Abstract
Purpose of review
JC polyomavirus (JCPyV) is a significant human pathogen that causes an asymptomatic infection in the kidney in the majority of the population. In immunosuppressed individuals, the virus can become reactivated and spread to the brain, causing the fatal, demyelinating disease progressive multifocal leukoencephalopathy (PML). There are currently limited treatment options for this fatal disease. Attachment to receptors and entry into host cells are the initiating events in JCPyV infection and therefore an attractive target for therapeutics to prevent or treat PML. This review provides the current understanding of JCPyV attachment and entry events and the potential therapeutics to target these areas.
Recent findings
JCPyV attachment and entry to host cells is mediated by α2,6-linked lactoseries tetrasaccharide c (LSTc) and 5-hydroxytryptamine receptors (5-HT2Rs), respectively, and subsequent trafficking to the endoplasmic reticulum is required for infection. Recently, vaccines, monoclonal antibodies, and small molecules have shown promise as anti-viral and PML therapies.
Summary
This review summarizes our current understanding of JCPyV attachment, entry, and trafficking and the development of potential PML therapeutics that inhibit these critical steps in JCPyV infection.
Keywords: JC Polyomavirus, Progressive Multifocal Leukoencephalopathy, Natalizumab, Serotonin Receptors, VP1
Introduction
JC polyomavirus (JCPyV) is the causative agent of the fatal, demyelinating disease progressive multifocal leukoencephalopathy (PML) [1]. Although PML is a rare disease, JCPyV infects the majority of the population [2, 3] and causes an asymptomatic infection in the kidney and B lymphocytes [4–7]. In severely immunosuppressed individuals, JCPyV can spread to the central nervous system (CNS) and infects astrocytes and oligodendrocytes [8–10], leading to severe demyelination and PML [11, 12].
JCPyV is a nonenveloped virus with a dsDNA genome ~5130 basepairs in size [12]. The viral capsid is comprised of three structural proteins: viral protein 1 (VP1), VP2, and VP3 [13]. VP1 is a pentameric protein present on the exterior of the capsid, and 72 VP1 pentamers interconnected through C-terminal extensions give rise to the ~40nm icosahedral capsid [14]. VP1 is the viral attachment protein that mediates binding to host cells to initiate infection [15–17]. JCPyV is internalized into host cells by endocytosis [18–20] and traffics through the endocytic compartment to the endoplasmic reticulum (ER) [21, 22], where the viral capsid is partially uncoated [23, 22]. The virus retrotranslocates from the ER to the nucleus where viral transcription and replication take place through temporally regulated gene expression [12].
The mechanisms of JCPyV attachment and entry have been the focus of research for the past several years with a particular interest in defining the cellular receptors that mediate viral attachment and entry into host cells and subsequent development of anti-viral therapies. Additionally, sequencing of viral isolates from PML patients revealed polymorphic changes that arise in the viral capsid attachment protein VP1 during viremia [24–28], representing a novel target for the development of therapeutic strategies for PML. As there are currently limited treatment options to manage JCPyV and PML, the development of an effective anti-JCPyV therapy would represent a major breakthrough. Several treatment strategies have been developed in recent years as potential methods to reduce PML disease burden including vaccines [29, 30], broadly neutralizing anti-JCPyV VP1 antibodies [31], and small molecule inhibitors [22, 32, 33]. This review summarizes the findings on JCPyV attachment, entry, and trafficking and illuminates the development of potential therapeutics targeted to block these early steps in the virus life cycle to prevent or treat PML.
Progressive Multifocal Leukoencephalopathy
JCPyV was originally isolated from the brain of an individual with PML [8, 9, 11, 34]. JCPyV infects the majority of the population [2, 3] and causes an asymptomatic infection in the kidney and can persist in B lymphocytes [4–7]. In healthy individuals, the virus remains in the kidney as a persistent infection and can be shed in the urine during times of viral reactivation [35, 36]. In individuals with severe immunosuppression, such as those with underlying immunosuppression, HIV-1 infection, or those receiving immunomodulatory therapies, JCPyV can become reactivated and spread to the CNS [27, 37] and infect astrocytes and oligodendrocytes [8, 9]. Viral-induced cell lysis of oligodendrocytes, the myelin-producing cells of the CNS, [38–40] and infection of astrocytes and glial progenitor cells (GPCs), results in severe demyelination and PML [10–12]. Additionally, JCPyV has been detected in cortical neurons and in the granule cell neurons of individuals with PML [41–43], indicating that neuronal infection can contribute to PML pathogenesis. Demyelination in individuals with PML results in neurological dysfunction and patients develop symptoms including cognitive disorders, motor weakness, language disturbances, and visual defects [44]. PML is a rapid, progressive disease that usually results in fatality within months to 1 year of symptom onset [45]. If the underlying immunosuppression is treated, such as with highly-active antiretroviral therapy (HAART) for HIV-1 infection or by removing the immunosuppressive therapy to restore immune function, the life span of the patient can be prolonged [45, 46]. However, individuals treated for immune reconstitution are at risk for development of immune reconstitution inflammatory syndrome (IRIS), which can lead to neurological worsening, and the patients are oftentimes severely debilitated [46].
HIV-1 infection accounts for 80% of PML cases and ~5% of HIV-1+ individuals develop PML, which is considered an AIDS-defining illness [47]. The majority of non-HIV-PML cases are due to the use of immunomodulatory therapies for immune-mediated diseases [48–50]. In the past decade, the number of PML cases has risen in patients receiving an FDA-approved drug, natalizumab (Tysabri®), for treatment of multiple sclerosis (MS) [49]. Natalizumab is a humanized monoclonal antibody (mAb) specific for very late antigen-4 (VLA-4) (α4β1 integrin) [50] that blocks autoreactive VLA-4+ T- and B-lymphocyte trafficking to the brain [51] to prevent the T cells from attacking the myelin in the CNS, the key feature of the disease etiology of MS [49, 52]. As of March 6, 2017, there have been 711 natalizumab-associated cases of PML [53], as well as PML cases in individuals receiving other humanized mAb treatments including rituximab, efalizumab, and infliximab [49, 50, 54–56]. Newly developed drugs including fingolimod [57], dimethyl fumarate drugs, and other fumaric acid ester-containing drugs have also caused cases of PML [58–61]. While some cases of PML have occurred in individuals for whom these drugs were prescribed for immune-mediated diseases including Crohns disease or rheumatic disease [62, 63], the majority of PML cases occurred when these drugs were prescribed for MS [49]. It is likely that the physiological aspects of this disease, damage to the CNS by autoreactive T cells [52], in combination with a mAb therapy that prevents immune surveillance in the CNS [49, 64], may present an environment that promotes JCPyV neuroinvasion. While these drugs have all been associated with the development of PML, the relative benefit to the patient combined with risk stratification of PML development is taken into consideration [65], and the prescribing information for drugs like Tysabri® contains a black box warning [53]. In fact, the drug ocrelizumab that was recently FDA-approved for both relapsing and primary progressive MS, targeting CD20+ B cells, has not resulted in any cases of PML during the Phase III clinical trials [66, 67], yet also comes with a warning about the potential risk of PML [68]. The rise in the development of immune-mediated therapies and the related increase in immunomodulatory therapy-associated PML cases together with HIV-1-related PML cases highlight a critical need for the development of improved PML treatments.
