Abstract
Objective
The Children's Oncology Group (COG) study AREN0534 aimed to improve EFS and OS while preserving renal tissue by intensifying pre-operative chemotherapy, completing definitive surgery by 12 weeks from diagnosis, and modifying post-operative chemotherapy based on histologic response.
Summary Background Data
No prospective therapeutic clinic trials in children with bilateral Wilms tumors (BWT) exist. Historical outcomes for this group were poor and often involved prolonged chemotherapy; on NWTS-5, 4-year event-free-survival (EFS) for all children with bilateral Wilms tumor was 56%.
Methods
Patients were enrolled and imaging studies were centrally reviewed to assess for bilateral renal lesions. They were treated with 3-drug induction chemotherapy (vincristine, dactinomycin and doxorubicin) for 6 or 12 weeks based on radiographic response followed by surgery and further chemotherapy determined by histology. Radiation therapy was provided for post-chemotherapy stage III and IV disease.
Results
189/208 patients were evaluable. 4-year EFS and OS were 82.1% (95% CI: 73.5%-90.8%) and 94.9% (95% CI: 90.1%-99.7%. 23 patients relapsed and 7 had disease progression. After induction chemotherapy 163/189 (84.0%) underwent definitive surgical treatment in at least one kidney by 12 weeks and 39% retained parts of both kidneys. Surgical approaches included: unilateral total nephrectomy with contralateral partial nephrectomy (48%), bilateral partial nephrectomy (35%), unilateral total nephrectomy (10.5%), unilateral partial nephrectomy (4%) and bilateral total nephrectomies (2.5%).
Conclusions
This treatment approach including standardized three-drug preoperative chemotherapy, surgical resection within 12 weeks of diagnosis and response and histology-based post-operative therapy improved EFS and OS and preservation of renal parenchyma compared to historical outcomes for children with BWT.
Introduction
The outcome for patients with unilateral favorable histology Wilms tumor is excellent with event free survival (EFS) greater than 85%.1-4 However, patients with bilateral Wilms tumor (BWT) have significantly poorer results. On National Wilms Tumor Study 5 (NWTS-5), the four-year EFS estimates for all patients with BWT was 56% (95% CI: 44.8% -66.6%); for patients with favorable histology, focal anaplastic and diffuse anaplastic BWT, four- year estimates were 65% (with retention of heterozygosity at 1p), 76% and 25%, respectively.5-7 The Société Internationaled' Oncologie Pédiatrique (SIOP) reported a 10-year overall survival (OS) of just 69% for patients with synchronous BWT treated with either preoperative radiotherapy and/or chemotherapy.8-10 Both also reported several late deaths from disease recurrence more than 3 years after diagnosis.11
End stage renal disease (ESRD) rates in children with BWT are also higher as compared to those with unilateral tumors.12, 13 The 20-year cumulative incidence of ESRD for survivors of unilateral WT was less than 1%; (0.6% in non-syndromic children), whereas the rate in children with BWT was 12% and much higher in children with genetic syndromes such as Denys Drash Syndrome (DDS, 75%) or the Wilms Tumor-Aniridia-Genitourinary Malformation-Mental Retardation Syndrome (WAGR, 50%). In the patients with BWT who had ESRD within 5 years after treatment the cause was a second nephrectomy in (30/32) 94% whereas it was only 50% of cases in the children who had ESRD after 5 years.12
Several factors are thought to contribute to the unfavorable outcomes in patients with BWT.14-16 These include under-staging resulting in under-treatment, delay in local disease control and increased incidence of anaplasia. Biopsy, both open wedge and core needle methods, often fails to identify focal or diffuse anaplastic histology.16 This has resulted in some patients being treated for extended periods of time without effect. Shamberger and colleagues showed that children with BWT received chemotherapy regimens that lasted between 37 and 50 weeks exposing them to both acute and long-term toxic events but with no effect on renal preservation or overall treatment outcome.14 It has also been shown that in some patients a tumor may not change in size on radiographic imaging or may slightly increase after chemotherapy because the tumor had differentiated or undergone rhabdomyomatous changes.17 While these patients have excellent survival, their tumors will not regress in size with further chemotherapy.
The success in treating unilateral WT has been the direct result of several large controlled trials by the research consortia in North America, the United Kingdom and Europe.3 These studies have defined surgical, radiotherapy and chemotherapy regimens in other stages of WT. However, no prospective clinical trial with defined treatment regimens for BWT has been reported.
To improve outcomes for children with BWT, the Children's Oncology Group (COG) opened study AREN0534 in 2009.18 The primary aims related to BWT were to improve four-year event-free survival (EFS) to 73% for patients with BWT, to prevent complete removal of at least one kidney in 50% of patients with BWT by using prenephrectomy three-drug chemotherapy with vincristine, dactinomycin and doxorubicin, and to have at least 75%of children with BWT undergo definitive surgical treatment by 12 weeks after initiation of chemotherapy. The purpose of this paper is to present the results of these primary aims.
Methods Section
Study
The COG study AREN0534, “Treatment for Patients with Bilateral, Multi-centric, or Bilaterally-Predisposed Unilateral Wilms Tumor” had three arms: one for treatment of patients with BWT, one for patients with unilateral tumors at high-risk for metachronous disease or multi-centric tumors, and one for patients with diffuse hyperplastic perilobar nephro blastomatosis (DHPLN).18 This report presents the results of children with BWT.
