Abstract
The incidence of melanoma and associated mortality rate from advanced disease in older adults is increasing over time. Checkpoint inhibitors have demonstrated a survival benefit for the treatment of stage IV or unresectable stage III disease and have become one of the standards of care. Data suggests that adults aged 65 and older benefit from treatment with checkpoint inhibitors without an increased incidence in adverse events. However, clinicians should be aware of the potential side effects of this class of medications and how to manage them in older adults.
Keywords: Melanoma, checkpoint inhibitors, ipilimumab, nivolumab, pembrolizumab, immune-related adverse events
1. Introduction
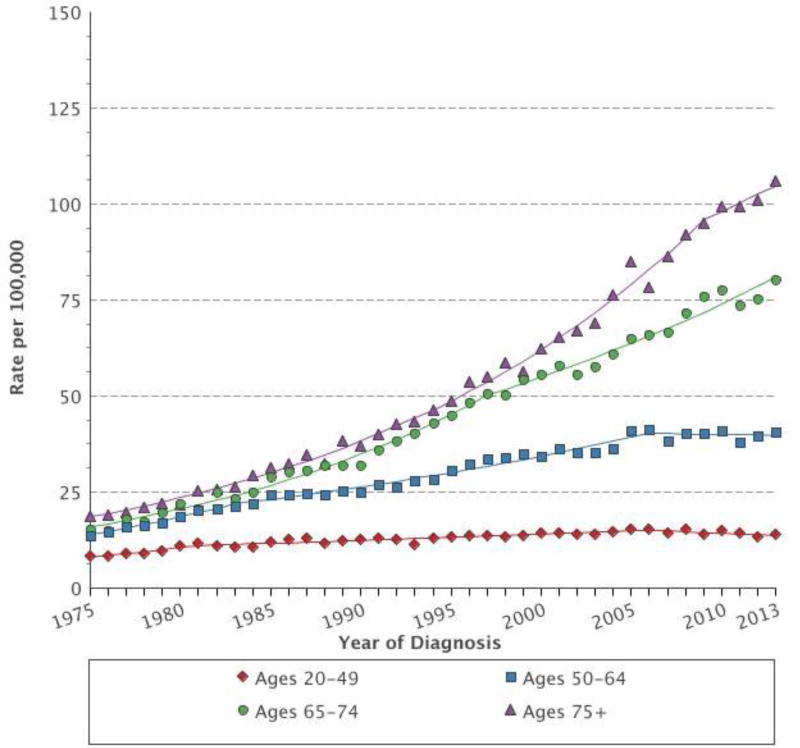
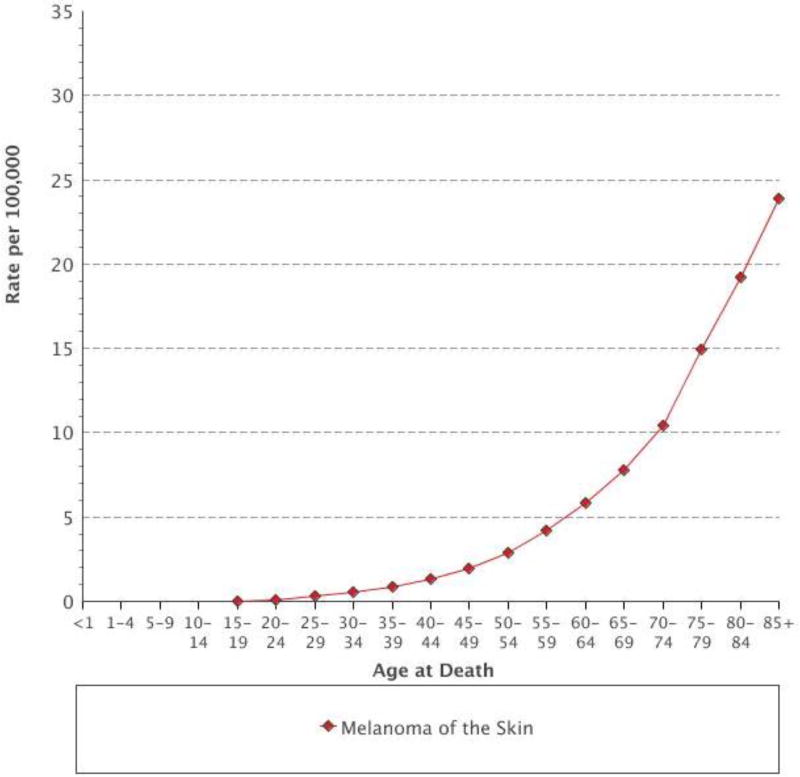
Advanced age is a known risk factor for developing cancer and is associated with a poorer prognosis.[1, 2] This is reflected in the incidence and mortality of advanced melanoma. Data from the SEER database demonstrate that the incidence of melanoma is highest in those aged 75 older and that the incidence rate in that age group has more than quadrupled since 1975 (Figure 1). Similarly, older adults have an increase in melanoma-associated mortality, peaking in those aged 85 and older (Figure 2).[3] In this article we provide perspective on the management of older patients with advanced melanoma, with an emphasis selecting appropriate immunotherapy and managing immune-related adverse events (irAE).