JC Polyomavirus Attachment to Host Cells
The initial attachment of viruses to host cells is mediated by cellular receptors, and JCPyV binds specifically to sialic acid containing receptors, a common feature shared by polyomaviruses and other viruses [69, 70]. A number of sialic acid containing receptors have been studied for their role in supporting JCPyV infection including α2,3- and α2,6-containing sialic acid receptors [16, 17, 27, 71–74]. A structural-functional approach demonstrated that JCPyV utilizes a specific α2,6-containing sialic acid receptor known as lactoseries tetrasaccharide c (LSTc), which was identified using a glycan array screen with purified VP1 pentamers of the JCPyV-1a strain Mad-1 [17] (Fig. 1). JCPyV VP1 did not bind to any other glycans included on the array, including any of the sialic acid-containing ganglioside receptors [17], which are utilized by all other known polyomaviruses [17, 75–78] and have been suggested as receptors for JCPyV [27, 72]. Interestingly, using other techniques JCPyV has been demonstrated to bind to ganglioside receptors, albeit with significantly reduced affinity, and gangliosides do not support infection [74]. The X-ray crystal structure of JCPyV VP1 in complex with LSTc demonstrated specific residues required for VP1 binding to LSTc (L54, N123, S266, and S268, also referred to L55, N124, S267, and S269 in conventions counting the Met start codon) [17]. Mutation of the residues critical for sialic acid binding in the context of an infectious viral clone and purified VP1 pentamers revealed that these residues are essential for growth and binding in glial cells [17]. Interestingly, mutations in the exact residues that are critical for LSTc attachment and infection of cells in vitro are found to arise in individuals with PML [25–28].
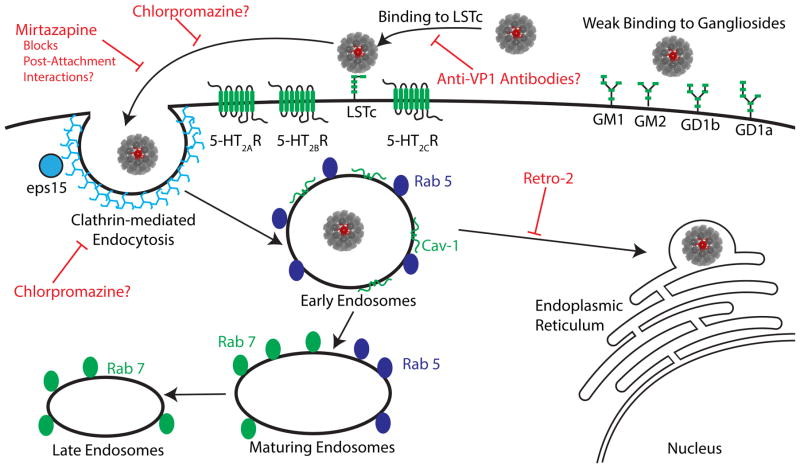
Fig. 1. Attachment and entry of JCPyV into host cells and potential targets for PML treatment.
JCPyV binds to α2,6 sialic acid-containing receptor lactoseries tetrasaccharide c (LSTc) through interactions with the viral capsid protein VP1 (pentamer in red) to initiate infection of susceptible cells. JCPyV binds with weak affinity to sialic acid-containing gangliosides, however, this interaction does not appear to lead to productive infection. Following interactions with LSTc, JCPyV enters cells through clathrin-mediated endocytosis in an EPS15-dependent manner that is sensitive to chlorpromazine treatment. The serotonin 2 subfamily (5-HT2A, 2B, 2C) of receptors play an important role in viral internalization, yet are not thought to contribute to virus binding. Mirtazapine and chlorpromazine interfere with viral infection, possibly by disrupting JCPyV interactions with serotonin receptors. Following endocytosis, JCPyV likely accumulates in Rab5-positive early endosomes. JCPyV also localizes with Cav-1 positive vesicles, but it is currently unclear whether these are also early endosomes. The virus then undergoes retrograde transport to the ER, a step that is sensitive to Retro-2 treatment.
PML-associated VP1 Mutations
Samples of the cerebral spinal fluid (CSF) from individuals with PML reveal that ~90% of the viral isolates have at least one point mutation or combinations of mutations in VP1 in residues L54, N123, S266, or S268 [25–28]. These VP1 mutations are not usually found in isolates from the urine, indicating that perhaps VP1-associated mutations are linked to viral spread to the CNS or favor PML onset [25–28]. Interestingly, PML-associated mutations in VP1 results in abolished binding to LSTc as determined by X-ray crystallography [79], and pseudoviruses engineered with PML-associated mutations were not capable of transducing a range of brain cell types or hemagglutinating human red blood cells indicating a loss of sialic acid binding [79]. These in vitro studies indicate that virions with PML-associated mutations would be non-infectious in the host [79]. However, these mutations were generated in the background of the Mad-1 laboratory prototype strain, which is of the viral genotype 1a, and, there are seven genotypes used to classify JCPyV strains based on differences in the VP1 amino acid sequences [80]. Introduction of PML-associated mutations into the background of the JCPyV-2a strain have resulted in viruses that are infectious in vitro in oligodendrocytes, astrocytes, and glial progenitor cells (GPCs) and in vivo in a chimeric mouse model with explanted GPCs [10]. Interestingly, viruses collected from mice after infection with wild type JCPyV-2a demonstrated PML-associated mutations D66G and S123C, which are within the sialic acid binding pocket and arise in human patients [17]. Further, JCPyV-2a pseudoviruses with PML-associated mutations such as S266F can transduce some cancer cell lines [29]. Thus, the specific genotypic background of PML strains seems to be an important factor for PML-associated mutations in PML progression in vivo and for growth in vitro. Further, alternate routes of receptor-independent viral entry and additional cell types need to be tested to determine the infectious nature of these mutants. It is possible that JCPyV binding to and infection of cells in the brain including, astrocytes, oligodendrocytes, and neurons, is sialic-acid-independent in an in vivo infection. Haley et al. demonstrated that astrocytes and oligodendrocytes from human brain tissues were negative for LSTc [81]. Therefore, viruses isolated from PML patients with mutations in VP1 within the sialic acid binding sites could possibly lead to neuroinvasion via a sialic acid-independent manner through interactions with an alternate receptor or through a receptor-independent invasion mechanism that has been demonstrated for other viruses [82, 83]. Further research is necessary to define whether viruses with mutations in VP1 are the infectious form of the virus or whether they arise during CNS invasion and contribute to PML pathogenesis through an alternative mechanism.