Enrollment and Eligibility
Local institutional review board or research ethics board approval of this study was obtained prior to enrolling patients. Patients had to be < 30 years old at the time of initial diagnosis and had to have synchronous bilateral renal masses 1 cm or greater on radiographic imaging. All patients were first enrolled on the COG biology and classification study AREN03B2. Patients could enroll without a diagnostic biopsy (the majority), with a diagnostic biopsy or after definitive surgery. Before enrollment on the therapeutic study AREN0534, real-time central review of diagnostic imaging, pathology (if obtained) and operative notes confirmed the status of BWT.19-21 Enrollment was required within 14 days of diagnosis or 7 days after starting therapy. Due to the difficulty of distinguishing WT from a nephrogenic rest based on imaging studies and percutaneous biopsies, the guideline was established that a child with a single renal lesion 1 cm or greater in size in the contralateral kidney or multiple lesions (of any size) in the contralateral kidney should be treated on the synchronous BWT stratum. Patients with an isolated lesion less than 1 cm in the contralateral kidney should be treated by nephrectomy with post-operative therapy based on the pathologic findings. These patients could have been enrolled in another therapeutic study.18, 22
Staging
Patients with BWT received both a local stage and an overall disease stage. The final local stage was based on the abdominal tumor burden, whereas the disease stage accounted for the presence of distant metastatic disease. The COG and SIOP staging systems have been well described.23 The highest local stage is III and disease stage is IV. Patients treated with preoperative chemotherapy without undergoing biopsy were staged according to the staging system used by SIOP.24
Treatment
The overall strategy of the study was to administer prenephrectomy chemotherapy with the goal to perform bilateral partial nephrectomies. Initial induction therapy included vincristine, dactinomycin and doxorubicin (regimen VAD) for two cycles three weeks per cycle (dosing and regimen in supplemental files). After six weeks, cross-sectional imaging was performed and a tumor response was assigned for each kidney (see response criteria below). If it was deamed feasible by the local institution to perform bilateral partial nephrectomies, surgery was to be undertaken. If the tumors achieved a partial response (PR) but were not yet amendable to bilateral partial nephrectomy, chemotherapy was continued for another two cycles. If tumors in either kidney did not achieve a PR, bilateral open renal biopsies were performed to assess the histologic reason for non-responsiveness. After four cycles of VAD (12 weeks), repeat cross sectional imaging was performed and definitive surgery was required.
Radiology Response Criteria
Criteria used to assess tumor response included reduction in size and the ability to perform a nephron-sparing procedure. Response was based on the Response Evaluation Criteria in Solid Tumor (RECIST 1.1) modified to include 3 lesions per kidney. 25 Target lesions were defined as lesions greater than 10 mm within the kidney. If multiple target lesions were present at least three of them were described. Overall response was not modified by extra renal target lesions nor non-target disease. Each kidney was assessed separately. PR was defined as at least a 30% decrease in the sum of the diameters of target lesions (which equals to a 50% decrease in volume), taking as reference the baseline sum diameters. Progressive disease (PD) was defined as at least a 20% increase in the sum of the diameters of target lesions and stable disease (SD) as neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD.
Chemotherapy
Adjuvant therapy was based on tumor stage and histologic response after either six or 12 weeks of chemotherapy. Table 1 describes the chemotherapy regimens and table 2(A, B, C) shows the treatment strategy for patients with BWT at initial diagnosis, week 6 and week 12 (dosing in supplemental files).The final risk stratification based on post-surgery and chemotherapy pathology used by SIOP.23 The details of the chemotherapy regimens have been used in prior studies, however in the recent studies the regimens changed with respect to mg/kg versus mg/m2 dosing (VAD, EE4A, DD4A, I and revised UH-1 (supplemental files).26-28 Treatment was based on the kidney with the highest stage. For example, if one kidney was stage I and the other stage III, chemotherapy treatment was based on the stage III.
Table 1.
| Regimen Name | Chemotherapy Drugs | Duration |
|---|---|---|
| VAD (induction therapy only) | Vincristine, dactinomycin, doxorubicin | 12 weeks maximum |
| EE-4A | Vincristine and dactinomycin | 19 weeks |
| DD-4A | Vincristine, dactinomycin, doxorubicin and radiation therapy | 25 weeks |
| Regimen I | Vincristine, dactinomycin doxorubicin cyclophosphamide and etoposide, as well as radiation therapy | 28 weeks |
| Revised UH-1 | Vincristine, dactinomycin doxorubicin cyclophosphamide carboplatin, etoposide and radiation | 31 weeks |
Flank radiation in most cases.
For specific dosing details please see supplemental files
Table 2A. Initial Therapy for Bilateral Wilms Tumor at Time of Enrollment.
| BILATERAL WILMS TUMOR | |
|---|---|
| INITIAL REGIMEN | |
| Imaging only (no histology) | VAD |
| Imaging and biopsy- Reveal favorable histology | VAD |
| Imaging and biopsy-Reveal diffuse anaplastic Wilms tumor | Revised UH-1 |
| Total or partial nephrectomy at diagnosis Treatment base don the highest assigned risk for either kidney and systemic metastasis | Therapy appropriate for stage and histology |
VAD= Vincristine, dactinomycin and doxorubicin
UH-1 = Vincristine, dactinomycin doxorubicin cyclophosphamide carboplatin, and etoposide
Table 2B. Treatment Regimens for Bilateral Wilms Tumor at Week 6.