Figure 1.
Age-adjusted SEER incidence rates of melanoma from 1975–2013 (SEER 9)
Figure 2.
Age-specific mortality rates from melanoma from 2009–2013
2. Characteristics of Primary Melanomas in the Older Adult
In general, older adults tend to present with higher-risk primary melanomas. They are the age group most likely to present with very thick (> 4 mm) primary tumors;[4] they also have a greater mean number of mitotic figures and are more likely to develop either local recurrence or in-transit metastases.[5] Interestingly, older adults are less likely to have a positive sentinel lymph node biopsy compared to their younger counterparts.[5]
3. What are Checkpoint Inhibitors?
Checkpoint inhibitors (CPI) enhance antitumor immunity by blocking negative regulators (checkpoints) of T cell function that exist both on immune and tumor cells. Although there are many T cell checkpoints that could be amenable to this approach, two particular targets, cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death-1 (PD-1) have been most significantly evaluated in large clinical trials. Ipilimumab (anti-CTLA-4)[6, 7] as well as nivolumab [8] and pembrolizumab [9, 10] (anti-PD1) are currently approved by the United States Food and Drug Administration (FDA) for the treatment of metastatic melanoma. The combination of nivolumab plus ipilimumab is also FDA-approved.[11, 12]
4. The Immune System of the Older Adult
It is has been hypothesized that older adults may benefit less from immunotherapy [13] given immunosenescence, the phenomenon of decreased immune function as a result of age-associated alterations to the immune system.[14] Mouse models have demonstrated that advancing age is associated with changes in both the innate and adaptive immune system.[15] Older mice demonstrate modifications in the cytokine production[16] and reduced cytotoxicity of CD8+ T cells.[17] In humans, advanced age is associated with decreased T cell diversity[18] and decreased CD28 expression which is a necessary co-stimulatory signal for T cell activation.[19]
5. What is known about using immunotherapy in the older adult
There is limited information about the efficacy and toxicity of CPI in older adults, defined as those aged 65 and older, mostly derived from subgroup analysis of larger clinical trials. Data from the FDA demonstrate that the age distribution of registration clinical trials is similar to the general oncology population. Adults aged 65 and older consisted of 28% of patients treated with ipilimumab, and 39% of patients treated with single agent nivolumab or pembrolizumab; 9% of patients treated with single agent nivolumab were aged 75 or older. Additional data comes from cross-trial meta-analyses, including one of 4725 patients with lung cancer, kidney cancer or melanoma treated on 8 phase II/III trials. This study found that CPI treatment prolonged overall survival (OS) in older adults, using an age cut-off of 65–70. A subgroup analysis of adults aged 75 and older found an OS advantage for treatment with anti-CTLA-4 agents but not with anti-PD1 antibodies.[20] Another meta-analysis of CPI randomized trials found that in 1244 older patients, CPI significantly improved OS (HR, 0.72 (95% CI 0.58–0.9); p=.004) in comparison with controls.[21]
Sileni et al specifically examined the efficacy and toxicity of ipilimumab in patients aged 70 and older with advanced melanoma and found that there was no difference in median OS between patients aged ≥ 70 years (8.9 months (95% CI 7.2–10.6)) and < 70 years (7.0 months (95% CI 6.1–7.9); p= 0.17); in addition, rates of immune related adverse events (irAE) were similar between the two groups.[22] The efficacy of the PD-1 blocking antibodies in older adults with advanced melanoma was also assessed in a number of randomized clinical trials. In CheckMate 066, when compared to dacarbazine, treatment with nivolumab was associated with a HR of 0.44 (0.24 – −0.81) in adults aged 65–75 and a HR of 0.25 (0.10 – −0.61) in adults aged >75 years.[8] In KEYNOTE-006, pembrolizumab dosed every 2 weeks or 3 weeks was associated with a HR of 0.56 (0.36 – −0.87) and 0.66 (0.44 – −1.01) respectively when compared to ipilimumab.[23] As for the combination of nivolumab plus ipilimumab, in CheckMate 069 the objective response rate was 64% in patients younger than 65 years compared to 53% in those aged 65 and older.[24] The FDA now requires the inclusion of a Geriatric Use subsection in the labeling for prescription drugs to provide relevant information for clinicians about the use of those products in older adults.[25] As per the package inserts for ipilimumab, nivolumab and pembrolizumab, no differences in safety or efficacy were reported for older adults. [26, 27]
