Abstract
The first total-body positron emission tomography (TB-PET) scanner represents a radical change for experimental medicine and diagnostic health care.
Current PET: Power and Limitations
Positron emission tomography (PET) is unrivaled as a highly specific and sensitive means for tomographic imaging of molecular interactions and pathways in humans, offering great utility for translational medicine. PET involves the administration of a specific radiolabeled molecule (radiotracer) to a patient and scanning of the body to measure photon emission after positron decay to localize and quantify the radiotracer. The principles underlying PET allow the study of many biological processes (1), and PET has been used extensively for research and clinical applications, particularly in the brain and heart and for various cancers. However, positron detection with current scanners is an inherently inefficient process, which limits the use of PET. Only ∼1% of the photons emitted from a human subject injected with radiotracer are actually detected because the axial field of view (FOV) and hence the length of the body that can be imaged at any one time is typically less than 25 cm (Fig. 1A). This limitation also means that any “full-body” scan must be constructed from serial scans. Moreover, the enormously rich information that may be obtained by measuring temporal changes in radiotracer distribution can only be accurately captured from one part of the body at a time. As a result, the full potential of PET as a translational research tool has not yet been realized.
Fig. 1. Seeing into our future.
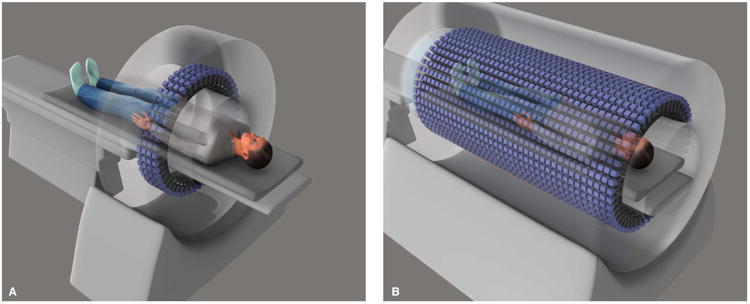
Illustration depicting (A) a conventional PET scanner and (B) total-body PET (TB-PET) scanner. An x-ray computed tomography (CT) scanner will be mounted on the front of the TB-PET gantry for anatomical coregistration to ensure optimal integration of anatomical imaging with molecular imaging.
There is a simple but radical solution to realize the full potential of PET—extending the FOV to cover the entire length of the body (Fig. 1B). In TB-PET, the vast majority of the emitted photons could be captured. This step change in technological evolution would mean simultaneous coverage of all the tissues and organs in the body, with an overall >40-fold gain in effective sensitivity and a >6-fold increase in signal-to-noise ratio compared with whole-body imaging on current PET scanners (2), offering profound advantages for a broad range of PET applications.
For example, the large increase in sensitivity would support imaging of “lower-density” systemic disease, such as cancer micrometastases, inflammation, and infection. Pharmacokinetic studies using radiolabeled drugs could be carried out over significantly longer time courses. On the basis of current levels of administered radioactivity, the radiotracer dynamic range would be extended from 3 to 4 to 7 to 9 radioactive half-lives—an important improvement when imaging with small-molecule radiotracers labeled with short-lived radionuclides such as carbon-11 (half-life, 20.1 min). Increased delay between radiotracer administration and imaging would also be possible, enabling enhanced clearance of nonspecific signal and hence higher contrast for detecting specific signal—an important improvement for studies that use slow-clearing macromolecule radiotracers, for example antibodies labeled with longer-life radionuclides such as zirconium-89 (half-life, 78.4 hours). Alternatively, the radiation doses needed for whole-body imaging could be significantly reduced. This would facilitate microdose radiotracer studies, allow multiple repeat scans in the same subject for longitudinal and interventional studies, and support expansion of PET investigations to populations such as children and pregnant women, in which radiation dose concerns have impeded its use.