| BWT with COMPLETE RESPONSE AFTER WEEK 6 IMAGING REVIEW | ||
| Stage | Chemotherapy Regimen | |
| CR by imaging after 6 weeks of chemotherapy, no biopsy done | Localized disease at initial imaging | EE4A |
| CR by imaging after 6 weeks of chemotherapy | Metastatic disease on initial imaging | DD4A |
| CR by imaging after 6 weeks of chemotherapy, initial biopsy performed | All Stages | DD-4A for favorable histology Revised UH-1 for anaplastic histology |
| SURGICAL RESECTION AT 6 WEEKS FURTHER CHEMOTHERAPY IS BASED ON HISTOLOGY OF REMOVED TUMOR AND HIGHEST TUMOR STAGE. | ||
| Histology | Stage | Regimen |
| Completely necrotic | I-II | EE-4A |
| Intermediate risk | I | EE-4A |
| Intermediaterisk | II | DD-4A |
| Intermediate risk | III-IV | DD-4A+XRT |
| Blastemal predominant | I | DD-4A |
| Diffuse anaplastic Wilms tumor | I | DD-4A+XRT |
| Completely necrotic | III-IV | DD-4A+XRT |
| Focal anaplastic Wilms tumor | I-III | D-4A+XRT |
| Blastemal predominant | II | Reg. I |
| Blastemal predominant | III-IV | Reg. I +XRT |
| Focal anaplastic Wilms tumor | IV | Revised UH-1+XRT |
| Diffuse anaplastic Wilms tumor | II-IV | Revised UH-1)+XRT |
| PARTIAL RESPONSE IN BOTH KIDNEYS BUT BILATERAL PARTIAL NEPHRECTOMY NOT FEASIBLE AT 6 WEEKS. NO SURGERY SHOULD BE PERFORMED. CONTINUE CHEMOTHERAPY. | ||
| Histology | Stage | Regimen |
| If initial therapy based on imaging only | All stages | 6 more weeks of VAD Reevaluate at 12 weeks. |
| If initial therapy based on imaging and biopsy revealed favorable histology | All stages | 6 more weeks of VAD |
| If initial therapy based on imaging and biopsy revealed anaplastic Wilms tumor | All stages | Continue with Revised UH-1 Reevaluate at 12 weeks. |
| LESS THAN PARTIAL RESPONSE IN EITHER KIDNEY AT 6 WEEKS. THESE PATIENTS REQUIRE BILATERAL OPEN BIOPSIES. | ||
| 6-week biopsy- Reveals anaplasia | All stages | Revised Regimen UH-1 and treat per stage. Reassess at 12 weeks. |
| 6-week biopsy- Reveals blastemal predominant | All stages | Regimen I. Reassess at 12 weeks. |
| 6-week biopsy- All other histology | All stages | Continue VAD for 6 weeks and reassess at 12 weeks. |
CR= Complete Response
XRT= Radiation therapy
VAD= Vincristine, dactinomycin and doxorubicin
EE4A= Vincristine and dactinomycin I= Vincristine, dactinomycin, doxorubicin, cyclophosphamide and etoposide
UH-1 = Vincristine, dactinomycin doxorubicin cyclophosphamide carboplatin and etoposide
Table 2C. Treatment Regimen from Bilateral Wilms Tumor at Week 12.
| BILATERAL WILMS TUMOR AFTER WEEK 12 IMAGING REVIEW | ||
|---|---|---|
| REGIMEN | ||
| IF DEFINITIVE SURGERY HAD OCCURRED AT END OF WEEK 6 | Continue with regimen as signed at the end of Week 6. | |
| ATEND OF WEEK 6, THERAPY WAS EITHER CONTINUED CHEMOTHERAPY OR BILATERAL OPEN BIOPSIES WERE PERFORMED. DEFINITIVE SURGERY REQUIRED EITHER PARTIAL OR TOTAL NEPHRECTOMY (UNLESS CR BY IMAGING AT WEEK 12). Treatment will be based on the highest assigned risk for either kidney and systemic metastasis: | ||
| HISTOLOGY | STAGE | REGIMEN |
| Complete response | DD-4A | |
| Completely necrotic | I-II | EE-4A( |
| Completely necrotic | III-IV | DD-4A+XRT |
| Intermediate risk | I | EE-4A |
| Intermediate risk | II | DD-4A |
| Intermediate risk | III-IV | DD-4A+XRT |
| Blastemal predominant | I | DD-4A |
| Diffuse anaplastic Wilms tumor | I | DD-4A+XRT |
| Focal anaplastic Wilms tumor | I-III | DD-4A+XRT |
| Blastemal predominant | II | Reg. I |
| Blastemal predominant | III-IV | Reg. I +XRT |
| Focal anaplastic Wilms tumor | IV | Revised H-1+XRT |
| Diffuse anaplastic Wilms tumor | II-IV | Revised UH-1+XRT |
CR= Complete Response
XRT= Radiation therapy
VAD= Vincristine, dactinomycin and doxorubicin
EE4A= Vincristine and dactinomycin
I= Vincristine, dactinomycin, doxorubicin, cyclophosphamide and etoposide
UH-1 = Vincristine, dactinomycin doxorubicin cyclophosphamide carboplatin and etoposide
Radiation Therapy
For favorable histology tumors that were classified as abdominal stage III, flank radiotherapy with 10.8 Gy was utilized (19.8 Gy for ≥16 years old). A difference from other COG studies for unilateral Wilms tumor was that although needle or biopsies prior to chemotherapy were considered as a criterion for stage III, these patients were not mandated to receive flank radiation therapy if there were no other reasons for stage III designation. Patients requiring whole abdomen irradiation due to preoperative tumor rupture, peritoneal metastases or a large intraoperative tumor spill affecting areas outside the tumor bed as determined by the surgeon received 10.5Gy. A boost of 10.8 Gy was given to patients with residual disease. For patients with stage I-II anaplastic histology, radiation doses were the same as for favorable histology. However, higher doses (19.8 Gy flank or 10.5 Gy whole abdomen with a 9 Gy boost to the flank for patients requiring whole abdomen irradiation) were provided to patients with stage III diffuse anaplastic histology. Patients ≤12 months old requiring whole abdomen XRT did not receive the boost to the flank. All patients with stage IV disease with pulmonary metastasis received whole lung irradiation to a dose of 12 Gy (10.5 Gy for patients < 12 months old).
