Abstract
Background/Aims
To assess the long-term effect of Helicobacter pylori eradication on symptomatic improvement according to the type of antibiotic and the duration of treatment in H. pylori-associated functional dyspepsia.
Methods
We searched Pubmed, Embase, CINAHL, and the Cochrane library databases for randomized controlled trials written in English and undertaken up to August 2016 that met our eligibility criteria. The search methodology used combinations of the following keywords: Helicobacter pylori OR H. pylori OR HP; dyspepsia OR functional dyspepsia OR non-ulcer dyspepsia; eradication OR cure OR treatment. The study outcome was the summary odds ratio (OR) for symptomatic improvement in H. pylori-associated functional dyspepsia with successful eradication therapy. Subgroup analyses were performed based on the type of antibiotic, and the duration of treatment, whether or not patients had symptoms of irritable bowel syndrome, and on race.
Results
Sixteen randomized controlled trials met the inclusion criteria. The summary OR for symptomatic improvement in patients in our eradication group was 1.33 (95% confidence interval [CI], 1.16–1.54; P < 0.01). In a subgroup analysis on type of antibiotic, symptomatic improvement with metronidazole-containing regimen (OR, 1.87; 95% CI, 1.26–2.77) was better than treatment with clarithromycin (OR, 1.29; 95% CI, 1.11–1.50). H. pylori eradication therapy given for 10–14 days was the more effective for symptom improvement than 7-day therapy. When the studies excluding irritable bowel syndrome cases were analyzed, there were no therapeutic effects of H. pylori eradication on symptomatic improvement.
Conclusions
In the clinical setting, the most effective H. pylori eradication regimen for functional dyspepsia to provide relief of symptoms is a metronidazole-based treatment regimen for at least 10 days. The explanation for this is that H. pylori-associated functional dyspepsia could be associated with dysbiosis.
Keywords: Eradication, Functional dyspepsia, Helicobacter pylori
Introduction
Functional dyspepsia (FD) is a common functional gastrointestinal disorder with reported prevalence rates of between 11% and 29.2%.1 Its symptoms are characterized by multifunctional disorders of the upper gastrointestinal (GI) tract mediated by altered GI motility,2 abnormal acid secretion,3 visceral hypersensitivity,4 an imbalance of the autonomic nervous system,5 psychological factors, and Helicobacter pylori infection.6 The role of H. pylori infection in functional dyspepsia is not fully elucidated, and the therapeutic gains and symptomatic improvements following H. pylori eradication have been matters of debate.
In the literature, the main mechanisms of symptom development in H. pylori-associated functional dyspepsia reported were altered GI motility and mucosal inflammation.6–12 Increasing eosinophils of the duodenum secondary to the exposure of gastric acid or food allergens to the duodenum might correlate with FD, and it has been suggested that there is a correlation between eosinophils and low-grade inflammation.13,14 Furthermore, it has been shown that the patients with microscopic duodenitis achieve greater symptomatic responses to H. pylori eradication than those without microscopic duodenitis.15 In addition to a direct inflammatory response of the GI tract mucosa, decreased gastric acid secretions are common in patients with H. pylori-associated FD.7,8 This could be closely related to dysbiosis of gut microbiota. Recently, sequencing analysis of gut microbiota showed that H. pylori was not alone in the stomach and the interaction of H. pylori with those microorganisms might play a role in H. pylori-associated functional dyspepsia.16,17 In this study, we hypothesize that H. pylori infected patients diagnosed as FD might have dysbiosis of the gut microbiota. Most of them would also have irritable bowel syndrome (IBS).
Previous meta-analyses reported that eradication therapy produced marginal therapeutic gain and relief of symptoms in H. pylori-associated FD, although results differed depending on trial design and study selection criteria and quality.10–12,18–20 However, there has been no systemic review of the differences in efficacy according to the eradication regimen. We hypothesized that we would find different therapeutic effects for different eradication regimens. Broad spectrum antibiotics and longer treatment durations that had a sufficient effect on gut microbial imbalance would result in better symptomatic improvements after H. pylori eradication. The aim of this study was to evaluate whether the therapeutic gain would be different in patients with H. pylori-associated FD according to the type of antibiotic and duration of treatment. When FD studies excluding IBS cases were analyzed, we compared the therapeutic effects of H. pylori eradication on symptomatic improvement. We also compared the therapeutic effects in Asian and non-Asian populations, because there is considerable heterogeneity of symptoms and pathophysiology based on ethnic differences.
Materials and Methods
Search Strategy
The meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement.21 The following procedure was used to collect clinical randomized controlled trials (RCTs) written in English undertaken up to August 2016 from the Pubmed, Embase, Cochrane library and CINAHL digital dissertation databases (undertaken by N.J.K., Medical Library, The Catholic University of Korea, Seoul, Korea). We used the following Medical Subject Heading (MeSH) terms and/or text words: H. pylori OR Helicobacter pylori OR HP AND dyspepsia OR functional dyspepsia OR non-ulcer dyspepsia OR FD AND eradication OR treatment OR cure. Two persons (Y.J.K. and W.C.C.) independently reviewed all potentially relevant material to determine whether or not it met the inclusion criteria.
Inclusion and Exclusion Criteria
To be included in our meta-analysis studies had to meet following criteria; (1) RCTs with at least a 12-month follow-up period, (2) study population of patients with dyspepsia (symptom-based Rome I, II, or III classification), (3) symptoms of dyspepsia defined as an outcome measurement of the study, (4) H. pylori infection verified by rapid urease test, 13C breath test, or histologic examination, (5) treatment group received triple or quadruple therapy for 7–14 days, and (6) control group who only received placebo as intervention and were not treated with any other antibiotics or bismuth.
Studies were excluded if they were available only as an abstract, a review study, a case report, a study without raw data available for retrieval, a duplicate publication or if they were not written in English. When the patients of enrolled studies complained of reflux-like symptoms only, those studies were excluded.
Study Outcomes
The primary outcome of this study was the weighted summary odds ratio (OR) of symptomatic improvement in patients with and without H. pylori eradication with 95% CI. All of these analyses were planned a priori. Additional post hoc analyses were performed based the type of antibiotic, duration of therapy, and racial differences. The secondary outcomes were to analyze and compare the OR for each eradication antibiotic and duration of treatment (1 week vs longer than 10 days). Racial differences were compared by comparing Asian and non-Asian study populations. To evaluate the association of H. pylori-associated FD and IBS, we tried to find out whether IBS was included in methods of the articles. Since it was not clear if that IBS included the all studies, we reviewed the articles which mentioned IBS exclusion definitely studies using criteria of Rome II/III and explicit mentioned studies of IBS exclusion.
Study Selection and Data Extraction
Two investigators (Y.J.K. and W.C.C.) independently assessed the titles and abstracts of all retrieved papers and assessed their eligibility for inclusion in our meta-analysis. Full articles were scrutinized if the title and abstract were ambiguous. The reviewers used a standardized approach to conduct the literature search, data extraction, and quality assessment. The data sought included the study population, baseline characteristics of the participants, details related to the protocol, year of publication, therapy regimen of H. pylori eradications, definition of symptom relief and study outcome. When the results were combined and any disagreement occurred, this was resolved by the investigators.
