Abstract
The endometrium maintains complex controls on proliferation and apoptosis as part of repetitive menstrual cycles that prepare the endometrium for the window of implantation and pregnancy. The reliance on inflammatory mechanisms for both implantation and menstruation, creates the opportunity in the setting of endometriosis for the establishment of chronic inflammation that is disruptive to endometrial receptivity, causing both infertility and abnormal bleeding. Clinically, there can be little doubt that the endometrium of women with endometriosis is less receptive to embryo implantation, and strong evidence exists to suggests that endometrial changes are associated with decreased cycle fecundity as a result of this disease. Here we provide unifying concepts regarding those changes and how they are coordinated to promote progesterone resistance and estrogen dominance through aberrant cell signaling pathways and reduced expression of key homeostatic proteins in eutopic endometrium of women with endometriosis.
Keywords: Endometriosis, endometrium, progesterone resistance, infertility, implantation, endometrial receptivity
Introduction
The endometrium maintains complex autocrine, paracrine and endocrine signaling involving sex steroids, cytokines and chemokines and intracellular signaling, culminating in receptivity to embryo implantation (1). In lieu of a successful pregnancy, the endometrium undergoes complex inflammatory changes that, in a non-scarring fashion, sloughs the lining so that it can be replaced following menstruation (2). While acute inflammation is required for both implantation and menstruation (3), chronic inflammation is disruptive and a major cause of infertility and menstrual bleeding disorders (4-6). Endometriosis affects millions of women, and a major cause of infertility and pelvic pain (7). Women with endometriosis are twice as likely to have infertility (8, 9) and pregnancy loss (10, 11). Changes in endometrial receptivity due to endometriosis has been well studied and several key studies support our argument that endometriosis affects the endometrium and reduces fertility. How these changes affect fertility is equally important, as a thorough understanding of the physiological mechanism will provide opportunities for both diagnosis and therapy for this common condition.
Endometriosis and Infertility – What is the Evidence?
In endometriosis, inflammation is centrally associated with the pathophysiology of this disorder, contributing to progesterone resistance and estrogen dominance (12). Endometriosis is a systemic and reversible inflammatory condition that alters endometrial function (13, 14). Clinical and animal studies support this association between endometriosis and infertility, including: 1) early prospective studies that show endometriosis patients are infertile (15, 16); 2) a recent large retrospective study demonstrating increased risk of infertility was associated with endometriosis (8), 3) reduced success rates in women with endometriosis in the setting of intrauterine insemination (IUI) has been repeatedly demonstrated (17-19), and 4) IUI results, in general, that find decreased fecundity comparing endometriosis to other diagnoses (20). Finally, 5) there is a high prevalence of endometriosis in women who have otherwise unexplained infertility (21).
Treatment of endometriosis has been shown to be beneficial for future fertility and improved pregnancy outcomes (22, 23). Studies from In Vitro Fertilization (IVF) cycles have documented a decreased pregnancy rate (24, 25), which can be improved with gonadotropin releasing hormone agonist (GnRHa) suppression (26), surgery (27) or aromatase inhibitor therapy (28). While early studies on donor oocytes has suggested the primary defect associated with endometriosis may reside in the ovary and oocyte quality (29), larger and more recent studies have documented that defective implantation is also likely involved (30). Prapas and co-workers studied the result of 240 cycles, placing sibling oocytes from the same donor into women with or without endometriosis. Adjusted odds ratio (95% CI) showed reduced implantation (0.78 (0.67-0.91)), clinical pregnancy rate (0.22 (0.08-0.57)), ongoing pregnancy (0.11 (0.03-0.35)), and live birth rate (0.19 (0.09-0.38)) for women with endometriosis (30).
Animal studies support clinical data suggesting that endometriosis leads to implantation defects, again implicating the endometrium. Induction of endometriosis in animals demonstrates similar phenotypes to human disease (31-34). The failure to implant embryos associated with endometriosis was transferrable in the peritoneal fluid (PF) in rabbits (35), as well as in mice who received human endometriotic PF (36). Induction of endometriosis in the baboon has been shown to be associated with gradual but profound alterations in the endometrium over time (37), suggesting that inflammation and the immune system may be involved in these changes.
Endometrial biomarkers are differentially expressed in the endometrium of women with endometriosis compared to normal women (38, 39) and studies over the years have refined and expanded these approaches to include miRNA arrays, proteomics and selected molecules including BCL6 (Fig 1). Early studies on endometrial proteins that participate in embryo attachment and invasion reported a decrease in expression of key proteins associated endometriosis (5, 40). Endometrial integrins are cell-surface receptors for extracellular matrix proteins that were first described in early 1990 (41, 42). We and others reported on specific key integrins including the ανβ3 integrin with a role in implantation (41, 43, 44) and this integrin was decreased in women with infertility and endometriosis (45) and unexplained infertility (46). L-selectin ligand, another extracellular ligand thought to be an attachment receptor on the endometrium for embryo-derived selectin, is decreased in the endometrium of endometriosis and unexplained infertility (47-49).
Figure 1.
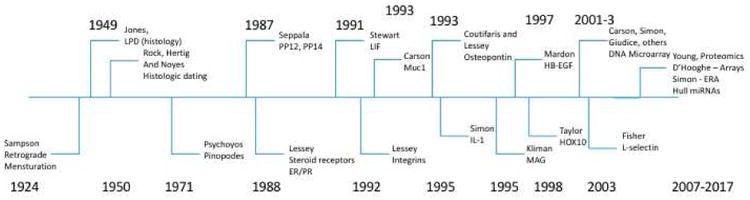
Timeline for major discoveries in endometriosis and related defects in endometrial receptivity. The history of discovery of endometrial changes associated with endometriosis is shown in this timeline. Pivotal research on endometriosis commenced with the work of Sampson, but the changes in endometrium were noted by representative investigators up to the present day. There are many other important contributions that may not be represented. (144-167)
The changes in endometrial gene expression associated with defective endometrial receptivity reflect a shift away from normal progesterone action (39) and toward excessive estrogen activity. Such alterations in the balance between estrogen and progesterone likely impact fertility and implantation while also promoting the pathogenesis of endometriosis as a disease (50). Progesterone receptor changes and down-stream effects of progesterone (51, 52) are noted in women with endometriosis (53). Park et al. suggested that the endometrium of women with endometriosis is more proliferative as a result of endometriosis (54) and we demonstrated the endometrium displays an inappropriate elevation in secretory phase estrogen receptors (ESR1) at the time of implantation (55). As ESR1 levels are down-regulated in almost all mammalian species studied at implantation and the primary role of progesterone,(56) this failure to down-regulate ESR1 is a single primary endpoint that may predict implantation failure.