The ability to simultaneously record dynamic data from all tissues of the body would be a major advance. Kinetic data analysis would be greatly enhanced by the increased statistical precision in computing quantitative rate constants of biomarker exchange, because currently, high statistical noise often hampers parametric component analyses. An additional pragmatic advantage would be the ability to derive high-quality tracer input function data from major arterial blood vessels in all scans, avoiding the need for patient arterial cannulation to gain dynamic input data from serial blood samples. Furthermore, the combined effects of an unprecedented increase in regional kinetic information on whole-body function, reduced noise of the underlying data, and application of novel tomographic reconstruction methodologies would result in the production of high-precision, high-resolution total-body parametric data. This would create an innovative new “multisystem” biology approach, enabling studies of systemic disease and inter-organ interactions.
The First TB-PET Scanner
Until recently, the promise of TB-PET and its projected applications remained a vision of the EXPLORER consortium (http://explorer.ucdavis.edu) and other investigators. The technical challenges of TB-PET have been extensively studied, and resources from the National Institutes of Health (NIH) facilitated detailed feasibility and performance simulation studies, which provided confidence and credibility of the expected advanced capabilities (2–4). However, the challenge was funding the construction of the first prototype machine, an expensive novel device, without having the initial proof-of-concept. This impasse was successfully overcome in September 2015 through funding from the NIH Transformative Research Award program, which recognizes high-risk, high-reward, paradigm-shifting innovative research (5). After a construction period of around 2 years, we anticipate that the world's first TB-PET scanner will be operational in 2018 as a research resource.
Research and Health Care Applications
The projected applications of TB-PET in translational medicine are many and varied. Here, we outline research areas in which TB-PET is perceived to be most impactful, in order of their likely introduction.
Oncology
A salient experimental area in which to exploit TB-PET's increased sensitivity is in the detection of small, low-density tumor deposits (micro-metastases). Currently, magnetic resonance imaging or combined 18-fluorine–labeled fluorodeoxyglucose (18F-FDG) PET/CT is used for clinical examinations and can detect individual subclinical localized metastatic deposits down to ∼5 mm3. However, smaller cancer deposits that may be present throughout the body are occult because of imaging sensitivity and specificity. For example, smaller or less-dense focal micrometastases or multiple microscopic tumor foci that have diffused to the surrounding microenvironment of a primary organ or disseminated at low density within a secondary organ are currently only detected with invasive or ex vivo histopathological techniques. Because some patients will undoubtedly have undetected micrometastatic disease, all patients with metastasis risk indicators are treated with toxic and expensive chemotherapy for potential meta-static disease, after which, it is not possible to determine treatment efficacy. The development of noninvasive imaging methods to detect micrometastases could thus serve several critical unmet needs in oncology: to enable staging of cancer patients, to support the rational prescription of neoadjuvant or adjuvant chemotherapy in individual patients, and to provide a means for empirical assessment of therapeutic response (6).
Parametric PET methods generated from dynamic acquisitions can provide additional valuable information for micrometastases detection, but the statistical noise from current PET is typically too high for the successful derivation of multiple individual kinetic parameters. In combination with parametric kinetic modeling methods, TB-PET is likely to substantially advance the capabilities of FDG-PET for micrometastases detection, with the added advantages of permitting delayed scanning to increase contrast and allow dynamic data acquisition (6). Because of spatial resolution limitations (partial volume effects), small micrometastases are unlikely to be individually visualized, even with TB-PET. Thus, the goal will be to detect the presence of micrometastatic deposits within a tissue region of interest by using dynamic imaging and quantitative modeling to measure the changes in the underlying tissue kinetics produced by the micrometastases. Initially, 18F-FDG methodologies could be explored to seek proof of principle. Subsequently, more specific imaging cancer biomarkers could be translated to highly sensitive TB-PET micrometastases detection as they emerge from ongoing preclinical studies—for example, radiolabeled nanobodies for breast cancer subtype detection (7). In turn, TB-PET would support the translational pipeline of new radiotracers. Following on from the study of micrometastases would be corresponding opportunities for similar investigations in infectious diseases (for example, to detect occult HIV, tuberculosis, and parasite deposits) and to detect low-grade inflammation, including atheromatous plaques.