Statistical Methods
The R language meta-package version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for the meta-analysis. The summary OR with 95% CI was evaluated for quantitative analyses. Heterogeneity among the studies was measured using Higgin’s I2 statistics and 95% CIs were calculated. When there was substantial heterogeneity (I2 > 50%), all analyses were based on the random-effect model (DerSimonian-Laird method); otherwise (I2 < 50%), the fixed effect model (inverse variance method) was used. To test for publication bias, we used a test for asymmetry of the funnel plot. A P-value of less than 0.05 was considered statistically significant. Meta-analysis of variance (ANOVA) was used for subgroup analysis based on type of antibiotic and duration of treatment regimen. All the statistical methods of this study were performed by S.H.K. and J.H.Y. from Bio-Age, Medical Development Institute, Seoul, Korea.
Results
Study Characteristics
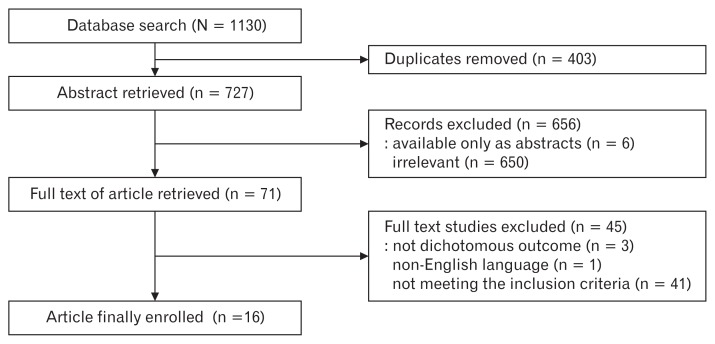
The initial search strategy identified 1130 articles. After removal of duplicates, the 727 remaining articles were screened. After reviewing the title and abstracts, 6 trials were available only as abstracts and 650 trials were irrelevant, leaving 71 potentially relevant studies. In the remaining articles, 1 study was excluded because the manuscript was not written in English, 3 trials did not use dichotomous outcomes, and 41 trials did not meet the eligibility criteria. Reviewing the full manuscripts, we identified 16 RCTs with a total of 1920 subjects that met the inclusion criteria (Table 1 and Fig. 1).22–37
Table 1.
The Baseline Characteristics of the Enrolled Studies
| Studies | Country | HP eradication rate | Age | Sex | Smoking | Drinking | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | Control | ||
| Ang et al,22 2006 | Singapore | 73.2% | 0% | 38.6 | 38.4 | 35.2% | 37.3% | ||||
| Blum et al,23 1998 | Multicenter | 79% | 2% | 47 | 47 | 39.6% | 43.3% | 18.3% | 16.5% | 28.0% | 25.6% |
| Froehlich et al,25 2001 | Switzerland | 75% | 4% | 43.6 ± 12.4 | 45.6 ± 14.2 | 49% | 40% | ||||
| Gisbert et al,26 2004 | Spain | 76% | 42 | 41 | 30% | 28% | 33% | 36% | |||
| Gwee et al,27 2009 | Singapore | 68.3% | 4.9% | 44.7 ± 11.4 | 36.1 ± 12.1 | 43.9% | 46.3% | 30.7% | 26.7% | 17.9% | 27.8% |
| Hsu et al,28 2001 | China | 78% | 0% | 50.3 ± 15.1 | 51.6 ± 16.4 | 49.4% | 47.6% | 15.7% | 14.6% | 7.2% | 4.9% |
| Koskenpato et al,29 2001 | Finland | 82% | 1% | 51.5 ± 9.5 | 51.8 ± 11.8 | 30% | 39% | 54% | 59% | ||
| Mazzoleni et al,31 2011 | Brazil | 88.6% | 7.4% | 46.1 ± 12.4 | 46.0 ± 12.2 | 23.4% | 19.2% | 45.3% | 39.4% | 15.4% | 14.3% |
| Mazzoleni et al,30 2006 | Brazil | 91.3% | 0% | 43.2 ± 11.9 | 39.2 ± 13.8 | 19.6% | 25.6% | 17.4% | 11.6% | 13.0% | 16.3% |
| McColl et al,32 1998 | UK | 88% | 5% | 42 ± 12 | 42.2 ± 13 | 51% | 47% | 34% | 33% | ||
| Sodhi et al,33 2013 | India | 66.6% | 6.6% | 46 | 43 | 29.3% | 35.7% | 33% | 20% | ||
| Talley et al,34 1999 | Australia | 85% | 4% | 51 ± 14 | 49 ± 13 | 38% | 33% | 20% | 27.5% | 38% | 36% |
| Talley et al,35 1999 | Australia | 90% | 2% | 46.3 | 46.5 | 43% | 48% | 27% | 26% | 41% | 41% |
| Veldhuyzen van Zanten et al,36 2003 | Canada | 82% | 6% | 47 ± 13 | 49 ± 13 | 41.3% | 50.0% | 34.7% | 26.8% | 20.6% | 23.2% |
| Bruley Des Varannes et al,24 2001 | France | 69% | 18% | 50 ± 16 | 52 ± 14 | 43% | 46% | 26% | 27% | 28% | 30% |
| Yazdanbod et al,37 2015 | Iran | 80.3% | 4.9% | ||||||||
HP, Helicobacter pylori.
Figure 1.
Flow diagram of the study.
Study Result
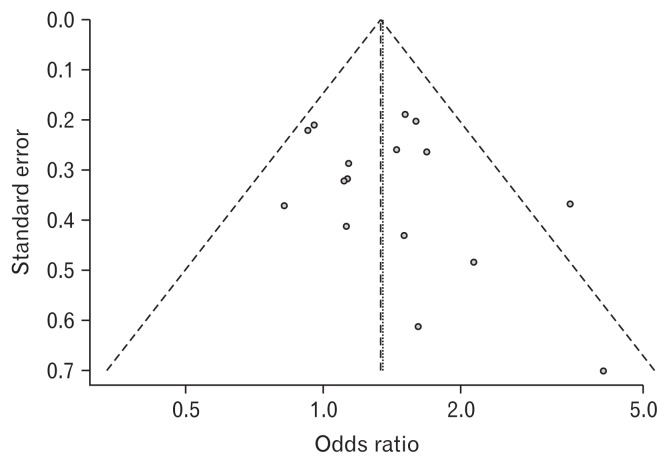
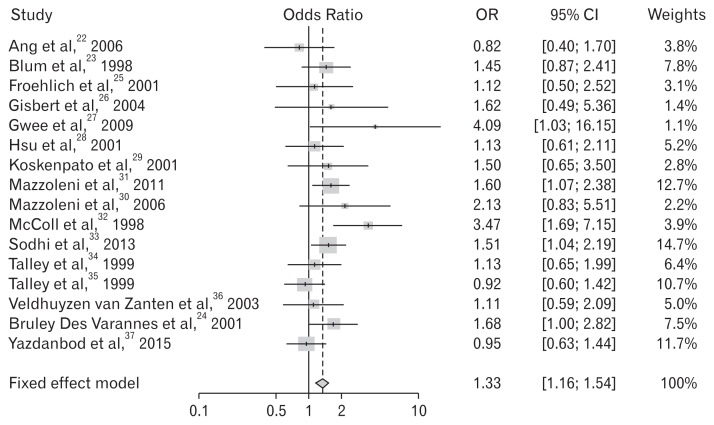
The cumulative eradication rate of H. pylori in the treatment group was 80.3% (Table 1). In the meta-analysis of all 16 RCTs, 13 trials reported successful symptom relief results. Figure 2 shows the existence of publication bias according to the test for the asymmetry of the funnel plot (P = 0.338). Because there was no significant heterogeneity (Higgins’ I2 = 27.0%), we summarized OR using a fixed effect model. The summary OR was 1.33 (95% CI, 1.16–1.54; P < 0.01), indicating that the patients with H. pylori-associated FD had a greater than 1.33-fold increased probability of symptom relief after eradication. (Table 2 and Fig. 3)
Figure 2.