Statistical Considerations
The study was monitored by an independent data safety monitoring board. A target accrual of 234 patients was required to enroll a minimum of 115 eligible patients with favorable histology BWT without blastemal features to meet the study's statistical considerations. This minimal sample size provided 80% power (testing [using the two-sample log-rank test] at the 5% level of statistical significance [1-sided]) to detect about a 43% reduction in the risk of failure in non-blastemal, favorable histology BWT (relative failure rate 0.57:1.00, corresponding to an increase in 4-year EFS to about 80%). Interim efficacy monitoring was done at 25%, 50% and 75% of the expected information using an O'Brien-Fleming boundary (truncated at 3 standard deviations).29 The other histological subsets were too small to detect definitively an improvement in their EFS and are presented using descriptive statistics. Statistical methods used included Fisher exact test and Kaplan-Meier estimates of the OS and EFS curves.30
Results
Patient characteristics
The study opened in July 2009 and closed in June 2015. Two hundred and forty-nine patients were enrolled and 7 were declared inevaluable (1 failure to sign proper consent, 2 with incorrect pathology, 4 went off study because of investigator choice). There were 99 males and 143 females. Eighty percent of children presented before 48 months. 21.1% of the patients had a genetic syndrome as an underlying diagnosis. There were nine children with hemi-hypertrophy, 7 Beckwith-Wiedemann Syndrome(BWS), 6 with WAGR syndrome and 3 with DDS and 16 with an isolated anomaly. One hundred and ninety–five enrolled on the BWT strata, of which 163 had non-blastemal type, 13 had blastemal-type and 19 unknown post-chemotherapy histology. Five of 195 were declared inevaluable due to protocol violations related to chemotherapy delivery and one was excluded because the patient had rhabdoid pathology. Median follow-up was 3.75 years. Twenty- six had at least one kidney with anaplasia, 9 had focal anaplasia and 17 had diffuse anaplasia. After induction chemotherapy and surgery, the local stage distribution(based on the kidney with the highest stage) was stage I (37.5%), stage II (14.4%) and stage III (48.1%). Twenty-seven (14.4%) of patients had stage IV disease (pulmonary metastasis in all cases). Thirteen patients had biopsies at time zero.
Tumor Response and Surgical Resection
The tumor response to pre-nephrectomy chemotherapy among 189 patients with BWT using the RECIST criteria for the least responsive tumors was CR (8), PR (121), SD (58) and PD (2). Of the 189 BWT patients, 163 (84.0%) underwent definitive surgical treatment (partial or complete nephrectomy or wedge resection in at least one kidney) by 12 weeks after initiation of chemotherapy(aim 3). Thirty percent of those patients who achieved definitive surgical resection within the 12 week specified aim did so by at the six-week evaluation point. Surgical approaches included: unilateral total nephrectomy with contralateral partial nephrectomy (48%), bilateral partial nephrectomy (35%), unilateral total nephrectomy (10.5%), unilateral partial nephrectomy (4%) and bilateral total nephrectomies (2.5%).
Clinical Outcomes
For the 189 patients with BWT, the 4-year EFS and OS (aim1) were82.1% (95% CI: 73.5%-90.8%) and 94.9% (95% CI: 90.1%-99.7%). (Figure 1) There were 23(12%) relapses with a median time to relapse of 15.21 ±10.7 (SD)months. 16/167 (9.5%) patients with favorable histology, 7/16(43%)patients with diffuse anaplastic histology, and 0/7 patients with focal anaplastic histology relapsed. Three relapses occurred at multiple sites, 10 in the remaining kidney(s) alone, seven in the lung alone, one in the liver alone, and two in the abdomen but not the renal fossa. Seven patients progressed on therapy, with one death.
Figure 1. Kaplan Meier Curve ARENO534 Event Free Survival and Overall Survival for all patients and histology.
