Abstract
The purpose of this project is to enhance the trans-membrane penetration of Δ8-Tetrahydrocannabinol (Δ8-THC) and to study the effect of various lipid based systems in delivering the compound, non-invasively, to anterior and posterior ocular chambers. Solid lipid nanoparticles (SLNs), fast gelling films were manufactured using high pressure homogenization and melt cast techniques, respectively. The formulations were characterized for drug content, entrapment efficiency, particle size and subsequently evaluated in vitro for trans-corneal permeation. In vivo, the drug disposition was tested via topical administration in albino rabbits. The eye globes were enucleated at the end of experiment and tissues were analyzed for drug content. All formulations showed favorable physicochemical characteristics in terms of particle size, entrapment efficiency and drug content. In vitro, the formulations exhibited a transcorneal flux that depended on the formulation’s drug load. An increase in drug load from 0.1% – 0.75% resulted in 12 to16-folds increase in permeation. In vivo, the film was able to deliver THC to all the tissues with high accumulations in cornea and sclera. The SLNs showed a greater ability in delivering THC to all the tissues, at a significantly lower drug load, due to their colloidal size range, which in turn enhanced corneal epithelial membrane penetration. The topical formulations evaluated in the present study were able to successfully deliver Δ8-THC in therapeutically meaningful concentrations (EC50 values for CB1: 6nM and CB2: 0.4nM) to all ocular tissues except the vitreous humor, with pronounced tissue penetration achieved using SLNs as a Δ8-THC delivery vehicle.
Keywords: Solid lipid nanoparticles, Ocular Films, Lipid Based Drug Delivery, Ocular Drug Delivery, Tetrahydrocannabinol
Introduction
Glaucoma is a significant cause of irreversible vision loss globally. The onset of this condition can be multifactorial, most often involving an increase in intraocular pressure (IOP) leading to loss of retinal ganglion cells (RGC) secondary to crush injury, ischemia, and apoptosis (1, 2). Studies have shown that this condition is widely prevalent globally with an estimated prevalence of two million people in the United States, and accounts for 17.8% of the medical costs attributed to major eye diseases. These numbers are expected to increase, with more than three million of the populace being afflicted by this disease by the year 2020 (1, 3). Internationally, the prevalence of glaucoma is expected to reach a staggering 79.3 million by 2020, with more than 11 million transitioning into a state of “complete loss of vision” (4).
Elevation of IOP, an important clinical factor in glaucoma, can be a result of altered aqueous humor flow and drainage dynamics caused by a change in the trabecular meshwork structure. Death of RGC has also been shown to have an effect along with elevated IOP on the ultimate loss of vision in glaucoma patients (5, 6). It has also been shown that a segment of patients, mostly of Asian ancestry, have been diagnosed with glaucoma with no elevated IOP or normo-pressure glaucoma (7). This shows that elevated IOP is a determining but not the only factor causing this condition. More recent studies focus on the loss of RGC due to apoptosis, a mechanism of programmed cell death, which is believed to be the main reason for neuronal damage, ultimately leading to RGC death (8, 9).
Δ9-Tetrahydrocannabinol (Δ9-THC) is the primary active constituents of Cannabis sativa, with its isomer Δ8-Tetrahydrocannabinol (Δ8-THC) being a very minor component. Δ9-THC has shown potential in the treatment of glaucoma through its IOP lowering and neuroprotective effects (10, 11). The mechanism of action is not completely understood, though it has been said to have an agonistic action on the cannabinoid receptors (CB1 and CB2) (12, 13). These receptors are expressed on the iris-ciliary, retina-choroid and the trabecular meshwork (14). Δ9-THC, through these receptors, causes relaxation of the trabecular meshwork which results in increased aqueous humor drainage and subsequent IOP reduction (11). Neuroprotective action of Δ9-THC was also recently studied by El-Remessy et al. in N-methyl-D-aspartate (NMDA) induced retinal toxicity (11), making Δ9-THC a promising candidate in glaucoma therapy. Previous reports from our group have demonstrated the physicochemical characteristics and permeability and in vivo disposition of Δ9-THC and it’s relatively water soluble prodrugs in the eye. The effects of ion pairing and micellar solutions on the disposition of Δ9-THC in the eye were studied in these previous reports (15, 16).
While all the above literature focuses completely on Δ9-THC, little has been said and reported about the potential of its isomer, Δ8-THC. This compound exhibits a stereochemistry similar to Δ9-THC and is also chemically more stable than the latter (17). In addition, the efficacy of Δ8-THC in reducing IOP has been demonstrated previously in rabbits with a long lasting IOP reducing effect evident even at the end of 8 hours(18).
Although Δ8-THC is chemically more stable than Δ9-THC, delivery of this compound to the deeper ocular tissues is challenging. Like Δ9-THC, this compound is also highly lipophilic and poorly soluble and resinous in nature. Table I compares the properties of these two isomers.
Table I.
Physicochemical Properties of Δ9 & Δ8-Tetrahydrocannabinol1
| Property | Δ8-Tetrahydrocannabinol | Δ9-Tetrahydrocannabinol |
|---|---|---|
| Molecular Weight | 314.4 | 314.4 |
| Log P | 7.53 ± 0.36 | 7.68 ± 0.35 |
| mLog P | 3.96 | 3.96 |
| Log D | 7.07 | 7.07 |
| pKa | 9.6 | 9.6 |
| Polar surface area | 29.46 | 29.46 |
| Solubility (μg/mL) | 0.26 ± 0.03 | 1 – 2 |
Solid lipid nanoparticles (SLNs) have been studied over the years as a platform for enhancing topical administration (19, 20). Ease of fabrication, stability, targeted delivery, non-toxicity, small size, prolonged release are some of the advantages of this delivery system. Additionally, the small size of the SLNs can enhance ocular delivery by increasing the corneal residence time and penetration (21–23). Ibrahim et al reported increased bioavailability of gatifloxacin using mucoadhesive nanoparticles synthesized using Eudargit RS 100 and hyaluronic acid (24). Cavalli et al. reported that ocular bioavailability of tobramycin increased 4-fold with incorporation of the drug into an SLN formulation. The authors attributed the increased bioavailability to the trapping of the nanoparticles in the epithelial mucus layer, thus, resulting in prolonged release of the drug into the aqueous humor for up to 6 hours (22). Although the utility of SLNs in ocular therapy in conditions pertaining to the anterior chamber has been investigated and reported, the same cannot be said for delivery to the posterior segment.
