Abstract
Objective
Current practice guidelines do not recommend benzodiazepines for acute management of anxiety disorders in pediatric patients. However, in procedural settings, benzodiazepines are commonly used to relieve acute pre-procedural stress. This meta-analysis examines the efficacy and tolerability of benzodiazepines as short-term anxiolytics in children.
Method
PubMed was searched for randomized controlled trials assessing the efficacy of benzodiazepines as short-term anxiolytics in pediatric patients. Twenty-one trials involving a total of 1,416 participants were included. A fixed effects model was used to examine the standardized mean difference of improvement in anxiety levels compared to control conditions. In stratified subgroup and meta-regression, the effect of the specific agent, dose, timing, and setting of benzodiazepine treatment was examined.
Results
A significant benefit was seen for benzodiazepines compared to control (standardized mean difference = 0.71 [95% confidence interval, 0.60–0.82], k = 24, z = 12.7, p<0.001). There was also funnel plot asymmetry in this meta-analysis, suggesting some evidence of publication bias. Moderator analyses found that when benzodiazepines were used in dental or non-operating room procedures, they were more effective than when they were used in operating room procedures (test for subgroup differences Q2 = 6.34, p=0.04). Tolerability analysis revealed there was no significant difference in the risk of developing irritability or behavioral changes between benzodiazepine and control groups.
Conclusions
Benzodiazepines are effective and well-tolerated when used as short-term anxiolytics in procedural settings for pediatric patients. Further research is needed to determine whether benzodiazepines are effective in pediatric anxiety disorders.
Keywords: benzodiazepines, anxiety, children, meta-analysis
Introduction
When confronted with acute stressors (e.g. surgical or dental procedures), healthy children may experience acute anxiety attacks, characterized by intense motor and visceral activation, as well as distortions of perception, loss of concentration and impaired memory resulting in temporary debilitation [34]. Preoperative anxiety has been associated with post-procedural psychological and behavioral problems in children, including separation anxiety, eating anxiety, apathy, withdrawal, sleep problems and aggression towards authority [26]. Though generally short-lived, these maladaptive behaviors are nevertheless distressing and counterproductive to the recovery process in immediate post-procedure periods.
While the use of benzodiazepines for the treatment of anxiety disorders and acute anxiety in adults is robust [1, 20, 31], limited research exists regarding the effects of benzodiazepines in pediatric populations. Due to the concern for developing dependence and the lack of established efficacy studies [32], the use of benzodiazepines for anxiety in children is limited mostly to the use of short-acting agents (e.g. midazolam) as sedatives prior to surgical or dental procedures. The goal of this meta-analysis is to examine the efficacy of benzodiazepines as acute anxiolytics in pediatric populations in randomized, placebo-controlled trials.
Methods
Search Strategy
Three reviewers searched PubMed (1965-Aug 22, 2016) for relevant citations. Within PubMed, the search strategy (“Benzodiazepines”[Mesh] AND “Anxiety”[Mesh] OR “Anxiety Disorders”[Mesh]) AND (Randomized Controlled Trial[ptyp] AND (“infant”[MeSH Terms] OR “child”[MeSH Terms] OR “adolescent”[MeSH Terms])) was used. There were no language limitations in this initial search stage.
Inclusion/Exclusion Criteria
Trials were included in the meta-analysis if they were blinded randomized controlled trials (RCT) that assessed the efficacy of benzodiazepines for short-term anxiety in the pediatric population (age less than 18 years). Trials that involved adult populations or mixed adult/pediatric populations were excluded. Included trials were required to have reported the mean numerical measurements of anxiety levels in both the treatment and control groups. Trials were required to measure anxiety specifically, as an outcome. Trials that used rating scales to measure sedation rather than anxiety (e.g. Ramsey Sedation Scale) were excluded. Trials included had to have examined the acute anxiolytic effects of benzodiazepines. Trials that examined the effects of benzodiazepines on anxiety for longer than one day were excluded. Additionally, all RCTs were included if an inactive control condition (placebo or no placebo) was present. Trials that included an active control condition (e.g. parental presence, clowns, behavioral training) not also given to the benzodiazepine group were excluded. Studies written in a language other than English were also excluded.
Meta-Analytic Procedures
To extract data from included articles, customized Microsoft Excel spreadsheets were used. Data extracted included the type of benzodiazepine (midazolam or not midazolam), the route of administration (intranasal, intravenous, oral or rectal), the dose (in mg/kg) and the diazepam dose equivalent per kg, the timing of administration (in minutes prior to stressful event), the indication for benzodiazepine use (dental, operating room or non-surgical procedure), sample size, mean age of treatment and control groups, the types of outcomes rating scales used, and the timing of the endpoint measurement.
The primary outcome measure was the endpoint score on the anxiety rating scale used in the trial. Rating scales that measured sedation rather than anxiety were not included in the meta-analysis. The difference in anxiety levels between the benzodiazepine groups and the control groups was calculated as the standardized mean difference (SMD) using Comprehensive Meta-Analysis version 2 (Biostat, Englewood, New Jersey). This measure was favored over weighted mean difference because rating scales differed between the included studies. As there was some evidence of publication bias, a fixed-effect model was used for this meta-analysis. A fixed-effects model is considered more conservative in the case of publication bias because it gives less weight to smaller studies that are more prone to publication bias. Publication bias was assessed by plotting the effect size against standard error for each trial (i.e. a funnel plot). In addition, publication bias was statistically tested by the Egger test. Heterogeneity between trials was determined by Q-statistic and I2 statistic.