Drug development
An obvious area for early exploitation of TB-PET is in the development of new pharmaceuticals (8, 9). For example, high-sensitivity total-body pharmacodynamic (PD) investigations could be carried out during phase 1 and 2 clinical trials. Through direct radiolabeling of drug compounds, early-phase total-body bio-distribution and pharmacokinetic investigations could be undertaken, either at microdoses or for significantly longer time courses than is currently possible. Similarly, PET-based functional studies to examine the PD effects of toxins could be conducted, and the biodistribution of potential toxic compounds, with an appropriate radio-label, could be assessed.
Cell tracking and function
Total-body high-sensitivity imaging could help support the development of stem cell therapies and cancer immunotherapies. For example, labeled stem cells or T cells could be administered to study their biodistribution over periods of days to weeks, and the future prospect of assessing the functional presence of therapeutic cell types could be supported via imaging of cytokine secretion using specific radiolabeled affibodies.
Maternal-fetal and pediatric studies
The high-sensitivity of TB-PET would enable ultra-low (near-background) radiation dose scanning, which could support the much-needed translation of basic research in maternal-fetal medicine to human in vivo investigations. For example, noninvasive and specific measurements of placental transport, fetal metabolism, inflammation, and infection could be carried out to advance knowledge of fetal distress and growth retardation (10). Similarly, the prospect of ultra-low radiation would also support expansion of PET investigations to pediatric disease, such as for in vivo brain-body studies that could advance our knowledge about developmental disorders and childhood obesity.
Multisystem disease
The whole-body imaging capabilities of TB-PET could be capitalized on in brain-body or multisystem diseases—such as brain-gut conditions, metabolic syndrome and endocrine homeostasis, placebo effects, anxiety, depression, psychosis, and neurodegenerative diseases. For these complex conditions, the simultaneous study of multiple body organs would advance our understanding of pathophysiology and spur translation of basic research.
Health care
For existing health care applications, TB-PET could have the immediate impact of better image quality, reduced radiation dose, reduced scanning time, or a combination of these. A compelling area for early clinical application of TB-PET is cancer imaging—the current seminal health care application of PET. For example, a total-body FDG-PET/CT examination could be completed in just 15 to 30 s— 40 times faster than current methods—thus enabling single breath-hold scanning and testing of many more patients per machine per day. However, the initial high projected cost of TB-PET (about five to six times that of a conventional scanner) will likely preclude installation in most health care centers, until either cost is reduced through technological developments or significant clinical advantage is proven. Nonetheless, for some hospitals around the globe where patient demand is high or space restrictions prevent multiple scanner installation, the need for increased throughput of cancer patients is anticipated to justify early investment in TB-PET. As with previous PET technology developments, advantages experienced in research and in the clinic will propel initiatives in academia and industry to optimize affordability, which in turn will support expansion of TB-PET to other sites and assist in the translation of TB-PET research applications into new health care procedures.
Impact and Challenges
The timely introduction of TB-PET offers a highly sensitive and specific “systems biology” framework for studying the human body, a seamless accelerated approach to drug development, assistance for the testing of cell-based therapies in first-in-human trials, a way to address the study of multisystem diseases, support for initiatives that integrate physical and mental health treatments, and a way to further the understanding of factors in maternal-fetal biology that affect adult health. The initial challenges of TB-PET will be to develop imaging paradigms that demonstrate its powerful and unique role, which extends to the development of imaging biomarkers that will be needed to enable advanced applications. Managing, deciphering, and synthesizing the large amounts of data that represent molecular function throughout the body is challenging. Thus, engagement with the international translational and clinical research community will be essential to define and introduce impactful applications and to develop optimal imaging paradigms and analysis methodologies in support of those applications.
On the basis of previous experience in the evolution of PET technology, we anticipate a self-fulfilling process from the interrelationships between clinical use, technology development, and research applications. Research and clinical experiences will stimulate the development of lower-cost TB-PET technology, thus offering a far-reaching future for TB-PET in translational medicine and health care.
Acknowledgments
We thank M. Green for manuscript preparation assistance. We also acknowledge the support for this initiative from clinical academics, scientists, and PET methodologists in the United States, United Kingdom and international PET manufacturers.