Endometriosis and Inflammation
Why would the endometrium and endometrial receptivity be altered in endometriosis? Inflammation is known to alter endometrial receptivity and has been associated specifically with endometriosis (57, 58). Endometriosis results in systemic and local cytokine expression changes that disrupts normal endometrial function (59-62), and is reversible by surgical removal of endometriosis (63). One of the hallmark changes seen in the endometrium of women with endometriosis is an induction of p450 aromatase expression (64, 65). While usually restricted to certain cell types including the ovary, placenta, and brain (66), over-expression in endometrium changes the dynamic of progesterone to estrogen activity, and favors development and growth of endometriosis (67). Further, inflammation has been shown to influence aromatase and steroid receptor expression (68). Brosens demonstrated that elevated aromatase expression is associated with poor IVF outcomes (69). Estrogen, perhaps locally produced, has been shown to inhibit key molecules in attachment of embryos including the ανβ3 integrin (70). Reduced integrin expression associated with reduced IVF outcomes that can be over-come with aromatase inhibitors given in the cycle of stimulation (28). Thus, this is a one example of a single biomarker that has been shown to predict IVF outcome and be amenable to therapy.
The pro-implantation cytokine, leukemia inhibitory factor (LIF), is essential for normal implantation (71), LIF was shown to peak at the time of implantation in the mouse and in animals with targeted LIF mutations, embryos would float within the uterus, but not implant. Exogenous LIF would result in implantation suggesting a soluble effect of this cytokine. Studies in the human have shown that LIF is present at the time of implantation(72) but its expression is reduced in women with endometriosis (73). Other key molecules that are required for normal endometrial receptivity such as HOXA10 (74), is reduced in endometriosis but restored after surgical resection of disease (75). Reduction in HOXA10 has been reported to be due to epigenetic changes associated with aberrant methylation of the HOXA10 promoter (76).
The inflammatory response seen in endometriosis is unusual and may be related to intrinsic programmatic endometrial responses to progesterone withdrawal (2). During the late phases of the menstrual cycle progesterone levels fall and inflammatory responses ensue in an orchestrated response required for menstruation (2, 77). Since progesterone action is impaired in the setting of endometriosis (78), this may mimic progesterone withdrawal and thereby stimulate a premature inflammatory (premenstrual) response (79). Rel-A (p65) is a subunit of NF-kB that is central to the inflammatory response. Rel-A inhibits progesterone receptor via the PR promoter (80) and therefore further contributes to progesterone resistance. ARID1A, an anti-inflammatory protein that is often mutated in ovarian and breast cancers (81, 82), is down-regulated in endometriosis (83), and appears to be a key regulator of the inflammatory response seen in this disease, by blocking Rel-A action on cytokine expression (82). Inflammatory responses are also exaggerated by the loss of other regulatory proteins including protein-inhibitor of STAT3 (PIAS3), which we recently reported was reduced in the endometrium of women with endometriosis (84).
These inflammatory responses of the endometrium of endometriosis has important downstream effects that impact fertility (85-90) and have been recently reviewed (5, 91). Interleukin-17 is central to many of the changes in endometriosis including the stimulating effect on cyclooxygenase-2 (Cox-2) activity, IL-8 and aromatase expression (92). IL-17 is specifically elevated in the blood and endometrium of women with endometriosis (61). COX-2 (93) and prostaglandins (94, 95) are central to eutopic endometrial changes associated with endometriosis. The shift toward estrogen dominance induces these factors that promote inflammation, angiogenesis, cell proliferation and immunosuppression. IL-17-induced IL-8 has been shown to target the PTEN/AKT signaling pathway which is aberrantly activated in endometriosis (96). IL-17 also induces the inflammatory cytokine IL-6 (61), that is elevated in the endometrium of endometriosis patients (97, 98). IL-17 expression is reduced following treatment of endometriosis (61).
We have reported that endometriosis is associated with sustained activation of STAT3 in eutopic endometrium, that is driven by IL-6 (13) and exacerbated by down-regulation of its primary inhibitor, protein-inhibitor of STAT3 (PIAS3) in women with endometriosis (84). STAT3 phosphorylation stabilizes hypoxia-induced factor 1-alpha (HIF1A)⍰(13) and stimulates expression of BCL6 expression (99). STAT3 activation appears to contribute to progesterone resistance and is central to inflammatory responses, including stimulation of these downstream effectors leading to the hallmark changes seen in endometriosis: proliferation, cell-survival and angiogenesis. HIF1A, which normally appears at menstruation is responsible for many down-stream effects including angiogenesis. Inflammation associated with endometriosis has been implicated in epigenetic change (100) as well as aberrant activation of signaling pathways.
Endometriosis and Infertility: Role of Eutopic Endometrium
Endometriosis has been described as a progesterone resistant disease due to the blunted or inadequate response to progesterone of both the eutopic and ectopic endometrial cells and tissue (101-103). This is demonstrated by low expression of PR (104), blunted expression of progesterone target genes (105-107), and an inadequate decidualization response (103, 105, 107). Progesterone resistance associated with endometriosis contributes to increased cell proliferation and survival (54) and elevated estrogen receptors (55). As progesterone plays a role in decreasing inflammation in the endometrium, the insensitivity to progesterone signaling results in a pro-inflammatory condition (108, 109). The consequences of this is far-reaching affecting estrogen driven mechanisms and differentiating capacity of the tissue.
A role for eutopic endometrium in endometriosis-related infertility has also been focused on the defects in decidualization, a change in endometrial morphology that is essential for pregnancy success (110-112). There are multiple pathways by which decidualization defects might arise, and many have been identified as aberrant in endometriosis and defects in decidual responses have been widely reported (103, 113, 114). The decidua is an important component of the maternal/embryo interface that provides nutrients to the embryo, protects the developing embryo from stress pathways and immune rejection, and regulates the invasion of the trophoblast. Thus, aberrant decidualization would lead to unfavorable effects on embryo implantation and pregnancy. Although progesterone is a key hormone involved in initiating and prolonging the decidualization process signaling pathways have been demonstrated to amplify this response, including the PKA pathway (115-119), while the AKT and MAPK pathways have been demonstrated to blunt decidualization. Impaired decidualization has been reported in both eutopic and ectopic tissues in endometriosis (103, 110, 120). Human endometrial stromal cells suppress AKT during decidualization (121) and increased activation of PI3K/AKT impedes decidualization (103). FOX01, required for decidualization, is inactivated by AKT pathway (122) while inhibition of PI3K and AKT increases nuclear FOXO1 and IGFBP1 expression in response to progestin and dibutyryl cAMP treatment (103). PI3K/AKT also activates estrogen receptor alpha (ESR1), increasing its activation (123). In addition, in keeping with clinical observations of decreased estrogen receptor beta (ESR2)(124) AKT has also been shown to down-regulate ESR2(124), with the net effect of enhancing estrogen action. The AKT pathway may also impact progesterone action; AKT can downregulate PR protein expression of PR in breast cancer and endometrial cancer cells, and in stromal cells derived from endometriosis (125-127). AKT has been shown to attenuate PR action in endometrial cancer cells by affecting recruitment of coregulators to PR on chromatin (128). AKT inhibitors increased cellular PR and decreased cell survival and increased apoptosis in endometriosis (127). NOTCH1 is another gene that is critical for decidualization of both mouse and human uterine stromal cells (129). Decreased Notch signaling is associated with endometriosis and contributes to impaired decidualization through the down-regulation of FOXO1 (129). Interestingly, NOTCH1 may be a target of SIRT1 (130).