Another ocular delivery platform that has promise is drug loaded films. These systems, have the advantage of providing increased contact time with the ocular surface and prolonged release, which reduces the dosing frequency. Depending on the mechanism of drug release post application, the films are classified as either soluble or insoluble. Soluble films are generally made of soluble or erodible polymers and therefore circumvent the need of removal from the eye. The polymer can be either natural or synthetic and release from these kinds of delivery systems is mainly by diffusion as the polymer undergoes gradual gelling followed by dissolution in the tear fluid. Hermans et al reported the improved bioavailability of cyclosporin A using chitosan films (25), while Attia et al used erodible gelatin films for improving the bioavilability of dexamethasone (26).
In this study we evaluate the efficacy of topically administered SLNs in delivering Δ8-THC to the posterior ocular tissues. Further, we also study the utility of a melt-cast film formulation. This film formulation technique, unlike previous reports, avoids the use of solvents, thereby eliminating the risks posed by residual solvents to the ocular tissues.
A comparative evaluation of the SLNs, films, in terms of their ability to deliver the drug to various ocular tissues, has been undertaken both at in vitro and in vivo levels.
Materials & Methods
Materials
Compritol® ATO 888 and Precirol® ATO 5 were gift samples from Gattefosse, France. Poloxamer® 188 was obtained from BASF, Chattanoga, TN. Lipoid® E 80 (Lipoid, Ludwigshafen, Germany) was a gift sample. Propofol, randomly methylated beta cyclodextrin, hydroxypropyl methyl cellulose (4000 cps), Polyethylene Oxide N10 were purchased from Sigma (St. Louis, MO). All other chemicals were purchased from Fisher Scientific (St. Louis, MO). Solvents used for analysis were of HPLC grade.
Animal Tissues
Whole eye globes of New Zealand Albino rabbits were purchased from Pel Freez Biologicals (Rogers, AK). Eyes were shipped overnight in Hanks Balanced Salt Solution (HBSS) over wet ice. Corneas were isolated and used immediately on receipt.
Animals
Male New Zealand White Albino Rabbits were procured from Harlan Labs (Indianapolis, IN). Animal experiments conformed to the tenets of the Association for Research in Vision and Ophthalmology statement on the Use of Animals in Ophthalmic and Vision Research and followed the University of Mississippi Institutional Animal Care and Use committee approved protocols.
Formulations
Solid Lipid Nanoparticles Containing Δ8-THC
SLNs were prepared as per previously established protocols (27), using a high speed & high pressure homogenization method. Δ8-THC was accurately weighed and melted along with Compritol® 888 ATO or Precirol® ATO 5 (Gattefosse, France) to obtain a clear lipid phase. An aqueous phase containing 0.25% Poloxamer® 188, 0.75% Tween® 80 and 2.25% Glycerin (w/v) in distilled water, was heated and added to the melted lipid phase under stirring. A coarse emulsion from this pre-mix was formed using an Ultra-Turrax®, followed by high pressure homogenization. The temperature during this entire process was maintained at 70°C. The hot emulsion was slowly cooled to room temperature to form Δ8-THC SLNs.
SLNs with randomly-methylated beta cyclodextrins (RMβCD) in the lipid phase were prepared by dissolving Δ8-THC & RMβCD in acetonitrile and keeping in a water bath for 24h at 25°C for complex formation. At the end of 24h, the organic solvent was evaporated under nitrogen and molten lipid was added to this Δ8-THC-RMβCD complex and the above procedure was repeated for SLN production. Alternately, Δ8-THC-RMβCD SLN formulation was prepared without the Δ8-THC -RMβCD complexation step. Instead, RMβCD was dissolved in the aqueous phase along with Poloxamer 188®, Glycerin, Tween® 80 and the procedure described above was followed for SLN production.
Film Formulation Containing Δ8–THC
Hot melt cast method was utilized to prepare the polymeric film. Polyethylene oxide (PEO N10: MW 100000 Daltons) was used as the matrix forming material. Δ8-THC (20% w/w) was dissolved in acetonitrile and dispersed in PEO N10 with adequate mixing. The mixture was placed in a vacuum chamber to evaporate the organic solvent. A 13 mm die was placed over a brass plate and the brass plate was heated to 70 °C using a hot plate. The drug-polymer mixture was placed in the center of the die, compressed and further heated for 2–3 min. Following cooling, 4 mm × 2 mm film segments were cut from the film.
The formulation compositions are presented in Table II
Table II.
Composition (%w/v) of the various Δ8-Tetrahydrocannabinol Formulations:
| Formulation Code | F0 | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|---|
| Formulation Type | Solid Lipid Nanoparticles (SLNs) (%w/v) | Film (%w/w) | ||||
| δ8-THC | 0.1 | 0.75 | 0.75 | 0.75 | 0.75 | 20 |
| Precirol | 3 | |||||
| Compritol | 3 | 3 | 3 | 3 | ||
| Glycerine | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | |
| Tween 80 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | |
| Poloxamer 188 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | |
| RMßCD |
3 (In aqueous Phase) |
3 (THC-CD complex) |
||||
| HPMC 4k | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | |
| PEO N10 | 80 | |||||
| IPBS (mL) | q.s | q.s | q.s | q.s | q.s | |
Determination of Particle Size and Polydispersity Index
Particle size and Polydispersity Index (PDI) of the SLNs were measured using a dynamic light scattering instrument, Zetasizer Nano ZS (Malvern Instruments Inc., Westbrough, MA), at 25 °C. A high concentration zeta cell was used to measure both mean particle size (z averaged) and PDI.
Drug Content Estimation Procedure for the Formulations
Δ8-THC content in the SLNs was measured according to the following procedure. An accurately measured amount of the formulation was extracted in 1 mL of ethanol and the suspension was centrifuged for 10 minutes at 13000 rpm. The supernatant was diluted in mobile phase and was analyzed for Δ8-THC content.
Δ8-THC content in the film was determined by placing the film in 10 mL of acetonitrile and sonicating for 15 min. The film dissolves completely in acetonitrile. This mixture was centrifuged at 13000 rpm for 10 min to separate the PEO N10 and supernatant with Δ8-THC was collected. The supernatant was then analyzed by HPLC. To determine the content uniformity of the film three separate sections from the same film were analyzed following the same procedure as described above.
