Abstract
Advances in cancer therapy in the past few years have include the development of medications that modulate immune checkpoint proteins. Cytotoxic T-lymphocyte antigen 4 (CTLA4) and programmed death 1 (PD1), are two coinhibitory receptors that are expressed on activated T cells to which therapeutic blocking antibodies have reached routine clinical use. Immune checkpoint blockade can induce inflammatory side effects, termed as immune-related adverse events (IRAEs), which resemble autoimmune disease. In this Review, we describe the current data regarding immune-related endocrinopathies, including hypophysitis, thyroid dysfunction and the development of diabetes mellitus. We discuss the clinical management of these endocrinopathies within the context of our current understanding of the mechanisms of IRAEs.
Introduction
The increased understanding of the human immune system and emergence of immune modulation techniques have led to a new era in cancer therapy, and the idea of using our own biology to treat cancer is a revolutionary area of oncology. To ensure the immune system does not cause harm the host when reacting to a foreign antigen, humans have evolved immune checkpoint proteins and mechanisms to quickly halt an immune response. However, in the case of cancer, malignant cells have developed many mechanisms to evade the human immune system 1,2, including the ability to limit immune responses through such immune checkpoints 3. New cancer therapies have made use of the accumulating knowledge regarding immune regulation and immune system checkpoints; for example, cytotoxic T-lymphocyte antigen 4 (CTLA4) and the programmed cell death 1 (PD1) pathway.
In resting T cells, CTLA4 resides intracellularly but is translocated to the plasma membrane shortly after T-cell activation 4. In an active immune response, CD28 on the T-cell surface binds to the B7 co-stimulatory ligand on antigen presenting cells to provide the second signal that allowing the T cell to mature 4. CTLA4 binds with high affinity to B7 and can compete with CD28 to further inhibit T-cell activity 5. This process prevents the second signal that supports T-cell activation and effectively stops the T-cell from maintaining an immune response 6 (FIG. 1). Monoclonal antibodies that target CTLA4, such as ipilimumab, have demonstrated efficacy in cancer treatment 7,8 (FIG. 1). The binding of these antibodies to CTLA4 results in the prevention of B7 binding; with B7 now accessible, CD28 enables the upregulation of T-cell activity4. CD28-initiated downstream activation of mitogen-activated protein kinase results in formation of activator protein 1 (AP-1) complex9; in conjunction with T-cell receptor-mediated nuclear factor of activated T-cells signal, the AP-1 complex induces IL-2 cytokines, which mediate T-cell growth 9. With CTLA4 blocked, activated T cells proliferate and achieve a persistent state of activation, which enables the targeting of otherwise poorly immunogenic tumour antigens to cancer cells 10.
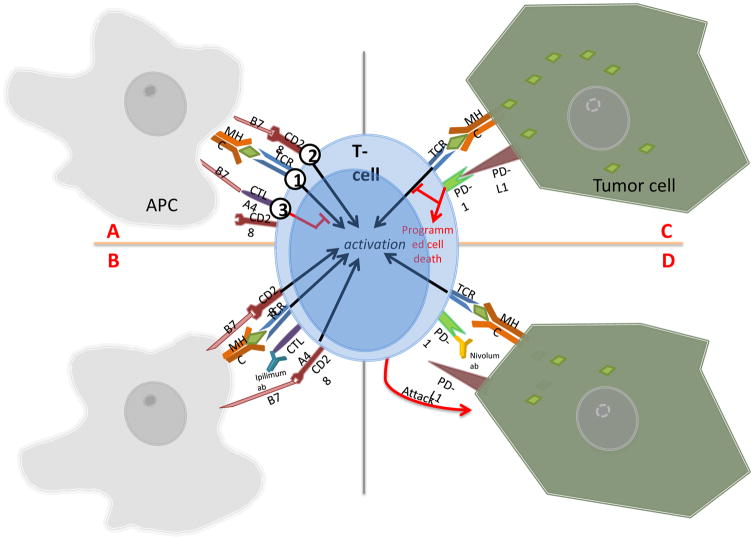
Figure 1.
A. Normal CTLA4 interaction with B7 costimulatory ligand. 1) First activation signal is initiated when T-cell receptor (TCR) binds to antigen presenting cell’s (APC) MHC presenting an antigen. 2) Second activation signal is fired when CD28 receptor binds to B7 costimulatory ligand on the APC. 3) CTLA4 receptors present on T-cell act as a checkpoint, and inhibits T-cell activation by outcompeting CD28 receptors to bind to B7 ligand. This negates the effect of second activation signal. B. Ipilimumab, an anti-CTLA4 antibody, indirectly increases T-cell activity by binding to the CTLA4 receptor. Second activation signal via B7 and CD28 connection is reactivated. C. By blocking either PD-1 or PD-L1 protein, Nivolumab enables the T-cell to detect tumor cells.
D. By blocking either PD-1 or PD-L1 protein, Nivolumab enables the T-cell to detect tumor cells.
PD1 is an immune cell-specific surface receptor 11,12, and ligands for PD1 (PDL1 and PDL2) are associated proteins found on antigen presenting cells as well as cancer cells 13,14,15,16. When bound to a ligand, PD1 lowers the threshold for apoptosis, induces anergy via blunted T-cell receptor signaling, and generally leads to T-cell depletion (FIG. 1) 5,17. In certain tumour cells, upregulation of PDL1 expression has been seen, which leads to increased inhibition of T-cell activity in favour of tumour cell survival 18,19. A monoclonal antibody against PD1 can block this pathway (that is a PD1–PDL1 interaction) and result in the upregulation of immune response and inhibition of tumour growth (FIG. 1) 20,21,22,23.
Suppressing these immune checkpoints results in immune-mediated antitumour activity in mouse models and clinical trials 24,20,25,7,8,15,26. Specifically, suppression of CTLA4 and PD1 pathway enables the expansion of tumour-specific T cells 5,20. However, immunotherapy has also led to immune-related adverse events (IRAEs) 27,28, which can range from mild to fatal, depending on the organ system and severity 27. Although endocrinopathies are not among the most common IRAEs reported, they can be particularly severe and, consequently, must be carefully monitored during treatment with immunotherapeutic agents 29.
The two main endocrinopathies observed with checkpoint blockade treatments include hypophysitis (typically present with CTLA4 antibodies) and primary hyperthyroidism or hypothyroidism (seen with antibodies against PD1, PDL1 and CTLA4) 30,31,32. The precise mechanisms remain unclear; however, possible pathophysiologies are currently being evaluated in mouse models 33. CTLA4 and PD1 monoclonal antibodies target different mechanisms5; while CTLA4 is involved in initial T-cell deactivation, PD1 targets the modulatory phase of the immune response 34,35, which might, in part, explain the differences in IRAEs between the two therapies.
Interestingly, a correlation seems to exist between overall patient survival and the incidence and severity of IRAEs 36,37. This trend might be due to the monitoring of patients for a longer period of time and the bias resulting from extended duration of symptomatic observation 38,39. However, the correlation could also be the result of autoimmunity being indicative of a nonspecifically overactive immune system resulting in increased antitumour efficacy 40. This notion is supported by a decrease in cancer-specific mortality in patients who also experience IRAEs, including endocrine IRAEs 40,36. With the increased clinical application of immunotherapeutics, understanding the prevalence, detection and management of IRAEs in the patient population is important.
In this Review, we focus on the endocrinopathies related to four immunotherapeutic agents for which the largest amount of safety data is available: ipilimumab (CTLA4), tremelimumab (CTLA4), nivolumab (PD1) and pembrolizumab (PD1). We also briefly discuss antibodies that block PDL1, which are in clinical development and have toxicities that generally resemble those of PD1 blocking antibodies 26.
CTLA4 antibodies
Ipilimumab (also known as MDX-010) was first shown to be efficacious in two different phase III trials in patients with metastatic melanoma 7,8 and was subsequently approved by the FDA for treatment of unresectable or metastatic melanoma 41. Tremelimumab (first known as CP-675,206) works through a similar mechanism to ipilimumab to block CTLA4 42,43,44.
The reported adverse effects of ipilimumab include dermatitis, enterocolitis, hepatitis and uveitis, some of which have been classified as severe grade 3–4 effects, principally due to colitis or hepatitis45. Endocrinopathies are of special interest, as they are considered rare in the general population, with idiopathic autoimmune hypophysitis only seen in one out of every 9 million individuals 46,47. Pituitary dysfunction is among the most commonly reported endocrinopathies (mean 9.1%; Table 1) associated with ipilimumab, with other reported endocrinopathies, including primary hypothyroidism, primary hyperthyroidism and primary adrenal sufficiency 31,32.
Table 1.