6. Incidence of irAEs
By blocking the negative regulators of T cell function that are normally important for maintaining self-tolerance, CPI treatment can be associated with distinctive inflammatory side effects known as irAEs. IrAEs are distinct both in mechanism and management from side effects commonly associated with chemotherapy.[28, 29] While the types of irAEs are similar across CPI treatments, the incidence varies based on the type of antibody selected. In general, PD-1 inhibitors have a lower incidence of irAEs compared to antibodies that block CTLA-4 such as ipilimumab; whereas the combination of nivolumab and ipilimumab has a higher rate of irAEs than either approach as monotherapy. For example, in a phase 3 study in patients with advanced melanoma receiving nivolumab, ipilimumab, or the combination of both, grade 3/4 treatment-related adverse events were observed in 16.3% of patients treated with nivolumab, 27.3% of patients treated with ipilimumab and 55% of patients treated with the combination.[11] Similar results were seen in a phase 3 study of pembrolizumab vs. ipilimumab in patients with melanoma with lower rates of grade 3/4 toxicity in patients receiving pembrolizumab.[10]
In patients treated with anti-PD-1 inhibitors, the most common irAEs are fatigue, rash and pruritus occurring in 20–35% of patients; the most common high grade toxicities are diarrhea, elevation in alanine amino-transferase (ALT) or aspartate amino-transferase (AST). Fortunately these grade 3–4 irAEs are rare in patients receive anti-PD-1 monotherapy (<2%).[10, 11] For patients receiving ipilimumab-based treatment, the most common irAEs are similar with an increased risk of diarrhea (~30–40%). Endocrinopathies are also observed in up to 10% of patients treated with CTLA-4 inhibition [30, 31], including hypophysitis (pituitary inflammation), hypothyroidism, and adrenal insufficiency. The frequency of endocrinopathy in patients treated with PD-1 agents is less well known, but appears to be less common at <1% in patients[10, 32]; there are additionally reports of autoimmune insulin-dependent diabetes.[33] Pneumonitis is a rare (<10%), but potentially life-threatening irAE seen in patients treated with CTLA-4 and PD-1 blocking antibodies.[8–10, 32, 34, 35] Fortunately, despite the rates of grade 3/4 toxicity, irAEs that lead to treatment-related death are exceedingly rare, ≤2%. [36]
The incidence of irAEs in older adults does not appear to be substantially different than in younger adults. In CheckMate 069, high grade irAEs were more commonly reported in patients receiving combination therapy, both in those younger than 65 (54%) and aged 65 and older (52%). In the PD-1 monotherapy arm, 15% of patients older than 65 years experienced a high grade irAE compared to 26% of patients younger than 65 years.[24] There is little known about steroid use in different age groups; however, in a small series of patients aged 80 and older presented at ASCO 2016, 28% of patients treated with CPI monotherapy required treatment with systemic steroids for irAEs. Early discontinuation of treatment for toxicity was common, occurring in 31% of ipilimumab patients, 20% of anti-PD-1 monotherapy patients and 50% of combination therapy patients.[37]
7. Treatment Decisions: Choosing Between Checkpoint Inhibitors
Treatment with CPI should be strongly considered for all older patients with advanced melanoma, including those who are very old (aged 80 and older). Data presented at the 2016 ASCO annual meeting demonstrated long term survival for very old patients with advanced melanoma treated with ipilimumab, with 20% of patients surviving at least 3 years.[37] Moreover, data from the head and neck literature suggest that in patients receiving anti-PD-1 monotherapy quality of life measures remain stable or even improve slightly across all ages.[38] In the frontline setting, clinicians should consider treatment with either nivolumab or pembrolizumab monotherapy or the combination of nivolumab plus ipilimumab. The decision to treat older patients with either combination or monotherapy is one that should be made in consultation with the patient and his or her family. Anti-PD-1 monotherapy offers a robust response rate (~40%) associated with a relatively low risk of high grade adverse events. The response rate is higher with combination therapy (~60%) but is also associated with at least a threefold increase in high grade adverse events. It should be noted that data for overall survival is still immature when trying to compare combination therapy to anti-PD-1 monotherapy.