For secondary analysis, a stratified subgroup analysis was conducted using Comprehensive Meta-Analysis to assess the effects of (1) type of benzodiazepine (midazolam, not midazolam), (2) type of control condition (placebo, no placebo), (3) route of administration (oral, intranasal, intravenous, rectal), (4) setting of administration (dental, operating room, non-operating room). For trials that examined oral midazolam, an additional meta-regression examining the effects of dose and timing of medication was performed. The threshold for statistical significance was selected to be P<0.05 for all analyses.
Results
Included Trials
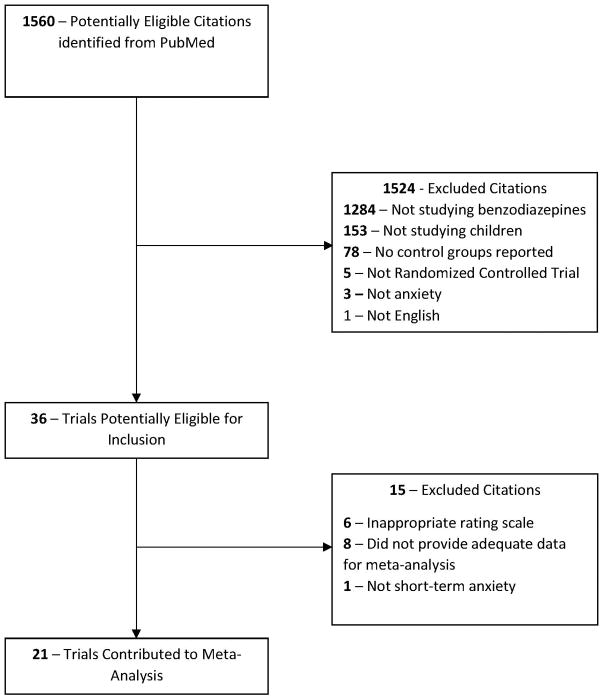
Twenty-one trials involving a total of 1,416 participants were included in this meta-analysis [9–15, 17–19, 21, 24, 25, 27, 29, 33, 35, 37, 39, 40, 42]. Figure 1 depicts the algorithm for selection of the included trials. A total of 24 treatment arms were included from the 21 eligible studies. Eighteen trials compared the use of a benzodiazepine with the use of a placebo, while three trials did not use a placebo [15, 24, 40]. All 21 trials used midazolam as the treatment medication. One of the trials also had a treatment arm using diazepam as the medication [42]. The setting of benzodiazepine use was dental procedure in 2 trials [9, 13], operating room procedure in 14 trials [11, 12, 14, 15, 21, 24, 25, 27, 29, 33, 35, 39, 40, 42], and non-operating room procedure in 5 trials [10, 17–19, 37].
Figure 1.
Selection of Studies.
Efficacy of Benzodiazepines in the Treatment of Acute Anxiety
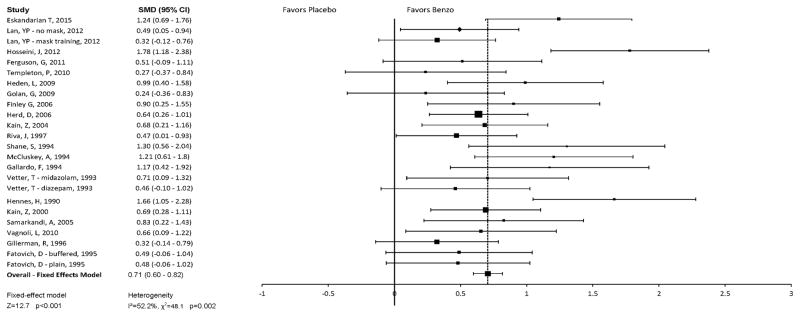
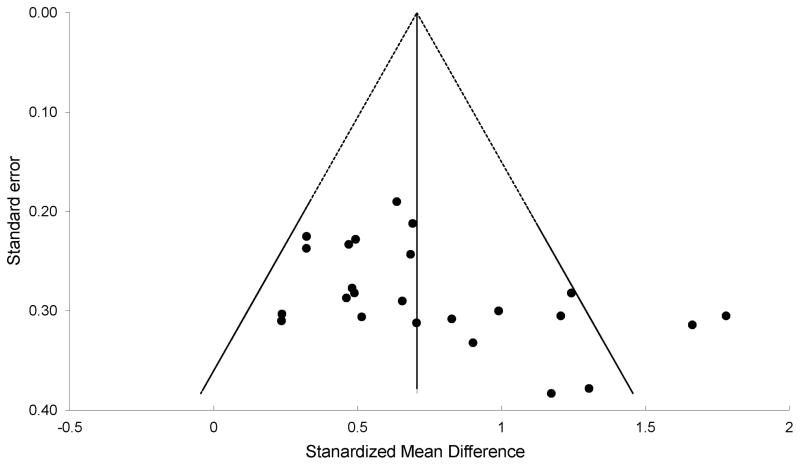
Figure 2 depicts a Forest plot of the reported efficacy of benzodiazepines compared with control conditions. Meta-analysis of all trials demonstrated a significant benefit of benzodiazepines compared to control (SMD = 0.71 [95% confidence interval (CI), 0.60–0.82], k = 24, z = 12.7, p<0.001) using a fixed-effects model. There was significant heterogeneity between trials (Q 23= 48.07, p = 0.002, I2 = 52.15%). Funnel plot asymmetry suggested evidence of publication bias [(Egger test intercept: 3.68 [95% CI, 0.49–6.88], t = 2.4, p = 0.03), see Figure 3 for funnel plot] although the results were unchanged when the trim-and-fill method was used to adjust for possible publication bias and funnel plot asymmetry. When a random-effects model was used in sensitivity analysis (which is less conservative in the case of publication bias), benzodiazepines still showed significant and similar benefits compared to placebo (SMD = 0.74 [95% CI: 0.58–0.90], k = 24, z = 9.02, p<0.001).