Funnel plot. P = 0.338.
Table 2.
The Results of Meta-analysis for the Effects of Helicobacter pylori Eradication on Functional Dyspepsia
| Studies | Treatment | Control | OR | 95% lower CI | 95% upper CI | Weights | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Improved patients (n) | Total (n) | Proportion | Improved patients (n) | Total (n) | Proportion | ||||||
| Ang et al,22 2006 | 45 | 71 | 0.634 | 40 | 59 | 0.678 | 0.822 | 0.397 | 1.704 | 3.8 | |
| Blum et al,23 1998 | 45 | 164 | 0.274 | 34 | 164 | 0.207 | 1.446 | 0.868 | 2.408 | 7.8 | |
| Froehlich et al,25 2001 | 15 | 92 | 0.163 | 13 | 88 | 0.148 | 1.124 | 0.501 | 2.521 | 3.1 | |
| Gisbert et al,26 2004 | 21 | 34 | 0.618 | 8 | 16 | 0.500 | 1.615 | 0.487 | 5.361 | 1.4 | |
| Gwee et al,27 2009 | 10 | 41 | 0.244 | 3 | 41 | 0.073 | 4.086 | 1.034 | 16.155 | 1.1 | |
| Hsu et al,28 2001 | 47 | 81 | 0.580 | 44 | 80 | 0.550 | 1.131 | 0.606 | 2.110 | 5.2 | |
| Koskenpato et al,29 2001 | 16 | 77 | 0.208 | 11 | 74 | 0.149 | 1.502 | 0.646 | 3.496 | 2.8 | |
| Mazzoleni et al,31 2011 | 94 | 201 | 0.468 | 72 | 203 | 0.355 | 1.598 | 1.072 | 2.383 | 12.7 | |
| Mazzoleni et al,30 2006 | 16 | 46 | 0.348 | 9 | 45 | 0.200 | 2.133 | 0.825 | 5.514 | 2.2 | |
| McColl et al,32 1998 | 33 | 160 | 0.206 | 11 | 158 | 0.070 | 3.472 | 1.686 | 7.152 | 3.9 | |
| Sodhi et al,33 2013 | 95 | 259 | 0.367 | 72 | 260 | 0.277 | 1.513 | 1.044 | 2.192 | 14.7 | |
| Talley et al,34 1999 | 32 | 133 | 0.241 | 31 | 142 | 0.218 | 1.135 | 0.646 | 1.991 | 6.4 | |
| Talley et al,35 1999 | 69 | 170 | 0.406 | 71 | 167 | 0.425 | 0.924 | 0.599 | 1.425 | 10.7 | |
| Veldhuyzen van Zanten et al,36 2003 | 44 | 75 | 0.587 | 46 | 82 | 0.561 | 1.111 | 0.590 | 2.093 | 5.0 | |
| Bruley Des Varannes et al,24 2001 | 55 | 129 | 0.426 | 38 | 124 | 0.306 | 1.682 | 1.003 | 2.821 | 7.5 | |
| Yazdanbod et al,37 2015 | 87 | 186 | 0.468 | 83 | 173 | 0.480 | 0.953 | 0.630 | 1.443 | 11.7 | |
| Total (Fixed effect model) | 1.334 | 1.157 | 1.537 | 100.0 | < 0.01 | ||||||
P-value of test of heterogeneity among studies = 0.152; Higgins’ I2 = 27.0% (0.0%, 60.0%).
Figure 3.
Forest plot for the effects of Helicobacter pylori eradication on the symptom relief in patients with functional dyspepsia.
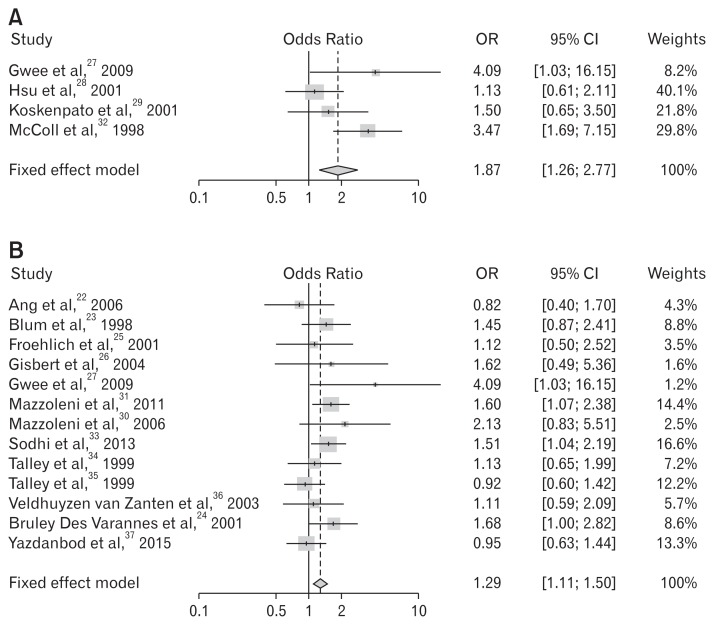
We analyzed symptom improvement according to the antibiotic type. The meta-ANOVA analysis showed more significant improvements with metronidazole (OR, 1.87; 95% CI, 1.26–2.77; P < 0.01) compared to clarithromycin (OR, 1.29; 95% CI, 1.11–1.50; P < 0.01) in the eradication group. Tinidazole was included in the metronidazole group as antibiotics of the same drug class, nitroimidazole antimicrobial. The metronidazole-based regimen produced more therapeutic gain than the clarithromycin-based regimen in H. pylori-associated FD (Table 3 and 4 and Fig. 4).
Table 3.