Studies have demonstrated that the MAPK pathway is also overactive in the eutopic endometrium from women with endometriosis (131). Microarray analysis in eutopic endometrium from endometriosis patients identified members of the MAPK and PI3K signaling pathways to be significantly regulated (132, 133). These genes included RON, SOS, 14-3-3 protein eta, and uPAR in epithelial cells and KSR and PI3K p85 regulatory subunit alpha in stromal cells. A recent GWAS analysis of Stage A endometriosis revealed a total of 14 pathways were enriched, including the Grb2-Sos, Wnt signaling p130Cas, and extracellular signal-regulated kinase (ERK)1/ERK2/MAPK pathways (134). Wu et al, (135) conducted a comprehensive profiling of gene expression differences between the ectopic and eutopic endometrium taken from women with endometriosis adjusted for menstrual phase and the location of the lesions. Regulators of the MAPK signaling pathway including DUSP5, AKT1, HSPB2, PDGFB, PDGFRA, PLA2G5, MAPK6, MAPK7, RAC1, RAF1, RPS6KA3, TGFB3, MKNK1 were altered. Global analysis of genes performed by Burney et al (136) of eutopic endometrium of women with endometriosis, identified genes associated with inactivation of MAPK signaling cascades such as ERBB receptor feedback inhibitor 1 (ERRFI1, also known as MIG-6), and regulators of G protein signaling 1 (RGS1), which is an activator of GTPases that rapidly turns off G-protein coupled receptor signaling pathways, were decreased in endometriosis. Velarde et al (131) showed that increased ERK1/2 activity in the eutopic endometrial stromal cells from women with endometriosis inhibited cAMP-mediated down-regulation of cyclin D1. FOXO1 an important mediator of decidualization of endometrial stromal cells can be phosphorylated and its function modified by ERK and p38 (137) as well as other kinases such as DYRK1a (138), CK1 (139), and SGK (140).
Summary: Clinical Correlates
While clinical studies support the concept of endometrial receptivity defects endometriosis, these observations need to be based on a physiological mechanism. In this review, we hope that the reader can better understand how those changes noted in the eutopic endometrium of women with infertility and endometriosis biologically impact endometrial receptivity. In vitro Fertilization (IVF) is taking on a larger role for the treatment of infertile couples and is a platform on which endometrial receptivity defects are increasingly being tested (141). Many of the defective pathways described here contribute to infertility and have therapeutic and diagnostic implications. Increasingly, it appears that endometriosis has a negative impact on IVF outcomes, and treatment strategies are evolving to address such defects. Endometrial receptivity defects should remain a relevant and vital part of the workup of couples with infertility.
Conclusions
Strong evidence supports the concept that endometrial defects exist in women with endometriosis. The inflammatory nature of this disease, accompanied by excess estrogen action that leads to a constellation of changes in the eutopic endometrium that interferes with normal embryo implantation. Signaling pathways associated with proliferation and cell survival are activated in endometriosis, while anti-proliferative progesterone pathways are being turned off. Progesterone resistance results in inadequate antagonism of estrogen action, increased inflammation, inadequate differentiation of the stroma and remodeling of the endometrium, all of which can lead to an non-receptive endometrium for embryo implantation. Inflammation appears to be central to these defects. For these reasons, it seems clear that the eutopic endometrium is a primary barrier to implantation in women with active endometriosis.
Figure 2.
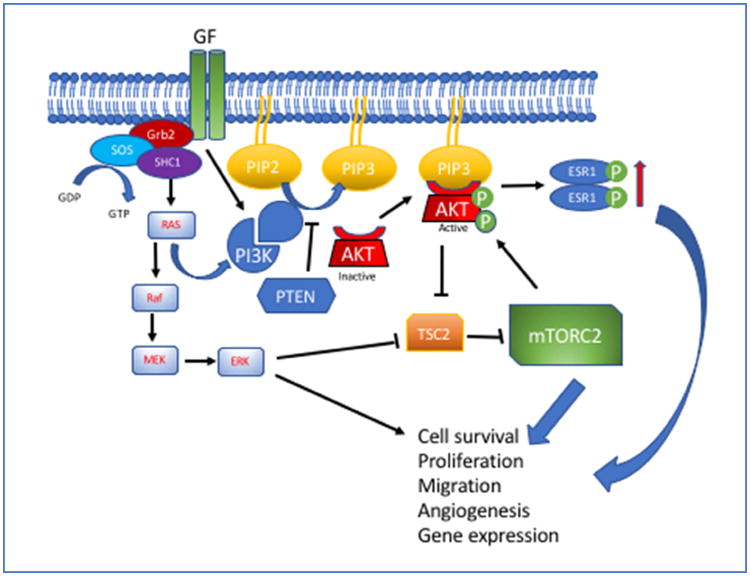
The PI3K/AKT pathway is triggered when receptor tyrosine kinases are activated by ligand binding (GF) subsequently activating PI3K and adapter proteins (Grb2, SOS and SHC1). PI3K converts intracellular PtdIns-4,5-P2 (PIP2) to PtdIns-3,4,5-P3 (PIP3). In the absence of PTEN, which antagonizes PI3K, PIP3 activates AKT as a primary kinase downstream of PI3K. AKT moves to the plasma membrane, is phosphorylated and activated by mTORC2. PTEN antagonizes PI3K activity by dephosphorylating PIP3, leading to its conversion back to PIP2. Kras also contributes to PI3K activation and triggers generation of ERK pathway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, et al. Embryo implantation. Developmental biology. 2000;223:217–37. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 2.Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Reviews in endocrine & metabolic disorders. 2012;13:277–88. doi: 10.1007/s11154-012-9223-7. [DOI] [PubMed] [Google Scholar]
- 3.Critchley HOD, Jones RL, Lea RG, Drudy TA, Kelly RW, Williams ARW, et al. Role of inflammatory mediators in human endometrium during progesterone withdrawal and early pregnancy. The Journal of clinical endocrinology and metabolism. 1999;84:240. doi: 10.1210/jcem.84.1.5380. [DOI] [PubMed] [Google Scholar]
- 4.Lessey BA, Young SL. Homeostasis imbalance in the endometrium of women with implantation defects: the role of estrogen and progesterone. Seminars in reproductive medicine. 2014;32:365–75. doi: 10.1055/s-0034-1376355. [DOI] [PubMed] [Google Scholar]
- 5.Lessey BA, Lebovic DI, Taylor RN. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Seminars in reproductive medicine. 2013;31:109–24. doi: 10.1055/s-0032-1333476. [DOI] [PubMed] [Google Scholar]
- 6.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nature reviews Endocrinology. 2014;10:261–75. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 7.Pfeifer S, Fritz MA, Goldberg J, McClure R, Lobo R, Thomas M, et al. Endometriosis and infertility: a committee opinion. Fertility and sterility. 2012;98:591–8. doi: 10.1016/j.fertnstert.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod. 2016 doi: 10.1093/humrep/dew085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hughes EG, Fedorkow DM, Collins JA. A quantitative overview of controlled trials in endometriosis-associated infertility. Fertility and sterility. 1993;59:963. [PubMed] [Google Scholar]
- 10.Daya S. Infertility and Reproductive Medicine Clinics of North America. Vol. 7. Philadelphia: W. B. Saunders Company; 1996. Endometriosis and Spontaneous Abortion; pp. 759–73. [Google Scholar]
- 11.Vercammen EE, D'Hooghe TM. Endometriosis and recurrent pregnancy loss. Seminars in reproductive medicine. 2000;18:363–8. doi: 10.1055/s-2000-13726. [DOI] [PubMed] [Google Scholar]