Figure 2. Forest plot: Efficacy of Benzodiazepines as Short-Term Anxiolytics in Pediatric Patients.
This plot shows mean standardized difference in anxiety levels of subjects given benzodiazepine pre-medication compared to subjects given the control condition. Overall, benzodiazepine treatment was more efficacious than control (SMD = 0.71 [95% confidence interval (CI), 0.60–0.82], k = 24, z = 12.7, p<0.001). There was significant heterogeneity between trials (Q 23= 48.07, p = 0.002, I2 = 52.15%) and some evidence of publication bias
Figure 3. Funnel Plot.
The funnel plot of trials included in this meta-analysis demonstrating asymmetry suggesting publication bias (Egger test intercept: 3.68 [95% CI, 0.49–6.88], t = 2.4, p = 0.03). Using the trim-and-fill method to adjust for asymmetry in the funnel plot did not change the overall estimate of the benefit of benzodiazepines compared to placebo.
Moderators of Benzodiazepine Efficacy in the Treatment of Acute Anxiety
The type of benzodiazepine was not significantly associated with the efficacy of therapy (test for subgroup differences: Q1 = 0.94, p = 0.33). Midazolam (SMD = 0.75, [95% CI, 0.59 – 0.92], z = 8.87, p<0.001) was not significantly more efficacious than diazepam (SMD = 0.46, [95% CI, −0.10 – 1.02], z = 1.61, p = 0.11).
The setting in which benzodiazepines were used was significantly associated with efficacy of therapy (test for subgroup differences: Q2 = 6.34, p=0.04). Trials done in the setting of dental (SMD = 1.22, [95% CI, 0.77 – 1.66], z = 5.36, p<0.001) or non-operating room procedures (SMD = 0.89, [95% CI, 0.52 – 1.25], z = 4.79, p<0.001) showed more efficacy than trials in the setting of the operating room (SMD = 0.64, [95% CI, 0.46 – 0.82], z = 6.95, p<0.001). However, when the analysis was restricted to trials involving midazolam only, the setting of use was not significantly associated with efficacy (test for subgroup differences Q2 = 5.86, p=0.054); dental and non-operating room settings remained more efficacious than operating room settings in trials involving midazolam only.
The route of administration was significantly associated with the efficacy of therapy (test for subgroup differences Q3 = 14.88, p = 0.002). Studies using intranasal (SMD = 1.50, [95% CI, 0.97 – 2.03], z = 5.59, p<0.001) and rectal (SMD = 1.30, [95% CI, 0.56 – 2.05], z = 3.45, p = 0.001) routes demonstrated greater efficacy than studies using oral (SMD = 0.67, [95% CI, 0.53 – 0.82], z = 8.92, p<0.001) or intravenous benzodiazepines (SMD = 0.41, [95% CI, 0.09 – 0.72], z = 2.54, p = 0.01). The findings remained unchanged when the analysis was restricted to the trials involving midazolam only.
The type of control condition was not significantly associated with the efficacy of therapy (test for subgroup differences Q1 = 1.42, p = 0.23). Studies that used a placebo for the control condition (SMD = 0.77, [95% CI, 0.59 – 0.95], z = 8.43, p<0.001) did not have a significantly different therapeutic efficacy than studies that did not use a placebo (SMD = 0.55, [95% CI, 0.24 – 0.86], z = 3.48, p < 0.001). The findings were similar when the analysis was restricted to the trials involving midazolam only.
The type of rating scale used to quantify anxiety levels was not significantly associated with the efficacy of therapy (test for subgroup differences Q2 = 2.51, p= 0.29). Studies that used the Visual Analog Scale (SMD = 1.11, [95% CI, 0.65 – 1.57], z = 4.73, p<0.001) or ordinal scales (SMD = 0.77, [95% CI, 0.52 – 1.02], z = 6.08, p<0.001) did not report a significantly greater medication efficacy than studies using the m-Yale pediatric anxiety scale (PAS) (SMD = 0.69, [95% CI, 0.45 – 0.94], z = 5.53, p<0.001). These results remained unchanged when the analysis was restricted to trials involving midazolam only.
In the subgroup of trials using oral midazolam, dose was not significantly associated with therapeutic efficacy (B =−0.50±0.48 [95% CI, −1.45 – 0.45], z = −1.0, p= 0.30). Timing of drug administration was also not significantly associated with efficacy (B = 0.003±0.005 [95% CI, −0.007 – 0.012], z = 0.5, p= 0.61).