Endocrine IRAEs in patients treated with ipilimumab
| Study | Cohort | Endocrinopathy | ||||||
|---|---|---|---|---|---|---|---|---|
| Age (range) | n | Hypophysitis | 2°/Other AI | 2°/Other HT | 1° HT | Thyroiditis | 1° AI | |
| Phan, et al(2003)64 | 52 (39–67) | 14 | 1 | 1 | 1 | NR | NR | NR |
| Attia, et al(2005)82 | 50.1 (21–67) | 56 | 1 | NR | NR | NR | NR | NR |
| Maker, et al(2006)62 | 48 (24–68) | 46 | 8 | NR | 1* | NR | NR | NR |
| Downey, et al(2007)86 | 50 (21–69) | 139 | 13 | NR | 3* | NR | NR | NR |
| Small, et al(2007)89 | 70.5 (56–79) | 14 | NR | NR | NR | NR | NR | NR |
| Yang, et al(2007)39 | 52–59 (31–70) | 61 | 2 | 1* | NR | NR | NR | NR |
| Weber, et al(2008)90 | 59 (29–87) | 88 | NR | 1* | NR | NR | NR | NR |
| Ansell, et al(2009)91 | 56 (37–79) | 18 | 1 | NR | NR | NR | NR | NR |
| O’Day, et al(2010)92# | 59 (26–85) | 155 | NR | NR | NR | NR | NR | NR |
| Hodi, et al(2010)7 | 55.6–56.8|| | 540 | 4 | 5* | 8* | NR | NR | NR |
| Hersh, et al(2011)93 | 61 (25–82) | 72 | NR | 1* | NR | NR | NR | NR |
| Ku et al (2010)87 | 62 (38–86) | 53 | 1ǂ | 2 | 1* | NR | NR | NR |
| Royal, et al(2010)94 | 55 (27–68) | 27 | 1 | NR | NR | NR | NR | NR |
| Robert, et al(2011)8 | 57.5|| | 250 | NR | NR | NR | NR | NR | NR |
| DiGiacomo, et al(2011)95 | 55 (23–77) | 27 | NR | NR | 2* | NR | 1 | NR |
| Eggermont, et al(2015)88 | 51 (20–84) | 475 | 86 | NR | 42* | NR | NR | NR |
| Faje, et al(2014)36† | 59.9–68.2|| | 154 | 17ǂ | 7 | 17 | 8 | NR | NR |
| Ryder, et al(2014)32†** | NR | 256 | 19 | 16 | 11 | 15 | 8 | 2 |
| Postow, et al(2015)131 | 67 (31–80) | 47 | 3 | 2* | 7* | NR | NR | NR |
| Larkin, et al(2015)132 | 61 (18–89) | 315 | 12 | NR | 13* | NR | NR | NR |
| Albarel, et al(2015)48†## | 55.5|| | 131 | 15 | 11 | 13 | NR | NR | NR |
| Total | 2938 | 184 (9.1%) | 37 (6.1%) | 42 (7.6%) | 23 (5.6%) | 9 (3.2%) | 2 (0.8%) | |
| Event/Totalǂǂ | - | 184/2017 | 37/608|||| | 42/555|||| | 23/410 | 9/283 | 2/256 | |
Unclear etiology (such as primary versus secondary)
Endocrinopathies (n=9) reported but no specifics given
Single patient within trial cohort with multiple events (hypophysitis)
Age range not given;
Retrospective review.
18 patients had secondary gondadotroph deficiency.
12 patients had secondary gondadotroph deficiency.
Percentage determined by total number of events divided from total number of patients only from studies reporting event.
Unclear etiologies were not included in calculation.
AI, adrenal insufficency; HT, hypothyroidism; NR, not reported
Note: Secondary gonadotroph deficiency was reported in 30 out of 401 patients (7.5%)
Hypophysitis
Hypophysitis related to CTLA4 antibody therapy has been reported to vary from between 0.4% and 17% in patients treated with this therapy31. This wide range in incidence has been associated with differences in hormonal monitoring, drug dose and improved recognition of anti-CTLA4-related hypophysitis, with low incidences being reported in initial studies 36,29. Two reports specifically studying anti-CTLA4-related hypophysitis and other endocrine dysfunction during ipilimumab therapy noted an 8–13% incidence of hypophysitis 48,36,32,49, and an 8.5–9% incidence of hypophysitis with ipilimumab combined with the PD1 monoclonal antibody, nivolumab has also been reported32,50. In our own analysis of clinical trials and retrospective reviews, we found an overall incidence of hypophysitis in 9.1% of patients (184 out of 2,017; Table 1); the incidence of secondary adrenal insufficiency, secondary hypothyroidism and secondary hypogonadism were 6.1%, 7.6%, and 7.5% respectively. Importantly, we only included instances with a clear secondary aetiology for adrenal insufficiency and hypothyroidism. Overall, many studies lacked specific endocrine data and further studies are, therefore, needed with in-depth hormonal studies.
The incidence of anti-CTLA4-related hypophysitis is associated with the dose of therapy received 51 although there have been conflicting reports regarding this finding 52,29,53,54,32,36,49. In addition, other investigators have reported that hypophysitis incidence was not significantly different between cohorts who received 3 mg/kg and 10 mg/kg 32,52. Some investigators argue that cumulative treatment effects might influence the timing of hypophysitis after treatment initiation, as many patients do not become symptomatic until ~11 weeks after the first dose 55,56,7. The time for the onset of hypophysitis symptoms can range from 6 to 12 weeks after CTLA4 antibody treatment initiation 31,36,29,32,49, but patients can present as early as week 4 and as late as week 16 56,57.
Hypohysitis related to CTLA4 antibody therapy may occur more commonly in men than in women. In one report, the prevalence of anti-CTLA4-related hypophysitis in men was 15.6% and 3.6% in women (P = 0.02; OR 4.73 (95% CI 1.27–30.79) 36. This finding is in contrast to idiopathic autoimmune hypophysitis, which is more common in women (men to women ratio 1:3) 46,30,58,59. This phenomena has been suggested to be the result of the increased prevalence of men with melanoma in these trials 32. In terms of age, one study reported a statistically significantly older population (mean age of 68.2 ± 2.4 years compared with 59.9 ± 1.0; P = 0.005) being affected by ipilimumab-induced hypophysitis 36.
Headache, fatigue and/or muscle weakness seem to be the most common (in 89% of those diagnosed hypophysitis) presenting symptoms of hypophysitis related to anti-CTLA4 therapy 32. However, these symptoms are nonspecific and could be misattributed to general symptoms related to cancer. Nausea, anorexia, weight loss, visual changes, alterations in mental status, temperature intolerance and arthralgias are also reported, but less frequently (10.5–21.1%) 36,29,32. Low levels of sodium (range of 113 to 134 mEq/l) have also been reported in patients with anti-CTLA4-related hypophysitis, with some studies reporting hyponatraemia in 47–56% of patients 36,32, although this was not reported in all studies 7,56,60. Morbidity attributed to anti-CTLA4-related hypophysitis is thought to be predominantly related to secondary adrenal insufficiency 27, which might be life threatening if not treated. Symptoms of adrenal crisis include hypotension, dehydration and electrolyte imbalance, and requires immediate attention 27. Notably, the symptoms of hypophysitis can improve in as quickly as days after starting corticosteroids 32.
Adrenocorticotropic hormone (ACTH) and/or TSH deficiency are the most common pituitary hormone abnormalities described in patients anti-CTLA4-related hypophysitis 36,32,61,62. These anterior pituitary hormone deficiencies are more prevalent than diabetes insipidus or posterior pituitary deficiency with only single case report of CTLA4-associated diabetes insipidus 55,56. Hypogonadotropic hypogonadism has also been reported although this might be confounded by the existence of illness-induced hypogonadism induced by severe illnesses, such as sepsis 29. Low levels of insulin-like growth factor 1 (IGF1) might also be present, but the growth hormone axis is less often evaluated, as treatment with growth hormone replacement is given to patients with active malignancy 36,29. Prolactin has been described as being both elevated and low in patients with anti-CTLA4-related hypophysitis 31,32,59.
Adrenal insufficiency associated with CTLA4-related hypophysitis is usually permanent 31,36,32,49, and these patients typically need life-long steroid replacement after developing this complication49. The recovery of secondary hypothyroidism can occur, but the frequency of this has been reported to vary from 6% to 64% 31,36,49,56. Gonadal axis recovery has also been noted to vary from 11% to 57% 31,36,59,55,57. Initial assessment of thyrotrope and gonadotrope function is complicated in an ill cancer patient undergoing cancer therapy, which can result in thyroid and gonadal lab values that are similar to values seen in hypopituitarism32; furthermore, recovery from illness can normalize these hormone levels (for example, sick euthyroid syndrome/sickness-induced hypogonadism) 32. Consequently, determining if recovery from thyroid or gonadal hormone deficiency was due to improvement in hypophysitis or due to simple recovery from the underlying illness is difficult.
Mild-to-moderate diffuse enlargement of the pituitary gland, with either homogenous or heterogeneous enhancement after contrast administration, is typically seen on sellar MRI in patients with CTLA4-related hypophysitis 31,36. The pituitary stalk might thicken and, although uncommon, pituitary enlargement could result in a mass effect on optic apparatus 36,49. In a retrospective review of MRI data, relative pituitary enlargement was seen to precede the clinical diagnosis of anti-CTLA4-related hypophysitis 36. This observation is further reiterated by the finding that the median time to onset of pituitary enlargement is 1 week before any biochemical evidence of hypopituitarism49. The pituitary gland is thought to decrease in size over ~4–12 weeks and subsequent atrophy of the gland can be seen 31,36,55,57,63,64. However, importantly a normal MRI does not rule out hypophysitis and management should be based on clinical presentation and evaluation of pituitary hormone levels 45.