- 12.Fox C, Morin S, Jeong JW, Scott RT, Jr, Lessey BA. Local and systemic factors and implantation: what is the evidence? Fertility and sterility. 2016;105:873–84. doi: 10.1016/j.fertnstert.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD, et al. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod. 2015;30:1069–78. doi: 10.1093/humrep/dev050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Human reproduction update. 2016;22:104–15. doi: 10.1093/humupd/dmv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strathy JH, Molgaard CA, Coulam CB, Melton LJ. Endometriosis and infertility: A laparoscopic study of endometriosis among fertile and infertile women. Fertility and sterility. 1982;38:667–72. doi: 10.1016/s0015-0282(16)46691-4. [DOI] [PubMed] [Google Scholar]
- 16.Verkauf BS. Incidence, symptoms, and signs of endometriosis in fertile and infertile women. JFlaMedAssoc. 1987;74:671–5. [PubMed] [Google Scholar]
- 17.Hammond MG, Jordan S, Sloan CS. Factors affecting pregnancy rates in a donor insemination program using frozen semen. American journal of obstetrics and gynecology. 1986;155:480. doi: 10.1016/0002-9378(86)90258-9. [DOI] [PubMed] [Google Scholar]
- 18.Omland AK, Tanbo T, Dale PO, èbyholm T. Artificial insemination by husband in unexplained infertility compared with infertility associated with peritoneal endometriosis. Hum Reprod. 1998;13:2602. doi: 10.1093/humrep/13.9.2602. [DOI] [PubMed] [Google Scholar]
- 19.Jansen RP. Minimal endometriosis and reduced fecundability: prospective evidence from an artificial insemination by donor program. Fertility and sterility. 1986;46:141–3. doi: 10.1016/s0015-0282(16)49474-4. [DOI] [PubMed] [Google Scholar]
- 20.Hughes EG. The effectiveness of ovulation induction and intrauterine insemination in the treatment of persistent infertility: a meta-analysis. Hum Reprod. 1997;12:1865. doi: 10.1093/humrep/12.9.1865. [DOI] [PubMed] [Google Scholar]
- 21.Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertility and sterility. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 22.Marcoux S, Maheux R, BÇrubÇ S, Langevin M, Graves G, Wrixon W, et al. Laparoscopic surgery in infertile, women with minimal or mild endometriosis. The New England journal of medicine. 1997;337:217–22. doi: 10.1056/NEJM199707243370401. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson TZ, Barlow DH, Koninckx PR, Olive D, Farquhar C. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev. 2002:CD001398. doi: 10.1002/14651858.CD001398. [DOI] [PubMed] [Google Scholar]
- 24.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertility and sterility. 2002;77:1148–55. doi: 10.1016/s0015-0282(02)03112-6. [DOI] [PubMed] [Google Scholar]
- 25.Hamdan M, Omar SZ, Dunselman G, Cheong Y. Influence of Endometriosis on Assisted Reproductive Technology Outcomes: A Systematic Review and Meta-analysis. Obstetrics and gynecology. 2015;125:79–88. doi: 10.1097/AOG.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 26.Sallam HN, Garcia-Velasco JA, Dias S, Arici A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst Rev. 2006:CD004635. doi: 10.1002/14651858.CD004635.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertility and sterility. 2005;84:1574–8. doi: 10.1016/j.fertnstert.2005.02.059. [DOI] [PubMed] [Google Scholar]
- 28.Miller PB, Parnell BA, Bushnell G, Tallman N, Forstein DA, Higdon HL, 3rd, et al. Endometrial receptivity defects during IVF cycles with and without letrozole. Hum Reprod. 2012;27:881–8. doi: 10.1093/humrep/der452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim¢n C, GutiÇrrez A, Vidal A, De los Santos MJ, Tarín JJ, Remohí J, et al. Outcome of patients with endometriosis in assisted reproduction: Results from in-vitro fertilization and oocyte donation. Hum Reprod. 1994;9:725. doi: 10.1093/oxfordjournals.humrep.a138578. [DOI] [PubMed] [Google Scholar]
- 30.Prapas Y, Goudakou M, Matalliotakis I, Kalogeraki A, Matalliotaki C, Panagiotidis Y, et al. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reproductive biomedicine online. 2012;25:543–8. doi: 10.1016/j.rbmo.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Schenken RS, Asch RH. Surgically induction of endometriosis in the rabbit: effects on fertility and concetnration of peritoneal fludi prostaglandins. Fertility and sterility. 1980;34:581–7. doi: 10.1016/s0015-0282(16)45199-x. [DOI] [PubMed] [Google Scholar]
- 32.D'Hooghe TM, Bambra CS, Raeymaekers BM, Riday AM, Suleman MA, Koninckx PR. The cycle pregnancy rate is normal in baboons with stage I endometriosis but decreased in primates with stage II and stage III-IV disease. Fertil Steril. 1996;66:809–13. [PubMed] [Google Scholar]
- 33.Vernon MW. Experimental endometriosis in laboratory animals as a research model. ProgClinBiolRes. 1990;323:49. [PubMed] [Google Scholar]
- 34.Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Seminars in reproductive medicine. 2010;28:75–80. doi: 10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- 35.Hahn DW, Carraher RP, Foldesy RG, McGuire JL. Experimental evidence for failure to implant as a mechanism of infertility associated with endometriosis. American journal of obstetrics and gynecology. 1986;155:1109–13. doi: 10.1016/0002-9378(86)90360-1. [DOI] [PubMed] [Google Scholar]
- 36.Illera MJ, Juan L, Stewart CL, Cullinan E, Ruman J, Lessey BA. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertility and sterility. 2000;74:41–8. doi: 10.1016/s0015-0282(00)00552-5. [DOI] [PubMed] [Google Scholar]
- 37.Braundmeier A, Jackson K, Hastings J, Koehler J, Nowak R, Fazleabas A. Induction of endometriosis alters the peripheral and endometrial regulatory T cell population in the non-human primate. Hum Reprod. 2012;27:1712–22. doi: 10.1093/humrep/des083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Human reproduction update. 2011;17:637–53. doi: 10.1093/humupd/dmr013. [DOI] [PubMed] [Google Scholar]
- 39.Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Seminars in reproductive medicine. 2010;28:51–8. doi: 10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- 40.Lessey BA. Implantation defects in infertile women with endometriosis. Annals of the New York Academy of Sciences. 2002;955:265–80. doi: 10.1111/j.1749-6632.2002.tb02787.x. discussion 93-5, 396-406. [DOI] [PubMed] [Google Scholar]
- 41.Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium Correlation with the normal and abnormal menstrual cycle. The Journal of clinical investigation. 1992;90:188–95. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lessey BA. A Model for Implantation Based on Integrin Expression in the Human Endometrium. 2nd World Conference on Implantation. 1994 Abst. [Google Scholar]
- 43.Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertility and sterility. 1994;62:497–506. [PubMed] [Google Scholar]
- 44.Tabibzadeh S. Patterns of expression of integrin molecules in human endometrium throughout the menstrual cycle. Hum Reprod. 1992;7:876–82. doi: 10.1093/oxfordjournals.humrep.a137753. [DOI] [PubMed] [Google Scholar]