PML Treatments Targeted to VP1
The incidence of PML-associated mutations in PML patients and in animal model systems indicate a correlation for PML-associated mutations and PML development. These findings further demonstrate that VP1 is a key target for antivirals and activation of humoral and cell-mediated immunity [31]. Recent studies have focused on vaccine or mAb therapies in combination with treatments to boost VP1-specific immunity (Table 1). For instance, two patients were treated under “compassionate use” with a vaccine consisting of JCPyV VP1 protein in combination with cytokine interleukin 7 (IL-7) treatment and a toll-like receptor (TLR) 7/9 agonist as an adjuvant [30]. This treatment led to JCPyV clearance with an undetectable viral load and patient recovery [30]. Interestingly, sera isolated from PML patients can effectively neutralize wild-type JCPyV, yet does not neutralize virus with PML-associated mutations, while sera from healthy patients can neutralize wild-type and PML variants [29, 31]. These findings suggest that patients who develop PML have antibody “recognition holes” during immunosuppression, and their antibodies cannot neutralize variants with PML-associated mutations such as L55F, S266F, and S269F [29, 31]. However, during immune reconstitution, the antibody titers in the CSF rise and the antibodies can recognize the PML-associated variants, which may be essential for neutralization and elimination of the virus [29, 31]. Thus, therapies to effectively neutralize JCPyV with PML-associated mutations and subsequently boost immunity are being developed [29, 31]. Vaccines generated from wild-type JCPyV-2a virus-like particles (VLPs) and administered to mice initially result in “recognition holes” that prevent neutralization of PML variants, yet these holes are closed when mice are administered a booster [29]. The “compassionate use” of a VLP vaccine in combination with IL-7 in a patient resulted in an increase in neutralizing antibody titer with antibody production specific for the PML-associated mutant found in the patient’s CSF and clinical improvements including reduced viremia and halted progression of PML lesions [29]. Furthermore, antibodies derived from memory B cells from healthy patients and patients with PML-IRIS were expanded through molecular cloning of antibodies capable of neutralizing JCPyV infection [31]. Antibodies from a natalizumab-PML-IRIS patient were found to have high neutralizing activity, high affinity for VP1, and recognition capacity for PML variants, providing a novel mAb-based strategy that could potentially neutralize JCPyV infection in PML patients [31]. This treatment could be particularly effective in individuals who develop PML-associated mutations during viremia that cannot be effectively neutralized by the compromised immune system and succumb to PML. Taken together, active or passive immunity with a VLP vaccine, VP1, or VP1-specific monoclonal antibodies in combination with treatments that boost host immunity represents a promising new target for PML treatment.
Table 1.
Potential treatments highlighted in this article that have been shown to reduce JCPyV infection and/or PML progression.
| Potential PML Treatment | FDA Licensed | Mechanism of Action | Tested in Clinical Setting |
|---|---|---|---|
| Mefloquine | Yes | Viral Replication Inhibitor | Yes |
| Mirtazapine | Yes | Serotonin Receptor Antagonist – Possible Receptor Competition | Yes |
| Retro-2 | No | Intracellular Transport Inhibitor | No |
| VP1 Subunit Vaccine | No | Active Immunization | Yes |
| VLP Vaccine | No | Active Immunization | Yes |
| Anti-VP1 mAb | No | Passive Immunization | Yes |
| LSTc competitive inhibitors | No | Receptor Competition | No |
Serotonin Receptors in JCPyV Entry
JCPyV enters host cells by clathrin-mediated endocytosis as JCPyV infection is significantly reduced by chlorpromazine, an inhibitor of clathrin-mediated endocytosis [18, 20]. Further, expression of dominant negative mutants of (epidermal growth factor receptor kinase substrate clone 15) eps15, a protein important for clathrin-mediated endocytic events, reduces infection [19]. Chlorpromazine is also a 5-hydroxytryptamine (5-HT) (serotonin) receptor antagonist that acts as an inhibitor of 5-HT2A serotonin receptor (5-HT2AR) [84], which led to the hypothesis that JCPyV utilizes 5-HT2AR as a proteinaceous receptor. Subsequently, the Atwood laboratory identified that 5-HT2AR is required for JCPyV infection [85], based on the findings that infection of glial cells was sensitive to the use of 5-HT2R-blocking antibodies specific to subtypes 2A and 2C, 5-HT2AR expression rendered HeLa cells permissive for infection, and JCPyV colocalized with 5-HT2AR-GFP at time points consistent with viral entry [85]. Furthermore, 5-HT2AR antagonists including ritanserin, ketanserin, mianserin, and mirtazapine reduced JCPyV infection in human glial cells [86]. Collectively, these data indicate that 5-HT2Rs are important for JCPyV infection and likely promote viral entry, most likely through clathrin-mediated endocytosis.
The 5-HT2Rs are seven-transmembrane-spanning G-protein coupled receptors widely expressed in the CNS and are commonly associated with physiologic and mood disorders [87]. Interestingly, 5-HT2Rs are expressed on a variety of cells in the CNS, including astrocytes and oligodendrocytes, and in the kidney, including the distal tubules and collecting ducts, all of which are sites of JCPyV infection [81, 88, 89]. Furthermore, 5-HT2Rs are expressed on neurons, and subtype 2A is found abundantly in the cerebral cortex [90] where JCPyV has been identified in sites of significant demyelination in individuals with PML [41, 43]. Interestingly, JCPyV is not able to infect microglia, cells that express 5-HT2Rs but lack expression of the JCPyV attachment factor LSTc, further indicating that 5-HT2Rs are not the sole requirement for viral infection [81].
Given that treatment of cells with inhibitors and antibodies specific for serotonin subtypes 5-HT2A and 5-HT2C have shown diminished JCPyV infection, it was speculated that multiple 5-HTR subtypes may be capable of conferring infection or multiple subtypes may play functionally redundant roles in infection. Expression of 5-HT2A, 5-HT2B, and 5-HT2C receptors increased the susceptibility of poorly permissive HEK293A cells, which express low levels of 5-HTRs, to JCPyV infection while other 5-HTR subtypes did not [20]. The presence of 5-HT2Rs in HEK293A cells did not impact viral attachment to the cell surface, but specifically enhanced viral entry that was blocked by chlorpromazine treatment [20]. These data demonstrate that the 5-HT2Rs subtypes are required for JCPyV entry [20], yet the direct entry mechanism remains unclear as chlorpromazine blocks clathrin-dependent endocytosis and is also a 5-HT2AR antagonist [84] (Fig. 1). Interestingly, JCPyV was demonstrated to infect human brain microvascular endothelial cells (HBMECs), which lack 5-HT2ARs, indicating that infection could occur in the absence of 5-HT2AR [91]. However, these findings predate evidence that JCPyV can also use 5-HTR subtypes 2B and 2C [20]. Furthermore, HBMECs are primary endothelial cells in contrast to the aforementioned HEKs, which are transformed epithelial cells. Therefore, entry strategies could vary in a tissue-specific manner or based on cellular transformation, and thus requires further investigation.
Given the evidence that JCPyV entry and infection are dependent on 5-HT2Rs and the abundance of on-market therapies that target the 5-HT2Rs, such as selective serotonin reuptake inhibitors (SSRIs) for use in depression treatment [87], clinicians have prescribed 5-HT2R antagonists, including mirtazapine, in an off-label treatment for PML. Mirtazapine is a serotonergic antagonist that selectively inhibits 5-HT2 and 5-HT3 receptors [92]. Interestingly, case reports have indicated varying degrees of efficacy following mirtazapine treatments in PML patients. Several studies show that treatment with mirtazapine dramatically improves prognosis for individuals diagnosed with PML, including decreased neurological deterioration and undetectable viral loads [93, 94]. However, other reports have described instances where treatment with mirtazapine had little beneficiary effect, as patients rapidly deteriorated regardless of 5-HT2R antagonist treatment [95, 96]. As mirtazapine blocks 5-HT2Rs it is tempting to speculate that treatment likely prevents JCPyV spread to other cells rather than treating established infection as MRI scans have remained unchanged in some cases [93, 95, 96]. Moreover, other reports indicate that mirtazapine, in combination with mefloquine, a treatment marketed for malaria, has been effective in treating some individuals with PML [97]. Treatment of cells with mefloquine decreased JCPyV infection by blocking viral replication, indicating that the combination treatment may be effective in treating PML [98]. While these reports are promising, conflicting evidence indicates that JCPyV-mediated infection by 5-HT2Rs requires further investigation as well as continued exploration of 5-HT2R antagonists as viable treatments for PML.