[H3] Mechanism
The mechanism of CTLA4 antibody-mediated endocrinopathy remains unclear. Autoantibodies targeting the pituitary have been described in patients (seven out of seven) with ipilimumab-induced hypophysitis; these antibodies were not found in an ipilimumab-treated cohort (13 of 13) who did not have pituitary abnormalities 33. Furthermore in ipilimumab-induced hypopituitarism, TSH-targeting antibodies were identified in all patients (n = 7); while other endocrine cell-targeting antibodies were also identified (FSH secreting cells in five patients and ACTH secreting cells in three patients) 33.
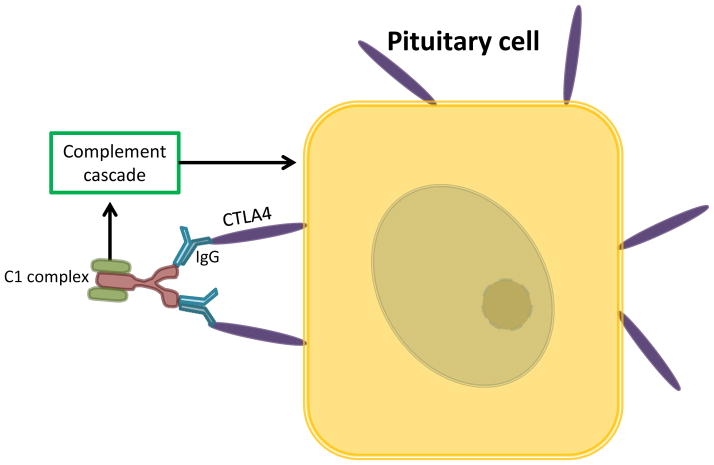
Investigators have suggested that hypophysitis is caused by complement activation from antibody immunity developed against the pituitary gland 33. Specifically, a type II hypersensitivity reaction to ectopic CTLA4 protein expressed on pituitary cells is thought to result in damage to the pituitary 65 (FIG. 2). Interestingly, patients treated with PD1 and/or PDL1 IgG4 antibodies instead of IgG1 used in ipilimumab rarely developed pituitary damage 26,66,67. This lead researchers to hypothesise that IgG1, which activates classical complement pathway, might be a possible mechanism of anti-CTLA4-related hypophysitis 33. In support of this hypothesis, the occurrence of hypophysitis in patients receiving ipilimumab (IgG1) is notably elevated (9.1%) compared with tremelimumab (IgG2b; 1.3%; Table 1 and 2)68,69,70,71. However, based on our review of the literature, tremelimumab (n = 773) was not clinically evaluated as robustly as ipilimumab (n = 2,938), and the direct comparison of IRAE incidences might not provide a definitive understanding of their toxicity.
Figure 2.
Pituitary tissues express ectopic CTLA4 protein. Binding to CTLA4 autoantibodies or Ipilimumab IgG1 is thought to lead to activation of classical complement pathway.
Table 2.
Endocrine IRAE in patients treated with tremelimumab
| Study | Cohort Characteristics | Endocrinopathy | ||||||
|---|---|---|---|---|---|---|---|---|
| Avg (range) | n | Hypophysitis | Adrenal insufficiency | Hypothyroidism | Hyperthyroidism | Thyroiditis | Other thyroid# | |
| Ribas et al (2005)68 | 54 (30–78) | 39 | 1 | NR | 1ǂ | 1 | 1 | NR |
| Camacho et al (2009)69 | 54–61 (20–83) | 90 | NR | NR | NR | NR | 1 | 1 |
| Ralph et al (2010)70 | 55.4 (37–75) | 18 | NR | NR | NR | NR | NR | NR |
| Kirkwood et al (2010)71 | 53 (18–89) | 246 | 1 | NR | NR | NR | NR | 8 |
| Chung et al (2010)44 | 62 (39–79) | 47 | NR | NR | 1ǂ | NR | NR | NR |
| Ribas et al (2013)43 | 57 (22–90) | 328 | 6 | 4ǂ | NR | NR | NR | 17 |
| Total | 773 | 8 (1.3%) | 4 (1.2%) | 2 (2.3%) | 1 (2.6%) | 2 (1.6%) | 26 (3.9%) | |
| Events/total§ | 8/613 | 4/328 | 2/86 | 1/39 | 2/129 | 26/664 | ||
Includes Grave’s disease, autoimmune thyroiditis and unspecified thyroid disorders.
Unspecified cause.
Percentage determined by total number of events divided from total number of patients only from studies reporting event.
NR, non reported; IRAE, immune-related adverse events.
[H3] Monitoring and management
Patients should be informed of the symptoms of anti-CTLA4-related hypophysitis, which can present between treatment and clinical visits. Baseline and follow-up thyroid function tests have been recommended following treatment with anti-CTLA4 therapy 38; however, in our experience screening for secondary adrenal insufficiency is often not a component of routine monitoring. Given that adrenal insufficiency can be life-threatening and the relatively high incidence of hypophysitis in patients treated with CTLA4 antibody therapy, routine monitoring with early morning ACTH and cortisol levels at baseline and during treatment should be considered 27. While receiving CTLA4 therapy, these tests can be performed monthly for the first 6 months given that anti-CTLA4-related hypophysitis tends to occur early in the course of treatment. If the tests are normal and if the patient is asymptomatic, testing can be done every 3 months for the next 6–12 months followed by every 6 months for the following 2 years. When patients have symptoms or signs of hypophysitis or hypopituitarism, they should have a prompt evaluation for these complications, which includes levels of early-morning ACTH, cortisol, TSH and free T4. If early morning readings are not feasible, or an urgent assessment is needed, samples can be taken at any time of the day. A very low random cortisol and ACTH level might be helpful in diagnosing secondary adrenal insufficiency. In the acute phase of pituitary damage, the adrenal glands might respond to ACTH stimulation normally because the adrenal glands have not yet atrophied from the chronic lack of ACTH stimulation 72. Consequently, a cosyntropin stimulation test is not as useful in diagnosing early secondary adrenal insufficiency. In patients with hypophysitis or hypopituitarism, gonadotropins and sex hormones should also be assessed. In those with secondary hypogonadism, prolactin levels can be measured
High-dose steroids can be used for those patients with critical illness, either related to hypophysitis or hypopituitarism, significant hyponatraemia, severe headache, visual abnormalities or significant pituitary enlargement that abuts or has mass effect on the optic apparatus. Glucocorticoid treatment can decrease pituitary size gradually with symptom relief 59,55,57. However, in a retrospective study, high dose steroids did not seem to reverse hypopituitarism, and the investigators suggest that secondary hormonal abnormalities should be treated instead of the hypopituitarism 49. By contrast, for idiopathic lymphocytic hypophysitis, spontaneous recovery of pituitary function as well as recovery after high dose steroids has been described in some patients 73,74. Low doses of glucocorticoids (for example, 15 mg to 25 mg of hydrocortisone in split doses or an equivalent dose of prednisone) can alleviate fatigue and headache and treat those with adrenal insufficiency 54. These regimens are also considered when low doses of steroids are needed for the patient to be eligible for clinical trials with immunotherapy drugs, as high dose steroids can be part of the exclusion criteria given their possible immunomodulatory effect. Fortunately, the anti-cancer effects of CTLA4 immunotherapy do not appear to be influenced by treatment of anti-CTLA4-related hypophysitis with glucocorticoids 53,75,76,77,78.
Levothyroxine can be used to treat secondary hypothyroidism; but glucocorticoid deficiency should be first treated to avoid any potential adrenal crisis that can be precipitated by replacing thyroid hormone first 79. Patients with central hypothyroidism or hypoadrenalism also often need long-term hormone replacement therapy 27,80,31,38,60. Hyponatraemia is typically short-lived, and improves after adrenal and thyroid hormone replacement 81. Testosterone might be used in patients who develop hypogonadotropic hypogonadism, but the treatment should be consistent with the current Endocrine Society guidelines 82. Estrogen replacement can also be considered in premenopausal women who have secondary hypogonadism 83.
Primary thyroid dysfunction
Distinguishing primary thyroid dysfunction (that is, related to thyroid gland dysfunction) from secondary to hypophysitis/pituitary dysfunction is important for treatment 27. Elevated levels of TSH with low-to-normal levels of free T4 (thyroxine) or T3 are seen in primary hypothyroidism84 Low to mid-normal levels of TSH with a low free T4 suggests hypothyroidism secondary to pituitary dysfunction 40. However, tests for levels of T3 in patients with any acute illnesses can be inaccurate 84. Other investigators have reported that the best assessment of primary thyroid dysfunction was by the measurement of TSH levels 40. In thyroiditis, primary hypothyroidism (high TSH and/or low free T4) might be preceded by a transient hyperthyroidism (low TSH, elevated free T4 and/or T3) 85. Unless thyroid dysfunction is subclinical and detected only via laboratory tests, hypothyroidism can be recognized by the presence of symptoms such as fatigue, muscle weakness, cold intolerance and bradycardia 85.