- 45.Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. The Journal of clinical endocrinology and metabolism. 1994;79:643–9. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 46.Lessey BA, Castelbaum AJ, Sawin SW, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertility and sterility. 1995;63:535–42. [PubMed] [Google Scholar]
- 47.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 48.Margarit L, Gonzalez D, Lewis PD, Hopkins L, Davies C, Conlan RS, et al. L-selectin ligands in human endometrium: comparison of fertile and infertile subjects. Hum Reprod. 2009;24:2767–77. doi: 10.1093/humrep/dep247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foulk RA, Zdravkovic T, Genbacev O, Prakobphol A. Expression of L-selectin ligand MECA-79 as a predictive marker of human uterine receptivity. Journal of assisted reproduction and genetics. 2007;24:316–21. doi: 10.1007/s10815-007-9151-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. The Journal of steroid biochemistry and molecular biology. 2002;83:149–55. doi: 10.1016/s0960-0760(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 51.Yoo JY, Shin H, Kim TH, Choi WS, Ferguson SD, Fazleabas AT, et al. CRISPLD2 is a target of progesterone receptor and its expression is decreased in women with endometriosis. PloS one. 2014;9:e100481. doi: 10.1371/journal.pone.0100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Seminars in reproductive medicine. 2010;28:5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- 53.Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertility and sterility. 2005;84:67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- 54.Park JS, Lee JH, Kim M, Chang HJ, Hwang KJ, Chang KH. Endometrium from women with endometriosis shows increased proliferation activity. Fertility and sterility. 2009;92:1246–9. doi: 10.1016/j.fertnstert.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 55.Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reproductive biology and endocrinology : RB&E. 2006;4(1):S9. doi: 10.1186/1477-7827-4-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Large MJ, Demayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Molecular and cellular endocrinology. 2012;358:155–65. doi: 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16:216–29. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecologic and obstetric investigation. 2006;62:139–47. doi: 10.1159/000093121. [DOI] [PubMed] [Google Scholar]
- 59.Ahn SH, Khalaj KL, Young SL, Lessey BA, Koti M, Tayade C. Nanostring gene profiling reveals unique immune-inflammation signatures in endometriosis patients. Fertility and sterility. 2016 doi: 10.1016/j.fertnstert.2016.07.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M, Tayade C. Immune-inflammation gene signatures in endometriosis patients. Fertility and sterility. 2016 doi: 10.1016/j.fertnstert.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn SH, Edwards AK, Singh SS, Young SL, Lessey BA, Tayade C. IL-17A Contributes to the Pathogenesis of Endometriosis by Triggering Proinflammatory Cytokines and Angiogenic Growth Factors. J Immunol. 2015;195:2591–600. doi: 10.4049/jimmunol.1501138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khoufache K, Michaud N, Harir N, Kibangou Bondza P, Akoum A. Anomalies in the inflammatory response in endometriosis and possible consequences: a review. Minerva endocrinologica. 2012;37:75–92. [PubMed] [Google Scholar]
- 63.Monsanto SP, Edwards AK, Zhou J, Nagarkatti P, Nagarkatti M, Young SL, et al. Surgical removal of endometriotic lesions alters local and systemic proinflammatory cytokines in endometriosis patients. Fertil Steril. 2016;105:968–77 e5. doi: 10.1016/j.fertnstert.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertility and sterility. 2006;85:1307–18. doi: 10.1016/j.fertnstert.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 65.Kitawaki J, Noguchi T, Amatsu T, Maeda K, Tsukamoto K, Yamamoto T, et al. Expression or aromatase cytochrome P450 protein and messenger ribonucleic acid in human endometriotic and adenomyotic tissues but not in normal endometrium. Biology of reproduction. 1997;57:514. doi: 10.1095/biolreprod57.3.514. [DOI] [PubMed] [Google Scholar]
- 66.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrine reviews. 1994;15:342–55. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 67.Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Zhou J, et al. Role of aromatase in endometrial disease. The Journal of steroid biochemistry and molecular biology. 2001;79:19–25. doi: 10.1016/s0960-0760(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 68.Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149:1190–204. doi: 10.1210/en.2007-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brosens J, Verhoeven H, Campo R, Gianaroli L, Gordts S, Hazekamp J, et al. High endometrial aromatase P450 mRNA expression is associated with poor IVF outcome. Hum Reprod. 2004;19:352–6. doi: 10.1093/humrep/deh075. [DOI] [PubMed] [Google Scholar]
- 70.Somkuti SG, Yuan L, Fritz MA, Lessey BA. Epidermal growth factor and sex steroids dynamically regulate a marker of endometrial receptivity in Ishikawa cells. The Journal of clinical endocrinology and metabolism. 1997;82:2192–7. doi: 10.1210/jcem.82.7.4102. [DOI] [PubMed] [Google Scholar]
- 71.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–9. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 72.Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:3115–20. doi: 10.1073/pnas.93.7.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franasiak JM, Holoch KJ, Yuan L, Schammel DP, Young SL, Lessey BA. Prospective assessment of midsecretory endometrial leukemia inhibitor factor expression versus alphanubeta3 testing in women with unexplained infertility. Fertility and sterility. 2014;101:1724–31. doi: 10.1016/j.fertnstert.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Du H, Taylor HS. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harbor perspectives in medicine. 2015;6:a023002. doi: 10.1101/cshperspect.a023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Celik O, Unlu C, Otlu B, Celik N, Caliskan E. Laparoscopic endometrioma resection increases peri-implantation endometrial HOXA-10 and HOXA-11 mRNA expression. Fertility and sterility. 2015;104:356–65. doi: 10.1016/j.fertnstert.2015.04.041. [DOI] [PubMed] [Google Scholar]
- 76.Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. American journal of obstetrics and gynecology. 2005;193:371–80. doi: 10.1016/j.ajog.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 77.Evans J, Salamonsen LA. Decidualized Human Endometrial Stromal Cells Are Sensors of Hormone Withdrawal in the Menstrual Inflammatory Cascade. Biology of reproduction. 2013 doi: 10.1095/biolreprod.113.108175. [DOI] [PubMed] [Google Scholar]