JCPyV Trafficking
Following internalization into host cells through a mechanism involving 5-HT2Rs, JCPyV enters the endocytic pathway and is observed in Rab5-positive early endosomes by 15 minutes post infection [21]. Rab proteins are small GTPases that play critical roles in controlling endocytic trafficking, sorting, motility, and fusion [99]. Localization of JCPyV to early endosomes appears to be a critical step, as overexpression of dominant-negative forms of Rab5 restrict JCPyV infection [21]. Localization to Rab5-positive early endosomes is in contrast to other polyomaviruses (Pys) such as Simian Virus 40 (SV40) and mouse polyomavirus (mPyV), which have been shown to accumulate within Rab7-positive maturing or late endosomes [100, 101]. Other Pys have been shown to initially interact with gangliosides on the surface of the cell, and the differences in entry pathways between JCPyV and other Pys may be due to JCPyV utilizing a secondary proteinaceous receptor for entry following sialic acid receptor interactions, differing from other Pys studied [20, 102–105]. JCPyV has also been observed in cholesterol rich and caveolin-1 (Cav-1) positive endosomes, and shRNA-mediated reduction of Cav-1 or sequestration of cholesterol with the drug Methyl-β-cyclodextrin reduces infection [21]. Although the entry pathway of JCPyV appears to be distinct from other Pys, they all appear to require caveolin-1+ vesicles for trafficking to the ER, implying overlapping events in endosomal and caveolin-mediated trafficking strategies [104–107]. As JCPyV has been shown to require tyrosine kinase activity and actin rearrangement, this suggests that microfilaments may be important for infection and trafficking within endosomal compartments following entry [19, 108].
Retrograde Transport of JCPyV
The actual site of egress from the endosomal-lysosomal system remains enigmatic for JCPyV. JCPyV has not been observed in Rab7-positive maturing or late endosomes, and overexpression of dominant-negative forms of Rab7 does not inhibit infectivity [19]. These observations suggest that JCPyV leaves the endosomal-lysosomal system from early endosomes (Fig. 1) [21]. The early endosome is a complex organelle that contains microdomains involved in endosomal maturation (vacuolar domain) and sorting (tubular endosomal network) (reviewed in [109]). Transport of cargo through the tubular endosomal network involves a number of proteins or protein complexes including clathrin, retromer, and the WASH complex [110, 111]. It is unclear whether JCPyV utilizes these host factors to initiate transport from the early endosome and future studies are needed to definitively identify the host factors responsible for JCPyV egress from the endosomal-lysosomal system.
Retrograde transport from endosomes results in movement of cargo to the trans-Golgi network [112]. However, colocalization between JCPyV virions (or other Pys) with the Golgi apparatus has not been described [23, 100, 101, 104]. It is currently unknown whether JCPyV undergoes direct endosome to ER transport, or transiently localizes to the Golgi prior to arrival in the ER. JCPyV accumulates in the host cell ER starting around 4–6 hours post infection [21, 23]. While the kinetics of JCPyV arrival to the ER is on par with other Pys, it is slow compared to other cargo targeting the ER [101, 113, 114]. Studies with other Pys have demonstrated that virus-receptor interactions are important for directing virions to the ER [100, 101, 115], and future quantitative studies using both virus and receptor labeling strategies may help to elucidate the retrograde transport pathway used by JCPyV.
Small Molecule-Mediated Inhibition of JCPyV Retrograde Transport
Presently, it is thought that JCPyV traffics to the ER where the virus interacts with components of the host cell quality control machinery, such as PDI and ERP57, to undergo partial uncoating of the viral capsid [23]. It is speculated that this partially uncoated capsid then undergoes retrotranslocation from the lumen of the ER into the cytosol using components of the endoplasmic reticulum associated degradation (ERAD) pathway [23]. Uncoating is a key step in the virus life cycle that results in release of the viral genome into the host nucleus, and therefore presents an attractive target for therapeutic development. Retro-2, a small molecule inhibitor of retrograde transport, was found to inhibit infection of JCPyV and other Pys [22]. Retro-2 acts on an unidentified host factor to inhibit retrograde transport from endosomes (Fig. 1) [116]. In addition, Retro-2 has also been shown to inhibit JCPyV pseudovirus transduction in several kidney and glial cell lines, suggesting that Retro-2 targets a conserved transport machinery. Small molecule inhibitors that target host cell factors run the risk of having deleterious side effects, but also reduce the likelihood that viruses will be able to generate escape mutations allowing infection [117–119]. Importantly, follow-up studies have produced chemical analogs of Retro-2 that inhibit viral infectivity at over 5-fold lower concentrations than Retro-2 [32]. These results suggest that continued development of Retro-2 or chemical analogs may eventually lead to drugs that provide effective treatment options for individuals suffering from JCPyV infection and PML. Future drug development studies will likely also need to examine the ability of Retro-2 or related compounds to cross the blood brain barrier in order to neutralize viral replication in the CNS.
Conclusions
JCPyV infection occurs in the majority of the population [2, 3] yet only results in PML in severely immunosuppressed individuals such as those with HIV-1 infection [47] or those under immunosuppressive therapies [49]. Viral attachment to host cells in culture is dependent on α2,6-sialic acid on LSTc via interactions with specific residues on VP1 [17]. Mutations arise in the sialic acid binding pocket of VP1, and these sites cannot be neutralized by antibodies isolated from individuals that are immunosuppressed [29, 31]. Individuals that recover from PML develop antibodies to PML-associated mutations that can lead to neutralization and recovery in combination with immune system activators [31]. VP1-based vaccine combination treatment to boost anti-VP1 immunity have led to clinical improvements in PML patients [29, 30], and VP1-specific mAbs represent a new potential treatment option [31]. Following attachment, JCPyV enters cells by 5-HT2Rs [20, 85], and although antagonists to 5-HT2Rs have shown some clinical promise [93, 94], there are other instances in which no clinical improvement has been observed [95, 96]. Novel small molecule inhibitor Retro-2 that blocks retrograde transport of the virus from the ER at lower concentrations shows promise in vitro [22, 32]. Taken together, viral attachment, entry, and trafficking represent novel targets for anti-viral therapeutics, presenting an opportunity for the continued development of improved PML treatments.