Entrapment Efficiency for Solid Lipid Nanoparticles
Entrapment efficiency was determined using AMICON® Ultra centrifugal filters with a 10000 KDa membrane. A measured amount of formulation was taken and placed in the centrifugal filter and the sample was spun at 13000 rpm for 30 min, following which the filtrate was collected and analyzed for free Δ8-THC content. Percentage Δ8-THC entrapped was calculated using the formula.
In vitro Release from Solid Lipid Nanoparticles
Slide-A-Lyzer® mini dialysis cassettes (0.5mL; 10k membranes) were used for studying release. The receiver medium, 18mL in volume, contained a solution of isotonic phosphate buffered saline (IPBS) with 2.5% w/v RMβCD at 37°C. A volume of 500 μL of the formulation was placed in the cassette. Samples, 1 mL aliquots, were drawn at regular intervals from the receiver which was then immediately replaced with an equal volume of fresh receiver solution. The samples were analyzed and the percentage drug released was determined. Experiments were carried out in triplicates.
In vitro Transcorneal Transport Studies
Corneas were excised from whole rabbit eye globes (Pel-Freez Biologicals; Rogers, AK). Briefly an incision was made about 2mm from the corneal-scleral junction and the cornea was excised by cutting radially along the sclera. The excised corneas were immediately mounted on side by side permeation cells (Permgear Inc, Hellertown, PA) (Fig. 1). A circulating water bath was used to maintain the temperature at 34°C during the transport studies. Receiver solution for all permeability studies consisted of 2.5% RMβCD solution in IPBS with pH adjusted to 7.4. The volume of the receiver solution was 3.2 mL, 0.2 mL more than the donor to maintain the natural curvature of the corneas, and the solution in the chamber was stirred continuously using magnetic stirrers. SLNs formulations were diluted in a 2:1 ratio with IPBS to yield donor solution for these studies. The initial donor concentrations were 0.66mg/mL in the case of F-0, 5mg/mL in the case of F-1, F-2, F-3, and F-4. Aliquots, 600μL, were withdrawn from the receiver chamber every thirty minutes for three hours and immediately replaced with an equal volume of the receiver solution. Samples were analyzed following the method described in the analytical methods section. All experiments were carried out in triplicate.
Fig. 1.
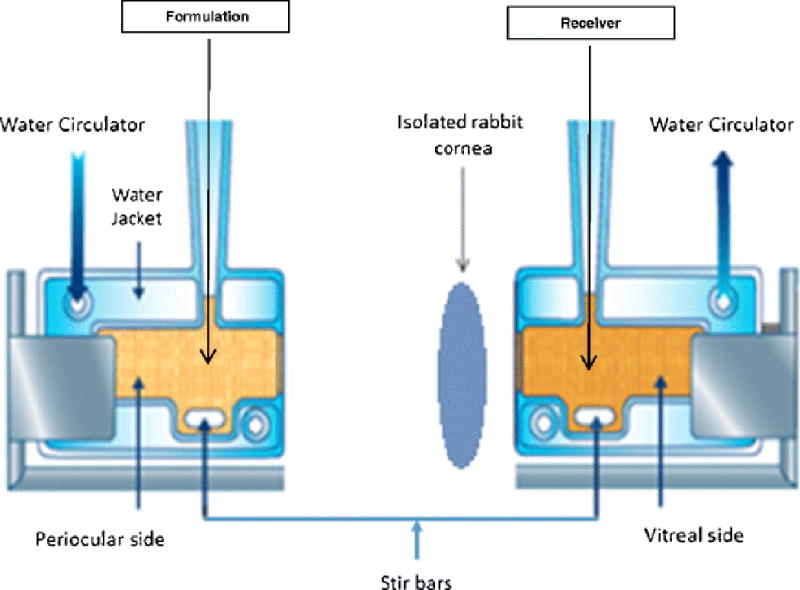
Side-by-side diffusion apparatus setup for studying in vitro trans-corneal transport of Δ8-Tetrahydrocannabinol from solid lipid nanoparticles, nanoemulsion formulations.
In vitro transcorneal flux of Δ8-THC from the matrix film was evaluated by sandwiching the film (4 mm × 2 mm × 2mm) in between a Spectra/Por® membrane (MWCO: 10,000 2 Daltons) and isolated rabbit cornea (Pel-Freez Biologicals; Rogers, AK). Corneas were excised from whole eye globes, with approximately 1 mm scleral portions remaining for ease of mounting. The membrane-film-cornea sandwich was then placed in between the side-by-side diffusion cells with the chamber towards the Spectra/Por® membrane representing the periocular surface and the chamber towards the cornea representing the aqueous humor (Fig. 2). The side-by-side diffusion cells were maintained at 34 °C using a circulating water bath. 2.5% RMβCD in IPBS (pH 7.4) was used as the receiver medium on both sides. The initial THC amount in this film section at the start of the experiment was 1.6 mg and weight of the film was 8 mg (original formulation has composition of 20% w/w THC and 80% w/w PEO N10) and aliquots, 600μL, were drawn every thirty minutes for three hours and replaced with an equal volume of receiver solution. Samples were analyzed following the method described in the analytical methods section. The experiment was carried out in triplicate.
Fig. 2.
