Abstract
Aminoacyl-tRNA synthetases (aaRSs) play essential roles in protein synthesis. As a member of the aaRS family, the tyrosyl-tRNA synthetase (TyrRS) in Escherichia coli has been shown in proteomic studies to be acetylated at multiple lysine residues. However, these putative acetylation targets have not yet been biochemically characterized. In this study, we applied a genetic-code-expansion strategy to site-specifically incorporate Nε-acetyl-L-lysine into selected positions of TyrRS for in vitro characterization. Enzyme assays demonstrated that acetylation at K85, K235, and K238 could impair the enzyme activity. In vitro deacetylation experiments showed that most acetylated lysine residues in TyrRS were sensitive to the E. coli deacetylase CobB but not YcgC. In vitro acetylation assays indicated that 25 members of the Gcn5-related N-acetyltransferase family in E. coli, including YfiQ, could not acetylate TyrRS efficiently, whereas TyrRS could be acetylated chemically by acetyl-CoA or acetyl-phosphate (AcP) only. Our in vitro characterization experiments indicated that lysine acetylation could be a possible mechanism for modulating aaRS enzyme activities, thus affecting translation.
Keywords: acetyltransferase, aminoacyl-tRNA synthetase, deacetylase, genetic code expansion, lysine acetylation
Graphical abstract
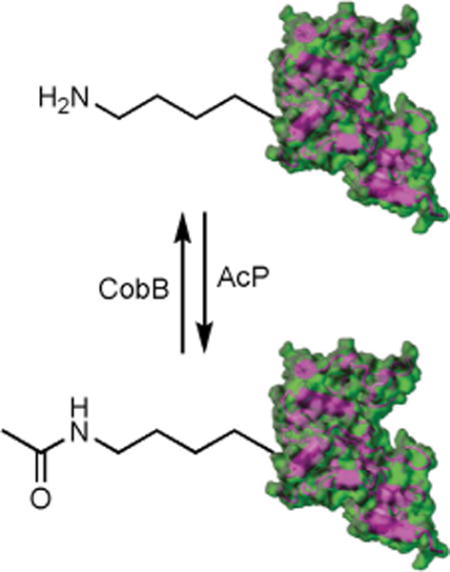
Reversible lysine acetylation: Site-specifically acetylated Escherichia coli tyrosyl-tRNA synthetase variants were generated by a genetic code expansion strategy. The acetylation of specific lysine residues abolished the aminoacylation activity. The lysine residues could be chemically acetylated by acetyl-phosphate (AcP) and deacetylated by CobB deacetylase in vitro.
Introduction
As the building blocks of ribosomal protein synthesis, aminoacyl-tRNAs are generated by a family of enzymes called aminoacyl-tRNA synthetases (aaRSs) through a two-step reaction: activation of amino acids, and aminoacylation of cognate tRNAs with activated amino acids.[1] Besides this classic function, aaRSs are also involved in a variety of biological processes, such as RNA splicing, translational and transcriptional regulation, signaling transduction, and cell cycles.[2] Over the past decade, aaRSs have attracted more and more attention due to their association with diseases[3] and applications in genetic-code-expansion strategies.[4]
Cells use post-translational modifications (PTMs) to modulate protein functions, structures, and interactions with other molecules, thus regulating a wide range of biological processes, such as gene transcription and cellular signaling.[5] Acetylation at lysine residues is one of the most abundant PTMs in both eukaryotes and prokaryotes.[6] Interestingly, proteomic studies have shown that aaRSs are favorable targets for lysine acetylation modifications.[7] Taking Escherichia coli as an example, acetylated lysine residues have been identified in all 20 aaRSs.[8] Recently, eukaryotic tyrosyl-tRNA synthetase (TyrRS) has been found to be acetylated under oxidative stress conditions. Acetylation of the lysine residue near the nuclear localization signal of TyrRS could inhibit its aminoacylation activity and induce conformational changes to promote the nuclear translocation of acetylated TyrRS. This acetylation could activate DNA repair genes downstream of transcription factor E2F1 to protect against DNA damage in mammalian cells and zebrafish.[9]
To facilitate studies of lysine acetylation, researchers have developed and optimized the orthogonal translation system to cotranslationally incorporate Nε-acetyl-L-lysine (AcK) into proteins at desired positions, thus producing homogeneously acetylated proteins to overcome the low stoichiometry of lysine acetylation in nature.[10] A recent study applied this strategy and showed that lysine acetylation could affect enzyme activities of LeuRS and ArgRS in E. coli.[11] Herein, we worked on the lysine acetylation of TyrRS, another member of class I aaRSs.[1e,12] Our results demonstrated that the effect of lysine acetylation on enzyme activities and the sensitivity of acetylated variants to the deacetylase CobB depended on the positions of acetylated lysine residues. Together with the previous study of LeuRS and ArgRS,[11] we proposed that lysine acetylation could be a general mechanism for modulating class I aaRS activities.