Acknowledgments
We thank all of our colleagues who contributed to the work reviewed in this manuscript and acknowledge the important contributions of those that we were not able to include due to space limitations. We thank Jeanne DuShane and Aaron Derdowski for reviewing the manuscript. This research was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103423 (MSM) and The University of Maine MEIF (MSM) and the SUNY Cortland Faculty Research Program Award (CDN).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
The authors declare that they have no conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
References
Recently published papers of particular interest have been highlighted as:
• Of importance
- 1.Weissert R. Progressive multifocal leukoencephalopathy. Journal of Neuroimmunology. 2011;231(1–2):73–7. doi: 10.1016/j.jneuroim.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 2.Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathogens. 2009;5(3):e1000363. doi: 10.1371/journal.ppat.1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egli A, Infanti L, Dumoulin A, Buser A, Samaridis J, Stebler C, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. The Journal of Infectious Diseases. 2009;199(6):837–46. doi: 10.1086/597126. [DOI] [PubMed] [Google Scholar]
- 4.Houff SA, Major EO, Katz DA, Kufta CV, Sever JL, Pittaluga S, et al. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. The New England Journal of Medicine. 1988;318(5):301–5. doi: 10.1056/NEJM198802043180507. [DOI] [PubMed] [Google Scholar]
- 5.Monaco MC, Atwood WJ, Gravell M, Tornatore CS, Major EO. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. Journal of Virology. 1996;70(10):7004–12. doi: 10.1128/jvi.70.10.7004-7012.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan CS, Dezube BJ, Bhargava P, Autissier P, Wuthrich C, Miller J, et al. Detection of JC virus DNA and proteins in the bone marrow of HIV-positive and HIV-negative patients: implications for viral latency and neurotropic transformation. The Journal of Infectious Diseases. 2009;199(6):881–8. doi: 10.1086/597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boukoum H, Nahdi I, Sahtout W, Skiri H, Segondy M, Aouni M. BK and JC virus infections in healthy patients compared to kidney transplant recipients in Tunisia. Microb Pathog. 2016;97:204–8. doi: 10.1016/j.micpath.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Silverman L, Rubinstein LJ. Electron microscopic observations on a case of progressive multifocal leukoencephalopathy. Acta Neuropathologica. 1965;5(2):215–24. doi: 10.1007/BF00686519. [DOI] [PubMed] [Google Scholar]
- 9.Zurhein G, Chou SM. Particles Resembling Papova Viruses in Human Cerebral Demyelinating Disease. Science. 1965;148:1477–9. doi: 10.1126/science.148.3676.1477. [DOI] [PubMed] [Google Scholar]
- 10*.Kondo Y, Windrem MS, Zou L, Chandler-Militello D, Schanz SJ, Auvergne RM, et al. Human glial chimeric mice reveal astrocytic dependence of JC virus infection. J Clin Invest. 2014;124(12):5323–36. doi: 10.1172/JCI76629. This work demonstrates the use of a chimeric mouse model as a tractable model system to study JCPyV infection and PML. The use of the mouse model established a greater role for astrocytes in PML than previously appreciated and reported that oligodendrocytes undergo apoptosis due to JCPyV infection. PML-associated variants arise in the mouse model confirming studies of PML patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astrom KE, Mancall EL, Richardson EP., Jr Progressive multifocal leuko-encephalopathy; a hitherto unrecognized complication of chronic lymphatic leukemia and Hodgkin’s disease. Brain. 1958;81(1):93–111. doi: 10.1093/brain/81.1.93. [DOI] [PubMed] [Google Scholar]
- 12.Ferenczy MW, Marshall LJ, Nelson CD, Atwood WJ, Nath A, Khalili K, et al. Molecular biology, epidemiology, and pathogenesis of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clinical Microbiology Reviews. 2012;25(3):471–506. doi: 10.1128/CMR.05031-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah KV, Fields BN, Knipe DM, Howley PM. Polyomaviruses. 3. Philadelphia: Lippincott-Raven Publishers; 1996. Fields virology. [Google Scholar]
- 14.Liddington RC, Yan Y, Moulai J, Sahli R, Benjamin TL, Harrison SC. Structure of simian virus 40 at 3.8-A resolution. Nature. 1991;354(6351):278–84. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 15.Chen BJ, Atwood WJ. Construction of a novel JCV/SV40 hybrid virus (JCSV) reveals a role for the JCV capsid in viral tropism. Virology. 2002;300(2):282–90. doi: 10.1006/viro.2002.1522. [DOI] [PubMed] [Google Scholar]
- 16.Gee GV, Tsomaia N, Mierke DF, Atwood WJ. Modeling a sialic acid binding pocket in the external loops of JC virus VP1. The Journal of Biological Chemistry. 2004;279(47):49172–6. doi: 10.1074/jbc.M409326200. [DOI] [PubMed] [Google Scholar]
- 17.Neu U, Maginnis MS, Palma AS, Stroh LJ, Nelson CD, Feizi T, et al. Structure-function analysis of the human JC polyomavirus establishes the LSTc pentasaccharide as a functional receptor motif. Cell Host Microbe. 2010;8(4):309–19. doi: 10.1016/j.chom.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pho MT, Ashok A, Atwood WJ. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. Journal of Virology. 2000;74(5):2288–92. doi: 10.1128/jvi.74.5.2288-2292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Querbes W, Benmerah A, Tosoni D, Di Fiore PP, Atwood WJ. A JC virus-induced signal is required for infection of glial cells by a clathrin- and eps15-dependent pathway. Journal of Virology. 2004;78(1):250–6. doi: 10.1128/JVI.78.1.250-256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assetta B, Maginnis MS, Gracia Ahufinger I, Haley SA, Gee GV, Nelson CD, et al. 5-Ht2 Receptors Facilitate JC Polyomavirus Entry. Journal of Virology. 2013;87(24):13490–8. doi: 10.1128/JVI.02252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Querbes W, O’Hara B, Williams G, Atwood W. Invasion of host cells by JC virus identifies a novel role for caveolae in endosomal sorting of noncaveolar ligands. Journal of Virology. 2006;80(19):9402–13. doi: 10.1128/JVI.01086-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson C, Carney D, Derdowski A, Lipovsky A, Gee G, O’Hara B, et al. A retrograde trafficking inhibitor of ricin and Shiga-like toxins inhibits infection of cells by human and monkey polyomaviruses. mBio. 2013;4(6):13. doi: 10.1128/mBio.00729-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson C, Derdowski A, Maginnis M, O’Hara B, Atwood W. The VP1 subunit of JC polyomavirus recapitulates early events in viral trafficking and is a novel tool to study polyomavirus entry. Virology. 2012;428(1):30–40. doi: 10.1016/j.virol.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng HY, Takasaka T, Noda K, Kanazawa A, Mori H, Kabuki T, et al. New sequence polymorphisms in the outer loops of the JC polyomavirus major capsid protein (VP1) possibly associated with progressive multifocal leukoencephalopathy. The Journal of General Virology. 2005;86(Pt 7):2035–45. doi: 10.1099/vir.0.80863-0. [DOI] [PubMed] [Google Scholar]
