Abstract
Reaction of thioamides (e.g., II) with benzynes generated by the hexadehydro-Diels–Alder (HDDA) cycloisomerization (e.g., I) produces dihydrobenzothiazines (e.g., III). It is postulated that the reaction proceeds via benzothietene (cf. IV) and o-thiolatoaryliminium (cf. V) intermediates and that the latter undergo intramolecular 1,3-hydrogen atom migration to produce the penultimate isomeric iminium zwitterions VI.
Graphical Abstract

Benzynes are versatile reactive intermediates, capable of being captured by many classes of nucleophiles. We recently reported some new modes of reaction between sulfides and benzynes produced by the hexadehydro-Diels–Alder (HDDA) reaction.1 For example, heating tetrayne 1 in the presence of 2-ethenyltetrahydrothiophene (2.3 equiv) and acetic acid (4.3 equiv) produced the arylsulfide 3 in a three-component reaction (Figure 1a). It is notable that acetic acid itself is a good trap of HDDA benzynes.2 However, none of the aryl acetate expected from AcOH addition was observed in the presence of the (more nucleophilic) sulfide. This underscores the reactivity pairing of the polarizable electrophilic alkyne in 2 with the soft sulfur-based nucleophile compared to an oxygen atom in the carboxylic acid. This multistep reaction cascade; passing through a zwitterion, ylide,3 and ion pair; exemplifies the type of complex reaction manifold that can be fueled by the substantial amount of potential energy afforded by the three alkynes that engage to produce the initial benzyne 2. We have also explored compounds containing other functional groups that possess a softly nucleophilic sulfur atom. We report here our results using various thioamides as the trapping agents.
Figure 1.

Examples of known reactions of benzyne with sulfur-containing nucleophiles.
Among the family of the common nitrogen-substituted thiono-containing functional groups—namely thioamide, thionocarbamate, dithiocarbamate, thiourea, and thioimide—we can locate examples of each of the last three having been reacted with an aryne. Greaney and coworkers have reported a unique thio-amidination reaction of Kobayashi-generated benzyne (i.e., o-TMSC6H4OTf + CsF) when trapped by a variety of thioureas (Figure 1b).4 Hwu et al. described an interesting addition-fragmentation reaction of a cyclic dithiocarbamate upon engaging o-benzyne (Figure 1c).5 Li et al. have reported an intriguing bis-benzyne reaction in which the sulfur atom in a thioimide initiated the process by attacking the benzyne (Figure 1d).6 We are unaware of previous reports of reactions of arynes with thioamides.
We initiated our study by attempting to trap the benzyne 5 with PhC(=S)NMe2 (6a, Figure 2). Heating 4 with 6a in benzene at 90 °C in a sealed vial for 12 hours led to formation of an unexpected product, isolated in 42% yield following chromatographic purification. The mass of the major product clearly indicated it to be a 1:1 adduct of the two reactants, but, surprisingly, the 1H NMR spectrum gave evidence of: only one N-methyl group, a methine (at δ 5.4 ppm), and an AB pattern indicative of an isolated methylene moiety (at δ 4.0 and 4.5 ppm), seemingly bearing a nitrogen substituent and having diastereotopic protons. Through analysis of 2D correlation spectra, we deduced the structure to be that of the dihydrobenzothiazine 7a. Although two regioisomeric modes of reaction are possible7 with respect to addition of the thioamide to the unsymmetrical benzyne 5, nucleophiles are known to undergo preferential addition to the carbon atom labeled with the red dot.1,8 This is consistent with the computed structure of 5,1 which shows a significantly larger internal bond angle at the red carbon atom vis-à-vis the one denoted with the blue dot; a larger internal angle correlates well with greater electrophilic character in arynes.9 This orientation of the attack was further supported by a nuclear Overhauser experiment with the analog 7b (Table 1). Species A–E represent a logical progression of intermediates that could carry reactants to product. The hydrogen atom migration leading from C/C* to E is both interesting and unusual, a point revisited computationally below (Figure 3).
Figure 2.