Meta-analysis on Symptomatic Improvement Following Helicobacter pylori Eradication by the Regimen Including Nitroimidazole Antimicrobial (Metronidazole and Tinidazole)
| Studies | Treatment | Control | OR | 95% lower CI | 95% upper CI | Weights | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Improved patients (n) | Total (n) | Proportion | Improved patients (n) | Total (n) | Proportion | ||||||
| Gwee et al,27 2009 | 10 | 41 | 0.244 | 3 | 41 | 0.073 | 4.086 | 1.034 | 16.155 | 8.2 | |
| Hsu et al,28 2001 | 47 | 81 | 0.580 | 44 | 80 | 0.550 | 1.131 | 0.606 | 2.110 | 40.1 | |
| Koskenpato et al,29 2001 | 16 | 77 | 0.208 | 11 | 74 | 0.149 | 1.502 | 0.646 | 3.496 | 21.8 | |
| McColl et al,32 1998 | 33 | 160 | 0.206 | 11 | 158 | 0.070 | 3.472 | 1.686 | 7.152 | 29.8 | |
| Total (Fixed effect model) | 1.870 | 1.260 | 2.774 | 100.0 | < 0.01 | ||||||
P-value of test of heterogeneity among studies = 0.223; Higgins’ I2 = 56.0% (0.0%, 85.4%).
Table 4.
Meta-analysis on Symptomatic Improvement Following Helicobacter pylori Eradication by the Regimen Including Clarithromycin
| Studies | Treatment | Control | OR | 95% lower CI | 95% upper CI | Weights | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Improved patients (n) | Total (n) | Proportion | Improved patients (n) | Total (n) | Proportion | ||||||
| Ang et al,22 2006 | 45 | 71 | 0.634 | 40 | 59 | 0.678 | 0.822 | 0.397 | 1.704 | 4.3 | |
| Blum et al,23 1998 | 45 | 164 | 0.274 | 34 | 164 | 0.207 | 1.446 | 0.868 | 2.408 | 8.8 | |
| Froehlich et al,25 2001 | 15 | 92 | 0.163 | 13 | 88 | 0.148 | 1.124 | 0.501 | 2.521 | 3.5 | |
| Gisbert et al,26 2004 | 21 | 34 | 0.618 | 8 | 16 | 0.500 | 1.615 | 0.487 | 5.361 | 1.6 | |
| Gwee et al,27 2009 | 10 | 41 | 0.244 | 3 | 41 | 0.073 | 4.086 | 1.034 | 16.155 | 1.2 | |
| Mazzoleni et al,31 2011 | 94 | 201 | 0.468 | 72 | 203 | 0.355 | 1.598 | 1.072 | 2.383 | 14.4 | |
| Mazzoleni et al,30 2006 | 16 | 46 | 0.348 | 9 | 45 | 0.200 | 2.133 | 0.825 | 5.514 | 2.5 | |
| Sodhi et al,33 2013 | 95 | 259 | 0.367 | 72 | 260 | 0.277 | 1.513 | 1.044 | 2.192 | 16.6 | |
| Talley et al,34 1999 | 32 | 133 | 0.241 | 31 | 142 | 0.218 | 1.135 | 0.646 | 1.991 | 7.2 | |
| Talley et al,35 1999 | 69 | 170 | 0.406 | 71 | 167 | 0.425 | 0.924 | 0.599 | 1.425 | 12.2 | |
| Veldhuyzen van Zanten et al,36 2003 | 44 | 75 | 0.587 | 46 | 82 | 0.561 | 1.111 | 0.590 | 2.093 | 5.7 | |
| Bruley Des Varannes et al,24 2001 | 55 | 129 | 0.426 | 38 | 124 | 0.306 | 1.682 | 1.003 | 2.821 | 8.6 | |
| Yazdanbod et al,37 2015 | 87 | 186 | 0.468 | 83 | 173 | 0.480 | 0.953 | 0.630 | 1.443 | 13.3 | |
| Total (Fixed effect model) | 1.286 | 1.106 | 1.497 | 100.0 | 0.001 | ||||||
P-value of test of heterogeneity among studies = 0.351; Higgins’ I2 = 9.5% [0.0%, 47.9%]
Figure 4.
Forest plots for the effects of Helicobacter pylori eradication on the symptom relief in patients with functional dyspepsia according to antibiotic use. (A) Metronidazole containing regimen. (B) Clarithromycin containing regimen.
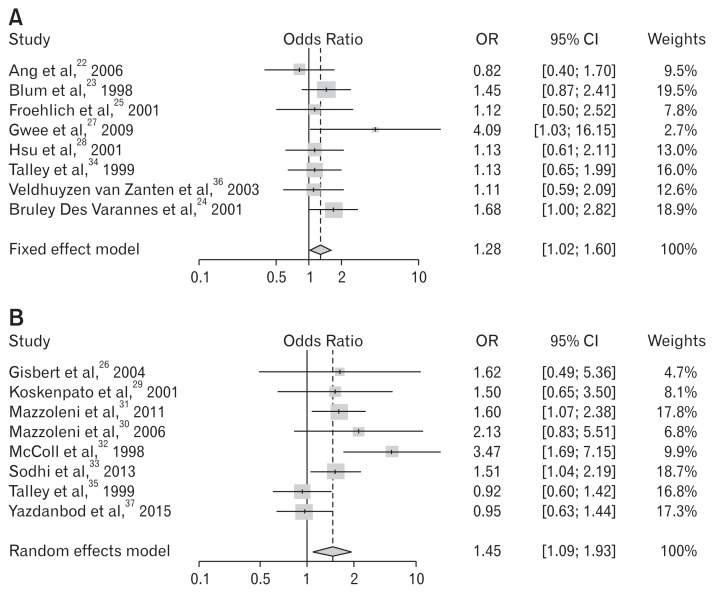
The selected studies used different treatment regimen durations (7, 10, or 14 day regimen). We divided the trials into 2 subgroups according to the duration of treatment regimen. Eight trials used a 7-day regimen and the remaining 8 trials administered a regimen of 10 days or more. Subgroup analysis confirmed that a regimen of 10 days or more yielded superior relief of symptoms (OR, 1.45; 95% CI, 1.09–1.93; P = 0.011). There was a significant difference between the 2 groups (Table 5 and Fig. 5).
Table 5.
Meta-analysis on Symptomatic Improvement Following Helicobacter pylori Eradication According to Duration
(A) 7-day regimen
| Studies | Treatment | Control | OR | 95% lower CI | 95% upper CI | Weights | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Improved patients (n) | Total (n) | Proportion | Improved patients (n) | Total (n) | Proportion | ||||||
| Ang et al,22 2006 | 45 | 71 | 0.634 | 40 | 59 | 0.678 | 0.822 | 0.397 | 1.704 | 9.5 | |
| Blum et al,23 1998 | 45 | 164 | 0.274 | 34 | 164 | 0.207 | 1.446 | 0.868 | 2.408 | 19.5 | |
| Froehlich et al,25 2001 | 15 | 92 | 0.163 | 13 | 88 | 0.148 | 1.124 | 0.501 | 2.521 | 7.8 | |
| Gwee et al,27 2009 | 10 | 41 | 0.244 | 3 | 41 | 0.073 | 4.086 | 1.034 | 16.155 | 2.7 | |
| Hsu et al,28 2001 | 47 | 81 | 0.580 | 44 | 80 | 0.550 | 1.131 | 0.606 | 2.110 | 13.0 | |
| Talley et al,34 1999 | 32 | 133 | 0.241 | 31 | 142 | 0.218 | 1.135 | 0.646 | 1.991 | 16.0 | |
| Veldhuyzen van Zanten et al,36 2003 | 44 | 75 | 0.587 | 46 | 82 | 0.561 | 1.111 | 0.590 | 2.093 | 12.6 | |
| Bruley Des Varannes et al,24 2001 | 55 | 129 | 0.426 | 38 | 124 | 0.306 | 1.682 | 1.003 | 2.821 | 18.9 | |
| Total (Fixed effect model) | 1.281 | 1.023 | 1.605 | 100.0 | 0.031 | ||||||
P-value of test of heterogeneity among studies = 0.532; Higgins’ I2 = 0.0% (0.0%, 62.6%).
