosine antagonists (theophylline), ondansetron, tegaserod, octreotide, gabapentin and pregabalin. All of them have been scarcely studied in functional esophageal disorders with some level of success. Thus far, none of those compounds was evaluated in patients with reflux hypersensitivity.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150:1262–1279. e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Drossman DA, Crazziari R, Talley NJ, Thompson WG, Whitehead WE the Rome II Multinational Working Teams. Rome II: the functional gastrointestinal disorders. 2nd ed. McLean: Degnon Associates, Inc; 2000. [Google Scholar]
- 3.Drossman DA. Rome III: the functional gastrointestinal disorders. 3rd ed. McLean: Degnon Associates, Inc; 2006. [Google Scholar]
- 4.Schmulson MJ, Drossman DA. What is new in Rome IV. J Neurogastroenterol Motil. 2017;23:151–163. doi: 10.5056/jnm16214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Esophageal disorders. Gastroenterology. 2016;150:1368–1379. doi: 10.1053/j.gastro.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Martinez SD, Malagon IB, Garewal HS, Cui H, Fass R. Non-erosive reflux disease (NERD)--acid reflux and symptom patterns. Aliment Pharmacol Ther. 2003;17:537–545. doi: 10.1046/j.1365-2036.2003.01423.x. [DOI] [PubMed] [Google Scholar]
- 7.Savarino E, Zentilin P, Tutuian R, et al. Impedance-pH reflux patterns can differentiate non-erosive reflux disease from functional heartburn patients. J Gastroenterol. 2012;47:159–168. doi: 10.1007/s00535-011-0480-0. [DOI] [PubMed] [Google Scholar]
- 8.Savarino E, Marabotto E, Zentilin P, et al. The added value of impedance-pH monitoring to Rome III criteria in distinguishing functional heartburn from non-erosive reflux disease. Dig Liver Dis. 2011;43:542–547. doi: 10.1016/j.dld.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Patel A, Sayuk GS, Gyawali CP. Prevalence, characteristics, and treatment outcomes of reflux hypersensitivity detected on pH-impedance monitoring. Neurogastroenterol Motil. 2016;28:1382–1390. doi: 10.1111/nmo.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roman S, Keefer L, Imam H, et al. Majority of symptoms in esophageal reflux PPI non-responders are not related to reflux. Neurogastroenterol Motil. 2015;27:1667–1674. doi: 10.1111/nmo.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickman R, Maradey-Romero C, Fass R. The role of pain modulators in esophageal disorders - no pain no gain. Neurogastroenterol Motil. 2014;26:603–610. doi: 10.1111/nmo.12339. [DOI] [PubMed] [Google Scholar]
- 12.Bruley des Varanness S, Shi G, Scarpignato C, Galmiche JP. Sensitivity to acid and distension in gastro oesophageal reflux disease (GORD) and the acid hypersensitive oesophagus. Gut. 1996;39(suppl 3):A182. [Google Scholar]
- 13.Hershcovici T, Fass R. Nonerosive reflux disease (NERD) - an update. J Neurogastroenterol Motil. 2010;16:8–21. doi: 10.5056/jnm.2010.16.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miwa H, Kondo T, Oshima T, Fukui H, Tomita T, Watari J. Esophageal sensation and esophageal hypersensitivity - overview from bench to bedside. J Neurogastroenterol Motil. 2010;16:353–362. doi: 10.5056/jnm.2010.16.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savarino E, Zentilin P, Tutuian R, et al. The role of nonacid reflux in NERD: lessons learned from impedance-pH monitoring in 150 patients off therapy. Am J Gastroenterol. 2008;103:2685–2693. doi: 10.1111/j.1572-0241.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- 16.Tamura Y, Funaki Y, Izawa S, et al. Pathophysiology of functional heartburn based on Rome III criteria in Japanese patients. World J Gastroenterol. 2015;21:5009–5016. doi: 10.3748/wjg.v21.i16.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao F, Gao Y, Chen X, Qian J, Zhang J. Comparison of oesophageal function tests between Chinese non-erosive reflux disease and reflux hypersensitivity patients. BMC Gastroenterol. 2017;17:67. doi: 10.1186/s12876-017-0624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frazzoni M, de Bortoli N, Frazzoni L, et al. Impairment of chemical clearance and mucosal integrity distinguishes hypersensitive esophagus from functional heartburn. J Gastroenterol. 2017;52:444–451. doi: 10.1007/s00535-016-1226-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Lee SK, Park JC, Shin SK, Lee YC. Effect of acid swallowing on esophageal contraction in patients with heartburn related to hypersensitivity. J Gastroenterol Hepatol. 2013;28:84–89. doi: 10.1111/j.1440-1746.2012.07258.x. [DOI] [PubMed] [Google Scholar]
- 20.Guarino MP, Cheng L, Ma J, et al. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterol Motil. 2010;22:746–751. e219. doi: 10.1111/j.1365-2982.2010.01514.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida N, Kuroda M, Suzuki T, et al. Role of nociceptors/neuropeptides in the pathogenesis of visceral hypersensitivity of nonerosive reflux disease. Dig Dis Sci. 2013;58:2237–2243. doi: 10.1007/s10620-012-2337-7. [DOI] [PubMed] [Google Scholar]