Clinicians should carefully consider the functional status of the patient and concomitant medical problems when deciding between monotherapy and combination therapy, especially those that are autoimmune in nature as these may be exacerbated by CPI. There are a number of geriatric assessment tools which can be utilized by the clinician in making this judgment. The Mini-Cognitive Assessment Instrument is validated as a screening test for cognitive dysfunction[39] which may make it difficult for older patients to report symptoms or comply with medications. The Timed Get up and Go Test (GUG) can be used to assess frailty and risk of falls; a prolonged GUG time has been associated with higher risk of early death in older patients with cancer.[40]
It is also essential to assess the social support available to older patients, including with whom the patient resides with whether or not they have reliable access to transportation. This is important when considering management of irAEs; in a recent series of patients treated with combination therapy at Memorial Sloan Kettering Cancer Center, 50% of patients required an emergency room visit and 36% of patients were hospitalized for treatment of an irAE.[41] If a patient lacks easy access to medical care, combination therapy may not be an appropriate choice. While combination therapy has been utilized in those aged 80 and older, it should be reserved for those patients with excellent performance status, minimal medical comorbidities and excellent social support.
8. Management of irAEs
Unfortunately, there are no prospective randomized trials to guide optimal management of irAEs; therefore, the management of irAEs is based upon clinical experience. Nevertheless, consensus guidelines regarding the treatment of the common irAEs including rash, colitis, hepatitis, endocrinopathies and pneumonitis has been established.[42] The mainstay of irAE treatment consists of immunosuppression with corticosteroids and, if necessary, other immunosuppressant agents such as infliximab. Fortunately, with appropriate management, most irAEs resolve (with the exception of endocrinopathies),[12] and temporary immunosuppression to treat an irAE does not appear to limit the efficacy of immune-checkpoint inhibition.[36, 43]
There are special considerations for management of irAEs in older adults. First, immune-related diarrhea may lead to high rates of dehydration due to the decline in renal function[44] and thirst perception in older adults[45], leading to an increase in mortality.[46] These patients should be monitored carefully and hospital admission for IV fluids and steroids may be warranted. Second, endocrinopathies in patients aged 65 and older may only present with nonspecific findings such as memory loss or cognitive decline. Clinicians must be vigilant to these subtle symptoms; a TSH and T4 should be checked with every CPI infusion and physicians should have a low threshold to assess other hormone levels as well as blood glucose given the increasing awareness of the risk of CPI-induced diabetes mellitus.[33, 47] Clinicians should also utilize the expertise of consultants for irAEs that are steroid refractory or otherwise challenging to manage.
Lastly, there are also considerations for administering prednisone for the treatment of irAEs in this age group. Older patients may be at higher risk for delirium or altered mental status caused by steroid therapy.[48] In addition, elderly adults are more likely to have co-morbid medical conditions such as cardiac disease or diabetes that may be worsened by steroid therapy. Clinicians should limit the duration of steroid usage as clinically indicated while monitoring for adverse affects. We also advise close coordination with the primary care physician in cases where medical comorbidities or polypharmacy may be challenging for the oncologist to manage.
9. Summary
Advanced melanoma is increasingly diagnosed amongst older adults with mortality rates increasing in those aged 75 and older. Treatment with CPI in this subgroup of patients is generally well tolerated with response rates that are comparable to those in younger people and evidence of durable benefit. Clinicians should be vigilant of irAEs to prevent morbidity and mortality, especially in this age group.