In clinical trials in which the patient received ipilimumab, the incidence of secondary hypothyroidism was 7.6% (4.3–11.0%) with primary hypothyroidism reported in 5.6% of patients (5.2–5.9%) (Table 1) 7,36,62,86,87,88,89,90,91,92,93,94. However, several of these trials did not include detailed information regarding the aetiology, diagnostic test values or management of the hypothyroidism. For example, different trials reported unspecified hypothyroidism in occurring in as few as 1.5% of patients and as high as 8.8% 7,36,62,86,87,88 (Table 1). The high occurrence of 8.8% might highlight the fact that this study enrolled patients who were free of radiographically detectable melanoma after surgery and received high dose (10 mg/kg) ipilimumab 88. The time to presentation of primary hypothyroidism following treatment with ipilimumab was not been clearly defined. In a retrospective review, in 154 patients had a normal baseline TSH before ipilimumab therapy, two developed transient thyrotoxicosis during treatment, followed by primary hypothyroidism, Six had newly elevated TSH levels during treatment or immediately after the conclusion of therapy 36. However, in this study, clinical presentation, thyroid autoantibody and thyroid ultrasonography data were not reported 36.
In a retrospective review of clinical trials using ipilimumab, primary hypothyroidism was identified in nine patients (who did not have concomitant PD1 blockade) 32. In these patients, hypothyroidism presentation ranged from as early as 5 months to 3 years, but thyroid autoantibody data was not presented. The most common presenting symptom was fatigue, which improved with thyroid hormone replacement; three patients also developed thyroiditis while receiving ipilimumab 32. The prevalence of subclinical primary hypothyroidism was best characterized in a retrospective study of 137 patients enrolled in the ipilimumab expanded access program where levels of TSH and free T4 were evaluated at baseline and during follow-up. Of these patients, six (4%) developed subclinical hypothyroidism, defined as a TSH level between 5 and 10 mIU/ml with normal levels of free T4 32. Two additional cases of ipilimumab-induced hypothyroidism out of 27 patients were also reported in an cohort of patients from Italy, one of whom transitioned to hypothyroidism from thyroiditis, which required hormone replacement therapy. 95.
Autoimmune thyroiditis and Grave’s ophthalmopathy have been described in some case reports. For example, three patients treated with ipilimumab (two in combination with bevacizumab) had autoimmune thyroiditis 96. A 51-year-old female on ipilimumab monotherapy initially presented with periorbital swelling and pain. Initial thyroid laboratory evaluation showed a normal TSH and free T4 with increased anti-thyroperoxidase antibodies (anti-TPO Abs; 662 IU/ml) and anti-thyroglobulin antibodies (148.5 IU/ml). Physical exam of the thyroid was normal but a CT scan confirmed Grave’s ophthalmopathy with swelling of extraocular muscles 96. In a patient treated with ipilimumab and bevacizumab combination therapy who presented with hand tremor, autoimmune thyroiditis was diagnosed with initial hyperthyroidism and positive anti-TPO and anti-thyroglobulin antibodies96. A third female patient was treated with ipilimumab and bevacizumab and developed painless thyroiditis during treatment. She initially presented with tachycardia without goiter or neck tenderness and a low TSH level (0.06 mIU/ml) and high normal free T4 level. In another report of Grave’s ophthalmopathy, a 51-year-old woman presented with extraocular muscle swelling and pain after ipilimumab treatment, MRI indicated potential Grave’s ophthalmopathy and levels of TSH-stimulating receptor, anti-microsomal and anti-thyroglobulin antibodies were elevated; levels of TSH and free T4 were normal 97. Clinicians should be able to differentiate the aetiology of the thyroid dysfunction for proper management of the IRAE.
[H3] Mechanism
In some reports, polymorphisms in CTLA4 have been shown to lead to a higher incidence of autoimmune disorders, such as Graves disease and Hashimoto thyroiditis 98,99. For example, in one study, 75% of patients with GG alleles at a single nucleotide polymorphism (SNP), JO33, developed adverse autoimmune effects, such as juvenile onset diabetes mellitus while 33% of those with AA or AG alleles presented with similar adverse effects88. However, this finding was not supported in other studies looking at the link between SNPs in the CTLA4 gene and risk for primary thyroid disorder 100,101. Although in a meta-analysis of studies evaluating 49A>G SNP, this polymorphism was associated with increased susceptibility to Grave’s ophthalmopathy in the general Chinese population, none of the polymorphisms evaluated within individual patients were confirmed to be associated with Grave’s ophthalmopathy 101.
[H3]Monitoring and management
No definitive recommendations regarding monitoring for primary hypothyroidism in patients undergoing immunotherapy have been reported. In our recommendation, monitoring patients for signs of hypothyroidism, such as fatigue, weight gain, and cold intolerance, is important. In addition to baseline thyroid function tests (TFTs; such as serum TSH and free T4) before initial immunotherapy, subsequent TFTs should be measured during treatment. When a patient notes any signs of thyroid dysfunction, TFTs should be measured. If evidence of hyperthyroidism or hypothyroidism is recorded, thyroid autoantibodies can also be measured 102. Primary hypothyroidism should be treated using levothyroxine hormone replacement therapy, while subclinical cases would favour further observation (such as in patients who are asymptomatic with levels of TSH <10 and normal levels free T4) 103. In severe thyrotoxicosis before progression to hypothyroidism, administering corticosteroid in those patients with severe symptoms might be prudent, while β blockers could be useful for the treatment of symptoms and signs of thyrotoxicosis, such as hand tremor and tachycardia 103. Those patients with mild symptoms of hyperthyroidism from thyroiditis can be observed and monitored for symptom progression as well as the development of permanent hypothyroidism.
Radioactive iodine uptake can be used to distinguish Grave’s disease from thyroiditis 104. Specifically, increased uptake of iodine is consistent with Grave’s disease while low uptake would be consistent with thyroiditis 104. However, given the frequent use of iodinated CT contrast in those patients undergoing cancer therapy, radioactive iodine uptake might not be a very sensitive test as the thyroid would be saturated with iodine resulting in low uptake regardless of the aetiology of hyperthyroidism 40. In addition, a high level of serum TSH-receptor antibody and the presence of ophthalmopathy would be consistent with Grave’s disease instead of thyroiditis 103.
Adrenalitis
Although extremely rare, adrenalitis and subsequent primary adrenal insufficiency believed to be associated with ipilimumab therapy have been reported 105,106. Bilateral adrenal gland enlargement after ipilimumab treatment has been reported in a patient who had normal sized-adrenal glands at baseline and simultaneous hypophysitis 105. The patient’s cortisol and aldosterone concentrations soon after the diagnosis of hypophysitis did not respond to cosyntropin stimulation, which indicates primary adrenal insufficiency; the size of the adrenal glands normalized within 6 weeks105. In a case report of a 79-year-old patient with metastatic melanoma, symmetric, smoothly enlarged, hypermetabolic adrenal glands were seen after 3 months of ipilimumab treatment 106. The patient had normal sized adrenal glands at baseline, which normalized in size and metabolic activity on follow-up scans performed after 8 months after the end of therapy. This patient also had an elevated cortisol level at 4 months when the adrenal gland enlargement was first noted. ACTH levels were unreported, cortisol levels were normal at 8 months 106. When adrenal enlargement is seen in patients, assessing adrenal function through the measurement of ACTH and cortisol levels and likely a cosyntropin stimulation test is important to rule out primary adrenal insufficiency 57.
PD1 antibodies
Pembrolizumab and nivolumab (formerly known as MK-3476 and MDX-1106, respectively) were approved by the FDA for the treatment of patients already treated with ipilimumab for unresectable or metastatic melanoma and disease progression107. Both antibodies inhibit the interaction between PD1 and its ligands and increases the immune response against cancer cells 108,20 and are efficacious against non-small cell lung cancer, renal cell cancer, bladder cancer and Hodgkin lymphoma 109,110,111. Unlike ipilimumab, in which hypophysitis is one of the more severe and frequent endocrine adverse effects, PD1 antibody therapy has not been linked to this same increased incidence of hypophysitis, which occurs in ≤1% of patients 12
Primary thyroid dysfuction
Some investigators have reported that between 39.0 and 54.2% of patients treated with PD1 antibodies experience an immune related adverse event 11. Of those patients, 4.7–6.0% had grade 3 or 4 endocrine adverse events based on the Common Terminology Criteria for Adverse Events 112). In our literature review, the most commonly reported endocrine adverse event with PD1 antibody therapy was hypothyroidism, with an incidence of 160 of 2,573 cases (~5.9%; Table 3) 11,12,26,66,67,109,107,113,114,115,116,117. Hyperthyroidism was recorded in 1.0–4.7% of patients (mean 3.3%; 71 of 2,153). As the specific clinical presentations, laboratory test results and subsequent management of thyroid dysfunction in these patients were not discussed in these clinical trials, we are unable to determine the precise aetiologies of these. The time to occurrence of thyroid abnormalities was not indicated in the trials we reviewed; however, primary hypothyroidism has been reported to present sometime between 5 months to 3 years after PD1 antibody treatment, but these data include a ipilimumab–nivolumab combination trial 32. In a report of 10 patients with nivolumab-related thyroiditis, individuals had abnormal TFTs ~3–8 weeks after the first dose of nivolumab 118. Six of these 10 cases of thyroiditis were seen during the transient thyrotoxic phase. TSH receptor antibodies were absent in all patients, but four individuals were positive for thyrotropin binding inhibitory immunoglobulins and/or TPO antibodies 118. Along with a low levels of TSH and elevated free T4 and T3, clinical presentations included fatigue and palpitations 3–6 weeks after the first anti-PD1 treatment. These patients all developed hypothyroidism, which required thyroid hormone replacement therapy. In the remaining four of 10 cases, hypothyroidism was found without the thyrotoxic phase at ~6–8 weeks after initial nivolumab treatment; of these, three patients also had anti-thyroglobulin and two anti-TPO antibodies present in their serum. This finding suggests that although both hypothyroidism groups have a common disease process, those with hypothyroidism but without thyrotoxicosis might have had a subclinical presentation of the thyrotoxic phase, which is, therefore, undetected.