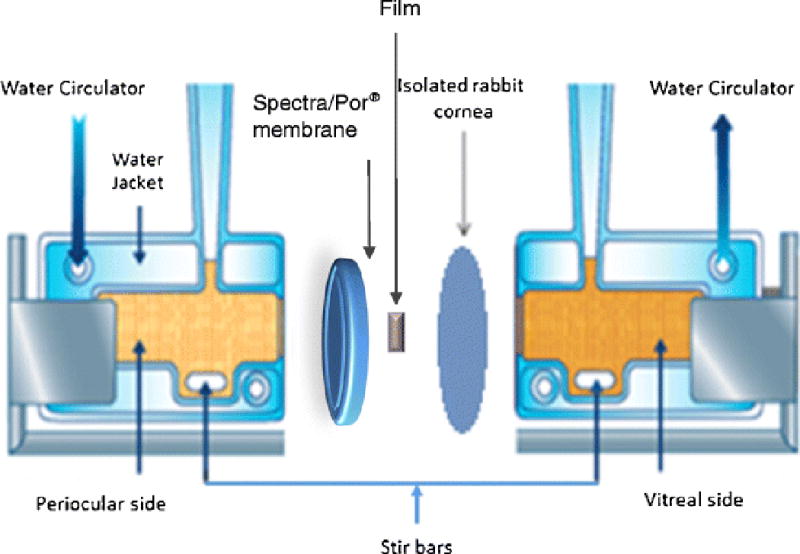
Side-by-side diffusion apparatus setup for studying in vitro trans-corneal transport of Δ8-Tetrahydrocannabinol from film formulation
In vivo Ocular Bioavailability Studies
Ocular bioavailability of Δ8–THC was evaluated from the SLN and film formulations, in male New Zealand albino rabbits weighing 2–2.5 Kg. Rabbits were anesthetized at the start of the experiment using a combination of ketamine (35 mg/kg) and xylazine (3.5 mg/kg) injected intramuscularly and were maintained under anesthesia throughout the experiment. Fifty microliters (375 μg dose) of the SLNs formulation or a 4×2×2 mm (1.6 mg dose) melt-cast film was instilled/placed topically into the conjunctival cul-de-sac of the rabbits. At the end of one hour after topical application, the rabbits were euthanized with an overdose of pentobarbital injected through the marginal ear vein under deep anesthesia. The eye was washed with ice cold IPBS and immediately enucleated and washed again. The ocular tissues were separated, weighed and stored at −80°C until further analysis. All experiments were carried out in triplicate.
Sample Preparation for Analysis
Standard Solutions
Stock solutions of Δ8 –THC were prepared in acetonitrile. Known quantities of Δ8-THC from these stock solutions were spiked in blank ocular tissues and allowed to stand for 10 minutes before protein precipitation using ice cold acetonitrile (1:1 ratio for aqueous, vitreous humor and 1 mL for all the other tissues). The samples were then centrifuged for 15 minutes at 4°C and 13000 rpm and the supernatant was collected. Standard curves were prepared in aqueous humor (10ng–200ng), vitreous humor (20ng–200ng), cornea (20ng–200ng), iris ciliary body (10ng–200ng), retina-choroid (10–200ng) and sclera (20–200ng). Propofol was used as the internal standard during the analysis.
Sample Preparation
One hundred microliters of aqueous humor and 500 microliters of vitreous humor were collected from each test eye into individual centrifugal tubes. All other tissues, retina-choroid, iris-ciliary, cornea & sclera, from each test eye were cut into very small pieces and placed into individual vials. Protein precipitation was carried out similar to the standard solution preparation and analyzed using the method described below.
Analytical Methods
Bio-analytical Method for in vivo Samples
A previously published bio-analytical method (16) using fluorescence detection was modified and used for analyzing THC content in the ocular tissues. HPLC system comprised of a Waters 600 pump, Waters 717 plus refrigerated autosampler and Waters 2475 fluorescence detector, set at an excitation wavelength of 220 nm and Δ8-THC was detected at emission wavelength of 305 nm. Emission units full scale (EUFS) was set at 150 and gain was set at 30. Phenomenex PFP(2) (5μM, 4.6 × 250 mM) column was used. The mobile phase consisted of 30% water containing 0.5% o-phosphoric acid and 70% acetonitrile, with a flow of 1mL/min. Injection volume was 50 μL. Retention time for propofol and THC were 7.0 min and 11.9 min, respectively.
The standard calibration curve was derived and the parameters such as coefficient of determination (r2), slope and Y-intercept were noted to establish the linearity of the method. The analytical method was also validated with respect to precision, accuracy, recovery and specificity. Limit of detection for Δ8-THC in various ocular tissues was, aqueous humor (5 ng), vitreous humor (10 ng), cornea (10 ng), iris ciliary body (5 ng), retina-choroid (5 ng) and sclera (10 ng) in the fluorescence method.
HPLC-UV Method for in vitro Samples
A Previously published Waters HPLC-UV system with a Phenomenex PFP(2) (5 μM, 4.6 × 250 mm) column was used for analysis (28). The mobile phase consisted of 18% v/v water, 0.75% v/v acetic acid, 30% v/v acetonitrile and 52% v/v methanol at a flow rate of 1.2 mL/min. The UV detector wavelength was set at 226 nm. Injection volume was 25 μl.
The standard calibration curve was derived and the parameters such as coefficient of determination (r2), slope and Y-intercept were noted to establish the linearity of the method. The analytical method was also validated with respect to precision, accuracy, recovery and specificity. Limit of detection and limit of quantification were found to be 10 ng/mL and 30 ng/mL, respectively, and the retention time for THC was about 13.1 min. The method was observed to be specific, precise and reproducible.
Results
Particle Size and Polydispersity Index of the Formulations
The particle size and PDI of the SLN formulations are as described in Table III.
Table III.
Particle size characterization & transcorneal Δ8-Tetrahydrocannabinol flux from various formulations. Transcorneal flux determined using side-by-side diffusion apparatus at 34°C. Data represents mean±SD (n=3)
| Code | Formulation | Particle Size (nm) | Polydispersity Index | Flux Across Isolated Corneas (μg/min/cm2) |
|---|---|---|---|---|
| F-0 | SLNs with Compritol - 0.1% Load) | 495 | 0.39 | 0.014 ± 0.005 |
| F-2 | SLNs with Compritol −0.75% Load | 390 | 0.32 | 0.17 ± 0.04 |
| F-3 | SLNs with Compritol & RMßCD in Aqueous Phase - 0.75% Load | 395 | 0.34 | 0.21 ± 0.009 |
| F-4 | SLNs with Compritol & RMßCD Complexed with THC- 0.75% Load | 410 | 0.34 | 0.23 ± 0.01 |
| F-5 | Film | – | – | 0.21 ± 0.05 |
Drug Content of the Various Formulations
Δ8-THC content in all the formulations ranged between 95 to 104%.
Entrapment Efficiency (EE) of the Solid Lipid Nanoparticles
The degree of entrapment varied in each formulation with highest EE shown by F-2, (92.5%), followed by F-1 (89.6%), F-4 (85.7%) and F-3 (82.4%).