(B) 10-day or more regimen
| Studies | Treatment | Control | OR | 95% lower CI | 95% upper CI | Weights | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Improved patients (n) | Total (n) | Proportion | Improved patients (n) | Total (n) | Proportion | ||||||
| Gisbert et al,26 2004 | 21 | 34 | 0.618 | 8 | 16 | 0.500 | 1.615 | 0.487 | 5.361 | 4.7 | |
| Koskenpato et al,29 2001 | 16 | 77 | 0.208 | 11 | 74 | 0.149 | 1.502 | 0.646 | 3.496 | 8.1 | |
| Mazzoleni et al,31 2011 | 94 | 201 | 0.468 | 72 | 203 | 0.355 | 1.598 | 1.072 | 2.383 | 17.8 | |
| Mazzoleni et al,30 2006 | 16 | 46 | 0.348 | 9 | 45 | 0.200 | 2.133 | 0.825 | 5.514 | 6.8 | |
| McColl et al,32 1998 | 33 | 160 | 0.206 | 11 | 158 | 0.070 | 3.472 | 1.686 | 7.152 | 9.9 | |
| Sodhi et al,33 2013 | 95 | 259 | 0.367 | 72 | 260 | 0.277 | 1.513 | 1.044 | 2.192 | 18.7 | |
| Talley et al,35 1999 | 69 | 170 | 0.406 | 71 | 167 | 0.425 | 0.924 | 0.599 | 1.425 | 16.8 | |
| Yazdanbod et al,37 2015 | 87 | 186 | 0.468 | 83 | 173 | 0.480 | 0.953 | 0.630 | 1.443 | 17.3 | |
| Total (random effect model) | 1.447 | 1.088 | 1.925 | 100.0 | 0.011 | ||||||
P-value of test of heterogeneity among studies = 0.046; Higgins’ I2 = 51.0% (0.0%, 78.1%).
Figure 5.
Forest plots for the effects of Helicobacter pylori eradication on the symptom relief in patients with functional dyspepsia according to duration of regimen. (A) Treatment period: 1 week. (B) Treatment period: longer than 1 week.
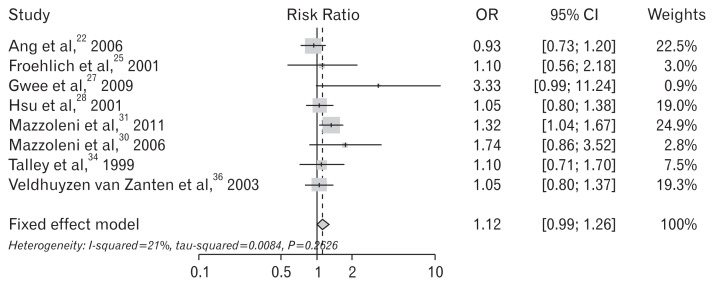
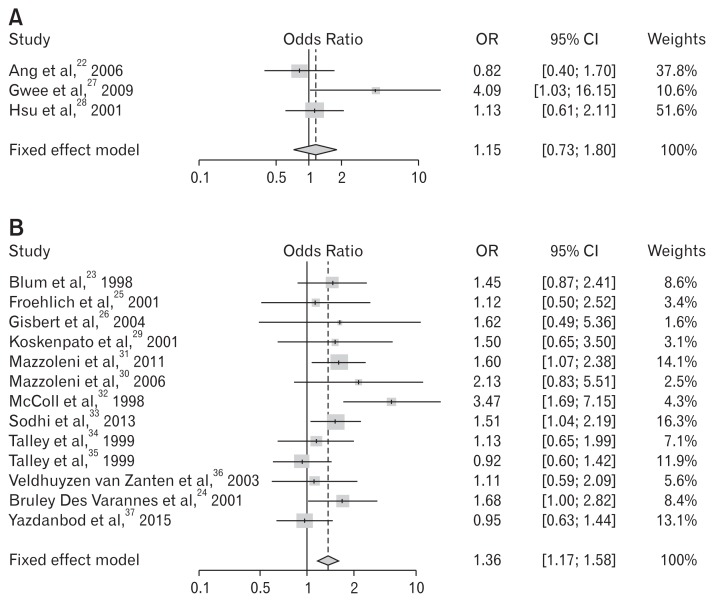
When exclusive diagnostic criteria for FD (excluding IBS) were adopted in the study enrollment, 8 studies were sub-analyzed - studies using criteria of Rome II/III24,30,31,34 and explicit mentioned studies of IBS exclusion.22,25,27,28 The summary OR was statistically insignificant (OR, 1.12; 95% CI, 0.99–1.26; P = 0.263) (Fig. 6). Thirteen studies that were performed on non-Asian populations showed statistically significant benefits for dyspeptic symptom relief, whereas the 3 trials performed on Asian populations revealed insignificant results (Table 6 and Fig. 7).
Figure 6.
Forest plots for the effect of Helicobacter pylori eradication on the symptom relief in patients with functional dyspepsia - its diagnostic criteria excluding irritable bowel syndrome was adopted.
Table 6.
Meta-analysis on Symptomatic Improvement Following Helicobacter pylori Eradication by Ethnic Difference
(A) Asian
| Studies | Treatment | Control | OR | 95% lower CI | 95% upper CI | Weights | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Improved patients (n) | Total (n) | Proportion | Improved patients (n) | Total (n) | Proportion | ||||||
| Ang et al,22 2006 | 45 | 71 | 0.634 | 40 | 59 | 0.678 | 0.822 | 0.397 | 1.704 | 37.8 | |
| Gwee et al,27 2009 | 10 | 41 | 0.244 | 3 | 41 | 0.073 | 4.086 | 1.034 | 16.155 | 10.6 | |
| Hsu et al,28 2001 | 47 | 81 | 0.580 | 44 | 80 | 0.550 | 1.131 | 0.606 | 2.110 | 51.6 | |
| Total (Fixed effect model) | 1.149 | 0.734 | 1.799 | 100.0 | 0.542 | ||||||
P-value of test of heterogeneity among studies = 0.152; Higgins’ I2 = 27.0% (0.0%, 60.0%).