- 25.Sunyaev SR, Lugovskoy A, Simon K, Gorelik L. Adaptive mutations in the JC virus protein capsid are associated with progressive multifocal leukoencephalopathy (PML) PLoS Genetics. 2009;5(2):e1000368. doi: 10.1371/journal.pgen.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delbue S, Branchetti E, Bertolacci S, Tavazzi E, Marchioni E, Maserati R, et al. JC virus VP1 loop-specific polymorphisms are associated with favorable prognosis for progressive multifocal leukoencephalopathy. Journal of Neurovirology. 2009;15(1):51–6. doi: 10.1080/13550280802425467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorelik L, Reid C, Testa M, Brickelmaier M, Bossolasco S, Pazzi A, et al. Progressive multifocal leukoencephalopathy (PML) development is associated with mutations in JC virus capsid protein VP1 that change its receptor specificity. The Journal of Infectious Diseases. 2011;204(1):103–14. doi: 10.1093/infdis/jir198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reid CE, Li H, Sur G, Carmillo P, Bushnell S, Tizard R, et al. Sequencing and analysis of JC virus DNA from natalizumab-treated PML patients. The Journal of Infectious Diseases. 2011;204(2):237–44. doi: 10.1093/infdis/jir256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29*.Ray U, Cinque P, Gerevini S, Longo V, Lazzarin A, Schippling S, et al. JC polyomavirus mutants escape antibody-mediated neutralization. Sci Transl Med. 2015;7(306):306ra151. doi: 10.1126/scitranslmed.aab1720. This report describes the development of a VLP-based JCPyV vaccine that was administered to mice and used in the clinical treatment of a PML patient. This work establishes the importance of neutralizing wild-type JCPyV and PML variants during PML progression and establishes a novel VLP-based vaccine as a potential PML treatment option. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30*.Sospedra M, Schippling S, Yousef S, Jelcic I, Bofill-Mas S, Planas R, et al. Treating progressive multifocal leukoencephalopathy with interleukin 7 and vaccination with JC virus capsid protein VP1. Clin Infect Dis. 2014;59(11):1588–92. doi: 10.1093/cid/ciu682. This report describes the clinical treatment of PML patients with a VP1-based JCPyV vaccine. The treatment led to clinical improvements suggesting that this is a viable treatment option to slow PML progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Jelcic I, Combaluzier B, Jelcic I, Faigle W, Senn L, Reinhart BJ, et al. Broadly neutralizing human monoclonal JC polyomavirus VP1-specific antibodies as candidate therapeutics for progressive multifocal leukoencephalopathy. Sci Transl Med. 2015;7(306):306ra150. doi: 10.1126/scitranslmed.aac8691. This study describes VP1-specific "recognition holes" in JCPyV immunity in immunocompromised patients. Through molecular cloning of B cells from PML patients, novel VP1-specific mAbs were tested for the ability to neutralize JCPyV infection and demonstrate clinical promise in vitro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carney DW, Nelson CD, Ferris BD, Stevens JP, Lipovsky A, Kazakov T, et al. Structural optimization of a retrograde trafficking inhibitor that protects cells from infections by human polyoma- and papillomaviruses. Bioorg Med Chem. 2014;22(17):4836–47. doi: 10.1016/j.bmc.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yatawara A, Gaidos G, Rupasinghe CN, O’Hara BA, Pellegrini M, Atwood WJ, et al. Small-molecule inhibitors of JC polyomavirus infection. J Pept Sci. 2015;21(3):236–42. doi: 10.1002/psc.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;1(7712):1257–60. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 35.Yogo Y, Kitamura T, Sugimoto C, Ueki T, Aso Y, Hara K, et al. Isolation of a possible archetypal JC virus DNA sequence from nonimmunocompromised individuals. Journal of Virology. 1990;64(6):3139–43. doi: 10.1128/jvi.64.6.3139-3143.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniel AM, Swenson JJ, Mayreddy RP, Khalili K, Frisque RJ. Sequences within the early and late promoters of archetype JC virus restrict viral DNA replication and infectivity. Virology. 1996;216(1):90–101. doi: 10.1006/viro.1996.0037. [DOI] [PubMed] [Google Scholar]
- 37.Dubois V, Dutronc H, Lafon ME, Poinsot V, Pellegrin JL, Ragnaud JM, et al. Latency and reactivation of JC virus in peripheral blood of human immunodeficiency virus type 1-infected patients. Journal of Clinical Microbiology. 1997;35(9):2288–92. doi: 10.1128/jcm.35.9.2288-2292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, et al. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17(4):607–15. doi: 10.1016/s0896-6273(00)80194-4. [DOI] [PubMed] [Google Scholar]
- 39.Spiegel I, Peles E. A new player in CNS myelination. Neuron. 2006;49(6):777–8. doi: 10.1016/j.neuron.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathologica. 2010;119(1):37–53. doi: 10.1007/s00401-009-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du Pasquier RA, Corey S, Margolin DH, Williams K, Pfister LA, De Girolami U, et al. Productive infection of cerebellar granule cell neurons by JC virus in an HIV+ individual. Neurology. 2003;61(6):775–82. doi: 10.1212/01.wnl.0000081306.86961.33. [DOI] [PubMed] [Google Scholar]
- 42.Wuthrich C, Cheng YM, Joseph JT, Kesari S, Beckwith C, Stopa E, et al. Frequent infection of cerebellar granule cell neurons by polyomavirus JC in progressive multifocal leukoencephalopathy. Journal of Neuropathology and Experimental Neurology. 2009;68(1):15–25. doi: 10.1097/NEN.0b013e3181912570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wuthrich C, Koralnik IJ. Frequent infection of cortical neurons by JC virus in patients with progressive multifocal leukoencephalopathy. Journal of neuropathology and experimental Neurology. 2012;71(1):54–65. doi: 10.1097/NEN.0b013e31823ede59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: consensus statement from the AAN Neuroinfectious Disease Section. Neurology. 2013;80(15):1430–8. doi: 10.1212/WNL.0b013e31828c2fa1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brew BJ, Davies NW, Cinque P, Clifford DB, Nath A. Progressive multifocal leukoencephalopathy and other forms of JC virus disease. Nat Rev Neurol. 2010;6(12):667–79. doi: 10.1038/nrneurol.2010.164. [DOI] [PubMed] [Google Scholar]
- 46.Hirsch HH, Kardas P, Kranz D, Leboeuf C. The human JC polyomavirus (JCPyV): virological background and clinical implications. APMIS. 2013;121(8):685–727. doi: 10.1111/apm.12128. [DOI] [PubMed] [Google Scholar]
- 47.Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M Progressive Multifocal Leukeoncephalopathy C. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8(6):255–73. doi: 10.1177/1756285615602832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carson KR, Focosi D, Major EO, Petrini M, Richey EA, West DP, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. The Lancet Oncology. 2009;10(8):816–24. doi: 10.1016/S1470-2045(09)70161-5. [DOI] [PubMed] [Google Scholar]
- 49.Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. The New England Journal of Medicine. 2012;366(20):1870–80. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 50.Hellwig K, Gold R. Progressive multifocal leukoencephalopathy and natalizumab. Journal of Neurology. 2011;258(11):1920–8. doi: 10.1007/s00415-011-6116-8. [DOI] [PubMed] [Google Scholar]
- 51.Kawamoto E, Nakahashi S, Okamoto T, Imai H, Shimaoka M. Anti-integrin therapy for multiple sclerosis. Autoimmune Dis. 2012;2012:357101. doi: 10.1155/2012/357101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–58. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 53.Biogen Idec. [Accessed April 11, 2017];2017 https://medinfo.biogen.com.