In vitro Release from the Solid Lipid Nanoparticles
Δ8-THC release was studied from F-1 and F-2 formulations in vitro. Similar release profiles were observed from both formulations till the end of 12 hours (f2 > 50), as shown in Fig. 3.
Fig. 3.
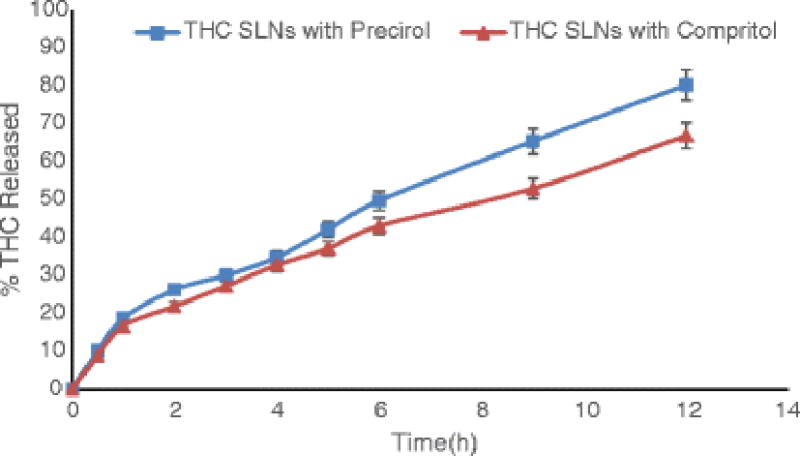
Release profiles of Δ8-Tetrahydrocannabinol from solid lipid nanoparticles (F1 and F2) using Slide-A-Lyzer® mini dialysis cassettes Data represents mean±SD (n=3).
In vitro Transcorneal Transport
Flux values across corneas were calculated for all the formulations (Table III) using the following formula:
where,dM/dt = Cumulative amount of drug in receiver solution with respect to timeA = Corneal surface available for permeation (0.636 cm2)
The film (F-5) formulation depicted higher flux in comparison to and SLN (F-0) formulations. Increasing the drug load from 0.1% to 0.75% in F-2, F-3 & F-4 resulted in several folds increase in the flux.
In vivo Ocular Bioavailability
The Δ8-THC levels in various ocular tissues, from the above formulations, one hour post topical administration, are illustrated in Table IV.
Table IV.
Ocular disposition of Δ8-Tetrahydrocannabinol one hour post topical administration of the selected formulations. Values expressed in μg/g of tissue. Data represents mean±SD (n=3)
| Tissue | SLN (Precirol) | SLN (Compitrol) | SLN with CD in Aq.Phase | SLN with THC-CD Complex | Film |
|---|---|---|---|---|---|
| Formulation Code | F-1 | F-2 | F-3 | F-4 | F-5 |
| Drug Load (%w/v) | 0.75 | 0.75 | 0.75 | 0.75 | 20% w/w |
| Dose | 375ug | 375ug | 375ug | 375ug | 1.6mg |
| Volume Instilled / film weight | 50ul | 50ul | 50ul | 50ul | 8mg |
| Aqueous Humor | 4.02±1.5 | 4.64±1.3 | 5.32± 0.3 | 4.91±2.1 | 0.14 ± 0.01 |
| Cornea | 9.25±0.5 | 8.44±1.9 | 10.72±1.7 | 10.12±0.8 | 22.45± 1.2 |
| Iris-Ciliary | 0.33±0.2 | 0.38±0.1 | 0.66±0.1 | 0.76± 0.2 | 0.26 ± 0.1 |
| Vitreous Humor | N.D | N.D | N.D | N.D | N.D |
| Retina-Choroid | 0.44±0.1 | 0.42±0.3 | 0.31±0.1 | 0.40± 0.2 | 0.12± 0.02 |
| Sclera | 1.00±0.4 | 0.80±0.2 | 1.20±0.6 | 1.24± 0.1 | 2.34 ± 0.5 |
With the SLN and film formulations, Δ8-THC was observed in all the ocular tissues except the vitreous humor. Comparatively, the SLNs were able to deliver higher concentrations of the drug to aqueous humor, iris-ciliary, retina choroid, while higher accumulations were observed in cornea and sclera with the film.
Discussion
Evidence of reduction in IOP through marijuana smoking was studied and reported as early as in the 70s (29). Over the years, there have been studies which portrayed the IOP reducing effect of Δ9-THC when given systemically (30). But, the same efficacy was not seen in studies, post topical administration, when Δ9-THC was administered as a mineral oil solution (31, 32). It was seen that even though there were sustained levels of Δ9-THC in the plasma for several hours, the IOP reducing effect was not marked and inconsistent. The authors in these studies, therefore concluded that to achieve a desired therapeutic effect, a substantial amount of the drug should be absorbed and maintained at the administration site, as the IOP reducing effect might be both localized and systemic. Another setback in these studies was the mineral oil solution, which had poor penetrating properties and therefore could not get past the corneal membrane (33). Previous reports have shown the ability of the relatively hydrophilic prodrug of Δ9-THC to reach into the iris-ciliary bodies, though no drug was seen to reach the retina-choroid (15, 16). The micellar solutions of the parent Δ9-THC on the other hand could not penetrate the surface tissues such as the cornea and sclera, highlighting the innate inability of the highly lipophilic molecule to permeate across the surface layers. Thus, either hydrophilic prodrug derivatization or design of formulation approaches which enhance ocular penetration will be needed for lipophilic therapeutic agents intended for topical ophthalmic application.