Tolerability
Side-effects of benzodiazepine treatment were sparsely reported by included trials and other trials using benzodiazepines in acute procedural settings. Individual studies reported increased rates of memory problems [17, 23, 44] and hiccups [17, 23] in subjects assigned to midazolam compared to placebo. Behavioral changes and irritability were examined in 5 trials involving 215 subjects [17, 29, 37, 39, 44]. Benzodiazepine use was associated with a similar risk of irritability/behavioral changes compared to placebo (RR=0.74, 95%CI: 0.51 – 1.06, z= −1.63, p= 0.10). Other adverse effects were also reported, including: nausea/vomiting [17, 23, 37] and respiratory depression [19], but were not significantly different between benzodiazepines and placebo arms in individual studies.
Discussion
This meta-analysis demonstrated that benzodiazepines are significantly more effective than control conditions in treating anxiety in procedural settings in children. These results are consistent with a prior systematic review (but not a meta-analysis) [7]. This meta-analysis extends upon the previous literature by suggesting that benzodiazepines (1) have a demonstrated large effect size for reducing anxiety in procedural situations compared to inactive control conditions or placebo; (2) appear more effective outside the operating room setting and (3) seem to be fairly well tolerated in short-term procedural contexts.
While this meta-analysis indicates that benzodiazepines are effective as a short-term anxiolytic in procedural settings, there is evidence in the literature to support the efficacy of behavioral treatments for the reduction of acute anxiety in procedural settings as well[2, 6, 8, 24]. Parental presence and the use of clowns (who are specially trained to perform music and magic in the hospital setting) were demonstrated to be effective in reducing a child’s preoperative anxiety level [5, 8, 15, 40]. Various methods of distraction including art therapy [8], video games [6], and music [2, 24], have also been examined as methods to reduce pre-procedural anxiety. When confronting acute psychiatric and intermittent anxiety conditions, behavioral treatments should be the preferred approach. In these settings, cognitive behavioral therapy (CBT) focuses on techniques for managing somatic reactions, identifying anxiety triggers, and desensitization to feared stimuli, and has been shown to be effective for children and adolescents with social anxiety disorder [36]. A 2015 review also found robust evidence for an effective acute response to CBT in children with various anxiety disorders [45]. However, the role of benzodiazepines as an adjunct pharmacotherapy to CBT for anxiety disorders has yet not been thoroughly investigated, thus it is yet unclear whether they would be useful as adjunctive therapy to behavioral treatments in acute procedural settings.
The results of this meta-analysis may have relevance for the treatment of anxiety disorders in children. Previous research on the effects of benzodiazepines as an anxiolytic in children with anxiety disorders is sparse and severely underpowered. Additionally, antiquated diagnostic criteria and rating scales have been used to examine anxiety outcomes [3, 16, 38]. Small, randomized, placebo-controlled trials have failed to demonstrate a significant benefit of alprazolam for anxiety in school-refusal (n=24) and overanxious or avoidant disorders (n=30)[38] and clonazepam for mixed anxiety disorders in children (n=15) [16]. These three trials have also reported generally mild, non-impairing side effects of benzodiazepines including dry mouth, drowsiness, fatigue, blurred vision, abdominal pain, headache, and dizziness, but have not demonstrated a statistically significant higher risk of such side effects compared to placebo [3, 16, 38]. The trial involving clonazepam reported 2 children discontinued the study medication due to worsened disinhibition, oppositional behavior and irritability [16]. Based on this sparse data, a Cochrane Review appropriately concluded that “no controlled evidence could be found for the efficacy of benzodiazepines to warrant their increased prescription for pediatric patients.” This review also voiced concerns regarding adverse effects (including disinhibition) and the potential for dependence with benzodiazepine use [22]. Thus, though the tolerability analysis of benzodiazepines used acutely in procedural settings has revealed no significant side effects, it is unclear how generalizable these findings will be to the treatment of acute pediatric anxiety and agitation where the dosing of benzodiazepines is likely to be more conservative and the medications may be used to treat anxiety repeatedly and for a longer duration of time.
Evidence-based treatment for Anxiety Disorders includes cognitive behavioral therapies (CBT) and pharmacotherapy, both of which have proven efficacy and are most effective when used in combination [43]. CBT for anxiety generally focus on desensitization to provocative stimuli and cognitive retraining to correct negative interpretations of stressors. Depending on the specific type of anxiety disorder, pharmacotherapy may involve long term use of selective serotonin reuptake inhibitors (SSRI) or selective norepinephrine reuptake inhibitors (SNRI). Although extremely effective, especially in children, both CBT and SSRI/SNRI pharmacotherapy take several months before the full treatment benefits accrue. Benzodiazepines, which are allosteric modulators of the GABAA receptors, have a much shorter time to onset than SSRIs or SNRIs, and are therefore potentially clinically useful for control in acute anxiety. In contrast to the data in pediatric populations, there exists fairly strong evidence for the use of benzodiazepines in the treatment of adult anxiety disorders, at least in the short term. One meta-analysis demonstrated that high potency benzodiazepines are effective and no different than antidepressants or psychological pain management techniques in the management of acute panic attacks [41]. Another meta-analysis found benzodiazepines to be significantly more efficacious than placebo in the short-term management of generalized anxiety disorder [28].