(B) Non-Asian
| Studies | Treatment | Control | OR | 95% lower CI | 95% upper CI | Weights | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Improved patients (n) | Total (n) | Proportion | Improved patients (n) | Total (n) | Proportion | ||||||
| Blum et al,23 1998 | 45 | 164 | 0.274 | 34 | 164 | 0.207 | 1.446 | 0.868 | 2.408 | 8.6 | |
| Froehlich et al,25 2001 | 15 | 92 | 0.163 | 13 | 88 | 0.148 | 1.124 | 0.501 | 2.521 | 3.4 | |
| Gisbert et al,26 2004 | 21 | 34 | 0.618 | 8 | 16 | 0.500 | 1.615 | 0.487 | 5.361 | 1.6 | |
| Koskenpato et al,29 2001 | 16 | 77 | 0.208 | 11 | 74 | 0.149 | 1.502 | 0.646 | 3.496 | 3.1 | |
| Mazzoleni et al,31 2011 | 94 | 201 | 0.468 | 72 | 203 | 0.355 | 1.598 | 1.072 | 2.383 | 14.1 | |
| Mazzoleni et al,30 2006 | 16 | 46 | 0.348 | 9 | 45 | 0.200 | 2.133 | 0.825 | 5.514 | 2.5 | |
| McColl et al,32 1998 | 33 | 160 | 0.206 | 11 | 158 | 0.070 | 3.472 | 1.686 | 7.152 | 4.3 | |
| Sodhi et al,33 2013 | 95 | 259 | 0.367 | 72 | 260 | 0.277 | 1.513 | 1.044 | 2.192 | 16.3 | |
| Talley et al,34 1999 | 32 | 133 | 0.241 | 31 | 142 | 0.218 | 1.135 | 0.646 | 1.991 | 7.1 | |
| Talley et al,35 1999 | 69 | 170 | 0.406 | 71 | 167 | 0.425 | 0.924 | 0.599 | 1.425 | 11.9 | |
| Veldhuyzen van Zanten et al,36 2003 | 44 | 75 | 0.587 | 46 | 82 | 0.561 | 1.111 | 0.590 | 2.093 | 5.6 | |
| Bruley Des Varannes et al,24 2001 | 55 | 129 | 0.426 | 38 | 124 | 0.306 | 1.682 | 1.003 | 2.821 | 8.4 | |
| Yazdanbod et al,37 2015 | 87 | 186 | 0.468 | 83 | 173 | 0.480 | 0.953 | 0.630 | 1.443 | 13.1 | |
| Total (fixed effect model) | 1.356 | 1.167 | 1.575 | 100.0 | < 0.001 | ||||||
P-value of test of heterogeneity among studies = 0.191; Higgins’ I2 = 25.0% (0.0 %, 61.0%).
Figure 7.
Forest plots for the effects of Helicobacter pylori eradication on the symptom relief in patients with functional dyspepsia according to ethnics. (A) Asian. (B) Non-Asian.
Discussion
It is still unclear whether H. pylori infection causes dyspeptic symptoms. Our meta-analysis is a comprehensive study focusing on a comparison of efficacy based on antibiotic type used and the duration of the eradication regimen on symptomatic improvements after H. pylori eradication. The strength of this meta-analysis was that it includes more recent trials and subgroup analysis according to H. pylori eradication regimens. Multiple complex factors are thought to be involved in the pathophysiology of FD such as gastro-duodenal inflammation by intestinal microbiota; our results support such a theory.
Several studies on H. pylori-associated FD have focused on changes in the intestinal environment.15–17,38 Intestinal microbiota play an important role in the maintenance of host health by providing energy, nutrients, and immunological protection and its imbalance can provoke various GI symptoms and disease.39 Because H. pylori-associated FD and intestinal dysbiosis could be shared with common pathogenesis such as impaired motility, there is a close relationship.14 Another possible pathogenesis is based on the decreased secretion of gastric acid by long-term infection of H. pylori. In patients with severe atrophy induced by H. pylori infection, acid secretion would be compromised and subsequently it results in bacterial overgrowth in the intestine. Previously, it has been demonstrated that patients who complained of dyspeptic symptoms would have an increased risk of intestinal dysbiosis.14,38,40 Further prospective evaluations are necessary to investigate whether patients with severe atrophic change would benefit on symptomatic improvement from H. pylori eradication.
Altered gut microbiota have been considered as a main pathophysiology of IBS and manipulation of gut microbiota represents a new strategy for the treatment of IBS. In the overlap syndrome consisting of FD and IBS, intestinal dysbiosis might be a common etiology and dominant pathogenesis. Recent clinical data revealed that intestinal dysbiosis was diagnosed in FD patients with refractory symptoms.38 Another study on H. pylori-negative Asian FD patients without IBS-overlap showed that 2-week therapy with rifaximin was efficient in a randomized placebo-controlled trial.41 This evidence suggested that low-level inflammation of the intestine caused by imbalance of intestinal microbiome might be one of mechanisms of functional dyspepsia. In the near future, new classification of functional GI disorder should be categorized according to the state of small intestinal microbiome. In addition, the concept of “colo-gastric reflex” is another pathophysiology of IBS. In recent human data, gastric accommodation regularly affects lower GI tract function and colonic gaseous distension or constipation decrease gastric accommodation or gastric emptying. It was called as the “colo-gastric reflex.”42 In other words, the relationship between dyspepsia and the colonic distension could be even more important.
We hypothesized that many patients with H. pylori-associated FD might have FD-IBS overlap and this concept was first applied. When all patients with IBS criteria were excluded, there was no statistical significance of symptomatic improvement after H. pylori eradication. However, it was insufficient to make a concrete conclusion because the different types of Rome criteria applied to the different studies and the diagnostic criteria of FD and IBS were mutually exclusive. It was not until recently that overlap syndrome was paid attention. In this point of view, H. pylori-associated FD could be a new disease category. Previous reports revealed that responders at 3 months and H. pylori eradicated status were associated with improvement of dyspeptic symptoms at 1 year, regardless of the overplayed IBS.43 Gastric mucosal inflammation induced by H. pylori infection was a crucial role in symptom generation, and the control of inflammation resulted in symptom improvement. Therefore, the Kyoto Global Consensus report insisted that H. pylori-associated dyspepsia was not as a FD, but a kind of infectious disease.44
Several antibiotics with various spectra of activity have been used for H. pylori eradication therapy. Clarithromycin is a representative of the macrolide antibiotics and these provide coverage of gram-positive bacteria, whereas metronidazole is the drug of choice for the treatment of anaerobic infection and the representative treatment drug for intestinal dysbiosis.45 Metronidazole-combined with these antibiotics are thought to be combinations that can cover broader antibacterial spectrum including anaerobes. Our results revealed that metronidazole-containing regimens were significantly more effective for symptom relief compared to clarithromycin-containing regimens. This was because the use of metronidazole in the treatment of H. pylori eradication brought about the secondary therapeutic effect of modifying the gut microbiota.
In FD, mucosal inflammation could play an important role and it was demonstrated that increased mast cells or eosinophils in the gastroduodenal mucosa were associated with symptom generation.46,47 However, it was questionable whether inflammatory cells were eliminated or lymphoid follicles healed completely after H. pylori eradication. Moreover, the influence of antibiotics on the intestinal flora could be temporary, and it would return to previous state after antibiotic therapy.48 Previously, several researchers insisted that at least 6 to 12 months of observation period should be required to judge the outcome of dyspeptic symptoms after successful eradication. Gastric mucosal inflammation activity and physiologic functions including acid secretion could recover or stabilize after 6 months.44 The restoration of gastric physiologic functions would have a possibility to modify intestinal dysbiosis.