Results
Expression of site-specifically acetylated TyrRS variants
Firstly, we chose specific positions of lysine residues to incorporate AcK. Although several proteomic studies of acetylation have been performed in E. coli,[8] the sets of identified acetylated lysine residues were distinct from each other due to differences in strains, growth stages, and detection methods. We selected four lysine residues that have been identified as being acetylated in more than three independent proteomic studies: K85, K144, K235, and K238. We also chose one lysine residue, K355, that has never been found to be acetylated, as a control. Then we utilized the engineered Methanosarcina barkeri pyrrolysyl-tRNA synthetase variant[10c] and our optimized tRNAPyl[10d] to cotranslationally incorporate AcK into selected sites whose corresponding positions in the TyrRS gene were mutated to TAG, individually. We used the BL21(DE3) strain as the expression host due to its significantly lower background of protein acetylation than that of K12-derived E. coli strains.[8d] Both western blotting (Figure 1A) and mass spectrometry (Figures S1–S5 in the Supporting Information) confirmed AcK incorporation at selected positions. After adding nicotinamine as the inhibitor of deacetylases in both growth media and purification buffers, we did not detect unmodified lysine residues at those selected positions.
Figure 1.
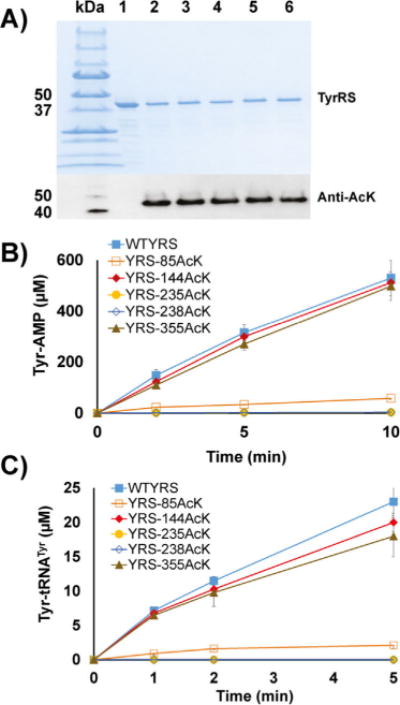
The expression and enzyme activities of TyrRS and its acetylated variants. A) SDS-PAGE and western blotting of purified TyrRS and its variants. Lane 1: wild-type TyrRS, lane 2: TyrRS-85AcK, lane 3: TyrRS-144AcK, lane 4: TyrRS-235AcK, lane 5: TyrRS-238AcK, lane 6: TyrRS-355AcK. The same volumes of elution fractions were loaded. B) ATP-PPi exchange assays of TyrRS and its variants. C) Aminoacylation assays of TyrRS and its variants. Mean values and standard deviations were calculated based on three replicates.
Effects of lysine acetylation on the TyrRS enzyme activity
Firstly, we measured tyrosine activation activities of TyrRS and its variants by ATP-PPi exchange assays (Figure 1B). Variants TyrRS-85AcK and-235AcK had dramatic decreases in activity. Variant TyrRS-238AcK had no detectable activity, while variants TyrRS-144AcK and -355AcK had similar activities to the wild-type TyrRS. We also performed steady-state kinetic analyses to determine kinetic parameters of tyrosine activation for TyrRS and its variants. The catalytic efficiency of the wild-type TyrRS was consistent with a previous report.[13] TyrRS-85AcK had a fivefold increase in the KM value for ATP, indicating its role in ATP binding. TyrRS-235AcK had slightly changed KM values for both ATP and tyrosine but a 200-fold decrease in catalytic efficiency, suggesting its role in catalysis (Table 1). Due to the undetectable activation activity of the variant TyrRS-238AcK, its kinetic parameters for tyrosine activation could not be determined.
Table 1.