Trapping of HDDA-generated benzyne 5 by N,N-dimethylthiobenzamide (6a) gives the dihydrobenzothiazine 7a.
Table 1.
Reactions of triyne 4 with thioamides 6b–6g,a leading to fluorenone derivatives 7
All reactions were carried out under the same reaction conditions (starting [4]o = 0.1 M, 1.5 equiv of 6); % yield is of chromatographically purified material.
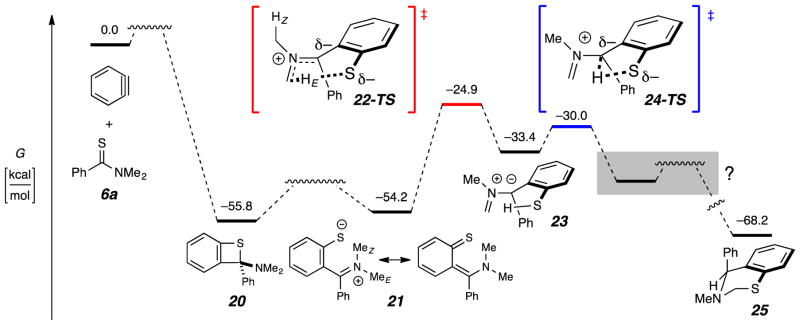
Figure 3.
Computed [SMD(benzene)//M06-2X/6-31+G(d,p)] reaction profile for the transformation of o-benzyne and N,N-dimethyl thiobenzamide (6a) to the parent dihydrobenzothiazine 25.
We proceeded to study the reactions of 4 (and 5) with a series of thiobenzoic acid amide analogs. The results are shown in Table 1. The reaction has considerable generality, as demonstrated by this series. An isopropyl group (6b to 7b) with a hindered methine proton will still undergo the C to D event. An N-aryl group (6c to 7c) is tolerated. Amides with two primary alkyl substituents (6d and 6e) give rise to a dihydrobenzothiazine with two stereocenters (7d and 7e). In these reactions we have seen no evidence for the formation of a second diastereomer. This highly preferential formation of the isomer with a trans relationship between the vinyl and phenyl groups in 7d was established from a single crystal X-ray analysis.10 In the solid state, the 1,3-thiazine ring adopts a half-chair conformation in which the vinyl group is disposed to be equatorial and the phenyl pseudoaxial. Presumably, maintaining minimal interaction between the large phenyl substituent on C1 and H11 along the reaction coordinate for the cyclization favors the transition state leading to diastereomer 7d.
The thioamide 6f, containing the electron withdrawing nitro-group, proceeded efficiently. On the other hand, the analogous amide with a donor 4-methoxy substituent instead, gave rise to a much less clean crude product mixture that we judged to contain a small amount of the methoxy version of product 7 (not shown) but was not otherwise further pursued. Finally, the nicotinic acid derived thioamide 6g proceeded well to give the pyridine-containing bicyclic compound 7g. The diastereomeric nature of 7e and 7g was assigned on the basis of similarities in 1H chemical shifts of several analogous protons in comparison to those in 7d. Moreover, since none of the second diastereomer was detected for any of these reaction products, a full reversal in the dr (>99:1 to <1:99) would be highly unlikely for any of these reactions that give products of such similar topology.
To establish that this transformation is not limited to some unidentified (and unanticipated) reactivity property of the benzyne 5, we explored the reactivity of five additional benzyne precursors (1 and 8a–8d, Table 2). Similarly, to demonstrate an expanded scope of thioamides that function in this reaction, we studied reactions using thioamides 6h–6o. There was no particular reason (nor, therefore, bias) for pairing the partners that are shown here. In a handful of instances, additional pairings were preliminarily explored, always with a very similar outcome in product type and reaction cleanliness.11 In other words, we know of no reason to think that any one of these benzynes (and myriad others) would be incompatible with any one of these aromatic12 thioamides.
Table 2.
Reactions of five other HDDA substrates (1 and 8a–d) with eight other thioamidesa
Reactions all performed between 75–95 °C for 12–24 h [see Supporting Information (SI) for details]
Notable aspects of this set of results include: (a) Sterically congested relationships in the products can be established (Ts and Ar in entries 1–4; gem-dimethyl and Boc with Ar in entries 8 and 9), (b) the electronic character of an N-aryl substituent makes little difference; (c) a thiourea proceeded to give an analogous product (entry 3); (d) heterocyclic substituents are accommodated [entries 3, 4, and 9 and product 7g (Table 1)]; (e) a cyclopropane ring survives the reaction (entry 5), suggesting the absence of a radical intermediate at the carbon from which hydrogen has been transferred; (f) benzo-fused bicyclic structures can arise (entries 4 and 6); and (g) functional groups capable of easy further transformation can be incorporated (allyl and vinyl in entries 1 and 7, ester in entry 7, and iodoarene in entry 8).
We have also used DFT calculations to probe aspects of the mechanism (cf. Figure 2) of this unusual transformation (Figure 3). The benzothietene 20 is the [2+2] adduct between benzyne and the thiocarbonyl group in 6a. This was seen to be similar in free energy to the isomeric, ring-opened, polarized thioquinone methide 21, and both were considerably more stable than the reactant pair. We did not attempt to locate transition structures for any processes interconverting benzyne and 6a with 20 or of 20 with 21. We explored possible pathways for the unimolecular isomerization of 21 to the product 25. This was fruitful once we allowed for the non-intuitive possibility of hydrogen atom removal by the basic sulfur atom from the iminium ion methyl group trans to the thiolated arene (cf. MeE in 21 and HE in 22-TS). This produces the azomethine ylide 23; 22-TS was the only transition structure that we could identify for this step. This TS lies ca. 30 kcal•mol−1 higher in energy than 21, which places that step in the category of being “feasible.” A very rapid (and highly exergonic) progression from 23 is computed to ensue. We could not identify an iminium thiolate like E (Figure 1) as a stable entity. Instead, the TS 24-TS collapsed directly to 25. A related, thiolate-mediated proton shift via an azomethine ylide like 23 was described both experimentally and computationally by Seidel, Houk, and coworkers.13
We then carried out a deuterium labeling experiment to probe the mechanism experimentally (Figure 4). Triyne 4 was heated with the tri-deuterated thiobenzamide 6ad3 to give a mixture of the products 26a and 26b in a ratio of 6:1. This clearly shows that a net, intramolecular 1,3-hydrogen migration is involved in this transformation and that it is most likely the rate-determining step (protium is preferentially transferred over deuterium) of the trapping sequence that converts 5 into 26. We did not observe any appreciable amount of the di- or the tetradeuterated analog of 16, which supports the view of the proton transfer as an intramolecular event, not requiring an external proton shuttle.
Figure 4.