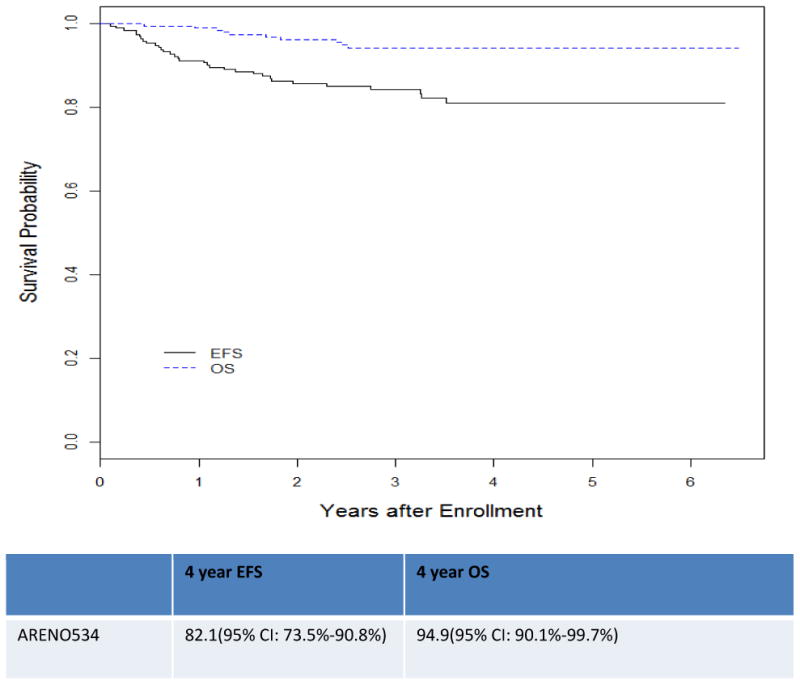
The 4-year EFS and OS according to histology and stage are listed in Table 3 Aim 2 was to prevent complete removal of at least one kidney in 50% of patients with BWT by using pre-nephrectomy 3-drug chemotherapy induction with vincristine, dactinomycin and doxorubicin. Of the 163 who underwent surgery by 12 weeks,143 had either 6 or 12 weeks of prenephrectomy chemotherapy. In this group 61% required complete nephrectomy of at least one kidney. The 20 other patients either had upfront surgery, surgery due to complications from disease progression or did not tolerate all the cycles of chemotherapy.
Table 3. ARENO534 Outcomes by Post-SurgicalStage and Histology.
| Histology | N | 4-year EFS (95% CI) | 4-year OS (95% CI) |
|---|---|---|---|
| FH/FH | 163 | 84.2% (75.2%–93.1%) | 97.3% (93.5%–100%) |
| FH/FH Stage I* | 66 | 85.4% (72.4%–98.5%) | 96.87% (90.7%–100%) |
| FH/FH Stage II* | 21 | 90.23% (69.3%–100%) | 100% (100%–100%) |
| FH/FH Stage III* | 59 | 84.34% (69.7%–99.0%) | 97.96% (92.6%–100%) |
| FH/FH Stage IV* | 17 | 76.5% (34.5%–100%) | 94.1% (71.8%–100%) |
| Both kidneys without blastemal features | 140 | 83.18% (73.2%–92.96%) | 977% (93.90%–100%) |
| At least one kidney with blastemal features | 11 | 81.8% (42.3%–100%) | 91% (64.1%–100%) |
| Anaplasia** | |||
| Diffuse Anaplasia | 17 | 58.2% (15.7%–100%) | 68.4% (24.9%–100%) |
| Focal Anaplasia | 8 | 87.5% (57.18%–100%) | 100% (100%–100%) |
| Stage I* | 4 | 100% | 100% |
| Stage II* | 3 | No cases | 66.67% |
| Stage III* | 15 | 72.7% (35.5%–100%) | 79% (43.2%–100%) |
| Stage IV* | 3 | No cases | No cases |
By the highest stage (either favorable histology focal or diffuse anaplasia present)
In at least one kidney
Toxicity
There were no grade 5 toxic events reports on the study. There were 4 cases of sinusoidal obstructive syndrome (SOS/VOD) in patients who received regimen DD4A as part of their post nephrectomy therapy. All 4 recovered.
Discussion
The AREN0534 study successfully improved outcomes for patients with BWT, with four-year EFS and OS estimates of 82.1% and 94.16%, as compared to four-year EFS and OS estimates of 56% and 80.8% on NWTS-5.7 Several interventions that were implemented on AREN0534 likely contributed to this improvement. First, chemotherapy was tailored according to post-chemotherapy histologic response. Patients with anaplastic and blastemal-type histology received more intensive chemotherapy compared to previous studies. Although the number of patients in each subgroup was small, the results suggest improvement for both favorable and diffuse anaplastic histology. Four-year EFS for favorable histology went from 65% (reported for patients with retention of heterozygosity at chromosome 1p) on NWTS-5 to 84.1% on AREN0534. For diffuse anaplastic histology, four-year EFS went from 25% on NWTS-5 to 58.2% on the current study. The four-year EFS and OS estimates for patients with blastemal-type tumors were 82% and 91%, respectively. Although outcomes in this small group of patients were not assessed on NWTS-5, this result is promising based on the adverse prognostic significance of blastemal type reported on the SIOP studies.31 It is possible that the survival estimates on AREN0534 will diminish over time based on observations from prior BWT reports that late relapses were common.18 However, thus far, the majority of patients relapsed within the first 18 months following diagnosis, which leads us to believe that the excellent outcomes on AREN0534 will persist.