Acknowledgments
Disclosures and Conflict of Interest Statements
JD Wolchok serves as a consultant for BMS, Merck, MedImmune, ZIOPHARM Oncology, Polynoma, Polaris, Jounce Therapeutics, Genentech, FStar, Beigene, Advaxis, Sellas Life Sciences, Lilly, Potenza Therapeutics, Tizona Therapeutics Inc, Amgen, AstraZeneca, and Chugai Pharma and has received honoraria from Regeneron. JDW has stock or other owndership with Potenza Pharmaceuticals and Vesuvius Pharmaceuticals. JD Wolchok and C Friedman have research funding from BMS.
References
- 1.Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14:13–22. doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- 2.Kendal WS. Dying with cancer: the influence of age, comorbidity, and cancer site. Cancer. 2008;112:1354–1362. doi: 10.1002/cncr.23315. [DOI] [PubMed] [Google Scholar]
- 3.Surveillance Research Program. National Cancer Institute; [Accessed on 11-4-2016]. Fast Stats: An interactive tool for access to SEER cancer statistics. http://seer.cancer.gov/faststats. [Google Scholar]
- 4.Jemal A, Saraiya M, Patel P, et al. Recent trends in cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol. 2011;65:S17–25. e11–13. doi: 10.1016/j.jaad.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald JB, Dueck AC, Gray RJ, et al. Malignant melanoma in the elderly: different regional disease and poorer prognosis. J Cancer Educ. 2011:2. doi: 10.7150/jca.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364 doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 10.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. New England Journal of Medicine. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 11.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. New England Journal of Medicine. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomihara K, Curiel TJ, Zhang B. Optimization of Immunotherapy in Elderly Cancer Patients. Critical reviews in oncogenesis. 2013;18:573–583. doi: 10.1615/critrevoncog.2013010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulop T, Larbi A, Witkowski JM, et al. Immunosenescence and Cancer. Critical reviews in oncogenesis. 2013;18:489–513. doi: 10.1615/critrevoncog.2013010597. [DOI] [PubMed] [Google Scholar]
- 15.Bandaranayake T, Shaw AC. Host Resistance and Immune Aging. Clinics in Geriatric Medicine. 2016;32:415–432. doi: 10.1016/j.cger.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kubo M, Cinader B. Polymorphism of age-related changes in interleukin (IL) production: differential changes of T helper subpopulations, synthesizing IL 2, IL3 and IL4. European Journal of Immunology. 1990;20:1289–1296. doi: 10.1002/eji.1830200614. [DOI] [PubMed] [Google Scholar]
- 17.Bloom ET, Umehara H, Bleackley RC, et al. Age-related decrement in cytotoxic T lymphocyte (CTL) activity is associated with decreased levels of mRNA encoded by two CTL-associated serine esterase genes and the perforin gene in mice. European Journal of Immunology. 1990;20:2309–2316. doi: 10.1002/eji.1830201021. [DOI] [PubMed] [Google Scholar]
- 18.Britanova OV, Putintseva EV, Shugay M, et al. Age-Related Decrease in TCR Repertoire Diversity Measured with Deep and Normalized Sequence Profiling. The Journal of Immunology. 2014;192:2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 19.Weng N-p, Akbar AN, Goronzy J. CD28− T cells: their role in the age-associated decline of immune function. Trends in Immunology. 2009;30:306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishijima TF, Muss HB, Shachar SS, Moschos SJ. Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A systematic review and meta-analysis. Cancer Treatment Reviews. 2016;45:30–37. doi: 10.1016/j.ctrv.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Funakoshi T, Muss H, Moschos S. Abstract A159: Comparison of efficacy of immune checkpoint inhibitors (ICIs) between younger and older patients: A meta-analysis of randomized controlled trials. Cancer Immunology Research. 2016;4:A159–A159. [Google Scholar]
- 22.Chiarion Sileni V, Pigozzo J, Ascierto PA, et al. Efficacy and safety of ipilimumab in elderly patients with pretreated advanced melanoma treated at Italian centres through the expanded access programme. Journal of Experimental & Clinical Cancer Research. 2014;33:1–7. doi: 10.1186/1756-9966-33-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elias R, Morales J, Rehman Y, Khurshid H. Immune Checkpoint Inhibitors in Older Adults. Current Oncology Reports. 2016;18:47. doi: 10.1007/s11912-016-0534-9. [DOI] [PubMed] [Google Scholar]