- 78.Osteen KG, Bruner-Tran KL, Keller NR, Eisenberg E. Progesterone-mediated endometrial maturation limits matrix metalloproteinase (MMP) expression in an inflammatory-like environment: a regulatory system altered in endometriosis. Annals of the New York Academy of Sciences. 2002;955:37–47. doi: 10.1111/j.1749-6632.2002.tb02764.x. discussion 86-8, 396-406. [DOI] [PubMed] [Google Scholar]
- 79.Li X, Large MJ, Creighton CJ, Lanz RB, Jeong JW, Young SL, et al. COUP-TFII Regulates Human Endometrial Stromal Genes Involved in Inflammation. Mol Endocrinol. 2013;27:2041–54. doi: 10.1210/me.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalkhoven E, Wissink S, van der Saag PT, van der Burg B. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. The Journal of biological chemistry. 1996;271:6217–24. doi: 10.1074/jbc.271.11.6217. [DOI] [PubMed] [Google Scholar]
- 81.Chandler RL, Damrauer JS, Raab JR, Schisler JC, Wilkerson MD, Didion JP, et al. Coexistent ARID1A-PIK3CA mutations promote ovarian clear-cell tumorigenesis through pro-tumorigenic inflammatory cytokine signalling. Nature communications. 2015;6:6118. doi: 10.1038/ncomms7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim M, Lu F, Zhang Y. Loss of HDAC-Mediated Repression and Gain of NF-kappaB Activation Underlie Cytokine Induction in ARID1A- and PIK3CA-Mutation-Driven Ovarian Cancer. Cell reports. 2016;17:275–88. doi: 10.1016/j.celrep.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim TH, Yoo JY, Wang Z, Lydon JP, Khatri S, Hawkins SM, et al. ARID1A Is Essential for Endometrial Function during Early Pregnancy. PLoS genetics. 2015;11:e1005537. doi: 10.1371/journal.pgen.1005537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoo JY, Jeong JW, Fazleabas AT, Tayade C, Young SL, Lessey BA. Protein Inhibitor of Activated STAT3 (PIAS3) Is Down-Regulated in Eutopic Endometrium of Women with Endometriosis. Biology of reproduction. 2016 doi: 10.1095/biolreprod.115.137158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dechaud H, Maudelonde T, Daures JP, Rossi JF, Hedon B. Evaluation of endometrial inflammation by quantification of macrophages, T lymphocytes, and interleukin-1 and -6 in human endometrium. Journal of assisted reproduction and genetics. 1998;15:612–8. doi: 10.1023/A:1020337528607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Othman Eel D, Hornung D, Salem HT, Khalifa EA, El-Metwally TH, Al-Hendy A. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. European journal of obstetrics, gynecology, and reproductive biology. 2008;137:240–6. doi: 10.1016/j.ejogrb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Kalu E, Sumar N, Giannopoulos T, Patel P, Croucher C, Sherriff E, et al. Cytokine profiles in serum and peritoneal fluid from infertile women with and without endometriosis. J Obstet Gynaecol Res. 2007;33:490–5. doi: 10.1111/j.1447-0756.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 88.Ulukus EC, Ulukus M, Seval Y, Zheng W, Arici A. Expression of interleukin-8 and monocyte chemotactic protein-1 in adenomyosis. Hum Reprod. 2005;20:2958–63. doi: 10.1093/humrep/dei154. [DOI] [PubMed] [Google Scholar]
- 89.Akoum A, Kong J, Metz C, Beaumont MC. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil Steril. 2002;77:989–94. doi: 10.1016/s0015-0282(02)03082-0. [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Velasco JA, Seli E, Arici A. Regulation of monocyte chemotactic protein-1 expression in human endometrial stromal cells by integrin-dependent cell adhesion. Biol Reprod. 1999;61:548–52. doi: 10.1095/biolreprod61.2.548. [DOI] [PubMed] [Google Scholar]
- 91.Miller JE, Ahn SH, Monsanto SP, Khalaj K, Koti M, Tayade C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget. 2017;8:7138–47. doi: 10.18632/oncotarget.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hirata T, Osuga Y, Takamura M, Saito A, Hasegawa A, Koga K, et al. Interleukin-17F increases the secretion of interleukin-8 and the expression of cyclooxygenase 2 in endometriosis. Fertility and sterility. 2011;96:113–7. doi: 10.1016/j.fertnstert.2011.04.060. [DOI] [PubMed] [Google Scholar]
- 93.Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16:561–6. doi: 10.1093/humrep/16.3.561. [DOI] [PubMed] [Google Scholar]
- 94.Wu MH, Lu CW, Chuang PC, Tsai SJ. Prostaglandin E2: the master of endometriosis? Exp Biol Med (Maywood) 2010;235:668–77. doi: 10.1258/ebm.2010.009321. [DOI] [PubMed] [Google Scholar]
- 95.Wu MH, Shoji Y, Chuang PC, Tsai SJ. Endometriosis: disease pathophysiology and the role of prostaglandins. Expert reviews in molecular medicine. 2007;9:1–20. doi: 10.1017/S146239940700021X. [DOI] [PubMed] [Google Scholar]
- 96.Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, et al. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27:2107–16. doi: 10.1093/humrep/des132. [DOI] [PubMed] [Google Scholar]
- 97.Ponce C, Torres M, Galleguillos C, Sovino H, Boric MA, Fuentes A, et al. Nuclear factor kappaB pathway and interleukin-6 are affected in eutopic endometrium of women with endometriosis. Reproduction. 2009;137:727–37. doi: 10.1530/REP-08-0407. [DOI] [PubMed] [Google Scholar]
- 98.Boutten A, Dehoux M, Edelman P, Seta N, Menard A, Madelenat P, et al. IL6 and acute phase plasma proteins in peritoneal fluid of women with endometriosis. ClinChimActa. 1992;210:187. doi: 10.1016/0009-8981(92)90204-4. [DOI] [PubMed] [Google Scholar]
- 99.Evans-Hoeker E, Lessey BA, Jeong JW, Savaris RF, Palomino WA, Yuan L, et al. Endometrial BCL6 Overexpression in Eutopic Endometrium of Women With Endometriosis. Reprod Sci. 2016;23:1234–41. doi: 10.1177/1933719116649711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bruner-Tran KL, Herington JL, Duleba AJ, Taylor HS, Osteen KG. Medical management of endometriosis: emerging evidence linking inflammation to disease pathophysiology. Minerva ginecologica. 2013;65:199–213. [PMC free article] [PubMed] [Google Scholar]
- 101.Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 102.Yin P, Lin Z, Cheng YH, Marsh EE, Utsunomiya H, Ishikawa H, et al. Progesterone receptor regulates Bcl-2 gene expression through direct binding to its promoter region in uterine leiomyoma cells. J Clin Endocrinol Metab. 2007;92:4459–66. doi: 10.1210/jc.2007-0725. [DOI] [PubMed] [Google Scholar]
- 103.Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab. 2012;97:E35–43. doi: 10.1210/jc.2011-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 105.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 106.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 107.Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83:529–37. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 108.Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017 doi: 10.1111/aogs.13156. [DOI] [PubMed] [Google Scholar]