Δ8-THC, being a highly lipophilic, poorly soluble and premeable compound (34), like Δ9-THC, poses significant challenges in its delivery across the eye into the deeper ocular tissues. Therefore, in this study we have utilized SLNs and films for improving the ocular penetration of Δ8-THC through the topical route. Initial in vitro transcorneal transport studies of the formulations was a comparison between F-0 (SLNs with Compritol®), F-5 (Film). The film formulation showed the highest flux in this study. SLNs on the other hand were not as good comparatively. The lower ability of the lipid based system, in particular the SLNs, to deliver more drug across the cornea could be because of the preference of Δ8-THC for the lipids, lack of lipases in the experimental set-up, decreased or absence of phagocytic uptake by the epithelial cells, low drug content in the SLNs (low Δ8-THC to lipid ratio). Moreover, the side-bi-side diffusion apparatus may not be a good system to study transcorneal flux from particulate systems because contact profile in vivo in the conjunctival sac would be different from the side-bi-side apparatus. A vertical diffusion apparatus may be more appropriate. Formulations F-2 (SLNs with Compritol®), F-3 (SLNs with RMβCD in the aqueous phase) and F-4 (SLNs with RMβCD complexed with the drug) had a drug load of 0.75%, as opposed to 0.1% in F-0. Significant improvement in flux, 12 to 16-folds increase, was observed. Permeability study was not performed on F-1. Since the entrapment efficiency was higher with Compritol®, it was decided that further formulations would be made using this lipid. Therefore, after F-3 and F-4 were fabricated, we made a comparative permeability evaluation of only the Compritol® based SLNs.
In order to test the effect of lipid on SLN release characteristics, two formulations were studied - one with Precirol® as the lipid (F-1) and the other with Compritrol® (F-2). The formulations were similar in all other aspects. In vitro drug release profiles of both these formulations were not very different, with the drug being released at almost similar rates (f2 > 50) from both formulations. Both formulations showed good characteristics in terms of their size, PDI and entrapment efficiencies (Table III).
Ocular inserts were reported as early as 1978 when Bloomfield et al suggested the use of collagen shields for delivery of gentamycin (35). Since then, a number of reports using these invasive techniques have appeared for various other drugs such as diclofenac sodium, cyclosporin A, dexamethasone, pilocarpine etc (36–40). While ocular inserts were effective in improving the bioavailability of the tested drugs, the fact they are required to be either surgically implanted or removed after a certain period of time makes them unattractive. In contrast, a topical film which gels on application, thereby providing a prolonged release platform and then ultimately dissolving in the tear fluid appears to be a more convenient system. Moreover, the melt cast method eliminates any concerns regarding the toxic effects of residual solvents associated with solvent cast films preparation technique and was found to be successful in producing films that had good physical characteristics. The process was fast and reproducible and the films produced were able to deliver the drug to the deeper ocular tissues. The formulation gelled in the tear fluid within 30–40 seconds on application and stayed in contact with the corneal surface. No irritation of ocular surface was observed and due to the higher residence time, it allowed the drug to permeate through cornea and was also able to deliver the drug into the retina-choroid. The ocular tissue concentrations obtained suggest involvement of the conjunctival-scleral diffusional pathway. Although conceptually very attractive, the observed ocular Δ8-THC levels from the film were much lower compared to that observed with the SLNs. This can be attributed to the fact that even though the film was able to achieve a longer residence time and sustained release, it had no effect on the inherent transmembrane permeability characteristics of Δ8-THC. As a result, Δ8-THC efficiently partitioned from the formulation into the corneal and scleral membranes but could not diffuse any further.
The SLNs, in contrast, were able to deliver a higher amount of Δ8-THC to all ocular tissues, except vitreous humor. Formulations F-1 & F-2, made with Precirol® and Compritol®, respectively, were very similar to each other. The observed Δ8-THC levels in the ocular tissues, particularly the retina-choroid, may be due to an uptake of these nanoparticles by the conjunctival and corneal membranes, as suggested in earlier studies(22). The advantages of SLNs and their utility in delivering a host of drugs such as ibuprofen, flurbiprofen, cyclosporine A, rapamycin, gatifloxacin, indomethacin etc. has been tested and published before (21, 23, 24, 27, 41–48). While most of the observations in these earlier reports have been made at in vitro and ex vivo studies, only a handful of the studies reported improved in vivo bioavailability and that too only to the anterior chamber delivery.
The efficacy of SLNs in posterior segment delivery, post topical administration, to our knowledge, has not been reported elsewhere and the results from this study are very encouraging. At almost a fifth of the dose, compared to the matrix film formulation, the SLNs were able to generate concentrations significantly greater than that obtained with the film formulation in the aqueous humor, retina-choroid and iris-ciliary bodies, of the anesthetized rabbits. Moreover, these concentrations are greater than the reported EC50 values for CB1 (6nM) and CB2 (0.4nM) receptors expressed on the iris-ciliary and retinal tissues, on which THC has an agonistic activity (49). The low dose also has the advantage of shielding the patient from any adverse systemic effects of THC.
Cyclodextrins were tested next in these formulations to investigate whether they would exert a permeability enhancing effect by virtue of its effect on the cell membrane, or whether cyclodextrin would entrap the delta-8 THC which could result in decreased release and ocular tissue levels but prolong the absorption phase. Therefore, RMβCD was added to the formulation by complexing with the API or by incorporating it into the aqueous phase. Cyclodextrin, however, did not have any effect in either case. It appears that the permeation enhancing or release control effect of the cyclodextrin, at the concentrations used (3% which is low), was minor in comparison to the phagocytosis supported uptake of the SLNs by the corneal epithelial cells. The presence of the surfactants could also have overshadowed the membrane permeabilization / inclusion complex formation. For some reason, however, the formulations containing cyclodextrins produced higher concentrations of THC in the iris-ciliary bodies. This needs to be investigated further.
The in vitro release and permeability data correlated well with the in vivo data. As predicted by the in vitro release studies both SLN formulations showed similar in vivo disposition profiles. The in vitro side-bi-side permeability set-up for the SLN formulations is good for comparison between SLN formulations but not to predict the permeability efficiency against a solution or film formulation. This is because the SLNs are not in constant contact with the corneal membrane, as would be the situation in vivo, and thus this in vitro set-up underestimates the transcorneal flux/permeability. The film on the other hand, in the modified permeability set-up, is juxtaposed to the corneal membrane and will thus be closer to the in vivo situation. The in vivo ocular disposition studies corroborate this concept as we see that the SLNs are superior to the films in terms of intraocular delivery of delta-8 THC. As mentioned above the film formulation is efficient in delivering the drug load to the outer tunic of the eye but the poor permeation characteristics of the delta-8 THC limited delivery into the inner ocular tissues. This is supported by results from an earlier study wherein transcorneal permeability of Δ8-THC had been observed to be negligible (34). The active uptake of the SLNs by the corneal and conjunctival epithelial cells, and lipase mediated release, helped improve the ocular distribution of delta-8 THC from these particulate formulations in vivo.