In 13 studies performed on a non-Asian population, there were statistically significant benefits for dyspeptic symptom relief, whereas the 3 trials performed on Asian populations revealed insignificant results. These differences in race-to-race efficacy are contrary to the predicted results. In Asia, H. pylori infection is common at a younger age, and it is more likely that atrophic gastritis has progressed over a longer period of time. In general, more advanced hypo-secretory stomach due to long-term H. pylori infection and atrophy of the gastric mucosa is known as a risk factor of dysbiosis. Therefore, Asian H. pylori-infected patients are more likely to have a more advanced hypo-secretory stomach. As a result, dysbiosis is more likely to be more prominent in Asian H. pylori-infected patients, and the potential for efficacy with antibiotic treatment may increase. However, in the all trials on Asian populations, the 7-day regimen was adopted, and there was a possibility of insufficient dose of antibiotic treatment. In addition, small sample size and a wide confidence interval limits the interpretation of the results. Brain-gut interaction was one of the important mechanisms of functional dyspepsia and it was unlikely that the Rome criteria completely reflect the various symptoms, especially in Asian patients with FD. Large-scale prospective studies should be needed in Asia, particularly in China and Korea which are well known areas of high prevalence of H. pylori.
There were potential limitations in this study. First, our literature search was restricted to English-language reports because of translation limitations, which could have introduced some bias. The relatively small number of studies on Asian populations is thought to have resulted in reduced reliability. Second, this study was not a direct pharmaceutical comparison, but a relative comparison via OR. However, based on Monson’s experience, an OR of 1.87 is highly effective for symptomatic improvement49; therefore, we do not think our comparison of the results was insufficient for our research purpose. Third, differences in enrollment criteria may have created heterogeneous H. pylori-associated FD populations in several clinical trials.
In conclusion, H. pylori eradication for FD could be an effective treatment strategy. When clinicians choose an eradication regimen for H. pylori-associated FD, administering metronidazole for 10 days or more would provide a great benefit for symptom improvement. In the future, more prospective RCTs are necessary for direct comparison of metronidazole and other antibiotic regimens for treating this disorder.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author contributions: Woo Chul Chung was responsible for the conception of the review question and final approval of manuscript; Na Jin Kim was responsible for the the acquisition; Soo Hwan Kim analyzed the data and interpreting data; Yeon-Ji Kim and Woo Chul Chung was responsible for the study selection, data extraction, and drafting the manuscript; Byung Wook Kim, Sung Soo Kim, and Jin Il Kim assisted in review design, interpretation and revision, and drafting of the manuscript; and Yeon-Ji Kim and Woo Chul Chung contributed to the revision of the manuscript.
References
- 1.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–2666. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talley NJ, Choung RS. Whither dyspepsia? A historical perspective of functional dyspepsia, and concepts of pathogenesis and therapy in 2009. J Gastroenterol Hepatol. 2009;24(suppl 3):S20–S28. doi: 10.1111/j.1440-1746.2009.06067.x. [DOI] [PubMed] [Google Scholar]
- 3.Tack J, Talley NJ. Functional dyspepsia--symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013;10:134–141. doi: 10.1038/nrgastro.2013.14. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049–1057. doi: 10.1136/gutjnl-2014-307843. [DOI] [PubMed] [Google Scholar]
- 5.Brook RA, Kleinman NL, Choung RS, Melkonian AK, Smeeding JE, Talley NJ. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol. 2010;8:498–503. doi: 10.1016/j.cgh.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168–174. doi: 10.1038/nrgastro.2013.9. [DOI] [PubMed] [Google Scholar]
- 8.Hurlimann S, Dür S, Schwab P, et al. Effects of Helicobacter pylori on gastritis, pentagastrin-stimulated gastric acid secretion, and meal-stimulated plasma gastrin release in the absence of peptic ulcer disease. Am J Gastroenterol. 1998;93:1277–1285. doi: 10.1111/j.1572-0241.1998.409_x.x. [DOI] [PubMed] [Google Scholar]
- 9.Koike T, Ohara S, Sekine H, et al. Helicobacter pylori infection prevents erosive reflux oesophagitis by decreasing gastric acid secretion. Gut. 2001;49:330–334. doi: 10.1136/gut.49.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moayyedi P, Soo S, Deeks JJ, et al. WITHDRAWN: eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2011;16:CD002096. doi: 10.1002/14651858.CD002096.pub5. [DOI] [PubMed] [Google Scholar]
- 11.Du LJ, Chen BR, Kim JJ, Kim S, Shen JH, Dai N. Helicobacter pylori eradication therapy for functional dyspepsia: systematic review and meta-analysis. World J Gastroenterol. 2016;22:3486–3495. doi: 10.3748/wjg.v22.i12.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao B, Zhao J, Cheng WF, et al. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48:241–247. doi: 10.1097/MCG.0b013e31829f2e25. [DOI] [PubMed] [Google Scholar]
- 13.Grace E, Shaw C, Whelan K, Andreyev HJ. Review article: small intestinal bacterial overgrowth--prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther. 2013;38:674–688. doi: 10.1111/apt.12456. [DOI] [PubMed] [Google Scholar]
- 14.Costa MB, Azeredo IL, Jr, Marciano RD, Caldeira LM, Bafutto M. Evaluation of small intestine bacterial overgrowth in patients with functional dyspepsia through H2 breath test. Arq Gastroenterol. 2012;49:279–283. doi: 10.1590/S0004-28032012000400009. [DOI] [PubMed] [Google Scholar]
- 15.Mirbagheri SS, Mirbagheri SA, Nabavizadeh B, et al. Impact of microscopic duodenitis on symptomatic response to Helicobacter pylori eradication in functional dyspepsia. Dig Dis Sci. 2015;60:163–167. doi: 10.1007/s10620-014-3285-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhong L, Shanahan ER, Raj A, et al. Dyspepsia and the microbiome: time to focus on the small intestine. Gut. 2016;66:1168–1169. doi: 10.1136/gutjnl-2016-312574. [DOI] [PubMed] [Google Scholar]
- 17.Holtmann G, Talley NJ. Functional dyspepsia. Curr Opin Gastroenterol. 2015;31:492–498. doi: 10.1097/MOG.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 18.Danesh J, Lawrence M, Murphy M, Roberts S, Collins R. Systematic review of the epidemiological evidence on Helicobacter pylori infection and nonulcer or uninvestigated dyspepsia. Arch Intern Med. 2000;160:1192–1198. doi: 10.1001/archinte.160.8.1192. [DOI] [PubMed] [Google Scholar]