Kinetic parameters for Tyr activation by TyrRS and its variants.
|
kcat [s−1] |
KM,ATP [mM] |
KM,Tyr [μM] |
kcat/KM,ATP [s−1mM−1] |
kcat/KM,Tyr [s−1μM−1] |
|
|---|---|---|---|---|---|
| wild-type | 14.2 ± 0.7 | 0.43 ± 0.07 | 7.13 ± 0.81 | 33.02 | 1.99 |
| 85AcK | 2.0 ± 0.3 | 2.23 ± 0.36 | 6.78 ± 0.65 | 0.90 | 0.29 |
| 144AcK | 12.3 ± 1.1 | 0.45 ± 0.03 | 7.82 ± 0.27 | 27.33 | 1.57 |
| 235AcK | 0.1 ± 0.0 | 0.71 ± 0.08 | 8.45 ± 1.02 | 0.14 | 0.01 |
| 238AcK | n.d.[a] | n.d. | n.d | n.d. | n.d. |
| 355AcK | 11.9 ±1.7 | 0.49 ± 0.11 | 6.69 ± 0.43 | 24.29 | 1.78 |
n.d.: not determined due to the undetectable activity of TyrRS-238AcK. Mean values and standard deviations were calculated based on three replicates.
Next, we tested aminoacylation activities of TyrRS and its variants (Figure 1C). Similar to TyrRS-235AcK, the variant TyrRS-85AcK had impaired aminoacylation activity. Variants TyrRS-235AcK and -238AcK had no detectable activities, whereas variants TyrRS-144AcK and -355AcK had slightly decreased activities compared to wild-type TyrRS. We also determined kinetic parameters of aminoacylation for TyrRS and its variants. The kcat value of wild-type TyrRS was 1.32 s−1, which was about twice the value previously reported.[14] The KM value for tRNATyr of wild-type TyrRS was within the range of those in previous reports.[15] KM values for tRNATyr of acetylated variants were not significantly affected at all positions (Table 2). Due to the undetectable aminoacylation activities of variants TyrRS-235Ack and -238AcK, their kinetic parameters for aminoacylation could not be determined.
Table 2.
Kinetic parameters for tRNATyr aminoacylation by TyrRS and its variants.
| kcat [s−1] | KM,tRNA [μM] | kcat/KM,tRNA [s−1μM−1] | |
|---|---|---|---|
| wild-type | 1.32 ± 0.23 | 0.37 ± 0.04 | 3.51 |
| 85AcK | 0.15 ± 0.06 | 0.45 ± 0.05 | 0.33 |
| 144AcK | 1.17 ± 0.37 | 0.42 ± 0.07 | 2.78 |
| 235AcK | n.d.[a] | n.d. | n.d. |
| 238AcK | n.d. | n.d. | n.d. |
| 355AcK | 1.09 ± 0.29 | 0.48 ± 0.14 | 2.27 |
n.d.: not determined due to undetectable activities of TyrRS-235AcK and -238AcK. Mean values and standard deviations were calculated based on three replicates.
Deacetylation of acetylated TyrRS variants in vitro
Lysine acetylation is a reversible modification process. Lysine deacetylases (KDACs), originally named as histone deacetylases, remove the acetyl group to leave the free ε-amino group of lysine residues in proteins.[16] Until now, there have only been two known KDACs in E. coli: CobB and YcgC.[17]
CobB is an NAD+-dependent deacetylase that has a wide range of protein substrates.[18] Thus, we first tested in vitro deacetylation of site-specifically acetylated TyrRS variants by the CobB protein. After incubating these variants with CobB for 1 h individually, western blotting was used to detect acetylation (Figure 2A). The results showed that CobB could deacetylate most of the acetylated lysine resides in TyrRS variants except position 144. Then, we tested aminoacylation activities of these acetylated variants after CobB treatment. The result indicated that CobB deacetylation could recover most of the impaired aminoacylation activities (Figure 2B).
Figure 2.