Conclusion
The SLN formulations hold great potential in the delivery of lipophilic compounds, such as Δ8-THC, to the deeper ocular tissues. Delivery from the film formulation, while achieving a sustained form that can be tailored with further optimization, depends on the physicochemical characteristics of the agent to diffuse across the tissues. The SLNs on the other hand penetrate the tissues on their own due to their size and deliver the drug into the deeper ocular tissues (anterior and posterior) at a significantly lower dose.
Acknowledgments
This publication was made possible by Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of the National Institutes of Health (NIH).
Footnotes
Data obtained from ACD/Structure Elucidator, version 12.01, Advanced Chemistry Development, Inc., Toronto, ON, Canada, www.acdlabs.com, 2014
Declaration of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–86. [PubMed] [Google Scholar]
- 2.Vasudevan SK, Gupta V, Crowston JG. Neuroprotection in glaucoma. Indian J Ophthalmol. 2011;59(Suppl):S102–13. doi: 10.4103/0301-4738.73700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phelps CD, Corbett JJ. Migraine and low-tension glaucoma. A case-control study. Invest Ophthalmol Vis Sci. 1985;26:1105–8. [PubMed] [Google Scholar]
- 4.Rein DB, Zhang P, Wirth KE, Lee PP, Hoerger TJ, McCall N, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–60. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 5.Ji J, Chang P, Pennesi ME, Yang Z, Zhang J, Li D, et al. Effects of elevated intraocular pressure on mouse retinal ganglion cells. Vision Res. 2005;45:169–79. doi: 10.1016/j.visres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci. 2002;43:402–10. [PubMed] [Google Scholar]
- 7.Leske MC, Wu SY, Honkanen R, Nemesure B, Schachat A, Hyman L, et al. Nine-year incidence of open-angle glaucoma in the Barbados Eye Studies. Ophthalmology. 2007;114:1058–64. doi: 10.1016/j.ophtha.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 8.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–81. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Bien A, Seidenbecher CI, Bockers TM, Sabel BA, Kreutz MR. Apoptotic versus necrotic characteristics of retinal ganglion cell death after partial optic nerve injury. J Neurotrauma. 1999;16:153–63. doi: 10.1089/neu.1999.16.153. [DOI] [PubMed] [Google Scholar]
- 10.Crandall J, Matragoon S, Khalifa YM, Borlongan C, Tsai NT, Caldwell RB, et al. Neuroprotective and intraocular pressure-lowering effects of (-)Delta9-tetrahydrocannabinol in a rat model of glaucoma. Ophthalmic Res. 2007;39:69–75. doi: 10.1159/000099240. [DOI] [PubMed] [Google Scholar]
- 11.El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, et al. Neuroprotective effect of (-)Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol. 2003;163:1997–2008. doi: 10.1016/s0002-9440(10)63558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He F, Song ZH. Molecular and cellular changes induced by the activation of CB2 cannabinoid receptors in trabecular meshwork cells. Mol Vis. 2007;13:1348–56. [PubMed] [Google Scholar]
- 13.Njie YF, Qiao Z, Xiao Z, Wang W, Song ZH. N-arachidonylethanolamide-induced increase in aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2008;49:4528–34. doi: 10.1167/iovs.07-1537. [DOI] [PubMed] [Google Scholar]
- 14.Porcella A, Maxia C, Gessa GL, Pani L. The human eye expresses high levels of CB1 cannabinoid receptor mRNA and protein. Eur J Neurosci. 2000;12:1123–7. doi: 10.1046/j.1460-9568.2000.01027.x. [DOI] [PubMed] [Google Scholar]
- 15.Hingorani T, Adelli GR, Punyamurthula N, Gul W, Elsohly MA, Repka MA, et al. Ocular disposition of the hemiglutarate ester prodrug of (9)-Tetrahydrocannabinol from various ophthalmic formulations. Pharm Res. 2013;30:2146–56. doi: 10.1007/s11095-013-1072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hingorani T, Gul W, Elsohly M, Repka MA, Majumdar S. Effect of ion pairing on in vitro transcorneal permeability of a Delta(9) -tetrahydrocannabinol prodrug: potential in glaucoma therapy. J Pharm Sci. 2012;101:616–26. doi: 10.1002/jps.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ethan B. Russo FG, ed The Handbook of Cannabis Therapeutics: From Bench to Bedside. New York: Haworth Integrative Healing Press; 2006. [Google Scholar]
- 18.Muchtar S, Almog S, Torracca MT, Saettone MF, Benita S. A submicron emulsion as ocular vehicle for delta-8-tetrahydrocannabinol: effect on intraocular pressure in rabbits. Ophthalmic Res. 1992;24:142–9. doi: 10.1159/000267160. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Purdon C, Smith E. Solid lipid nanoparticles for topical drug delivery. American Journal of Drug Delivery. 2006;4:215–20. [Google Scholar]
- 20.Zhou H-Y, Hao J-L, Wang S, Zheng Y, Zhang W-S. Nanoparticles in the ocular drug delivery. International Journal of Ophthalmology. 2013;6:390–6. doi: 10.3980/j.issn.2222-3959.2013.03.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Campos AM, Sanchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm. 2001;224:159–68. doi: 10.1016/s0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 22.Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238:241–5. doi: 10.1016/s0378-5173(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 23.Yuan X-b, Li H, Yuan Y-b. Preparation of cholesterol-modified chitosan self-aggregated nanoparticles for delivery of drugs to ocular surface. Carbohydrate Polymers. 2006;65:337–45. [Google Scholar]
- 24.Ibrahim HK, El-Leithy IS, Makky AA. Mucoadhesive nanoparticles as carrier systems for prolonged ocular delivery of gatifloxacin/prednisolone bitherapy. Mol Pharm. 2010;7:576–85. doi: 10.1021/mp900279c. [DOI] [PubMed] [Google Scholar]