A second intervention implemented on AREN0534 was to decrease the time from diagnosis to surgical resection. Shamberger and colleagues studied 38 patients from NWTS 4 with progressive or non-responsive disease.14 Preoperative chemotherapy was given for a median of 7 months (range, 2-29 months) before definitive resection. Thirty-six children went on to a second regimen, and of these, 21 children received a third regimen before resection. Ten of the 38 patients lacked histology data and of the remaining 28 patients, 15 had either rhabdomyomatous differentiation, complete necrosis or stromal differentiation. A tumor may also be non-responsive because it has anaplastic histology which may be chemo-insensitive and requires early definitive surgery.14 Furthermore, a review by the German Society of Pediatric Oncology and Hematology (GPOH) of patients with BWT reported that maximum tumor shrinkage occurred in the first 12 weeks of chemotherapy.32 Based on these considerations, an aim of AREN0534 was for 75% of patients to undergo definitive surgery by 12 weeks. To facilitate earlier surgical resection, all patients received three-drug therapy with vincristine/dactinomycin/doxorubicin rather than starting with just vincristine/dactinomycin and escalating if response was inadequate. The choice to use three drugs was based on data from SIOP studies showing greater tumor shrinkage with three-drug compared to two-drug therapy.23 Additionally, Paulino et al. found that a three-drug regimen resulted in a significantly lower relapse rate (8%) compared to a two-drug regimen (42%) in patients with synchronous BWT.33 AREN0534 exceeded its aim to expedite surgical resection, with 84% of patients having definitive surgical treatment by 12 weeks, with the majority of patients having a PR to chemotherapy.
Compared to patients with unilateral WT, children with BWT have poorer EFS and are at higher risk for later effects such as renal failure.11, 12, 34, 35 An overriding goal was to improve EFS balanced with conservation of renal tissue. Our goal was to have at least 50% of patients undergo bilateral nephron-sparing surgery (NSS). We did not reach this threshold, with only 39% successfully treated with bilateral NSS. Davidoff and associates reported a 13-year experience of 42 patients with BWT where he was able to perform bilateral NSS in 92% of patients.36 Comparison of these two study populations without imaging has its limits but the data does suggest that surgeon experience may affect the chance of undergoing NSS. The aim might have been ambitious for a cooperative group-wide study. However, the NSS rate on AREN0534 was greater than what would be expected based on the NSS experience with unilateral WT. Interestingly, the Surveillance, Epidemiology and End Results Program (SEER) from the NCI reports a similar renal sparing rate. SIOP protocols routinely use pre-nephrectomy chemotherapy, with up to 85% of tumors responding to some degree but not necessarily resulting in NSS in unilateral lesions.37-39 In the SIOP 2001 study, partial nephrectomies for unilateral WT were permitted if the tumor was polar or non-infiltrating by cross-sectional imaging, but only 3% of the total cases enrolled met these criteria for a partial nephrectomy after pre-nephrectomy chemotherapy.40 In patients with bilateral lesions there is an increased effort at renal preservation to avoid renal failure, in contrast with patients with unilateral lesions in which there is much less concern for this issue. A recent technical paper on longitudinal partial nephrectomy by Fuchs to address central renal lesions may also increase the rate of NSS.41 Other factors that may increase the NSS rates include regionalization of this patients to centers with more experience since these are rare cases and central review that includes technical guidance.
COG renal tumor studies require pathology prior to treatment and study enrollment. This study was an exception because patients could enroll based on imaging alone. The primary reasons to perform a biopsy at initial presentation are to avoid misdiagnosis of Wilms tumor and to detect anaplasia. The rate of misdiagnosis of Wilms tumor is low, between 1.6% to 5.5 % for unilateral renal tumors on SIOP studies.42 The rate is estimated to be even lower for bilateral renal tumors because bilateral non-Wilms renal tumors are very uncommon, though rare patients with bilateral rhabdoid tumor and renal cell carcinoma have been reported. 43 Regarding the detection of anaplasia, review of data from NWTS 4 showed that needle biopsy failed to detect anaplasia and open biopsy only detected them in 1/3 of cases.15 On NWTS-5, among 25 patients with bilateral anaplastic Wilms tumor for whom biopsy and nephrectomy histology were available for review, only two (8%) had anaplasia detected in the initial biopsy sample.5 Based on these considerations, patients could enroll on AREN0534 based on imaging alone with the caveat that in an unusual clinical situation that may suggest a diagnosis other than Wilms tumor, such as age > 10 years or atypical imaging features obtaining a tissue diagnosis before starting therapy should be considered.
The limitations of the study are inherent in its design. Although the EFS and OS reported here are improved compared to historical reports, this was not a randomized controlled trial and it is therefore difficult to pinpoint which factors played the greatest role in improving outcome. While radiographic criteria were well defined for this study, we cannot be certain that some of the smaller lesions which resolved with neoadjuvant therapy may have been nephrogenic rests. This may have resulted in the inclusion of patients with unilateral WT in the patient cohort. To mitigate this, we excluded patients with lesions less than a centimeter. It is difficult to estimate how this may affect results. In addition, a core or needle biopsy cannot discriminate between a rests ad tumor unless a rim of capsule or normal tissue is included in the sample. Wilms tumors arise from rests that become hyperplastic and can be considered premalignant. We do not feel that the main reason for the improved EFS and OS was that we were treating benign lesions Second, the study is not yet mature enough to determine the impact of this treatment strategy on late effects such as renal failure or anthracycline toxicity. Patients will be followed for 10 years to track the renal failure rates. Future study will need to also focus on the impact of initial tumor burden to predict response, common factors predicting relapse including biomarkers such as 1q gain or LOH 1p and 16q, impact of novel agents to improve tumor response and directing further strategies toward renal preserving surgery
The results of AREN0534 indicate that improved EFS and OS for patients with BWT can be achieved with real-time central review of imaging, surgery and pathology, standardized three-drug preoperative chemotherapy, surgical resection within 12 weeks of diagnosis and histology-based post-operative therapy. While the results provide benchmark outcomes for future studies, EFS can be improved and nephron-sparing surgery rates were suboptimal. In future studies, central review incorporating technical guidance for patients with BWT prior to definitive surgical may be useful.