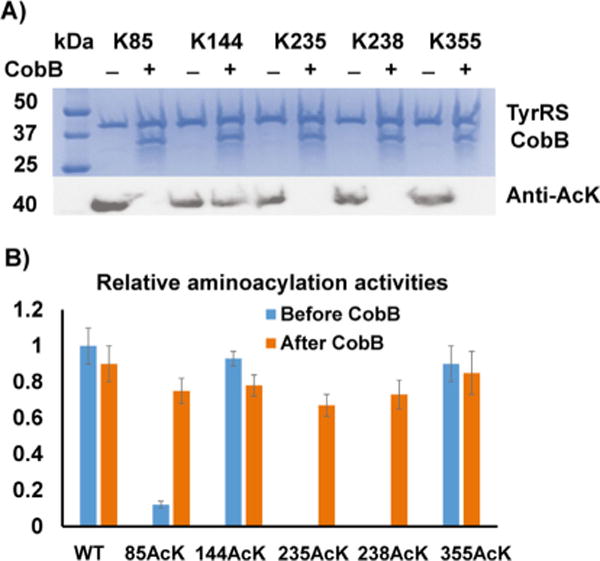
Deacetylation of acetylated TyrRS variants by CobB. A) SDS-PAGE and western blotting of acetylated TyrRS variants with and without CobB treatment. The same amounts of proteins were loaded. B) Relative aminoacylation activities of TyrRS and its variants before and after CobB treatment. The activity of wild-type TyrRS before CobB treatment was set as 1. The time for the aminoacylation assay was 1 min. The mean values and standard deviations were calculated based on three replicates.
YcgC is a recently discovered prokaryotic deacetylase[17c] that was previously known as a component in the dihydroxyacetone kinase complex.[19] We also tested in vitro deacetylation of site-specifically acetylated TyrRS variants by YcgC (Figure S6A). We used the known substrate of YcgC, RutR,[17c] as a control to show that YcgC was active in this study (Figure S6B). After incubating these variants with YcgC individually, western blotting results indicated that TyrRS was not the substrate of YcgC, consistent with the conclusion that YcgC targets a distinct set of substrates from those of CobB.[17c]
Enzymatic acetylation of TyrRS in vitro
In E. coli, there are two mechanisms to acetylate lysine residues in proteins. One is the classic enzymatic acetyl-CoA-dependent acetylation, which is catalyzed by a family of enzymes called lysine acetyltransferases (KATs).[20] The other is a newly discovered nonenzymatic acetyl-phosphate (AcP)-dependent acetylation, which predominates in E. coli.[8b,d]
There are five groups of KATs: the Gcn5-related N-acetyltransferase (GNAT) family, the MYST family, the CBP/p300 coactivators, the SRC family of coactivators, and the TAFII group of transcription factors.[21] The GNAT family is the most widely distributed group, with 25 putative members in E. coli.[21d] Among them, YfiQ is the only known protein KAT and was first identified and characterized in Salmonella.[22] Thus, we firstly tested the acetylation activity of YfiQ for TyrRS in vitro. The wild-type TyrRS protein purified from BL21(DE3) cells that had no detect able acetylation was incubated with acetyl-CoA only or with acetyl-CoA and YfiQ for 1 h (Figure 3). Interestingly, the acetyl-CoA itself could acetylate TyrRS, and YfiQ treatment only increased the acetylation level slightly, indicating YfiQ could not acetylate TyrRS efficiently in vitro. This result is consistent with previous proteomic studies showing that deletion of the yfiQ gene did not affect the acetylation level of TyrRS in vivo.[8b,d] As a control, we used the known substrate of YfiQ, RcsB,[23] to demonstrate that YfiQ was active in this study (Figure S7).
Figure 3.
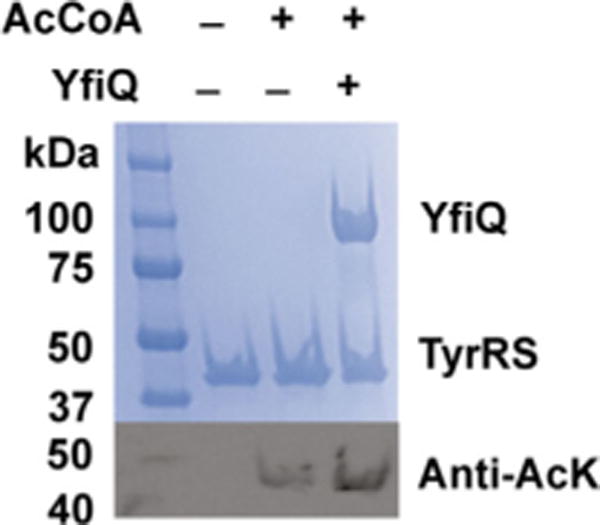
Acetylation of TyrRS by YfiQ. SDS-PAGE and western blotting of TyrRS with and without YfiQ treatment. The same amounts of proteins were loaded.