- 25.Hermans K, Van den Plas D, Kerimova S, Carleer R, Adriaensens P, Weyenberg W, et al. Development and characterization of mucoadhesive chitosan films for ophthalmic delivery of cyclosporine A. Int J Pharm. 2014;472:10–9. doi: 10.1016/j.ijpharm.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Attia MA, Kassem MA, Safwat SM. In vivo performance of [3H]dexamethasone ophthalmic film delivery systems in the rabbit eye. International Journal of Pharmaceutics. 1988;47:21–30. [Google Scholar]
- 27.Hippalgaonkar K, Adelli GR, Repka MA, Majumdar S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: development, characterization, and in vitro evaluation. J Ocul Pharmacol Ther. 2013;29:216–28. doi: 10.1089/jop.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thumma S, Majumdar S, ElSohly MA, Gul W, Repka MA. Preformulation Studies of a Prodrug of Δ(9)-Tetrahydrocannabinol. AAPS PharmSciTech. 2008;9:982–90. doi: 10.1208/s12249-008-9136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flom MC, Adams AJ, Jones RT. Marijuana smoking and reduced pressure in human eyes: drug action or epiphenomenon? Invest Ophthalmol. 1975;14:52–5. [PubMed] [Google Scholar]
- 30.ElSohly MA, Harland EC, Benigni DA, Waller CW. Cannabinoids in glaucoma II: the effect of different cannabinoids on intraocular pressure of the rabbit. Curr Eye Res. 1984;3:841–50. doi: 10.3109/02713688409000797. [DOI] [PubMed] [Google Scholar]
- 31.Jay WM, Green K. Multiple-drop study of topically applied 1% delta 9-tetrahydrocannabinol in human eyes. Arch Ophthalmol. 1983;101:591–3. doi: 10.1001/archopht.1983.01040010591012. [DOI] [PubMed] [Google Scholar]
- 32.Green K, Roth M. Ocular effects of topical administration of delta 9-tetrahydrocannabinol in man. Arch Ophthalmol. 1982;100:265–7. doi: 10.1001/archopht.1982.01030030267006. [DOI] [PubMed] [Google Scholar]
- 33.Meisner D, Pringle J, Mezei M. Liposomal ophthalmic drug delivery. III. Pharmacodynamic and biodisposition studies of atropine. International Journal of Pharmaceutics. 1989;55:105–13. [Google Scholar]
- 34.Hippalgaonkar K, Gul W, ElSohly MA, Repka MA, Majumdar S. Enhanced solubility, stability, and transcorneal permeability of delta-8-tetrahydrocannabinol in the presence of cyclodextrins. AAPS PharmSciTech. 12:723–31. doi: 10.1208/s12249-011-9639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloomfield SE, Miyata T, Dunn MW, Bueser N, Stenzel KH, Rubin AL. Soluble gentamicin ophthalmic inserts as a drug delivery system. Arch Ophthalmol. 1978;96:885–7. doi: 10.1001/archopht.1978.03910050487020. [DOI] [PubMed] [Google Scholar]
- 36.Baeyens V, Kaltsatos V, Boisrame B, Varesio E, Veuthey JL, Fathi M, et al. Optimized release of dexamethasone and gentamicin from a soluble ocular insert for the treatment of external ophthalmic infections. J Control Release. 1998;52:215–20. doi: 10.1016/s0168-3659(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 37.Bensinger R, Shin DH, Kass MA, Podos SM, Becker B. Pilocarpine ocular inserts. Invest Ophthalmol. 1976;15:1008–10. [PubMed] [Google Scholar]
- 38.Khurana G, Arora S, Pawar PK. Ocular insert for sustained delivery of gatifloxacin sesquihydrate: Preparation and evaluations. Int J Pharm Investig. 2012;2:70–7. doi: 10.4103/2230-973X.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sankar AKCV, Durga S. Design and evaluation of diclofenac sodium ophthalmic inserts. Acta Pharm Sci. 2006;48:5–10. [Google Scholar]
- 40.Theng JT, Ti SE, Zhou L, Lam KW, Chee SP, Tan D. Pharmacokinetic and toxicity study of an intraocular cyclosporine DDS in the anterior segment of rabbit eyes. Invest Ophthalmol Vis Sci. 2003;44:4895–9. doi: 10.1167/iovs.02-1112. [DOI] [PubMed] [Google Scholar]
- 41.Calvo P, Vila-Jato JL, Alonso MJ. Comparative in vitro evaluation of several colloidal systems, nanoparticles, nanocapsules, and nanoemulsions, as ocular drug carriers. J Pharm Sci. 1996;85:530–6. doi: 10.1021/js950474+. [DOI] [PubMed] [Google Scholar]
- 42.Contreras-Ruiz L, de la Fuente M, Parraga JE, Lopez-Garcia A, Fernandez I, Seijo B, et al. Intracellular trafficking of hyaluronic acid-chitosan oligomer-based nanoparticles in cultured human ocular surface cells. Mol Vis. 2011;17:279–90. [PMC free article] [PubMed] [Google Scholar]
- 43.de la Fuente M, Seijo B, Alonso MJ. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Invest Ophthalmol Vis Sci. 2008;49:2016–24. doi: 10.1167/iovs.07-1077. [DOI] [PubMed] [Google Scholar]
- 44.Enriquez de Salamanca A, Diebold Y, Calonge M, Garcia-Vazquez C, Callejo S, Vila A, et al. Chitosan nanoparticles as a potential drug delivery system for the ocular surface: toxicity, uptake mechanism and in vivo tolerance. Invest Ophthalmol Vis Sci. 2006;47:1416–25. doi: 10.1167/iovs.05-0495. [DOI] [PubMed] [Google Scholar]
- 45.Pignatello R, Bucolo C, Ferrara P, Maltese A, Puleo A, Puglisi G. Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur J Pharm Sci. 2002;16:53–61. doi: 10.1016/s0928-0987(02)00057-x. [DOI] [PubMed] [Google Scholar]
- 46.Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–55. doi: 10.1016/s0142-9612(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 47.Vega E, Egea MA, Valls O, Espina M, Garcia ML. Flurbiprofen loaded biodegradable nanoparticles for ophtalmic administration. J Pharm Sci. 2006;95:2393–405. doi: 10.1002/jps.20685. [DOI] [PubMed] [Google Scholar]
- 48.Yuan XB, Yuan YB, Jiang W, Liu J, Tian EJ, Shun HM, et al. Preparation of rapamycin-loaded chitosan/PLA nanoparticles for immunosuppression in corneal transplantation. Int J Pharm. 2008;349:241–8. doi: 10.1016/j.ijpharm.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 49.Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. British journal of pharmacology. 2007;152:1092–101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]