- 109.Patel B, Elguero S, Thakore S, Dahoud W, Bedaiwy M, Mesiano S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Human reproduction update. 2015;21:155–73. doi: 10.1093/humupd/dmu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertility and sterility. 2006;85:564–72. doi: 10.1016/j.fertnstert.2005.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Molecular human reproduction. 2007;13:323–32. doi: 10.1093/molehr/gam005. [DOI] [PubMed] [Google Scholar]
- 112.Minici F, Tiberi F, Tropea A, Orlando M, Gangale MF, Romani F, et al. Endometriosis and human infertility: a new investigation into the role of eutopic endometrium. Hum Reprod. 2008;23:530–7. doi: 10.1093/humrep/dem399. [DOI] [PubMed] [Google Scholar]
- 113.Lee CH, Kim TH, Lee JH, Oh SJ, Yoo JY, Kwon HS, et al. Extracellular signal-regulated kinase 1/2 signaling pathway is required for endometrial decidualization in mice and human. PloS one. 2013;8:e75282. doi: 10.1371/journal.pone.0075282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ahn JI, Yoo JY, Kim TH, Kim YI, Ferguson SD, Fazleabas AT, et al. cAMP-Response Element-Binding 3-Like Protein 1 (CREB3L1) is Required for Decidualization and its Expression is Decreased in Women with Endometriosis. Current molecular medicine. 2016;16:276–87. doi: 10.2174/1566524016666160225153659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine. 1997;6:301–7. doi: 10.1007/BF02820507. [DOI] [PubMed] [Google Scholar]
- 116.Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–20. doi: 10.1210/endo.140.10.7070. [DOI] [PubMed] [Google Scholar]
- 117.Telgmann R, Maronde E, Tasken K, Gellersen B. Activated protein kinase A is required for differentiation-dependent transcription of the decidual prolactin gene in human endometrial stromal cells. Endocrinology. 1997;138:929–37. doi: 10.1210/endo.138.3.5004. [DOI] [PubMed] [Google Scholar]
- 118.Tierney EP, Tulac S, Huang ST, Giudice LC. Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiological genomics. 2003;16:47–66. doi: 10.1152/physiolgenomics.00066.2003. [DOI] [PubMed] [Google Scholar]
- 119.Takano M, Lu Z, Goto T, Fusi L, Higham J, Francis J, et al. Transcriptional cross talk between the forkhead transcription factor forkhead box O1A and the progesterone receptor coordinates cell cycle regulation and differentiation in human endometrial stromal cells. Mol Endocrinol. 2007;21:2334–49. doi: 10.1210/me.2007-0058. [DOI] [PubMed] [Google Scholar]
- 120.Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic Enzyme and Key Decidualization Marker Dysregulation in Endometrial Stromal Cells from Women with Versus Without Endometriosis. Biology of reproduction. 2008 doi: 10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoshino O, Osuga Y, Hirota Y, Koga K, Yano T, Tsutsumi O, et al. Akt as a possible intracellular mediator for decidualization in human endometrial stromal cells. Molecular human reproduction. 2003;9:265–9. doi: 10.1093/molehr/gag035. [DOI] [PubMed] [Google Scholar]
- 122.Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor-binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biology of reproduction. 2005;73:833–9. doi: 10.1095/biolreprod.105.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. The Journal of biological chemistry. 2001;276:9817–24. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 124.Sanchez M, Sauve K, Picard N, Tremblay A. The hormonal response of estrogen receptor beta is decreased by the phosphatidylinositol 3-kinase/Akt pathway via a phosphorylation-dependent release of CREB-binding protein. The Journal of biological chemistry. 2007;282:4830–40. doi: 10.1074/jbc.M607908200. [DOI] [PubMed] [Google Scholar]
- 125.Cui X, Zhang P, Deng W, Oesterreich S, Lu Y, Mills GB, et al. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003;17:575–88. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]
- 126.Pant A, Lee, Lu Z, Rueda BR, Schink J, Kim JJ. Inhibition of AKT with the orally active allosteric AKT inhibitor, MK-2206, sensitizes endometrial cancer cells to progestin. PloS one. 2012;7:e41593. doi: 10.1371/journal.pone.0041593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eaton JL, Unno K, Caraveo M, Lu Z, Kim JJ. Increased AKT or MEK1/2 activity influences progesterone receptor levels and localization in endometriosis. The Journal of clinical endocrinology and metabolism. 2013;98:E1871–9. doi: 10.1210/jc.2013-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee, Maniar K, Lydon JP, Kim JJ. Akt regulates progesterone receptor B-dependent transcription and angiogenesis in endometrial cancer cells. Oncogene. 2016;35:5191–201. doi: 10.1038/onc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Su RW, Strug MR, Joshi NR, Jeong JW, Miele L, Lessey BA, et al. Decreased Notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. The Journal of clinical endocrinology and metabolism. 2015;100:E433–42. doi: 10.1210/jc.2014-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Marcel N, Perumalsamy LR, Shukla SK, Sarin A. The lysine deacetylase Sirtuin 1 modulates the localization and function of the Notch1 receptor in regulatory T cells. Science signaling. 2017:10. doi: 10.1126/scisignal.aah4679. [DOI] [PubMed] [Google Scholar]
- 131.Velarde MC, Aghajanova L, Nezhat CR, Giudice LC. Increased mitogen-activated protein kinase kinase/extracellularly regulated kinase activity in human endometrial stromal fibroblasts of women with endometriosis reduces 3′,5′-cyclic adenosine 5′-monophosphate inhibition of cyclin D1. Endocrinology. 2009;150:4701–12. doi: 10.1210/en.2009-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsuzaki S, Canis M, Vaurs-Barriere C, Boespflug-Tanguy O, Dastugue B, Mage G. DNA microarray analysis of gene expression in eutopic endometrium from patients with deep endometriosis using laser capture microdissection. Fertility and sterility. 2005;84(2):1180–90. doi: 10.1016/j.fertnstert.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 133.Matsuzaki S, Canis M, Pouly JL, Botchorishvili R, Dechelotte PJ, Mage G. Differential expression of genes in eutopic and ectopic endometrium from patients with ovarian endometriosis. Fertility and sterility. 2006;86:548–53. doi: 10.1016/j.fertnstert.2006.02.093. [DOI] [PubMed] [Google Scholar]
- 134.Uimari O, Rahmioglu N, Nyholt DR, Vincent K, Missmer SA, Becker C, et al. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum Reprod. 2017:1–14. doi: 10.1093/humrep/dex024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wu Y, Kajdacsy-Balla A, Strawn E, Basir Z, Halverson G, Jailwala P, et al. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology. 2006;147:232–46. doi: 10.1210/en.2005-0426. [DOI] [PubMed] [Google Scholar]