The other 24 GNAT family members either catalyze different reactions (ArgA, AstA, CitC, MnaT, PanM, PhnO, RimI, RimJ, RimL, SpeG, TmcA, and WecD) or have unknown functions (ElaA, YafP, YedL, YhbS, YhhY, YiaC, YiiD, YjaB, YjdJ, YjgM, YjhQ, and YpeA).[20f] Although some work has been done to identify general protein motifs that can be recognized by KATs,[6f,24] they may have distinct specificities for different protein substrates individually. Thus, we further tested in vitro acetylation of TyrRS by all 24 GNAT family members (Figure S8). The wild-type TyrRS expressed and purified from BL21(DE3) was treated with individual purified GNAT family members and acetyl-CoA for 1 h. Western blotting results showed that there was no obvious enhanced acetylation with treatment of any GNAT family members compared to that with acetyl-CoA treatment only, thus indicating that acetylation of TyrRS may follow the non-enzymatic mechanism.
Nonenzymatic acetylation of TyrRS in vitro
Next, we tested nonenzymatic acetylation of TyrRS by AcP in vitro. The wild-type TyrRS purified from BL21(DE3) cells was treated with AcP only. Western blotting was used to determine acetylation levels (Figure 4A). The results showed that AcP itself could acetylate TyrRS chemically. And the acetylation level increased with incubation time. Aminoacylation assays demonstrated that AcP treatment could decrease TyrRS enzyme activities in a time-dependent manner (Figure 4B), which is consistent with the conclusion that accumulation of protein acetylation by AcP in vivo could be a general mechanism for modulating protein functions in E. coli.[8c]
Figure 4.
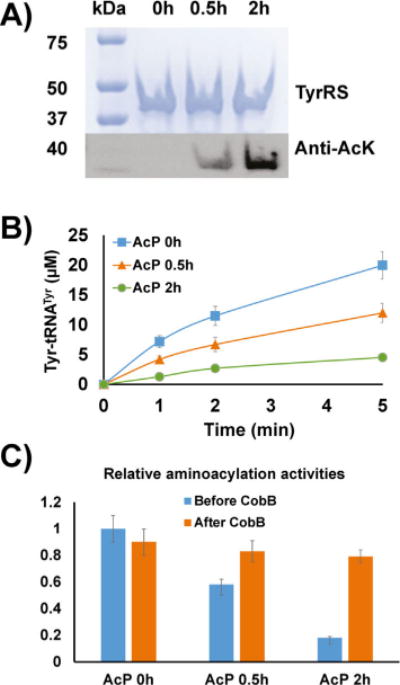
Acetylation of TyrRS by AcP. A) SDS-PAGE and western blotting of TyrRS incubated with AcP (5 mM). The same amounts of proteins were loaded. B) Aminoacylation assays of TyrRS after incubation with AcP. C) Relative aminoacylation activities of AcP-treated TyrRS before and after CobB treatment. The activity of TyrRS without AcP treatment before CobB treatment was set as 1. The time for the aminoacylation assay was 1 min. Mean values and standard deviations were calculated based on three replicates.
In earlier experiments, we demonstrated that CobB treatment could recover impaired enzyme activities of acetylated variants (Figure 2B). Here, our results also showed that CobB could recover impaired enzyme activities caused by nonenzymatic acetylation (Figure 4C). It indicated that AcP-acetylated lysine residues could also be substrates for CobB, supporting the previous proteomic study.[18]
We further analyzed non-enzymatic acetylation sites in TyrRS with AcP treatment by mass spectrometry. Nine acetylated lysine residues were found in three independent experiments (K67, K85, K90, K144, K230, K235, K238, K321, and K377; Figures S9–S17). However, based on the lysine acetylation database, there are a total of 18 putative acetylation sites in E. coli TyrRS (K8, K67, K85, K90, K103, K144, K154, K230, K235, K238, K249, K250, K255, K321, K377, K390, K399, and K416).[25] This might be caused by different E. coli strains, growth conditions, and mass spectrometry methods. It could also imply that the lysine acetylation at other sites in TyrRS might need additional cofactors, coenzymes, or unknown KATs.