- 22.Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591–599. [PubMed] [Google Scholar]
- 23.Jang SH, Ryu HS, Choi SC, Lee SY. Psychological factors influence the overlap syndrome in functional gastrointestinal disorders and their effect on quality of life among firefighters in South Korea. J Dig Dis. 2016;17:236–243. doi: 10.1111/1751-2980.12330. [DOI] [PubMed] [Google Scholar]
- 24.Fass R, Naliboff BD, Fass SS, et al. The effect of auditory stress on perception of intraesophageal acid in patients with gastroesophageal reflux disease. Gastroenterology. 2008;134:696–705. doi: 10.1053/j.gastro.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Naliboff BD, Mayer M, Fass R, et al. The effect of life stress on symptoms of heartburn. Psychosom Med. 2004;66:426–434. doi: 10.1097/01.psy.0000124756.37520.84. [DOI] [PubMed] [Google Scholar]
- 26.Farré R, De Vos R, Geboes K, et al. Critical role of stress in increased oesophageal mucosa permeability and dilated intercellular spaces. Gut. 2007;56:1191–1197. doi: 10.1136/gut.2006.113688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lind T, Havelund T, Lundell L, et al. On demand therapy with omeprazole for the long-term management of patients with heartburn without oesophagitis--a placebo-controlled randomized trial. Aliment Pharmacol Ther. 1999;13:907–914. doi: 10.1046/j.1365-2036.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 28.Fass R. Erosive esophagitis and nonerosive reflux disease (NERD): comparison of epidemiologic, physiologic, and therapeutic characteristics. J Clin Gastroenterol. 2007;41:131–137. doi: 10.1097/01.mcg.0000225631.07039.6d. [DOI] [PubMed] [Google Scholar]
- 29.Bilgi MM, Vardar R, Yıldırım E, Veznedaroğlu B, Bor S. Prevalence of psychiatric comorbidity in symptomatic gastroesophageal reflux subgroups. Dig Dis Sci. 2017;62:984–993. doi: 10.1007/s10620-016-4273-4. [DOI] [PubMed] [Google Scholar]
- 30.de Bortoli N, Frazzoni L, Savarino EV, et al. Functional heartburn overlaps with irritable bowel syndrome more often than GERD. Am J Gastroenterol. 2016;111:1711–1717. doi: 10.1038/ajg.2016.432. [DOI] [PubMed] [Google Scholar]
- 31.Dickman R, Fass R. Functional heartburn. Curr Treat Options Gastroenterol. 2005;8:285–291. doi: 10.1007/s11938-005-0021-0. [DOI] [PubMed] [Google Scholar]
- 32.Marrero JM, de Caestecker JS, Maxwell JD. Effect of famotidine on oesophageal sensitivity in gastro-oesophageal reflux disease. Gut. 1994;35:447–450. doi: 10.1136/gut.35.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Stanley S, Ciociola AA, Zubaidi S, Proskin HM, Miner PB., Jr A single dose of ranitidine 150 mg modulates oesophageal acid sensitivity in patients with functional heartburn. Aliment Pharmacol Ther. 2004;20:975–982. doi: 10.1111/j.1365-2036.2004.02217.x. [DOI] [PubMed] [Google Scholar]
- 34.Watson RG, Tham TC, Johnston BT, McDougal NI. Double blind cross-over placebo controlled study of omeprazole in the treatment of patients with reflux symptoms and physiological levels of acid reflux--the “sensitive oesophagus”. Gut. 1997;40:587–590. doi: 10.1136/gut.40.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mainie I, Tutuian R, Agrawal A, Adams D, Castell DO. Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg. 2006;93:1483–1487. doi: 10.1002/bjs.5493. [DOI] [PubMed] [Google Scholar]
- 36.Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Effects of anti-reflux surgery on weakly acidic reflux and belching. Gut. 2011;60:435–441. doi: 10.1136/gut.2010.224824. [DOI] [PubMed] [Google Scholar]
- 37.Varia I, Logue E, O’connor C, et al. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J. 2000;140:367–372. doi: 10.1067/mhj.2000.108514. [DOI] [PubMed] [Google Scholar]
- 38.Doraiswamy PM, Varia I, Hellegers C, et al. A randomized controlled trial of paroxetine for noncardiac chest pain. Psychopharmacol Bull. 2006;39:15–24. [PubMed] [Google Scholar]
- 39.Spinhoven P, Van der Does AJ, Van Dijk E, Van Rood YR. Heart-focused anxiety as a mediating variable in the treatment of noncardiac chest pain by cognitive-behavioral therapy and paroxetine. J Psychosom Res. 2010;69:227–235. doi: 10.1016/j.jpsychores.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Broekaert D, Fischler B, Sifrim D, Janssens J, Tack J. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2006;23:365–370. doi: 10.1111/j.1365-2036.2006.02772.x. [DOI] [PubMed] [Google Scholar]
- 41.Ostovaneh MR, Saeidi B, Hajifathalian K, et al. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: double-blind placebo-controlled trial. Neurogastroenterol Motil. 2014;26:670–678. doi: 10.1111/nmo.12313. [DOI] [PubMed] [Google Scholar]
- 42.Viazis N, Keyoglou A, Kanellopoulos AK, et al. Selective serotonin reuptake inhibitors for the treatment of hypersensitive esophagus: a randomized, double-blind, placebo-controlled study. Am J Gastroenterol. 2012;107:1662–1667. doi: 10.1038/ajg.2011.179. [DOI] [PubMed] [Google Scholar]