- 136.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 137.Asada S, Daitoku H, Matsuzaki H, Saito T, Sudo T, Mukai H, et al. Mitogen-activated protein kinases, Erk and p38, phosphorylate and regulate Foxo1. Cell Signal. 2007;19:519–27. doi: 10.1016/j.cellsig.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 138.Woods YL, Rena G, Morrice N, Barthel A, Becker W, Guo S, et al. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem J. 2001;355:597–607. doi: 10.1042/bj3550597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rena G, Bain J, Elliott M, Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60–5. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–65. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Opoien HK, Fedorcsak P, Omland AK, Abyholm T, Bjercke S, Ertzeid G, et al. In vitro fertilization is a successful treatment in endometriosis-associated infertility. Fertility and sterility. 2012;97:912–8. doi: 10.1016/j.fertnstert.2012.01.112. [DOI] [PubMed] [Google Scholar]
- 142.Nakagawa K, Ohgi S, Horikawa T, Kojima R, Ito M, Saito H. Laparoscopy should be strongly considered for women with unexplained infertility. The journal of obstetrics and gynaecology research. 2007;33:665–70. doi: 10.1111/j.1447-0756.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 143.Tsuji I, Ami K, Miyazaki A, Hujinami N, Hoshiai H. Benefit of diagnostic laparoscopy for patients with unexplained infertility and normal hysterosalpingography findings. The Tohoku journal of experimental medicine. 2009;219:39–42. doi: 10.1620/tjem.219.39. [DOI] [PubMed] [Google Scholar]
- 144.Sampson JA. Benign and malignant endometrial implants in the peritoneal cavity and their relation to certain ovarian tumors. SurgGynecolObstet. 1924;38:287–311. [Google Scholar]
- 145.Jones GS. Some newer aspects of management of infertility. JAMA : the journal of the American Medical Association. 1949;141:1123–9. doi: 10.1001/jama.1949.02910160013004. [DOI] [PubMed] [Google Scholar]
- 146.Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertility and sterility. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 147.Psychoyos A, Mandon P. Etude de la surface de l'epithelium uterin au microscope electronique a balayage. CRHebdSeances AcadSciParis. 1971;272:2723–5. [PubMed] [Google Scholar]
- 148.Rutanen EM, Koistinen R, Seppala M, Julkunen M, Suikkari AM, Huhtala ML. Progesterone-associated proteins PP12 and PP14 in the human endometrium. JSteroidBiochem. 1987;27:25. doi: 10.1016/0022-4731(87)90290-1. [DOI] [PubMed] [Google Scholar]
- 149.Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. The Journal of clinical endocrinology and metabolism. 1988;67:334–40. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- 150.Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. ProcNatlAcad SciUS A. 1991;88:11408–12. doi: 10.1073/pnas.88.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lessey BA. Integrin cell adhesion molecules: Immunohistochemical evaluation of endometrial integrin receptor subunits and extracellular matrix components in endometriosis. AmFertSocAnnMtg. 1992;O-19:S8. [Google Scholar]
- 152.Surveyor GA, Gendler SJ, Pemberton L, Spicer AP, Carson DD. Differential expression of Muc-1 at the apical cell surface of mouse uterine epithelial cells. FASEB J. 1993;7:1151a. [Google Scholar]
- 153.Coutifaris C, Lessey BA. SocGynecolInvest. Toronto: 1993. Co-expression of endometrial osteopontin and its receptor, the av•3 integrin, define the window of human receptivity to embryo implantation; p. S135. [Google Scholar]
- 154.Apparao KB, Murray MJ, Fritz MA, Meyer WR, Chambers AF, Truong PR, et al. Osteopontin and its receptor alphavbeta(3) integrin are coexpressed in the human endometrium during the menstrual cycle but regulated differentially. The Journal of clinical endocrinology and metabolism. 2001;86:4991–5000. doi: 10.1210/jcem.86.10.7906. [DOI] [PubMed] [Google Scholar]
- 155.Simon C, Piquette GN, Frances A, Polan ML. Localization of interleukin-1 type I receptor and interleukin-1 beta in human endometrium throughout the menstrual cycle. The Journal of clinical endocrinology and metabolism. 1993;77:549–55. doi: 10.1210/jcem.77.2.8345061. [DOI] [PubMed] [Google Scholar]
- 156.Simon C, Piquette GN, Frances A, El-Danasouri I, Irwin JC, Polan ML. The effect of interleukin-1· (IL-1·) on the regulation of IL-1 receptor type I messenger ribonucleic acid and protein levels in cultured human endometrial stromal and glandular cells. The Journal of clinical endocrinology and metabolism. 1994;78:1-. doi: 10.1210/jcem.78.3.8126141. [DOI] [PubMed] [Google Scholar]
- 157.Kliman HJ, Feinberg RF, Schwartz LB, Feinman MA, Lavi E, Meaddough EL. A mucin-like glycoprotein identified by MAG (mouse ascites Golgi) antibodies: Menstrual cycle-dependent localization in human endometrium. The American journal of pathology. 1995;146:166. [PMC free article] [PubMed] [Google Scholar]
- 158.Yoo HJ, Barlow DH, Mardon HJ. Temporal and spatial regulation of expression of heparin-binding epidermal growth factor-like growth factor in the human endometrium: a possible role in blastocyst implantation. Developmental genetics. 1997;21:102–8. doi: 10.1002/(SICI)1520-6408(1997)21:1<102::AID-DVG12>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 159.Taylor HS, Arici A, Olive D, Igarashi P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. The Journal of clinical investigation. 1998;101:1379–84. doi: 10.1172/JCI1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Carson DD. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Molecular human reproduction. 2002;8:871–9. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- 161.Dominguez F, Remohi J, Pellicer A, Simon C. Human endometrial receptivity: a genomic approach. Reproductive biomedicine online. 2003;6:332–8. doi: 10.1016/s1472-6483(10)61853-6. [DOI] [PubMed] [Google Scholar]
- 162.Kao LC, Yang J, Lessey BA, Giudice LC. Microarray expression profiling reveals candidate genes for human uterine receptivity. Fertility and sterility. 2001;76:S59. [Google Scholar]
- 163.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299:405–8. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]
- 164.Fassbender A, Vodolazkaia A, Saunders P, Lebovic D, Waelkens E, De Moor B, et al. Biomarkers of endometriosis. Fertility and sterility. 2013;99:1135–45. doi: 10.1016/j.fertnstert.2013.01.097. [DOI] [PubMed] [Google Scholar]
- 165.Scotchie JG, Fritz MA, Mocanu M, Lessey BA, Young SL. Proteomic analysis of the luteal endometrial secretome. Reprod Sci. 2009;16:883–93. doi: 10.1177/1933719109337165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Diaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martinez-Conejero JA, Alama P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertility and sterility. 2013;99:508–17. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 167.Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, et al. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol. 2009;23:265–75. doi: 10.1210/me.2008-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]