Table 3.
Endocrine IRAEs with PD1 and PDL1 antibodies
| PD1 antibodies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Antibody | Cohort | Endocrinopathy | ||||||
| Age (range) | n | Hypothyroidism | Hyperthyroidism | Adrenal insufficency | Hypophysitis | Other thyroid# | T1DM | ||
| Topalian, et al(2012)12 | Nivolumab | 63 (29–85) | 296 | 7ǂ | 3 | NR | 2§ | NR | NR |
| Topalian, et al(2014)11 | Nivolumab | 61 (29–85) | 107 | 6ǂ | 2 | NR | 1 | 1 | NR |
| Motzer, et al(2015)109|| | Nivolumab | 61 (SD 9) | 168 | 10ǂ | NR | NR | NR | NR | NR |
| Gettinger, et al(2015)67## | Nivolumab | 65 (38–85) | 129 | NR | NR | NR | NR | NR | NR |
| Rizvi, et al(2015)113 | Nivolumab | 65 (57–71) | 117 | 3ǂ | NR | 1ǂ | NR | 1 | NR |
| Brahmer, et al(2015)114 | Nivolumab | 62 (39–85) | 135 | 5ǂ | NR | NR | NR | NR | NR |
| Robert, et al(2015)107 | Nivolumab | 64 (18–86) | 210 | 9ǂ | 7ǂ | NR | 1 | NR | 1 |
| Larkin, et al(2015)132 | Nivolumab | 59 (25–90) | 316 | 27ǂ | 13ǂ | NR | 2 | NR | NR |
| Robert, et al(2014)66 | Pembrolizumab | 59 (18–88) | 173 | 7ǂ | 3ǂ | NR | 2 | NR | NR |
| Robert, et al(2015)115 | Pembrolizumab | 61–63 (18–89) | 556 | 52ǂ | 27ǂ | NR | 3 | NR | 2 |
| Garon, et al(2015)116 | Pembrolizumab | 64 (28–93) | 495 | 34ǂ | 9ǂ | NR | NR | NR | NR |
| Total | 2702 | 160 (5.9%) | 71 (3.3%) | 2 (1.7%) | 10 (0.6%) | 1 (1.3%) | 2 (0.4%) | ||
| Event/Totalǂǂ | 160/2573 | 71/2153 | 2/117 | 10/1658 | 3/224 | 3/766 | |||
| PDL1 antibodies | |||||||||
| Study | Antibody | Cohort | Endocrinopathy | ||||||
| Age (range) | n | Hypothyroidism | Hyperthyroidism | Adrenal insufficency | Hypophysitis | Other thyroid# | T1DM | ||
| Brahmer, et al(2012) | MDX-1105 | 63 (29–83) | 207 | 6ǂ | NR | 2ǂ | NR | 2 | NR |
| McDermott, et al(2016) | Atezolizumab | 61 (33–81) | 70 | 6ǂ | NR | NR | NR | NR | NR |
| Total | 277 | 12 (4.3%) | NA | 2 (1.0%) | NA | 2 (1.0%) | NA | ||
| Event/Totalǂǂ | 12/277 | NA | 2/207 | NA | 2/207 | NA | |||
Includes autoimmune thyroiditis, unspecified thyroid disorders.
Unspecified cause.
Reported as <1% of 296 patients, but exact number of event not reported;
18 endocrinopathies reported by no specifics given other than 10 hypothyroidism.
Eight endocrinopathies reported but no specifics given.
Percentage determined by total number of events divided from total number of patients only from studies reporting event. IRAE, immune-related adverse event; NA, not applicable; NR, none reported; T1DM, type 1 diabetes mellitus
In another report, two possible cases of subclinical autoimmune thyroid dysfunction in patients undergoing nivolumab therapy were detailed 119. Both cases reported thyroid ultrasound findings that were consistent with Hashimoto’s thyroiditis with elevated levels of anti-thyroglobulin and TPO antibodies. One of these patients developed worsening primary hypothyroidism after the second administration of nivolumab while the other patient presented with initial hyperthyroidism immediately prior to the second administration of this anti-PD1 therapy. Unfortunately, this latter patient had limited follow-up and, therefore, this patient’s ultimate thyroid status is unknown 119.
Painless thyroiditis has also been reported in a 55-year-old woman who developed dyspnea, anxiety, diarrhea and palpitations 3 weeks after her second nivolumab treatment; levels of TSH were undetectable and levels of free T4 (2.06 ng/dl) and T3 (554.2 pg/dl) were high 120. Thyroid autoantibody assays showed the patient had normal levels of TPO antibodies and normal thyroid-stimulation immunoglobulins with elevated thyroglobulin antibodies which indicated a level of thyroid autoimmunity; ultrasonography, which usually is not a diagnostic test for thyroiditis and can appear normal in mild cases, showed normal vasculature and density within the thyroid gland 120. This patient returned to a euthyroid state after therapy with β-blockers and withdrawal of immunotherapy, but it is important to note that thyrotoxicosis can still progress to a hypothyroid state as described above 118. Given this finding, nivolumab-induced hypothyroidism are likely to be sequela of thyroiditis.
[H3] Mechanism
The mechanism responsible for nivolumab-induced thyroid dysfunction is unclear. In a case series of patients presenting with painless thyroiditis and hypothyroidism following PD1 antibody therapy, 8 of 10 patients were positive for anti-thyroglobulin and anti-thyroid peroxidase antibodies, and all were negative for thyrotropin binding inhibitory immunoglobulins 118. While not verified, the investigators hypothesized that polymorphic variants in the PD1 gene in some individuals might predispose them to an increased risk of endocrine dysfunction 118. Future studies to understand the mechanism by which PD1 antibodies affect the thyroid tissue are necessary.
[H3] Management
When faced with transient thyrotoxicosis, physicians should act rapidly to ensure the best outcomes for their patients. Supportive therapy with β blockers can help to alleviate adrenergic symptoms and signs of hyperthyroidism, and immunotherapy could be held if severe symptoms are present 120. Radioactive iodine uptake can be inaccurate given the frequency with which iodine contrast-enhanced imaging is used in cancer patients 40. In the thyrotoxic phase of thyroiditis, TSH is expected to be low with elevated levels of free T4 or T3. Immunoassays to detect levels of TPO and thyroglobulin antibodies can be used to understand the autoimmune aetiology of thyroiditis 118. Certain TSH receptor antibodies, such as thyrotropin-stimulating immunoglobulins, can be used to distinguish Hashimoto’s thyroiditis from Grave’s disease to direct appropriate management 121.
If thyroiditis is in the thyrotoxic phase, the patient should be monitored for symptoms, signs and laboratory test abnormalities consistent with progression to hypothyroidism. Levothyroxine hormone replacement should be administered to treat overt hypothyroidism 12. In patients that have yet to experience this IRAE, patients should be monitored for any thyroid dysfunction. Although no established schedule for monitoring for thyroid dysfunction in those undergoing anti-PD1 therapy exists, close observation for signs of hyperthyroidism or hypothyroidism during follow-up visits is recommended. We further recommend that TFTs are monitored in those undergoing anti-PD1 therapy. Given that primary thyroid dysfunction can present as early as 3 weeks and as late as 3 years after treatment 120,32, TFTs should be monitored monthly for the first 6 months of treatment. For the management with CTLA4 immunotherapy, we recommend that for those receiving PD1 antibodies, if thyroid function is normal and the patient is asymptomatic, testing could be done every 3 months for 6–12 months followed by every 6 months for years 2 and 3. When patients have symptoms or signs of thyroid dysfunction, they should have a prompt evaluation for anti-PD1-induced thyroid dysfunction, especially given that fatigue is the most common overall adverse event in patients receiving PD1 blockade.
Hyperglycaemia
Although rare, PD1 pathway blockade can lead to diabetes mellitus 122,123,124,125. Anti-PD1 therapy was responsible for eight cases of type 1 diabetes mellitus (T1DM) with an additional case observed in a patient treated with anti-PDL1 therapy. Seven out of nine patients with T1DM initially presented with diabetic ketoacidosis (DKA) with the remaining two patients presenting with severe hyperglycemia122,123,124,125. Overall, the presence of GAD65 antibodies, a marker of T1DM along with DKA were found in five patients undergoing nivolumab treatment while three patients with had DKA or were GAD65 positive 124,122,123,125.
While the mechanism underlying the onset of T1DM in patients receiving PD1 immunotherapy is not well understood, the modulation of T-cell regulatory function has been suggested to be responsible for this IRAE 124. Specifically, three of five patients were found to have T1DM-specific autoantibodies (GAD65), and 40% presenting with upregulation of CD8+ T-cell response to a T1DM antigen 124.
Although patients with PD1 immunotherapy-related T1DM should be treated with insulin, no screening guidelines have been established to detect T1DM in these patients. Given the morbidity related to DKA and hyperglycaemia, clinicians managing patients undergoing anti-PD1 therapy should carefully monitor patients for elevated levels of blood sugar.