Discussion
AaRS can be divided into two classes based on their featured motifs.[12,26] TyrRS is a member of the class I aaRSs with a Rossmann-fold domain and conserved HIGH and KMSKS motifs.[26–27] K235 and K238 of TyrRS characterized in this study are the two lysine residues in the KMSKS motif. Previous structural and mutational studies of bacterial TyrRSs have illustrated that the KMSKS motif is involved in both tyrosine activation and tRNA binding.[28] Specifically, the second lysine residue (K238 in E. coli TyrRS) binds to ATP directly, while the first lysine residue (K235 in E. coli TyrRS) binds to the transition state and pyrophosphate.[28a] In our enzyme activity assays, variant TyrRS-238AcK had no detectable activity in either tyrosine activation and tRNA aminoacylation steps, possibly due to its direct binding with both ATP and tRNA, whereas the variant TyrRS-235AcK retained tyrosine activation activity, albeit with a 200-fold decrease. As for the other acetylation-affected site, TyrRS-85AcK had a decreased turnover number and ATP binding ability; this is consistent with a previous mutational study showing its binding with both the ATP-bound and transition states.[28a]
Recently, the Wang group characterized lysine acetylation of LeuRS and ArgRS, which are both class I aaRSs.[11] By mutational analyses and a genetic incorporation approach, they showed that acetylation on K619 of LeuRS (the first lysine residue in the KMSKS motif) and K126 of ArgRS (the compensating residue for the second lysine residue in the KMSKS motif) significantly impaired enzyme activities. Thus, we made a list of MS-identified lysine acetylation in the KMSKS motif of E. coli class I aaRSs (Table S2).[8] With the exception of MetRS, the other aaRSs all have at least one acetylated lysine residue in the KMSKS motif. Based on the previous study of LeuRS and ArgRS[11] and this study on TyrRS, we propose that acetylation of lysine reside(s) in the KMSKS motif could be a general mechanism to regulate activities of class I aaRSs in E. coli.
Previous proteomic studies demonstrated that deletion of the cobB gene increased the acetylation level of TyrRS by ≈ 15-fold in vivo.[8b,d] Our in vitro deacetylation results support these in vivo studies. To demonstrate the structure–function relationship of lysine acetylation of TyrRS, characterized lysine residues in this study were mapped onto the structure of TyrRS[28c] (Figure 5). This TyrRS structure only has the catalytic domain, so K355 was not included. K85, K235, and K238 are all at the catalytic site and on the surface of TyrRS, which are accessible for CobB, consistent with previous studies proposing that CobB-sensitive acetylated lysine residues tend to be located near the surface of proteins for easy access.[18] K144 is close to the dimer interface, so CobB cannot approach this position easily in order to remove the acetyl group; this might explain the resistance of K144 to CobB (Figure 2A). Moreover, these three CobB-sensitive sites (K85, K235, and K238) are also the ones that are most affected by lysine acetylation, indicating that these sites could be physiologically relevant acetylation sites and that the CobB protein plays important roles in regulating TyrRS functions. The Wang group also demonstrated that ArgRS and LeuRS were sensitive to both CobB and AcP.[11] Thus, aaRSs could also share similar mechanisms for acetylation and deacetylation.
Figure 5.
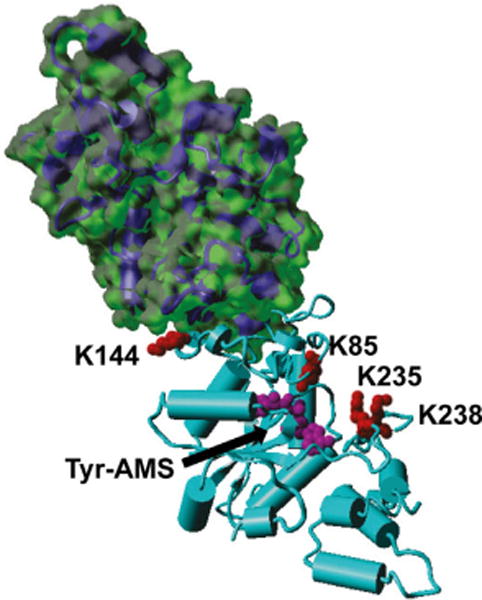
Mapping of acetylated lysine residues on the crystal structure of TyrRS (PDB ID: 1VBM). Selected lysine residues in this study were labeled on one subunit of TyrRS, and the surface of the other subunit was illustrated. Tyr-AMS: 5′-O-[N-(L-tyrosyl)sulfamoyl] adenosine, a tyrosyl-adenylate analogue.