- 54.Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113(20):4834–40. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar D, Bouldin TW, Berger RG. A case of progressive multifocal leukoencephalopathy in a patient treated with infliximab. Arthritis and Rheumatism. 2010;62(11):3191–5. doi: 10.1002/art.27687. [DOI] [PubMed] [Google Scholar]
- 56.Kothary N, Diak IL, Brinker A, Bezabeh S, Avigan M, Dal Pan G. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. Journal of the American Academy of Dermatology. 2011;65(3):546–51. doi: 10.1016/j.jaad.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 57.D’Amico E, Zanghi A, Leone C, Tumani H, Patti F. Treatment-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: A Comprehensive Review of Current Evidence and Future Needs. Drug Saf. 2016;39(12):1163–74. doi: 10.1007/s40264-016-0461-6. [DOI] [PubMed] [Google Scholar]
- 58.Ermis U, Weis J, Schulz JB. Case reports of PML in patients treated for psoriasis. The New England Journal of Medicine. 2013;369(11):1081. doi: 10.1056/NEJMc1307680. [DOI] [PubMed] [Google Scholar]
- 59.van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. The New England Journal of Medicine. 2013;368(17):1658–9. doi: 10.1056/NEJMc1215357. [DOI] [PubMed] [Google Scholar]
- 60.Nieuwkamp DJ, Murk JL, van Oosten BW, Cremers CH, Killestein J, Viveen MC, et al. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. The New England Journal of Medicine. 2015;372(15):1474–6. doi: 10.1056/NEJMc1413724. [DOI] [PubMed] [Google Scholar]
- 61.Hoepner R, Faissner S, Klasing A, Schneider R, Metz I, Bellenberg B, et al. Progressive multifocal leukoencephalopathy during fumarate monotherapy of psoriasis. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e85. doi: 10.1212/NXI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Assche G, Van Ranst M, Sciot R, Dubois B, Vermeire S, Noman M, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. The New England Journal of Medicine. 2005;353(4):362–8. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 63.Fleischmann RM. Progressive multifocal leukoencephalopathy following rituximab treatment in a patient with rheumatoid arthritis. Arthritis and Rheumatism. 2009;60(11):3225–8. doi: 10.1002/art.24906. [DOI] [PubMed] [Google Scholar]
- 64.Beltrami S, Gordon J. Immune surveillance and response to JC virus infection and PML. Journal of Neurovirology. 2014;20(2):137–49. doi: 10.1007/s13365-013-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sorensen PS, Bertolotto A, Edan G, Giovannoni G, Gold R, Havrdova E, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Multiple Sclerosis. 2012;18(2):143–52. doi: 10.1177/1352458511435105. [DOI] [PubMed] [Google Scholar]
- 66.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. The New England Journal of Medicine. 2017;376(3):209–20. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 67.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. The New England Journal of Medicine. 2017;376(3):221–34. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 68.Genetech Inc, Roche. OCREVUS prescribing information. 2017. [Google Scholar]
- 69.Stroh LJ, Stehle T. Glycan Engagement by Viruses: Receptor Switches and Specificity. Annual Review of Virology. 2014;1:285–306. doi: 10.1146/annurev-virology-031413-085417. [DOI] [PubMed] [Google Scholar]
- 70.Stencel-Baerenwald JE, Reiss K, Reiter DM, Stehle T, Dermody TS. The sweet spot: defining virus-sialic acid interactions. Nature Reviews Microbiology. 2014;12(11):739–49. doi: 10.1038/nrmicro3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu CK, Wei G, Atwood WJ. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal alpha(2–6)-linked sialic acids. Journal of Virology. 1998;72(6):4643–9. doi: 10.1128/jvi.72.6.4643-4649.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Komagome R, Sawa H, Suzuki T, Suzuki Y, Tanaka S, Atwood WJ, et al. Oligosaccharides as receptors for JC virus. Journal of Virology. 2002;76(24):12992–3000. doi: 10.1128/JVI.76.24.12992-13000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dugan AS, Gasparovic ML, Atwood WJ. Direct correlation between sialic acid binding and infection of cells by two human polyomaviruses (JC virus and BK virus) Journal of Virology. 2008;82(5):2560–4. doi: 10.1128/JVI.02123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stroh LJ, Maginnis MS, Blaum BS, Nelson CD, Neu U, Gee GV, et al. The Greater Affinity of JC Polyomavirus Capsid for alpha2,6-Linked Lactoseries Tetrasaccharide c than for Other Sialylated Glycans Is a Major Determinant of Infectivity. Journal of Virology. 2015;89(12):6364–75. doi: 10.1128/JVI.00489-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neu U, Woellner K, Gauglitz G, Stehle T. Structural basis of GM1 ganglioside recognition by simian virus 40. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(13):5219–24. doi: 10.1073/pnas.0710301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Erickson KD, Garcea RL, Tsai B. Ganglioside GT1b is a putative host cell receptor for the Merkel cell polyomavirus. Journal of Virology. 2009;83(19):10275–9. doi: 10.1128/JVI.00949-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qian M, Tsai B. Lipids and proteins act in opposing manners to regulate polyomavirus infection. Journal of Virology. 2010;84(19):9840–52. doi: 10.1128/JVI.01093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neu U, Allen SA, Blaum BS, Liu Y, Frank M, Palma AS, et al. A structure-guided mutation in the major capsid protein retargets BK polyomavirus. PLoS Pathogens. 2013;9(10):e1003688. doi: 10.1371/journal.ppat.1003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maginnis MS, Stroh LJ, Gee GV, O’Hara BA, Derdowski A, Stehle T, et al. Progressive multifocal leukoencephalopathy-associated mutations in the JC polyomavirus capsid disrupt lactoseries tetrasaccharide c binding. mBio. 2013;4(3):e00247–13. doi: 10.1128/mBio.00247-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cubitt CL, Cui X, Agostini HT, Nerurkar VR, Scheirich I, Yanagihara R, et al. Predicted amino acid sequences for 100 JCV strains. Journal of Neurovirology. 2001;7(4):339–44. doi: 10.1080/13550280152537201. [DOI] [PubMed] [Google Scholar]
- 81.Haley SA, O’Hara BA, Nelson CD, Brittingham FL, Henriksen KJ, Stopa EG, et al. Human polyomavirus receptor distribution in brain parenchyma contrasts with receptor distribution in kidney and choroid plexus. The American Journal of Pathology. 2015;185(8):2246–58. doi: 10.1016/j.ajpath.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Malik S, Eugenin EA. Mechanisms of HIV Neuropathogenesis: Role of Cellular Communication Systems. Curr HIV Res. 2016;14(5):400–11. doi: 10.2174/1570162x14666160324124558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar A, Kim JH, Ranjan P, Metcalfe MG, Cao W, Mishina M, et al. Influenza virus exploits tunneling nanotubes for cell-to-cell spread. Scientific Reports. 2017;7:40360. doi: 10.1038/srep40360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki H, Gen K, Inoue Y. Comparison of the anti-dopamine D(2) and anti-serotonin 5-HT(2A) activities of chlorpromazine, bromperidol, haloperidol and second-generation antipsychotics parent compounds and metabolites thereof. J Psychopharmacol. 2013;27(4):396–400. doi: 10.1177/0269881113478281. [DOI] [PubMed] [Google Scholar]