PDL1 antibodies
Endocrine-related IRAEs in response to PDL1 antibodies should also be managed using hormone replacement therapies, and reintroduction to treatment, when appropriate 26. Mechanistically, polymorphisms in genes coding for PDL1 or PD1 might increase the susceptibility to autoimmune disease 126,127,128. In a phase I clinical trial of the PDL1 antibody, MDX-1105, adverse events occurred in 39% of the 207 patient cohort with only a small number (n = 10; 5%) presenting with incidences above grade 3 26. Hypothyroidism was seen in six out of 207 patients (3%), adrenal insufficiency in two patients (1%) and autoimmune thyroiditis in another 1% of the cohort 26. In these studies clinical presentation or laboratory test results were not reported to determine if hypothyroidism was secondary to thyroiditis, if adrenal insufficiency was primary or secondary or the nature of the autoimmune thyroiditis. Phase I trials of atezolizumab and durvalumab also had elevated incidences of IRAEs compared with those seen in trials of MDX-1105. Specifically, hypothyroidism was reported in six of 70 patients in the atezolizumab cohort (9%) and 10 out of 99 in the durvalumab cohort (10.1%) 129,117. Although the elevated incidence of hypothyroidism with durvalumab might be partially explained by the combined administration with tremelimumab in this trial, why atezolizumab would have an increased incidence of hypothyroidism is unclear. Further, specific clinical and biochemical presentations were not reported, which makes determining the aetiology of the hypothyroidism difficult.
When comparing the adverse effects of PDL1 and PD1 antibody therapy, the overall adverse event rates (41% in PD1, 30% in PDL1) and endocrine-related rates (4% for both PD1 and PDL1) were comparable 26,12. However, hypothyroidism was reported in 4.3% of the PDL1 cohort compared with 5.9% in those receiving PD1 26,12,11. Although reports of thyroiditis were rare (~1%) in patients from PDL1 cohort and limited to individual case reports in PD1 cohort 26,12,11,119,120, this finding could be due to patients being misclassified as having hypothyroidism and not as thyroiditis. The incidence of thyroiditis might, therefore, be higher than that reported in the literature.
PDL1 also binds CD80 in addition to its interaction with PD1 receptors on activated T cells, which might explain differences in the reactions to PDL1 and PD1 inhibitors. Specifically, PDL1 antibodies would affect its target ligand’s interaction with both CD80 and PD1 receptors, while blockade of PD1 will result in inhibition of its interaction with PDL1 and PDL2 (a subtype of B7 family ligands related to PDL1) ligands 123. This complex set of ligand––receptor interactions by PD1 and PDL1 proteins might account for the differences in incidence of endocrine-related adverse effects. 130.
Combination therapies
Combined CTLA4 and PD1 blockade has been explored in preclinical models and clinical trials in metastatic melanoma 50,131,132,50,133,5. In our review of combination therapy clinical trials, the incidence of hypothyroidism was 13.9% (64 out of 462 cases) and hypophysitis at 8.0% (37 out of 462 cases; Table 4) in patients who received this double therapy. In a phase I trial, the combination of ipilimumab and nivolumab had incidences of 13% primary hypothyroidism (6 out of 45 patients), 9% thyroiditis (4 out of 45), and 9% hypophysitis (4 out of 45) 32. These data suggest that additive effects of the two therapies might contribute to certain IRAEs. Moreover, 53% of the cohort undergoing concurrent administration of nivolumab and ipilimumab presented with grades 3 to 4 incidences of all treatment-related adverse events, compared with only 18% of the cohort who received the treatment sequentially, 20% in ipilimumab only study, and 15% in nivolumab only study 50,7,12. In a phase II trial of concurrent administration of nivolumab and ipilimumab, a significantly higher rate of objective response (61%) in combination group than in the ipilimumab monotherapy group (11%) was reported (P <0.001) 131. As seen in previous combination therapy trials, the incidence of grade 3 to 4 adverse events were elevated at 54% in combination cohort versus 24% of monotherapy group. Specifically, hypophysitis was observed in 12% (7% in monotherapy group), and hypothyroidism in 16% (15% in monotherapy group). Similarly, in a subgroup analysis of CheckMate 067 phase III trial evaluating the efficacy of nivolumab and ipilimumab an increased incidence of overall grade 3 to 4 adverse events in combination group (55%) compared with 16.3% in nivolumab only group and 27.3% in ipilimumab group was seen134. In those patients who received more than one immunotherapeutic agent, we recommend the clinician assessed thyroid, pituitary and adrenal function, and the presence of hyperglycaemia as described for monotherapy earlier in the text. The additive side effects of combination checkpoint blockade therapy should be further examined.
Table 4.
Endocrine IRAEs with PD1–CTLA4 combination therapy
| Study | Cohort Characteristics | Endocrinopathy | |||||
|---|---|---|---|---|---|---|---|
| Age (range) | n | Hypothyroidism | Hyperthyroidism | Adrenal Insufficiency | Hypophysitis | Other thyroid# | |
| Wolchok et al (2013)50 | 58 (22–79) | 53ǂ | 2§ | 2§ | 2§ | 2§ | 3§ |
| Postow et al (2015)131 | 64 (27–87) | 95 | 15§ | 4§ | 6§ | 11§ | 22§ |
| Larkin et al (2015)132 | 59 (18–88) | 314 | 47§ | 31§ | NR | 24§ | NR |
| Total | 462 | 64 (13.9%) | 37 (8.0%) | 8 (5.4%) | 37 (8.0%) | 25 (16.9%) | |
| Event/total|| | 64/462 | 37/462 | 8/148 | 37/462 | 25/148 | ||
Includes autoimmune thyroiditis, unspecified thyroid disorders.
Only concurrent combo therapy reported. Sequenced treatrment arm (n = 33) was not.
Unspecified cause.
Percentage determined by total number of events divided from total number of patients only from studies reporting event. IRAE, immune-related adverse events. NR, not reported.
Adverse events and treatment reponse
The clinical response to immunotherapy has been associated with the occurrence of IRAEs 64,38. In one study, all three of the 14 patients in the cohort who had a clinical response to CTLA4 and IL-2 combination therapy also had grade 3–4 IRAE toxicities 64. Furthermore, other investigators describe of two adjuvant ipilimumab trials for patients with stage 3/4 melanoma in which a significant correlation exists between relapse free survival and presentation of IRAEs 38,135. Finally, patients presenting with ipilimumab-related hypophysitis have been reported to have a median survival time of 19.4 months compared with 8.8 months for those not presenting with hypophysitis (P = 0.05) 36. While encouraging, the investigators postulate that this analysis might be biased, as those who do not survive long enough during the trial do not get the prolonged observation of those patients with IRAEs36. Further analysis of the relationship between IRAEs and clinical outcomes must be conducted to understand benefits, if any, of such phenomena.
Conclusions
Major advances in the understanding of the immune response in cancer have led to rapid progress in clinical immunotherapy trials in the past decade. Although immunotherapies lack the traditional profile of chemotherapy-related adverse effects, a rare, yet major set of IRAEs has emerged. In clinical trials, an increased susceptibility to hypophysitis in those treated with CTLA4-targeted immunotherapy has been revealed. PD-1-targeted treatments have been predominately linked with primary thyroid dysfunction with a few rare cases of type 1 diabetes mellitus. Despite the current clinical understanding of endocrine IRAEs, which has led to effective treatment strategies with hormonal replacement, additional studies are needed to further understand the clinical characteristics and chronology of these adverse effects and clarify the mechanism by which immunotherapy results in endocrinopathies.
Key points.
The emergence of cancer immunotherapy has revolutionized cancer treatment, but is associated with serious immune-related adverse effects (IRAEs)
CTLA4-targeted immunotherapy is associated with increased susceptibility to hypophysitis and primary thyroid dysfunction
PD1-targeted immunotherapy is associated with primary thyroid dysfunction and type 1 diabetes mellitus
CTLA4 and PD1 combination therapy has an elevated incidence of hypothyroidism and possibly comparable incidence rates of hypophysitis to monotherapy with CTLA4 antibodies
IRAEs might be associated with improved clinical response of immunotherapy to tumours, but further studies are needed to evaluate this possible effect
Biographies
David J. Byun is currently a research fellow at the Department of Radiation Oncology at Memorial Sloan Kettering Cancer Center, and an MD candidate at Weill Cornell Medical College, both New York, USA. His primary research interests are in optimization of radiotherapy in treating prostate cancer, and in evaluating the abscopal phenomenon associated with combining radiation with immunotherapy.
Jedd D. Wolchok is the Lloyd J. Old/Virginia and Daniel K. Ludwig Chair in Clinical Investigation, and Chief of Melanoma & Immunotherapeutics Service at Memorial Sloan Kettering Cancer Center, New York, USA. He received his MD and PhD from New York University, where he also completed his residency program. Following his fellowship training at Memorial Sloan Kettering, he remained on the faculty. Dr Wolchok is a leader in cancer immunotherapy research as a clinician-scientist. As a principal investigator of several important clinical trials, his studies have contributed to the approval of ipilimumab and nivolumab for use in advanced melanoma.