There are two forms of TyrRSs in human: cytosolic TyrRS and mitochondrial TyrRS. The latter has a higher homology to E. coli TyrRS. K284 in mitochondrial TyrRS (the counterpart of K238 in E. coli TyrRS) was found to be modified by ubiquitination rather than acetylation.[29] As mentioned previously, lysine acetylation of cytosolic TyrRS was shown to promote its nuclear translocation to prevent oxidative damage.[9] Together, these facts imply that lysine acetylation should have different functions and regulation mechanisms in different organisms.
Conclusion
In summary, we have applied a genetic-code-expansion strategy to produced site-specifically acetylated E. coli TyrRS variants for in vitro biochemical characterization. Enzyme activity assays showed that acetylation at certain lysine residues could significantly impair enzyme activities. The CobB protein could deacetylate most of the acetylated lysine residues in TyrRS, both by genetic incorporation and nonenzymatic acetylation, whereas the only known E. coli KAT, YfiQ, could not acetylate TyrRS efficiently in vitro. Our in vitro biochemical experiments nicely complement proteomic studies to provide direct biochemical evidence of the impact of lysine acetylation on TyrRS function and imply a possible regulatory mechanism of aaRSs.
Experimental Section
Chemicals and materials
Non-radioactive chemicals were purchased from Sigma–Aldrich, BDH Chemicals (Radnor, PA) or Chem-Impex (Wood Dale, IL). Radioactive compounds were purchased from PerkinElmer. Primers were synthesized by Integrated DNA Technologies (Coralville, IA). DNA sequencing were performed by Eurofins Scientific (Louisville, KY). Ni-NTA resins and plasmid purification kits were purchased from Qiagen. Nucleic acid and protein electrophoresis systems were purchased from Bio-Rad. BL21(DE3) cells, DH5α cells, Q5 site-directed mutagenesis kits, DNA assembly kits, and restriction enzymes were purchased from New England Biolabs.
General molecular biology
DH5α cells were used for cloning. Plasmids were constructed from PCR fragments by using DNA assembly kits. Stop codon mutations were made by using Q5 site-directed mutagenesis kits. Strains and plasmids used in this study are listed in Table S1. For western blotting, purified E. coli TyrRS and its variants were fractionated by SDS-PAGE and transferred onto a PVDF membrane. The membrane was incubated at room temperature with gentle shaking in Tris-buffered saline and Tween-20 (TTBS) and 5% BSA blocking buffer for 60 min. HRP-conjugated acetylated lysine (Ac-K2-100) rabbit antibody (Cell Signaling Technology, Beverly, MA) was diluted 1:1000 and was used to soak the membrane overnight at 4°C. The membrane was prepared for detection using Pierce ECL western blotting substrates (Thermo Scientific). E. coli proteins CobB, YcgC, RutR, RcsB, and 25 GNAT family members were purified from the ASKA strain collection by Ni-NTA affinity chromatography.[30]
Expression and purification of TyrRS and its variants
The genes of TyrRS and its variants were cloned into the pCDF-1b plasmid with a C-terminal His6-tag and cotransformed into BL21(DE3) cells, with the pTech plasmid harboring the AcK incorporation system for expression.[10d] The expression strain was grown in lysogeny broth (1 L) supplemented with streptomycin (100 μg mL−1) and chloramphenicol (50 μg mL−1) at 37°C to an absorbance of 0.6–0.8 at 600 nm. Protein expression was induced by the addition of IPTG (0.5 mM) and supplemented with AcK (5 mM) and nicotinamine (NAM, a deacetylase inhibitor, 20 mM). Cells were incubated at 30°C for an additional 12 h and harvested by centrifugation for 10 min at 5000g and 4°C. The cell paste was suspended in lysis buffer [15 mL: Tris (50 mM pH 7.5), NaCl (300 mM), imidazole (20 mM), NAM (20 mM)] and sonicated. The crude extract was centrifuged for 25 min at 20000g and 4°C. The soluble fraction was loaded onto a column containing Ni-NTA resin (2 mL) previously equilibrated with lysis buffer (20 mL). The column was washed with washing buffer [20 mL; Tris (50 mM, pH 7.5), NaCl (300 mm), imidazole (50 mM), NAM (20 mM)]. The protein bound to the column was then eluted with elution buffer [2 mL: Tris (50 mM, pH 7.5), NaCl (300 mM), imidazole (200 mM), NAM (20 mM)]. The purified protein was dialyzed with HEPES·KOH (50 mM, pH 7.5), NaCl (10 mM), DTT (1 mM), and glycerol (50%), then stored at −80°C for further studies.