- 19.Jaakkimainen RL, Boyle E, Tudiver F. Is Helicobacter pylori associated with non-ulcer dyspepsia and will eradication improve symptoms? A meta-analysis. BMJ. 1999;319:1040–1044. doi: 10.1136/bmj.319.7216.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laine L, Schoenfeld P, Fennerty MB. Therapy for Helicobacter pylori in patients with nonulcer dyspepsia. A meta-analysis of randomized, controlled trials. Ann Intern Med. 2001;134:361–369. doi: 10.7326/0003-4819-134-5-200103060-00008. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ang TL, Fock KM, Teo EK, et al. Helicobacter pylori eradication versus prokinetics in the treatment of functional dyspepsia: a randomized, double-blind study. J Gastroenterol. 2006;41:647–653. doi: 10.1007/s00535-006-1818-x. [DOI] [PubMed] [Google Scholar]
- 23.Blum AL, Talley NJ, O’Moráin C, et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. Omeprazole plus clarithromycin and amoxicillin effect one year after treatment (OCAY) study group. N Engl J Med. 1998;339:1875–1881. doi: 10.1056/NEJM199812243392602. [DOI] [PubMed] [Google Scholar]
- 24.Bruley Des Varannes S, Flejou JF, Colin R, Zaim M, Meunier A, Bidaut-Mazel C. There are some benefits for eradicating Helicobacter pylori in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther. 2001;15:1177–1185. doi: 10.1046/j.1365-2036.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- 25.Froehlich F, Gonvers JJ, Wietlisbach V, et al. Helicobacter pylori eradication treatment does not benefit patients with nonulcer dyspepsia. Am J Gastroenterol. 2001;96:2329–2336. doi: 10.1111/j.1572-0241.2001.04037.x. [DOI] [PubMed] [Google Scholar]
- 26.Gisbert JP, Cruzado AI, Garcia-Gravalos R, Pajares JM. Lack of benefit of treating Helicobacter pylori infection in patients with functional dyspepsia. Randomized one-year follow-up study. Hepatogastroenterology. 2004;51:303–308. [PubMed] [Google Scholar]
- 27.Gwee KA, Teng L, Wong RK, Ho KY, Sutedja DS, Yeoh KG. The response of Asian patients with functional dyspepsia to eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2009;21:417–424. doi: 10.1097/MEG.0b013e328317b89e. [DOI] [PubMed] [Google Scholar]
- 28.Hsu PI, Lai KH, Tseng HH, et al. Eradication of Helicobacter pylori prevents ulcer development in patients with ulcer-like functional dyspepsia. Aliment Pharmacol Ther. 2001;15:195–201. doi: 10.1046/j.1365-2036.2001.00903.x. [DOI] [PubMed] [Google Scholar]
- 29.Koskenpato J, Farkkilä M, Sipponen P. Helicobacter pylori eradication and standardized 3-month omeprazole therapy in functional dyspepsia. Am J Gastroenterol. 2001;96:2866–2872. doi: 10.1111/j.1572-0241.2001.04240.x. [DOI] [PubMed] [Google Scholar]
- 30.Mazzoleni LE, Sander GB, Ott EA, et al. Clinical outcomes of eradication of Helicobacter pylori in nonulcer dyspepsia in a population with a high prevalence of infection: results of a 12-month randomized, double blind, placebo-controlled study. Dig Dis Sci. 2006;51:89–98. doi: 10.1007/s10620-006-3090-6. [DOI] [PubMed] [Google Scholar]
- 31.Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929–1936. doi: 10.1001/archinternmed.2011.533. [DOI] [PubMed] [Google Scholar]
- 32.McColl K, Murray L, El-Omar E, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med. 1998;339:1869–1874. doi: 10.1056/NEJM199812243392601. [DOI] [PubMed] [Google Scholar]
- 33.Sodhi JS, Javid G, Zargar SA, et al. Prevalence of Helicobacter pylori infection and the effect of its eradication on symptoms of functional dyspepsia in Kashmir, India. J Gastroenterol Hepatol. 2013;28:808–813. doi: 10.1111/jgh.12178. [DOI] [PubMed] [Google Scholar]
- 34.Talley NJ, Janssens J, Lauritsen K, Rácz I, Bolling-Sternevald E. Eradication of Helicobacter pylori in functional dyspepsia: randomised double blind placebo controlled trial with 12 months’ follow up. The optimal regimen cures helicobacter induced dyspepsia (ORCHID) study group. BMJ. 1999;318:833–837. doi: 10.1136/bmj.318.7187.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talley NJ, Vakil N, Ballard ED, 2nd, Fennerty MB. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N Engl J Med. 1999;341:1106–1111. doi: 10.1056/NEJM199910073411502. [DOI] [PubMed] [Google Scholar]
- 36.Veldhuyzen van Zanten S, Fedorak RN, Lambert J, Cohen L, Vanjaka A. Absence of symptomatic benefit of lansoprazole, clarithromycin, and amoxicillin triple therapy in eradication of Helicobacter pylori positive, functional (nonulcer) dyspepsia. Am J Gastroenterol. 2003;98:1963–1969. doi: 10.1016/S0002-9270(03)00432-5. [DOI] [PubMed] [Google Scholar]
- 37.Yazdanbod A, Salimian S, Habibzadeh S, Hooshyar A, Maleki N, Norouzvand M. Effect of Helicobacter pylori eradication in Iranian patients with functional dyspepsia: a prospective, randomized, placebo-controlled trial. Arch Med Sci. 2015;11:964–969. doi: 10.5114/aoms.2015.54851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimura S, Ishimura N, Mikami H, et al. Small intestinal bacterial overgrowth in patients with refractory functional gastrointestinal disorders. J Neurogastroenterol Motil. 2016;22:60–68. doi: 10.5056/jnm15116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simrén M, Barbara G, Flint HJ, et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159–176. doi: 10.1136/gutjnl-2012-302167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbara G, Feinle-Bisset C, Ghoshal UC, et al. The intestinal microenviroment and functional gastrointestinal disorders. Gastroenterol. 2016;150:1305–1318. e8. doi: 10.1053/j.gastro.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 41.Tan VP, Liu KS, Lam FY, Hung IF, Yuen MF, Leung WK. Randomised clinical trial: rifaximin versus placebo for the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2017;45:767–776. doi: 10.1111/apt.13945. [DOI] [PubMed] [Google Scholar]
- 42.Sjölund K, Ekman R, Lindgren S, Rehfeld JF. Disturbed motilin and cholecystokinin release in the irritable bowel syndrome. Scand J Gastroenterol. 1996;31:1110–1114. doi: 10.3109/00365529609036895. [DOI] [PubMed] [Google Scholar]
- 43.Kim SE, Park YS, Kim N, et al. Effect of Helicobacter pylori eradication on functional dyspepsia. J Neurogastroenterol Motil. 2013;19:233–243. doi: 10.5056/jnm.2013.19.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah SC, Day LW, Somsouk M, Sewell JL. Meta-analysis: antibiotic therapy for small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2013;38:925–934. doi: 10.1111/apt.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut. 2014;63:262–271. doi: 10.1136/gutjnl-2012-303857. [DOI] [PubMed] [Google Scholar]
- 47.Hall W, Buckley M, Crotty P, O’Morain CA. Gastric mucosal mast cells are increased in Helicobacter pylori-negative functional dyspepsia. Clin Gastroenterol Hepatol. 2003;1:363–369. doi: 10.1053/S1542-3565(03)00184-8. [DOI] [PubMed] [Google Scholar]
- 48.Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209–240. doi: 10.1007/s12263-011-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monson R. Occupational epidemiology. 2nd Ed. Boca Raton, Florida: CRC Press Inc; 1990. [Google Scholar]