- 85.Elphick G, Querbes W, Jordan J, Gee G, Eash S, Manley K, et al. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science. 2004;306(5700):1380–3. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 86.O’Hara BA, Atwood WJ. Interferon beta1-a and selective anti-5HT(2a) receptor antagonists inhibit infection of human glial cells by JC virus. Virus Research. 2008;132(1–2):97–103. doi: 10.1016/j.virusres.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71(4):533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 88.Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, et al. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. British Journal of Pharmacology. 1995;115(4):622–8. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bockaert J, Claeysen S, Becamel C, Dumuis A, Marin P. Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell and Tissue Research. 2006;326(2):553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- 90.Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27(1):79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 91.Chapagain ML, Verma S, Mercier F, Yanagihara R, Nerurkar VR. Polyomavirus JC infects human brain microvascular endothelial cells independent of serotonin receptor 2A. Virology. 2007;364(1):55–63. doi: 10.1016/j.virol.2007.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Szegedi A, Schwertfeger N. Mirtazapine: a review of its clinical efficacy and tolerability. Expert Opinion on Pharmacotherapy. 2005;6(4):631–41. doi: 10.1517/14656566.6.4.631. [DOI] [PubMed] [Google Scholar]
- 93.Cettomai D, McArthur JC. Mirtazapine use in human immunodeficiency virus-infected patients with progressive multifocal leukoencephalopathy. Archives of Neurology. 2009;66(2):255–8. doi: 10.1001/archneurol.2008.557. [DOI] [PubMed] [Google Scholar]
- 94.Kurmann R, Weisstanner C, Kardas P, Hirsch HH, Wiest R, Lammle B, et al. Progressive multifocal leukoencephalopathy in common variable immunodeficiency: mitigated course under mirtazapine and mefloquine. Journal of Neurovirology. 2015;21(6):694–701. doi: 10.1007/s13365-015-0340-4. [DOI] [PubMed] [Google Scholar]
- 95.Marzocchetti A, Tompkins T, Clifford DB, Gandhi RT, Kesari S, Berger JR, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology. 2009;73(19):1551–8. doi: 10.1212/WNL.0b013e3181c0d4a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Crossley KM, Agnihotri S, Chaganti J, Rodriguez ML, McNally LP, Venna N, et al. Recurrence of progressive multifocal leukoencephalopathy despite immune recovery in two HIV seropositive individuals. Journal of Neurovirology. 2016;22(4):541–5. doi: 10.1007/s13365-015-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schroder A, Lee DH, Hellwig K, Lukas C, Linker RA, Gold R. Successful management of natalizumab-associated progressive multifocal leukoencephalopathy and immune reconstitution syndrome in a patient with multiple sclerosis. Archives of Neurology. 2010;67(11):1391–4. doi: 10.1001/archneurol.2010.157. [DOI] [PubMed] [Google Scholar]
- 98.Brickelmaier M, Lugovskoy A, Kartikeyan R, Reviriego-Mendoza MM, Allaire N, Simon K, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrobial Agents and Chemotherapy. 2009;53(5):1840–9. doi: 10.1128/AAC.01614-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wandinger-Ness A, Zerial M. Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol. 2014;6(11):a022616. doi: 10.1101/cshperspect.a022616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Engel S, Heger T, Mancini R, Herzog F, Kartenbeck J, Hayer A, et al. Role of endosomes in simian virus 40 entry and infection. Journal of Virology. 2011;85(9):4198–211. doi: 10.1128/JVI.02179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qian M, Cai D, Verhey K, Tsai B. A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathogens. 2009;5(6):e100465. doi: 10.1371/journal.ppat.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Richterova Z, Liebl D, Horak M, Palkova Z, Stokrova J, Hozak P, et al. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. Journal of Virology. 2001;75(22):10880–91. doi: 10.1128/JVI.75.22.10880-10891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsai B, Gilbert J, Stehle T, Lencer W, Benjamin T, Rapoport T. Gangliosides are receptors for murine polyoma virus and SV40. The EMBO Journal. 2003;22(17):4346–55. doi: 10.1093/emboj/cdg439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gilbert J, Benjamin T. Uptake pathway of polyomavirus via ganglioside GD1a. Journal of Virology. 2004;78(22):12259–67. doi: 10.1128/JVI.78.22.12259-12267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Low J, Magnuson B, Tsai B, Imperiale M. Identification of gangliosides GD1b and GT1b as receptors for BK virus. Journal of Virology. 2006;80(3):1361–6. doi: 10.1128/JVI.80.3.1361-1366.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Norkin LC, Anderson HA, Wolfrom SA, Oppenheim A. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. Journal of Virology. 2002;76(10):5156–66. doi: 10.1128/JVI.76.10.5156-5166.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Richards AA, Stang E, Pepperkok R, Parton RG. Inhibitors of COP-mediated transport and cholera toxin action inhibit simian virus 40 infection. Mol Biol Cell. 2002;13(5):1750–64. doi: 10.1091/mbc.01-12-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ashok A, Atwood WJ. Contrasting roles of endosomal pH and the cytoskeleton in infection of human glial cells by JC virus and simian virus 40. Journal of Virology. 2003;77(2):1347–56. doi: 10.1128/JVI.77.2.1347-1356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saimani U, Kim K. Traffic from the endosome towards trans-Golgi network. Eur J Cell Biol. 2017;96(2):198–205. doi: 10.1016/j.ejcb.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 110.Seaman M. The retromer complex - endosomal protein recycling and beyond. Journal of Cell Science. 2012;125(Pt 20):4693–702. doi: 10.1242/jcs.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seaman M, Gautreau A, Billadeau D. Retromer-mediated endosomal protein sorting: all WASHed up! Trends in Cell Biology. 2013;23(11):522–528. doi: 10.1016/j.tcb.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;135(7):1175–87. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 113.Schelhaas M, Malmstrom J, Pelkmans L, Haugstetter J, Ellgaard L, Grunewald K, et al. Simian Virus 40 depends on ER protein folding and quality control factors for entry into host cells. Cell. 2007;131(3):516–29. doi: 10.1016/j.cell.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 114.Fujinaga Y, Wolf A, Rodighiero C, Wheeler H, Tsai B, Allen L, et al. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulm. Molecular Biology of the Cell. 2003;14(12):4783–93. doi: 10.1091/mbc.E03-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Campanero-Rhodes M, Smith A, Chai W, Sonnino S, Mauri L, Childs R, et al. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. Journal of Virology. 2007;81(23):12846–58. doi: 10.1128/JVI.01311-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stechmann B, Bai S-K, Gobbo E, Lopez R, Merer G, Pinchard S, et al. Inhibition of retrograde transport protects mice from lethal ricin challenge. Cell. 2010;141(2):231–42. doi: 10.1016/j.cell.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 117.Provencher V, Coccaro E, Lacasse J, Schang L. Antiviral drugs that target cellular proteins may play major roles in combating HIV resistance. Current Pharmaceutical Design. 2004;10(32):4081–101. doi: 10.2174/1381612043382422. [DOI] [PubMed] [Google Scholar]
- 118.Bonavia A, Franti M, Pusateri Keaney E, Kuhen K, Seepersaud M, Radetich B, et al. Identification of broad-spectrum antiviral compounds and assessment of the druggability of their target for efficacy against respiratory syncytial virus (RSV) Proceedings of the National Academy of Sciences of the United States of America. 2011;108(17):6739–44. doi: 10.1073/pnas.1017142108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krumm S, Ndungu J, Yoon J-J, Dochow M, Sun A, Natchus M, et al. Potent host-directed small-molecule inhibitors of myxovirus RNA-dependent RNA-polymerases. PloS ONE. 2011;6(5) doi: 10.1371/journal.pone.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]