Lynne M. Rosenberg is currently an MD candidate at Weill Cornell Medical College in New York, USA. She completed her undergraduate studies in Integrative Biology at University of California, Berkeley, USA. Her research interests are in understanding the role of cancer immunotherapy in the pediatric population.
Monica Girotra currently serves as the endocrinologist for the Pituitary and Skull Based Tumor Center at Memorial Sloan Kettering Cancer Center, New York, USA. Following her medical education and internal medicine residency at Weill Cornell Medical College, she completed her endocrinology fellowship at Columbia University Medical Center, both New York, USA. Her research focuses are in pituitary disorders, metabolic bone disease, and endocrine complications of immunotherapy.
Footnotes
Competing interests
J.D.W is a consultant and receives research funding from AstraZeneca, Bristol-Myers Squibb, Genentech, Merck and Medimmune. M.G. has been a consultant for AstraZeneca and Bristol-Myers Squibb. The author authors declare no competing interests.
Author contributions
D.J.B. and M.G. researched data for the article, made substantial contribution to discussion of the content, wrote and reviewed/edited the manuscript before submission. J.D.W. made substantial contribution to discussion of the content and reviewed/edited the manuscript before submission. L.M.R. researched data for the article and wrote the manuscript.
References
- 1.Pandolfi F, et al. Strategies to overcome obstacles to successful immunotherapy of melanoma. Int J Immunopathol Pharmacol. 2008;21:493–500. doi: 10.1177/039463200802100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart TJ, Smyth MJ. Improving cancer immunotherapy by targeting tumor-induced immune suppression. Cancer Metastasis Rev. 2011;30:125–140. doi: 10.1007/s10555-011-9280-5. [DOI] [PubMed] [Google Scholar]
- 4.Linsley PS, et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr Opin Immunol. 2006;18:206–213. doi: 10.1016/j.coi.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 9.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 10.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 14.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 17.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 21.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 22.Taube JM, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow LQ. Exploring novel immune-related toxicities and endpoints with immune-checkpoint inhibitors in non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2013 doi: 10.1200/EdBook_AM.2013.33.e280. [DOI] [PubMed] [Google Scholar]
- 24.Peggs KS, Quezada SA, Allison JP. Cell intrinsic mechanisms of T-cell inhibition and application to cancer therapy. Immunol Rev. 2008;224:141–165. doi: 10.1111/j.1600-065X.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- 25.Herbst RS, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 26.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 28.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–5283. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 29.Torino F, et al. Endocrine side-effects of anti-cancer drugs: mAbs and pituitary dysfunction: clinical evidence and pathogenic hypotheses. Eur J Endocrinol. 2013;169:R153–164. doi: 10.1530/EJE-13-0434. [DOI] [PubMed] [Google Scholar]
- 30.Torino F, Barnabei A, De Vecchis L, Salvatori R, Corsello SM. Hypophysitis induced by monoclonal antibodies to cytotoxic T lymphocyte antigen 4: challenges from a new cause of a rare disease. Oncologist. 2012;17:525–535. doi: 10.1634/theoncologist.2011-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corsello SM, et al. Endocrine side effects induced by immune checkpoint inhibitors. J Clin Endocrinol Metab. 2013;98:1361–1375. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 32.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–381. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwama S, et al. Pituitary expression of CTLA-4 mediates hypophysitis secondary to administration of CTLA-4 blocking antibody. Sci Transl Med. 2014;6:230ra245. doi: 10.1126/scitranslmed.3008002. [DOI] [PubMed] [Google Scholar]
- 34.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer. 2011;11:805–812. doi: 10.1038/nrc3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 36.Faje AT, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99:4078–4085. doi: 10.1210/jc.2014-2306. [DOI] [PubMed] [Google Scholar]
- 37.Eatrides J, et al. AACR advances in melanoma: from biology to therapy. Philadelphia, PA: 2014. [Google Scholar]
- 38.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 39.Yang JC, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamnvik OP, Larsen PR, Marqusee E. Thyroid dysfunction from antineoplastic agents. J Natl Cancer Inst. 2011;103:1572–1587. doi: 10.1093/jnci/djr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichert JM. Marketed therapeutic antibodies compendium. MAbs. 2012;4:413–415. doi: 10.4161/mabs.19931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribas A, et al. Phase III, open-label, randomized, comparative study of tremelimumab (CP-675,206) and chemotherapy (temozolomide [TMZ] or dacarbazine [DTIC]) in patients with advanced melanoma. ASCO Annual Meeting Proceedings. 2008;26:LBA9011. [Google Scholar]
- 43.Ribas A, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung KY, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol. 2010;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 45.Voskens CJ, et al. The price of tumor control: an analysis of rare side effects of anti-CTLA-4 therapy in metastatic melanoma from the ipilimumab network. PLoS One. 2013;8:e53745. doi: 10.1371/journal.pone.0053745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caturegli P, et al. Autoimmune hypophysitis. Endocr Rev. 2005;26:599–614. doi: 10.1210/er.2004-0011. [DOI] [PubMed] [Google Scholar]
- 47.Chodakiewitz Y, Brown S, Boxerman JL, Brody JM, Rogg JM. Ipilimumab treatment associated pituitary hypophysitis: clinical presentation and imaging diagnosis. Clin Neurol Neurosurg. 2014;125:125–130. doi: 10.1016/j.clineuro.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Albarel F, et al. Long-term follow-up of ipilimumab-induced hypophysitis, a common adverse event of the anti-CTLA-4 antibody in melanoma. Eur J Endocrinol. 2015;172:195–204. doi: 10.1530/EJE-14-0845. [DOI] [PubMed] [Google Scholar]
- 49.Min L, et al. Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: a retrospective cohort study. Clin Cancer Res. 2015;21:749–755. doi: 10.1158/1078-0432.CCR-14-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landek-Salgado MA, Leporati P, Lupi I, Geis A, Caturegli P. Growth hormone and proopiomelanocortin are targeted by autoantibodies in a patient with biopsy-proven IgG4-related hypophysitis. Pituitary. 2012;15:412–419. doi: 10.1007/s11102-011-0338-8. [DOI] [PubMed] [Google Scholar]
- 52.Faje A. Immunotherapy and hypophysitis: clinical presentation, treatment, and biologic insights. Pituitary. 2016;19:82–92. doi: 10.1007/s11102-015-0671-4. [DOI] [PubMed] [Google Scholar]
- 53.Boasberg P, Hamid O, O’Day S. Ipilimumab: unleashing the power of the immune system through CTLA-4 blockade. Semin Oncol. 2010;37:440–449. doi: 10.1053/j.seminoncol.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Kaehler KC, et al. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin Oncol. 2010;37:485–498. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Juszczak A, Gupta A, Karavitaki N, Middleton MR, Grossman AB. Ipilimumab: a novel immunomodulating therapy causing autoimmune hypophysitis: a case report and review. Eur J Endocrinol. 2012;167:1–5. doi: 10.1530/EJE-12-0167. [DOI] [PubMed] [Google Scholar]
- 56.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 57.Min L, Vaidya A, Becker C. Association of ipilimumab therapy for advanced melanoma with secondary adrenal insufficiency: a case series. Endocr Pract. 2012;18:351–355. doi: 10.4158/EP11273.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falorni A, Minarelli V, Bartoloni E, Alunno A, Gerli R. Diagnosis and classification of autoimmune hypophysitis. Autoimmun Rev. 2014;13:412–416. doi: 10.1016/j.autrev.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 59.Blansfield JA, et al. Cytotoxic T-lymphocyte-associated antigen-4 blockage can induce autoimmune hypophysitis in patients with metastatic melanoma and renal cancer. J Immunother. 2005;28:593–598. doi: 10.1097/01.cji.0000178913.41256.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber JS, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 61.Glezer A, Bronstein MD. Pituitary autoimmune disease: nuances in clinical presentation. Endocrine. 2012;42:74–79. doi: 10.1007/s12020-012-9654-7. [DOI] [PubMed] [Google Scholar]
- 62.Maker AV, et al. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother. 2006;29:455–463. doi: 10.1097/01.cji.0000208259.73167.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carpenter KJ, Murtagh RD, Lilienfeld H, Weber J, Murtagh FR. Ipilimumab-induced hypophysitis: MR imaging findings. AJNR Am J Neuroradiol. 2009;30:1751–1753. doi: 10.3174/ajnr.A1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quirk SK, Shure AK, Agrawal DK. Immune-mediated adverse events of anticytotoxic T lymphocyte-associated antigen 4 antibody therapy in metastatic melanoma. Transl Res. 2015;166:412–424. doi: 10.1016/j.trsl.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robert C, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 67.Gettinger SN, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ribas A, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 69.Camacho LH, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J Clin Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 70.Ralph C, et al. Modulation of lymphocyte regulation for cancer therapy: a phase II trial of tremelimumab in advanced gastric and esophageal adenocarcinoma. Clin Cancer Res. 2010;16:1662–1672. doi: 10.1158/1078-0432.CCR-09-2870. [DOI] [PubMed] [Google Scholar]
- 71.Kirkwood JM, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res. 2010;16:1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell AL, et al. Programmed death ligand 1 (PD-L1) gene variants contribute to autoimmune Addison’s disease and Graves’ disease susceptibility. J Clin Endocrinol Metab. 2009;94:5139–5145. doi: 10.1210/jc.2009-1404. [DOI] [PubMed] [Google Scholar]
- 73.Kristof RA, Van Roost D, Klingmuller D, Springer W, Schramm J. Lymphocytic hypophysitis: non-invasive diagnosis and treatment by high dose methylprednisolone pulse therapy? Journal of neurology, neurosurgery, and psychiatry. 1999;67:398–402. doi: 10.1136/jnnp.67.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chico A, et al. Reversible endocrine dysfunction and pituitary stalk enlargement. Journal of endocrinological investigation. 1998;21:122–127. doi: 10.1007/BF03350326. [DOI] [PubMed] [Google Scholar]
- 75.Hinrichs CS, Palmer DC, Rosenberg SA, Restifo NP. Glucocorticoids do not inhibit antitumor activity of activated CD8+ T cells. J Immunother. 2005;28:517–524. doi: 10.1097/01.cji.0000177999.95831.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harmankaya K, et al. Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Med Oncol. 2011;28:1140–1144. doi: 10.1007/s12032-010-9606-0. [DOI] [PubMed] [Google Scholar]
- 77.Amin A, et al. Evaluation of the effect of systemic corticosteroids for the treatment of immune-related adverse events (irAEs) on the development or maintenance of ipilimumab clinical activity. 2009 ASCO Annual Meeting. 2009;27 [Google Scholar]
- 78.Grob JJ, Hamid O, Wolchok J. Proceedings of the Joint ECCO 15–34th ESMO Multidisciplinary Congress; Berlin, Germany. 2009. [Google Scholar]
- 79.Di Giacomo AM, Biagioli M, Maio M. The emerging toxicity profiles of anti-CTLA-4 antibodies across clinical indications. Semin Oncol. 2010;37:499–507. doi: 10.1053/j.seminoncol.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 80.YERVOY (ipilimumab): serious and fatal immune-mediated adverse reactions. Ipilimumab US prescribing information: risk evaluation and mitigation strategy (REMS) 2012 < http://www.yervoy.com/hcp/rems.aspx>.