Supplementary Material
Acknowledgments
This project was supported by grants CA98543 (Children's Oncology Group Chair's grant) and CA98413 (COG SDC grant) from the National Institutes of Health. U10CA180899
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
The authors have NO conflicts of interest
References
- 1.Dome JS, Fernandez CV, Mullen EA, et al. Children's Oncology Group's 2013 blueprint for research: renal tumors. Pediatr Blood Cancer. 2013;60(6):994–1000. doi: 10.1002/pbc.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dome JS, Perlman EJ, ML R, et al. Renal Tumors. In: PA P, DG P, editors. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams and Wilkins; 2015. pp. 753–772. [Google Scholar]
- 3.Dome JS, Graf N, Geller JI, et al. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J Clin Oncol. 2015;33(27):2999–3007. doi: 10.1200/JCO.2015.62.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dome JS, Perlman EJ, Graf N. Risk stratification for wilms tumor: current approach and future directions. Am Soc Clin Oncol Educ Book. 2014:215–223. doi: 10.14694/EdBook_AM.2014.34.215. [DOI] [PubMed] [Google Scholar]
- 5.Dome JS, Cotton CA, Perlman EJ. Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J Clin Oncol. 2006;24(15):2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 6.Grundy PE, Breslow N, S L, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23(29):7312–7321. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- 7.Grigoriev Y, Ou S, Breslow N. National Wilms Tumor Study 5 Statisitical Report 2002. 2002 [Google Scholar]
- 8.Coppes MJ, de Kraker J, van Dijken PJ. Bilateral Wilms' tumor: long-term survival and some epidemiological features. J Clin Oncol. 1989;7(3):310–315. doi: 10.1200/JCO.1989.7.3.310. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Fitzgerald R, Breatnach F. Conservative surgical management of bilateral Wilms tumor: results of the United Kingdom Children's Cancer Study Group. J Urol. 1998;160(4):1450–3. doi: 10.1016/s0022-5347(01)62588-6. [DOI] [PubMed] [Google Scholar]
- 10.Paulino AC. Re: Conservative surgical management of bilateral Wilms tumor: results of the United Kingdom Children's Cancer Study Group. J Urol. 1999;162(1):167. doi: 10.1097/00005392-199907000-00046. [DOI] [PubMed] [Google Scholar]
- 11.Grundy P. Outocmes of patients with bilateral Wilms Tumor from NWTS-5 study 2005 [Google Scholar]
- 12.Breslow N, Collins AJ, Ritchey ML, et al. End stage renal failure in patients with Wilms tumor: Results from the national wilms tumor study group and the United States renal data system. J Urol. 2005;174:1972–1975. doi: 10.1097/01.ju.0000176800.00994.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paulino AC, Wen BC, Brown CK, et al. Late effects in children treated with radiation therapy for Wilms tumor. Int J Rad Oncol. 2000;46(5):1239–1246. doi: 10.1016/s0360-3016(99)00534-9. [DOI] [PubMed] [Google Scholar]
- 14.Shamberger RC, Haase G, Argani P. Bilateral Wilms' tumors with progressive or nonresponsive disease. J Pediatr Surg. 2006;41(4):652–657. doi: 10.1016/j.jpedsurg.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton TE, Green DM, Perlman EJ, et al. Bilateral Wilms' tumor with anaplasia: lessons from the National Wilms' Tumor Study. J Pediatr Surg. 2006;41(10):1641–1644. doi: 10.1016/j.jpedsurg.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton TE, Ritchey ML, Haase G, et al. The management of synchronous bilateral Wilms tumor: a report from the National Wilms Tumor Study Group. Ann Surg. 2011;253:1004–1010. doi: 10.1097/SLA.0b013e31821266a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson JR, Slater O, McHugh K, et al. Response without shrinkage in Bilateral Wilms Tumor: Significance of Rhabdomyomatous Histiology. J Pediatr Hem Oncol. 2002;24:31–34. doi: 10.1097/00043426-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich PF, Dome JS, Shamberger RC, et al. Treatment for Patients with Bilateral, Multicentric, or Bilaterally-Predisposed Unilateral Wilms Tumor. [Accessed 2017];Journal [serialonline] 2006 Available from: Childrens Oncology Group. [Google Scholar]
- 19.Mullen EM, Geller J, Ehrlich PF, et al. Real Time Central Review and Risk Stratification is feasible improves study quality and risk based therapy. A report from the Children's Oncology Group (COG) Renal Tumor Biology and Risk Stratifications Protocol ARENO3B2. J ClinOncol. 2014;32(15) [Google Scholar]