- 24.Hodi FS, Postow MA, Chesney JA, et al. Clinical response, progression-free survival (PFS), and safety in patients (pts) with advanced melanoma (MEL) receiving nivolumab (NIVO) combined with ipilimumab (IPI) vs IPI monotherapy in CheckMate 069 study. ASCO Meeting Abstracts. 2015;33:9004. [Google Scholar]
- 25.FDA. Guidance for Industry: Content and Format for Geriatric Labeling. 2001 [Google Scholar]
- 26.Opdivo (R) [package insert] Bristol-Myers Squibb; 2014. [Google Scholar]
- 27.Yervoy (R) [package insert] Bristol-Myers Squibb; 2011. [Google Scholar]
- 28.Weber JS, Kähler KC, Hauschild A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. Journal of Clinical Oncology. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 29.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and Its Toxicities: A Multidisciplinary Approach. The Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corsello SM, Barnabei A, Marchetti P, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361–1375. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 31.Ryder M, Callahan M, Postow MA, et al. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015 doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin-Liberal J, Furness AJ, Joshi K, et al. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunology, Immunotherapy. 2015;64:765–767. doi: 10.1007/s00262-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamid O, Schmidt H, Nissan A, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber JS, Antonia SJ, Topalian SL, et al. Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): A pooled analysis. ASCO Meeting Abstracts. 2015;33:9018. [Google Scholar]
- 37.Friedman CF, Horvat TZ, Minehart J, et al. Efficacy and safety of checkpoint blockade for treatment of advanced melanoma (mel) in patients (pts) age 80 and older (80+) ASCO Meeting Abstracts. 2016;34:10009. [Google Scholar]
- 38.Ferris RL, Blumenschein GJ, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. New England Journal of Medicine. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borson S, Scanlan J, Brush M, et al. The mini-cog: a cognitive 'vital signs' measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15:1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of Early Death Risk in Older Patients Treated With First-Line Chemotherapy for Cancer. Journal of Clinical Oncology. 2012;30:1829–1834. doi: 10.1200/JCO.2011.35.7442. [DOI] [PubMed] [Google Scholar]
- 41.Friedman CF, Navid-Azarbaijani P, Shoushtari AN, Chapman PB. Toxicity associated with ipilimumab and nivolumab (Ipi+Nivo) combination therapy in melanoma patients (pts) treated at a single-institution under an expanded-access program (EAP) ASCO Meeting Abstracts. 34:9519. [Google Scholar]
- 42.Yervoy Risk Evaluation and Mitigation Strategy (REMS) 2011 [Google Scholar]
- 43.Horvat TZ, Adel NG, Dang T-O, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. Journal of Clinical Oncology. 2015;33:3193–3198. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malmrose LC, Gray SL, Pieper CF, et al. Measured versus estimated creatinine clearance in a high-functioning elderly sample: MacArthur Foundation Study of Successful Aging. J Am Geriatr Soc. 1993;41:715–721. doi: 10.1111/j.1532-5415.1993.tb07459.x. [DOI] [PubMed] [Google Scholar]
- 45.Davies I, O'Neill PA, McLean KA, et al. Age-associated alterations in thirst and arginine vasopressin in response to a water or sodium load. Age Ageing. 1995;24:151–159. doi: 10.1093/ageing/24.2.151. [DOI] [PubMed] [Google Scholar]
- 46.Bourdel-Marchasson I, Proux S, Dehail P, et al. One-year incidence of hyperosmolar states and prognosis in a geriatric acute care unit. Gerontology. 2004;50:171–176. doi: 10.1159/000076775. [DOI] [PubMed] [Google Scholar]
- 47.Mellati M, Eaton KD, Brooks-Worrell BM, et al. Anti–PD-1 and Anti–PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care. 2015;38:e137–e138. doi: 10.2337/dc15-0889. [DOI] [PubMed] [Google Scholar]
- 48.Dubovsky AN, Arvikar S, Stern TA, Axelrod L. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53:103–115. doi: 10.1016/j.psym.2011.12.007. [DOI] [PubMed] [Google Scholar]