Benzodiazepines are commonly used in adult populations to provide anxiolytic effect in the first weeks of SSRI therapy when the therapeutic SSRI dose has not yet been reached. Because of the risk of dependence and of cognitive impairment with chronic use, benzodiazepines are generally not recommended as a standing medication for the long-term treatment of anxiety [34]. Given similar risks in pediatric populations, benzodiazepines are additionally not recommended for the long-term treatment of anxiety in children. Benzodiazepines, however, may have two other potential uses, including: (1) an adjunctive as-needed medication to manage crippling intermittent anxiety which leads to severe adverse consequences in children refractory to evidence-based pharmacotherapy and psychotherapy, and (2) to manage acute anxiety and aggressive behavior in a severely impaired pediatric anxiety population, especially on an inpatient setting as an alternative to off-label use of atypical antipsychotics. Based on the limited tolerability data in this analysis, benzodiazepines appear to have a more favorable side effect profile than atypical antipsychotics, as studies have shown atypical antipsychotics to be associated with weight gain, new-onset diabetes, increased risk of arrhythmia, and extra-pyramidal symptoms [30]. In pediatric patients, the risks of such side effects must be weighed carefully, as greater inter-patient heterogeneity in pharmacokinetics makes it difficult to predict the bioavailability of the antipsychotic medications [4]. However, given that reporting of benzodiazepine side-effect data is sparse and inconsistent in available trials and that when reported, side-effects were mostly assessed in a subjective manner, a more methodically rigorous, high-powered RCT would be needed to clearly elucidate the safety of short-term benzodiazepine use as an alternative to antipsychotics and other augmentation strategies in SSRI-refractory children with anxiety disorders. In the interim, we would recommend that for procedures likely to produce significant anxiety that clinicians consider a safe and effective treatment. Additionally, clinicians treating children experiencing acute anxiety symptoms (e.g. such as short-term treatment of severe panic attacks or separation anxiety or OCD symptoms) that benzodiazepines be viewed as a possibly beneficial treatment option that can be considered (especially in lieu of antipsychotic medications or primarily sedating medications (diphenhydramine or trazodone). We would recommend that behavioral therapies and techniques be used preferentially to medications as they have better evidence of efficacy and do not have the side-effect concerns of these other agents (including benzodiazepines).
Given the important findings of this meta-analysis, it is important to acknowledge several limitations in this study. The data consisted of a fairly small number of trials, especially those involving benzodiazepines other than midazolam. Even though a difference in effect size between midazolam and other benzodiazepines utilized was not shown, the small number of non-midazolam studies limits the generalizability of these findings to other agents. There was also funnel plot asymmetry in this meta-analysis, suggesting some evidence of publication bias. The effects of benzodiazepines as acute anxiolytics remained significant even after controlling for funnel plot asymmetry. Additionally, there was a large amount of heterogeneity between trials. Several subgroup analyses and meta-regressions were conducted to understand the sources of this heterogeneity, but given the small number of trials, the associations were underpowered.
This meta-analysis suggests that benzodiazepine use is quite effective as a short-term anxiolytic for children in procedural settings and is well tolerated in this context. Trials examining the use of benzodiazepines in pediatric anxiety disorders are quite sparse, underpowered and subject of antiquated diagnostic constructs of anxiety. Further research would be useful to examine the acute effects of benzodiazepines for anxiety in inpatient settings and as an adjunctive as-needed treatment in children with severe anxiety that has failed to improve substantially with optimal medication management and CBT. It would also be worth investigating whether benzodiazepines significantly interfere with the efficacy of CBT in the pediatric population, as existing literature suggests there is a detrimental effect of high potency benzodiazepines on CBT efficacy for anxiety in adults [46]. The data surrounding the strong discouragement of benzodiazepine use in pediatric anxiety disorders seems weak when considering (1) the strong data for allied pediatric professions suggesting potential efficacy and reasonable tolerability demonstrated in this meta-analysis and (2) that often off-label use of antipsychotics is the alternative available treatment in these children with acute anxiety.
Table 1. Characteristics of Included Trials.
Abbreviations: ROA= Route of Administration, mg/kg=milligrams per kilogram, min before=minutes before stressful event, OR= operating room.