- 20.Gow K, Barnhart DC, Hamilton TE, et al. Primary Nephrectomy and Intraoperative Tumor Spill: Report from the Children's Oncology Group (COG) Renal Tumors Committee. J Pediatr Surg. 2013;4834(1):38. doi: 10.1016/j.jpedsurg.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton TE, Barnhart D, Gow K, et al. Inter-rater reliability of surgical reviews for AREN03B2: a COG renal tumor committee study. J Pediatr Surg. 2014;49(1):154–8. doi: 10.1016/j.jpedsurg.2013.09.047. discussion 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchey ML, Shamberger RC, Hamilton TE, et al. Fate of bilateral renal lesions missed on preoperative imaging: a report from the National Wilms Tumor Study Group. J Urol. 2005;174(4 Pt 2):1519–1521. doi: 10.1097/01.ju.0000179536.97629.c5. [DOI] [PubMed] [Google Scholar]
- 23.Dome JS, Perlman EJ, Graf N. Risk stratification for wilms tumor: current approach and future directions. Am Soc Clin Oncol Educ Book. 2014:215–23. doi: 10.14694/EdBook_AM.2014.34.215. [DOI] [PubMed] [Google Scholar]
- 24.J dK, N G, F P, et al. Socitey of International Pediatric Oncology (SIOP) Wilms Tumor Protocol 2001. 2001 [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Green DM, Breslow N, D'Angio GJ, et al. The treatment of children with unilateral Wilms' tumor. J Clin Oncol. 1993;11:1009–1010. doi: 10.1200/JCO.1993.11.6.1009. [DOI] [PubMed] [Google Scholar]
- 27.Green DM, Thomas PRM, Schocat S. The treatment of Wilms tumor. Results of the National Wilms Tumor Studies. Hematol Oncol Clin North Am. 1995;9(6):1267–1274. [PubMed] [Google Scholar]
- 28.Green DM, Evans I, Breslow N, et al. Relationship between dose schedule and charges for treatment on National Wilms' tumor Study-4. A report from the National Wilms' Tumor Study Group. Journal of the National Cancer Institute Monographs. 1995;19:19–25. [PubMed] [Google Scholar]
- 29.O'Brien PC, Fleming TR. A Multiple Testing Procedure for Clinical Trials. Biometrics. 1979;35:549–546. [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;33:457–481. [Google Scholar]
- 31.Vujanic G, Sandstedt B, Harms D. Revised International Society of Paediatric Oncology (SIOP)Working Classification of Renal Tumors of Childhood. Med Pediatr Oncol. 2002;38(2):79–82. doi: 10.1002/mpo.1276. [DOI] [PubMed] [Google Scholar]
- 32.Graf N. 2010 [Google Scholar]
- 33.Paulino AC, Wilimas J, Marina N, et al. Local control in synchronous bilateral Wilms tumor. Int J Radiat Oncol BiolPhys. 1996;36(3):541–8. doi: 10.1016/s0360-3016(96)00377-x. [DOI] [PubMed] [Google Scholar]
- 34.Ehrlich PF. Bilateral Wilms' tumor: the need to improve outcomes. Expert Rev Anticancer Ther. 2009;9(7):963–73. doi: 10.1586/era.09.50. [DOI] [PubMed] [Google Scholar]
- 35.Sudour H, Audry G, Schleimacher G, et al. Bilateral Wilms tumors (WT) treated with the SIOP 93 protocol in France: epidemiological survey and patient outcome. Pediatr Blood Cancer. 2012;59(1):57–61. doi: 10.1002/pbc.24059. [DOI] [PubMed] [Google Scholar]
- 36.Kieran K, Davidoff AM. Nephron-sparing surgery for bilateral Wilms tumor. Pediatr Surg Int. 2015;31(3):229–236. doi: 10.1007/s00383-015-3668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weirich A, Ludwig R, Graf N. Survival in nephroblastoma treated according to the trial and study SIOP-9/GPOH with respect to relapse and morbidity. Ann Oncol. 2004;15(5):808–20. doi: 10.1093/annonc/mdh171. (15):5-808. [DOI] [PubMed] [Google Scholar]
- 38.Burger D, Moorman-Voestermans CGM, Mildenberger H, et al. The advantages of preoperative therapy in Wilms' tumor: a summarized report on clinical trials conducted by the lnternational Society of Paediatric Oncology (SIOP) Z Kinderchir. 1985;40:170–175. doi: 10.1055/s-2008-1059738. [DOI] [PubMed] [Google Scholar]
- 39.Lemerle J, Voute PA, Tournade MF. Effectiveness of preoperative chemotherapy in Wilms' tumor: Results of an International Society of Pediatric Oncology (SIOP) clinical trial. J Clin Oncol. 1983;1:604–609. doi: 10.1200/JCO.1983.1.10.604. [DOI] [PubMed] [Google Scholar]
- 40.Wilde JC, Aronson DC, Sznajder B, et al. Nephron sparing surgery (NSS) for unilateral wilms tumor (UWT): the SIOP 2001 experience. Pediatr Blood Cancer. 2014;61(12):2175–9. doi: 10.1002/pbc.25185. [DOI] [PubMed] [Google Scholar]
- 41.Fuchs J, Szavay P, Scheel-Walter H, et al. Longitudinal partial nephrectomy for stage V nephroblastoma with extension into the renal hilus. Pediatr Blood and Cancer. 2009;53(3):715–716. [Google Scholar]
- 42.Tournade MF, Com-Nougue C, de Kraker J, et al. Optimal duration of preoperative chemotherapy in unilateral non metasttic Wilms tumor in children older then six months. Results of the ninth International Society of Pediatric Oncology tumor trial. J Clin Oncol. 2001;19:488–500. doi: 10.1200/JCO.2001.19.2.488. [DOI] [PubMed] [Google Scholar]
- 43.Tomlinson GE, Breslow N, Dome JS, et al. Rhabdoid tumor of the kidney in the National Wilms' Tumor Study: age at diagnosis as a prognostic factor. J Clin Oncol. 2005;23(30):7641–7645. doi: 10.1200/JCO.2004.00.8110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