Funding: This work was supported by University of California, Davis, Research Investments in Science and Engineering (RISE) program and NIH grants R01 CA170874 and R01 CA206187 (with support from the National Cancer Institute, the National Institute of Biomedical Imaging and Bioengineering, and the NIH Office of the Director).
Footnotes
Author contributions: The EXPLORER project was conceived of by S.R.C. and R.D.B. with subsequent input from T.J., W.W.M., and J.S.K. T.J. and P.P. took the lead on developing application areas. The manuscript was drafted by T.J. with subsequent input and revision from all authors.
Competing interests: S.R.C. and W.W.M. have served as consultants to the medical imaging industry, including Siemens Medical Solutions (S.R.C. and W.W.M.) and GE Healthcare (S.R.C.).
References and Notes
- 1.Bailey DL, Townsend DW, Valk PE, Maisey MN, editors. Positron Emission Tomography Basic Sciences. Springer; 2005. [Google Scholar]
- 2.Cherry SR, Karp J, Moses WW, Qi J, Bec J, Berg E, Choong WS, Huber J, Krishnamoorthy S, Peng Q, Poon J, Surti S, Zhang X, Zhou J, Jones T, Badawi R. EXPLORER: An ultra-sensitive total-body PET scanner for biomedical research. Proceedings of the IEEE Medical Imaging Conference; Seoul, Korea. 27 October to 2 November 2013; www.nss-mic.org/2013/program/ListProgramDB.asp?session=M03. [Google Scholar]
- 3.Badawi RD, Poon JK, Zhang X, Karp JS, Moses WW, Qi J, Graham MM, Mankoff DA, Wahl RL, Jagust WJ, Budinger TF, Jones T, Cherry SR. EXPLORER: An ultrasensitive total-body PET scanner: Application feasibility simulations. Proceedings of the World Molecular Imaging Congress; Savannah, GA,. 18 to 21 September 2013; www.wmis.org/abstracts/2013/data/papers/LBAP125.htm. [Google Scholar]
- 4.Poon JK, Dahlbom ML, Moses WW, Balakrishnan K, Wang W, Cherry SR, Badawi RD. Optimal whole-body PET scanner configurations for different volumes of LSO scintillator: A simulation study. Phys Med Biol. 2012;57:4077–4094. doi: 10.1088/0031-9155/57/13/4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Institutes of Health (NIH) Common Fund, NIH Director's Transformative Research Award. http://commonfund.nih.gov/TRA/.
- 6.Price PM, Badawi RD, Cherry SR, Jones T. Ultra staging to unmask the prescribing of adjuvant therapy in cancer patients: The future opportunity to image micrometastases using total-body 18F-FDG PET scanning. J Nucl Med. 2014;55:696–697. doi: 10.2967/jnumed.113.133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keyaerts M, Xavier C, Heemskerk J, Devoogdt N, Everaert H, Ackaert C, Vanhoeij M, Duhoux FP, Gevaert T, Simon P, Schallier D, Fontaine C, Vaneycken I, Vanhove C, De Greve J, Lamote J, Caveliers V, Lahoutte T. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nucl Med. 2016;57:27–33. doi: 10.2967/jnumed.115.162024. [DOI] [PubMed] [Google Scholar]
- 8.Saleem A, Price PM. In: Cancer Drug Discovery and Development In Vivo Imaging of Cancer Therapy. Shields AF, Price PM, editors. Humana Press Inc.; 2007. pp. 169–204. [Google Scholar]
- 9.Matthews PM, Rabiner EA, Passchier J, Gunn RN. Positron emission tomography molecular imaging for drug development. Br J Clin Pharmacol. 2012;73:175–186. doi: 10.1111/j.1365-2125.2011.04085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones T, Budinger TF. The potential for low-dose functional studies in maternal-fetal medicine using PET/MR imaging. J Nucl Med. 2013;54:2016–2017. doi: 10.2967/jnumed.113.123919. [DOI] [PubMed] [Google Scholar]