| Study | Benzodiazepine | ROA | Dose (mg/kg) | Dose (diazepam equivalents/kg) | Med Timing (min before) | Setting | Control | Mean Age | Blinding |
|---|---|---|---|---|---|---|---|---|---|
| Eskandarian 2015 | Midazolam | IN | 0.2 | 0.2 | 20 | Dental | Placebo | 3.16 | yes |
| Lan 2012 | Midazolam | IV | 0.08 | 0.25 | 3 | OR | Placebo | 5.05 | yes |
| Hosseini 2012 | Midazolam | IN | 0.2 | 0.2 | 15 | OR | Placebo | 4.2 | yes |
| Ferguson 2011 | Midazolam | PO | 0.5 | 0.5 | 15 | OR | Placebo | 3.52 | yes |
| Templeton 2010 | Midazolam | PO | 0.2 | 0.2 | 30 | OR | Placebo | 7.05 | yes |
| Heden 2009 | Midazolam | PO | 0.3 | 0.3 | 30 | non-OR procedure | Placebo | 8.55 | yes |
| Golan 2009 | Midazolam | PO | 0.5 | 0.5 | 30 | OR | No placebo | 4.5 | yes |
| Finley 2006 | Midazolam | PO | 0.5 | 0.5 | 60 | OR | Placebo | 5.46 | yes |
| Herd 2006 | Midazolam | PO | 0.5 | 0.5 | 30 | non-OR procedure | Placebo | 3.5 | yes |
| Kain 2004 | Midazolam | PO | 0.5 | 0.5 | 30 | OR | No placebo | 5.3 | yes |
| Riva 1997 | Midazolam | PO | 0.75 | 0.75 | OR | Placebo | 6.25 | yes | |
| Shane 1994 | Midazolam | RECTAL | 0.45 | 0.45 | 15 | non-OR procedure | Placebo | 2.4 | yes |
| McCluskey 1994 | Midazolam | PO | 0.5 | 0.5 | 45 | OR | Placebo | 5.1 | yes |
| Gallardo 1994 | Midazolam | PO | 7.5mg | 7.5mg | Dental | Placebo | yes | ||
| Vetter 1993 | Midazolam | PO | 0.6 | 0.6 | OR | Placebo | 3.4 | yes | |
| Diazepam | 0.3 | 0.3 | |||||||
| Hennes 1990 | Midazolam | PO | 0.2 | 0.2 | 37.5 | non-OR procedure | Placebo | 2.455 | yes |
| Kain 2000 | Midazolam | PO | 0.5 | 0.5 | 5 | OR | Placebo | 7.5 | yes |
| Midazolam | PO | 0.5 | 0.5 | 10 | yes | ||||
| Midazolam | PO | 0.5 | 0.5 | 20 | yes | ||||
| Samarkandi 2005 | Midazolam | PO | 0.1 | 0.1 | 60 | OR | Placebo | 3.7 | yes |
| Midazolam | PO | 0.25 | 0.25 | ||||||
| Midazolam | PO | 0.5 | 0.5 | ||||||
| Vagnoli 2010 | Midazolam | PO | 0.5 | 0.5 | 45 | OR | No placebo | 7.7 | no |
| Gillerman 1996 | Midazolam | PO | 0.5 | 0.5 | 30 | OR | Placebo | 5.24 | yes |
| Fatovich 1995 | Midazolam | PO | 0.3 | 0.3 | 37.5 | non-OR procedure | Placebo | 4.73 | yes |
| 4.81 |
Acknowledgments
Disclosures: Dr. Bloch receives research support from Biohaven Pharmaceuticals and Therapix Biosciences. The authors also acknowledge the support of the National Institutes of Health 1K23MH091240 (MHB), the Tourette Association of America (MHB), NARSAD (MHB), the Patterson Foundation (MHB) and UL1 RR024139 from the National Center for Research Resources, a component of the National Institutes of Health, and NIH roadmap for Medical Research (MHB). Dr. Bloch is supported in part by the State of Connecticut, Department of Mental Health and Addiction Services, but this publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors.
Role of Funding Agency: Funders did not provide any direct support for this work.
Contributor Information
Ms. Heide Kuang, Medical student at Yale University School of Medicine.
Ms. Jessica A. Johnson, Yale Child Study Center (New Haven, CT).
Ms. Jilian M. Mulqueen, Boston College School of Social Work.
Dr. Michael H. Bloch, Yale Child Study Center and the Department of Psychiatry of Yale University (New Haven, CT).
References
- 1.Bandelow B, Reitt M, Rover C, Michaelis S, Gorlich Y, et al. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30(4):183–92. doi: 10.1097/YIC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 2.Berbel P, Moix J, Quintana S. Music versus diazepam to reduce preoperative anxiety: a randomized controlled clinical trial. Rev Esp Anestesiol Reanim. 2007;54(6):355–8. [PubMed] [Google Scholar]
- 3.Bernstein GA, Garfinkel BD, Borchardt CM. Comparative studies of pharmacotherapy for school refusal. J Am Acad Child Adolesc Psychiatry. 1990;29(5):773–81. doi: 10.1097/00004583-199009000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Caccia S. Safety and pharmacokinetics of atypical antipsychotics in children and adolescents. Paediatr Drugs. 2013;15(3):217–33. doi: 10.1007/s40272-013-0024-6. [DOI] [PubMed] [Google Scholar]
- 5.Capurso M, Ragni B. Psycho-educational preparation of children for anaesthesia: A review of intervention methods. Patient Educ Couns. 2016;99(2):173–85. doi: 10.1016/j.pec.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Chow CH, Van Lieshout RJ, Schmidt LA, Dobson KG, Buckley N. Systematic Review: Audiovisual Interventions for Reducing Preoperative Anxiety in Children Undergoing Elective Surgery. J Pediatr Psychol. 2016;41(2):182–203. doi: 10.1093/jpepsy/jsv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox RG, Nemish U, Ewen A, Crowe MJ. Evidence-based clinical update: does premedication with oral midazolam lead to improved behavioural outcomes in children? Can J Anaesth. 2006;53(12):1213–9. doi: 10.1007/BF03021583. [DOI] [PubMed] [Google Scholar]