- 81.Oelkers W. Hyponatremia and inappropriate secretion of vasopressin (antidiuretic hormone) in patients with hypopituitarism. N Engl J Med. 1989;321:492–496. doi: 10.1056/NEJM198908243210802. [DOI] [PubMed] [Google Scholar]
- 82.Attia P, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller KK, et al. Androgen deficiency in women with hypopituitarism. J Clin Endocrinol Metab. 2001;86:561–567. doi: 10.1210/jcem.86.2.7246. [DOI] [PubMed] [Google Scholar]
- 84.Kaplan MM, et al. Prevalence of abnormal thyroid function test results in patients with acute medical illnesses. Am J Med. 1982;72:9–16. doi: 10.1016/0002-9343(82)90565-4. [DOI] [PubMed] [Google Scholar]
- 85.Agabegi SS, Derby EA. Step-up To Medicine. 3. Lippincott Williams & Wilkins; 2013. pp. 170–171. [Google Scholar]
- 86.Downey SG, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ku GY, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eggermont AM, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 89.Small EJ, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 90.Weber JS, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 91.Ansell SM, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–6453. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Day SJ, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 93.Hersh EM, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 94.Royal RE, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–833. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Di Giacomo AM, et al. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy) Cancer Immunol Immunother. 2011;60:467–477. doi: 10.1007/s00262-010-0958-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Min L, Vaidya A, Becker C. Thyroid autoimmunity and ophthalmopathy related to melanoma biological therapy. Eur J Endocrinol. 2011;164:303–307. doi: 10.1530/EJE-10-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Borodic G, Hinkle DM, Cia Y. Drug-induced graves disease from CTLA-4 receptor suppression. Ophthal Plast Reconstr Surg. 2011;27:e87–88. doi: 10.1097/IOP.0b013e3181ef72a1. [DOI] [PubMed] [Google Scholar]
- 98.Ueda H, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 99.Sanderson K, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 100.Bednarczuk T, Gopinath B, Ploski R, Wall JR. Susceptibility genes in Graves’ ophthalmopathy: searching for a needle in a haystack? Clin Endocrinol (Oxf) 2007;67:3–19. doi: 10.1111/j.1365-2265.2007.02854.x. [DOI] [PubMed] [Google Scholar]
- 101.Han S, et al. CTLA4 polymorphisms and ophthalmopathy in Graves’ disease patients: association study and meta-analysis. Hum Immunol. 2006;67:618–626. doi: 10.1016/j.humimm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 102.Sinclair D. Clinical and laboratory aspects of thyroid autoantibodies. Ann Clin Biochem. 2006;43:173–183. doi: 10.1258/000456306776865043. [DOI] [PubMed] [Google Scholar]
- 103.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 104.Sarkar SD. Benign thyroid disease: what is the role of nuclear medicine? Semin Nucl Med. 2006;36:185–193. doi: 10.1053/j.semnuclmed.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 105.Min L, Ibrahim N. Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol. 2013;1:e15. doi: 10.1016/S2213-8587(13)70031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bacanovic S, Burger IA, Stolzmann P, Hafner J, Huellner MW. Ipilimumab-Induced Adrenalitis: A Possible Pitfall in 18F-FDG-PET/CT. Clin Nucl Med. 2015;40:e518–519. doi: 10.1097/RLU.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 107.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 108.Blank C, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 109.Motzer RJ, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 112.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009 < https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf>.
- 113.Rizvi NA, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brahmer J, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Robert C, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 116.Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 117.McDermott DF, et al. Atezolizumab, an Anti-Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates From a Phase Ia Study. J Clin Oncol. 2016;34:833–842. doi: 10.1200/JCO.2015.63.7421. [DOI] [PubMed] [Google Scholar]
- 118.Orlov S, Salari F, Kashat L, Walfish PG. Induction of painless thyroiditis in patients receiving programmed death 1 receptor immunotherapy for metastatic malignancies. J Clin Endocrinol Metab. 2015;100:1738–1741. doi: 10.1210/jc.2014-4560. [DOI] [PubMed] [Google Scholar]
- 119.Narita T, et al. Serological aggravation of autoimmune thyroid disease in two cases receiving nivolumab. J Dermatol. 2016;43:210–214. doi: 10.1111/1346-8138.13028. [DOI] [PubMed] [Google Scholar]
- 120.Verma I, Modi A, Tripathi H, Agrawal A. Nivolumab causing painless thyroiditis in a patient with adenocarcinoma of the lung. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nielsen CH, Hegedus L, Leslie RG. Autoantibodies in autoimmune thyroid disease promote immune complex formation with self antigens and increase B cell and CD4+ T cell proliferation in response to self antigens. Eur J Immunol. 2004;34:263–272. doi: 10.1002/eji.200324413. [DOI] [PubMed] [Google Scholar]
- 122.Gaudy C, et al. Anti-PD1 Pembrolizumab Can Induce Exceptional Fulminant Type 1 Diabetes. Diabetes Care. 2015;38:e182–183. doi: 10.2337/dc15-1331. [DOI] [PubMed] [Google Scholar]
- 123.Mellati M, et al. Anti-PD-1 and Anti-PDL-1 Monoclonal Antibodies Causing Type 1 Diabetes. Diabetes Care. 2015;38:e137–138. doi: 10.2337/dc15-0889. [DOI] [PubMed] [Google Scholar]
- 124.Hughes J, et al. Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care. 2015;38:e55–57. doi: 10.2337/dc14-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Martin-Liberal J, et al. Anti-programmed cell death-1 therapy and insulin-dependent diabetes: a case report. Cancer Immunol Immunother. 2015;64:765–767. doi: 10.1007/s00262-015-1689-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prokunina L, et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32:666–669. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- 127.Nielsen C, Hansen D, Husby S, Jacobsen BB, Lillevang ST. Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens. 2003;62:492–497. doi: 10.1046/j.1399-0039.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 128.Prokunina L, et al. Association of the PD-1.3A allele of the PDCD1 gene in patients with rheumatoid arthritis negative for rheumatoid factor and the shared epitope. Arthritis Rheum. 2004;50:1770–1773. doi: 10.1002/art.20280. [DOI] [PubMed] [Google Scholar]
- 129.Antonia S, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016;17:299–308. doi: 10.1016/S1470-2045(15)00544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Messal N, Serriari NE, Pastor S, Nunes JA, Olive D. PD-L2 is expressed on activated human T cells and regulates their function. Mol Immunol. 2011;48:2214–2219. doi: 10.1016/j.molimm.2011.06.436. [DOI] [PubMed] [Google Scholar]
- 131.Postow MA, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Larkin J, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Korman A, et al. Activity of anti-PD-1 in murine tumor models: role of “host” PD-L1 and synergistic effect of anti-PD-1 and anti-CTLA-4. J Immunol. 2007;178 [Google Scholar]
- 134.Larkin J, et al. 3303 Efficacy and safety in key patient subgroups of nivolumab (NIVO) alone or combined with ipilimumab (IPI) versus IPI alone in treatment-naïve patients with advanced melanoma (MEL) (CheckMate 067) European Journal of Cancer. 2015;51:S664– S665. [Google Scholar]
- 135.Weber JS, et al. Phase II trial of extended dose anti-CTLA-4 antibody ipilimumab (formerly MDX-010) with a multipeptide vaccine for resected stages IIIC and IV melanoma. 2009 ASCO Annual Meeting. 2009;27 [Google Scholar]