Mono-CD3-labeled thioamide 6ad3 shows preference for 1,3-migration of the H- over D-atom.
In conclusion, aromatic thioamides react with (thermally generated) benzynes to form dihydrobenzothiazine derivatives that we propose arise by an unusual, thiolate-assisted, 1,3-hydrogen atom migration. These mechanistic views are supported by a deuterium labeling experiment and DFT calculations. A wide range of thioamides participate in such a transformation to produce structurally diverse dihydrobenzothiazines in a regioselective and diastereoselective manner.
Supplementary Material
Acknowledgments
Support for this research was provided by the National Institutes of General Medical Sciences of the U.S. Department of Health and Human Services (GM65597). V.P. appreciates support from a Gleysteen-Heisig fellowship for undergraduate students. Some of the NMR data were obtained with an instrument acquired with funds from the NIH Shared Instrumentation Grant program (S10OD011952).
Footnotes
Notes
The authors declare no competing financial interests.
Supporting Information. The Supporting Information is available free of charge on the ACS Publications website.
Experimental details for the preparation of new compounds; spectroscopic data for their characterization, including copies of 1H and 13C NMR spectra; and computed (DFT) geometries and energies of benzyne intermediates and transition states (PDF).
References
- 1.Chen J, Palani V, Hoye TR. J Am Chem Soc. 2016;138:4318–4321. doi: 10.1021/jacs.6b01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karmakar K, Ghorai S, Xia Y, Lee D. Molecules. 2015;20:15862–15880. doi: 10.3390/molecules200915862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.See: Nakayama J. J Sulfur Chem. 2009;30:393–468.Iwamura H, Iwamura M, Nishida T, Yoshida M, Nakayama J. Tetrahedron Lett. 1971;12:63–66.
- 4.Greaney MF, Biswas K. Org Lett. 2011;13:4946–4949. doi: 10.1021/ol202049v. [DOI] [PubMed] [Google Scholar]
- 5.Hwu JR, Hsu YC. Chem Eur J. 2011;17:4727–4731. doi: 10.1002/chem.201001735. [DOI] [PubMed] [Google Scholar]
- 6.Shi J, Qiu D, Wang J, Xu H, Li Y. J Am Chem Soc. 2015;137:5670–5673. doi: 10.1021/jacs.5b02566. [DOI] [PubMed] [Google Scholar]
- 7.Indeed, 7a was formed along with a coeluting constitutional isomer in a regioisomeric ratio (rr) of 21:1 (see Supporting Information); similar results were seen for 7b, 7d, and 7e.
- 8.(a) Hoye TR, Baire B, Niu DW, Willoughby PH, Woods BP. Nature. 2012;490:208–212. doi: 10.1038/nature11518. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Willoughby PH, Niu D, Wang T, Haj MK, Cramer CJ, Hoye TR. J Am Chem Soc. 2014;136:13657–13665. doi: 10.1021/ja502595m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Hamura T, Ibusuki Y, Sato K, Matsumoto T, Osamura Y, Suzuki K. Org Lett. 2003;5:3551–3554. doi: 10.1021/ol034877p. [DOI] [PubMed] [Google Scholar]; (b) Cheong PHY, Paton RS, Bronner SM, Im GYJ, Garg NK, Houk KN. J Am Chem Soc. 2010;132:1267–1269. doi: 10.1021/ja9098643. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Garr AN, Luo D, Brown N, Cramer CJ, Buszek KR, VanderVelde D. Org Lett. 2010;12:96–99. doi: 10.1021/ol902415s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Details can be found at the Cambridge Crystallographic Data Centre (CCDC deposition number 1508652).
- 11.These reactions were typically not further explored (and, therefore, are not shown in Table 2) largely for pragmatic reasons. In some instances the product(s) coeluted with unreacted thioamide; in others, mixtures of products from regioisomeric modes of trapping (cf. entries 6 and 7) of the benzyne complicated the purification.
-
12.We studied several aliphatic thioamides; the presence of an acidic α-proton diverted the reaction course to provide a benzothiophene, a process involving spontaneous (air?) oxidation. For example:

- 13.Jarvis CL, Richers MT, Breugst M, Houk KN, Seidel D. Org Lett. 2014;16:3556–3559. doi: 10.1021/ol501509b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.