- 8.Dionigi A, Gremigni P. A Combined Intervention of Art Therapy and Clown Visits to Reduce Preoperative Anxiety in Children. J Clin Nurs. 2016 doi: 10.1111/jocn.13578. [DOI] [PubMed] [Google Scholar]
- 9.Eskandarian T, Arabzade Moghadam S, Reza Ghaemi S, Bayani M. The effect of nasal midazolam premedication on parents-child separation and recovery time in dental procedures under general anaesthesia. Eur J Paediatr Dent. 2015;16(2):135–8. [PubMed] [Google Scholar]
- 10.Fatovich DM, I, Jacobs G. A randomized, controlled trial of oral midazolam and buffered lidocaine for suturing lacerations in children (the SLIC Trial) Ann Emerg Med. 1995;25(2):209–14. doi: 10.1016/s0196-0644(95)70326-8. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson GG, Chen C, Yan Y, Royer ME, Campigotto M, et al. The efficacy of oral midazolam for decreasing anxiety in children undergoing voiding cystourethrogram: a randomized, double-blind, placebo controlled study. J Urol. 2011;185(6 Suppl):2542–6. doi: 10.1016/j.juro.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Finley GA, Stewart SH, Buffett-Jerrott S, Wright KD, Millington D. High levels of impulsivity may contraindicate midazolam premedication in children. Can J Anaesth. 2006;53(1):73–8. doi: 10.1007/BF03021530. [DOI] [PubMed] [Google Scholar]
- 13.Gallardo F, Cornejo G, Borie R. Oral midazolam as premedication for the apprehensive child before dental treatment. J Clin Pediatr Dent. 1994;18(2):123–7. [PubMed] [Google Scholar]
- 14.Gillerman RG, Hinkle AJ, Green HM, Cornell L, Dodge CP. Parental presence plus oral midazolam decreases frequency of 5% halothane inductions in children. J Clin Anesth. 1996;8(6):480–5. doi: 10.1016/0952-8180(96)00113-4. [DOI] [PubMed] [Google Scholar]
- 15.Golan G, Tighe P, Dobija N, Perel A, Keidan I. Clowns for the prevention of preoperative anxiety in children: a randomized controlled trial. Paediatr Anaesth. 2009;19(3):262–6. doi: 10.1111/j.1460-9592.2008.02903.x. [DOI] [PubMed] [Google Scholar]
- 16.Graae F, Milner J, Rizzotto L, Klein RG. Clonazepam in childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 1994;33(3):372–6. doi: 10.1097/00004583-199403000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Heden L, von Essen L, Frykholm P, Ljungman G. Low-dose oral midazolam reduces fear and distress during needle procedures in children with cancer. Pediatr Blood Cancer. 2009;53(7):1200–4. doi: 10.1002/pbc.22233. [DOI] [PubMed] [Google Scholar]
- 18.Hennes HM, Wagner V, Bonadio WA, Glaeser PW, Losek JD, et al. The effect of oral midazolam on anxiety of preschool children during laceration repair. Ann Emerg Med. 1990;19(9):1006–9. doi: 10.1016/s0196-0644(05)82564-8. [DOI] [PubMed] [Google Scholar]
- 19.Herd DW, McAnulty KA, Keene NA, Sommerville DE. Conscious sedation reduces distress in children undergoing voiding cystourethrography and does not interfere with the diagnosis of vesicoureteric reflux: a randomized controlled study. AJR Am J Roentgenol. 2006;187(6):1621–6. doi: 10.2214/AJR.05.1216. [DOI] [PubMed] [Google Scholar]
- 20.Hidalgo RB, Tupler LA, Davidson JR. An effect-size analysis of pharmacologic treatments for generalized anxiety disorder. J Psychopharmacol. 2007;21(8):864–72. doi: 10.1177/0269881107076996. [DOI] [PubMed] [Google Scholar]
- 21.Hosseini Jahromi SA, Hosseini Valami SM, Adeli N, Yazdi Z. Comparison of the effects of intranasal midazolam versus different doses of intranasal ketamine on reducing preoperative pediatric anxiety: a prospective randomized clinical trial. J Anesth. 2012;26(6):878–82. doi: 10.1007/s00540-012-1422-6. [DOI] [PubMed] [Google Scholar]
- 22.Ipser JC, Stein DJ, Hawkridge S, Hoppe L. Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev. 2009;(3):CD005170. doi: 10.1002/14651858.CD005170.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Isik B, Baygin O, Bodur H. Premedication with melatonin vs midazolam in anxious children. Paediatr Anaesth. 2008;18(7):635–41. doi: 10.1111/j.1460-9592.2008.02608.x. [DOI] [PubMed] [Google Scholar]
- 24.Kain ZN, Caldwell-Andrews AA, Krivutza DM, Weinberg ME, Gaal D, et al. Interactive music therapy as a treatment for preoperative anxiety in children: a randomized controlled trial. Anesth Analg. 2004;98(5):1260–6. doi: 10.1213/01.ane.0000111205.82346.c1. table of contents. [DOI] [PubMed] [Google Scholar]
- 25.Kain ZN, Hofstadter MB, Mayes LC, Krivutza DM, Alexander G, et al. Midazolam: effects on amnesia and anxiety in children. Anesthesiology. 2000;93(3):676–84. doi: 10.1097/00000542-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Kain ZN, Mayes LC, O’Connor TZ, Cicchetti DV. Preoperative anxiety in children. Predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150(12):1238–45. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- 27.Lan YP, Huang ZH, Finley GA, Zuo YX. Effects of the combination of mask preconditioning with midazolam pretreatment on anxiety and mask acceptance during pediatric inhalational induction and postoperative mask fear in children. Chin Med J (Engl) 2012;125(11):1908–14. [PubMed] [Google Scholar]
- 28.Martin JL, Sainz-Pardo M, Furukawa TA, Martin-Sanchez E, Seoane T, et al. Benzodiazepines in generalized anxiety disorder: heterogeneity of outcomes based on a systematic review and meta-analysis of clinical trials. J Psychopharmacol. 2007;21(7):774–82. doi: 10.1177/0269881107077355. [DOI] [PubMed] [Google Scholar]
- 29.McCluskey A, Meakin GH. Oral administration of midazolam as a premedicant for paediatric day-case anaesthesia. Anaesthesia. 1994;49(9):782–5. doi: 10.1111/j.1365-2044.1994.tb04451.x. [DOI] [PubMed] [Google Scholar]
- 30.McDonagh M, Peterson K, Carson S, Fu R, Thakurta S. Drug Class Review: Atypical Antipsychotic Drugs: Final Update 3 Report. Oregon Health & Science University; Portland, Oregon 97239: 2010. Drug Class Reviews. All rights reserved.: Portland (OR) [PubMed] [Google Scholar]
- 31.Nardi AE, Perna G. Clonazepam in the treatment of psychiatric disorders: an update. Int Clin Psychopharmacol. 2006;21(3):131–42. doi: 10.1097/01.yic.0000194379.65460.a6. [DOI] [PubMed] [Google Scholar]
- 32.Riddle MA, Bernstein GA, Cook EH, Leonard HL, March JS, et al. Anxiolytics, adrenergic agents, and naltrexone. J Am Acad Child Adolesc Psychiatry. 1999;38(5):546–56. doi: 10.1097/00004583-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Riva J, Lejbusiewicz G, Papa M, Lauber C, Kohn W, et al. Oral premedication with midazolam in paediatric anaesthesia. Effects on sedation and gastric contents. Paediatr Anaesth. 1997;7(3):191–6. doi: 10.1046/j.1460-9592.1997.d01-75.x. [DOI] [PubMed] [Google Scholar]
- 34.Sadock BJ, Sadock VA, Ruiz P. Kaplan and Sadock’s Synopsis of Psychiatry: behavioral sciences/clinical psychiatry. New York: Wolters Kluwer; 2015. [Google Scholar]
- 35.Samarkandi A, Naguib M, Riad W, Thalaj A, Alotibi W, et al. Melatonin vs. midazolam premedication in children: a double-blind, placebo-controlled study. Eur J Anaesthesiol. 2005;22(3):189–96. doi: 10.1017/s0265021505000335. [DOI] [PubMed] [Google Scholar]
- 36.Scaini S, Belotti R, Ogliari A, Battaglia M. A comprehensive meta-analysis of cognitive-behavioral interventions for social anxiety disorder in children and adolescents. J Anxiety Disord. 2016;42:105–12. doi: 10.1016/j.janxdis.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Shane SA, Fuchs SM, Khine H. Efficacy of rectal midazolam for the sedation of preschool children undergoing laceration repair. Ann Emerg Med. 1994;24(6):1065–73. doi: 10.1016/s0196-0644(94)70235-7. [DOI] [PubMed] [Google Scholar]
- 38.Simeon JG, Ferguson HB, Knott V, Roberts N, Gauthier B, et al. Clinical, cognitive, and neurophysiological effects of alprazolam in children and adolescents with overanxious and avoidant disorders. J Am Acad Child Adolesc Psychiatry. 1992;31(1):29–33. doi: 10.1097/00004583-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Templeton P, Burton D, Cullen E, Lewis H, Allgar V, et al. Oral midazolam for removal of Kirschner wires in the children’s orthopaedic outpatient department: a randomized controlled trial. J Pediatr Orthop. 2010;30(2):130–4. doi: 10.1097/BPO.0b013e3181ced3ae. [DOI] [PubMed] [Google Scholar]
- 40.Vagnoli L, Caprilli S, Messeri A. Parental presence, clowns or sedative premedication to treat preoperative anxiety in children: what could be the most promising option? Paediatr Anaesth. 2010;20(10):937–43. doi: 10.1111/j.1460-9592.2010.03403.x. [DOI] [PubMed] [Google Scholar]
- 41.van Balkom AJ, Bakker A, Spinhoven P, Blaauw BM, Smeenk S, et al. A meta-analysis of the treatment of panic disorder with or without agoraphobia: a comparison of psychopharmacological, cognitive-behavioral, and combination treatments. J Nerv Ment Dis. 1997;185(8):510–6. doi: 10.1097/00005053-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Vetter TR. A comparison of midazolam, diazepam, and placebo as oral anesthetic premedicants in younger children. J Clin Anesth. 1993;5(1):58–61. doi: 10.1016/0952-8180(93)90090-2. [DOI] [PubMed] [Google Scholar]
- 43.Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–66. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan K, Jing Q, Zhao JZ. Evaluation of oral midazolam as conscious sedation for pediatric patients in oral restoration. Chin Med Sci J. 2006;21(3):163–6. [PubMed] [Google Scholar]
- 45.Wehry AM, Beesdo-Baum K, Hennelly MM, Connolly SD, Strawn JR. Assessment and treatment of anxiety disorders in children and adolescents. Curr Psychiatry Rep. 2015;17(7):52. doi: 10.1007/s11920-015-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westra HA, Stewart SH. Cognitive behavioural therapy and pharmacotherapy: complementary or contradictory approaches to the treatment of anxiety? Clin Psychol Rev. 1998;18(3):307–40. doi: 10.1016/s0272-7358(97)00084-6. [DOI] [PubMed] [Google Scholar]