Mass spectrometry (MS) analysis
TyrRS variants were digested by trypsin with a standard in-gel digestion protocol and analyzed by LC-MS/MS on an LTQ Orbitrap XL equipped with a nanoACQUITY UPLC system. A Symmetry C18 trap column (180 μm × 20 mm) and a nanoACQUITY UPLC column (1.7 μm, 100 μm × 250 mm, 35°C) were used for peptide separation. Trapping was performed at 15 μLmin−1 in 99% buffer A (0.1% formic acid) for 1 min. Peptide separation was performed at 300 nLmin−1 with buffer A and buffer B (CH3CN containing 0.1% formic acid). A linear gradient was used from 5→50% buffer B over 50 min, increasing to 85% buffer B at 51 min. MS data were acquired by using an Orbitrap with one microscan and a maximum inject time of 900 ms, followed by data-dependent MS/MS acquisitions in the ion trap. The Mascot search algorithm was used to search for the appropriate acetylation substitution.
ATP-pyrophosphate (PPi) exchange assay
The ATP-PPi exchange assay was modified from previous experiments.[31] Each reaction (25 μL) contained the following components: HEPES·KOH (100 mM, pH 7.5), KCl (30 mM), MgCl2 (10 mM), DTT (2 mM), KF (2 mM), NaPPi (2 mM), ATP (5 mM), tyrosine (5 mM), [γ-32P]-ATP (2 μCi/μL), and TyrRS or its variants (0.1 μm). Reaction mixtures were incubated at 37°C. Time points were taken at 2, 5, and 10 min after transferring aliquots (1 μL) from reaction mixtures immediately to PEI-cellulose plates (Merck). For each sample, the blank reaction (containing no enzyme) was set as background. Reaction mixtures were separated on PEI-cellulose plates in urea (1M) and monopotassium phosphate (1M). Plates were then scanned in a Molecular Dynamics Storm 860 phosphorimager (Amersham Biosciences). Kinetic parameters were derived from plotting the initial velocity of a series of reactions which contained varied concentrations of Tyr or ATP and analyzed by nonlinear regression with software GraFit (Erithacus Software, Horley, UK).
In vitro transcription and purification of tRNATyr
E. coli tRNATyr was generated by modified protocols from previous experiments.[31] The template plasmid containing the tRNATyr gene was purified with the plasmid maxi kit, digested with BstNI, purified, and resolved in water. The transcription reaction [50 mM Tris (50 mM, pH 8); each of UTP, CTP, GTP, and ATP (5 mM, pH 7.0); MgCl2 (25 mM); spermidine (2 mM); DTT (10 mM); pyrophosphatase (0.5 μgmL−1, Roche); BstNI digested DNA template (60 μgmL−1), T7 RNA polymerase (0.5 μgmL−1, purified by Ni-NTA affinity chromatography)] was performed in a reaction volume of 40 mL overnight at 37°C. The tRNA was purified on 12% denaturing polyacrylamide gel containing urea (8M) and TBE buffer [Tris (90 mM, pH 8.3), boric acid (90 mM), and EDTA (2 mM)]. UV shadowing illuminated the pure tRNA band, which was excised and extracted three times with sodium acetate (1M, pH 5.3) at 4°C. The tRNA extractions were then precipitated in ethanol, dissolved in RNase-free distilled water, pooled, and finally desalted using a Biospin 30 column (Bio-Rad).
Aminoacylation assay
The aminoacylation assay was modified from previous experiments.[32] A reaction mixture (50 μL) containing HEPES·KOH (100 mM, pH 7.2), KCl (30 mM), MgCl2 (10 mM), ATP (5 mM, pH 7.0), DTT (5 mM), [3H]tyrosine (25 μM) plus unlabeled tyrosine (75 μM), tRNATyr (10 μM), and TyrRS or its variants (100 nM) was incubated at 37°C. Reaction mixtures (10 μL) were taken at 1, 2, and 5 min and spotted on Whatman 3MM filter paper pre-soaked with trichloroacetic acid (5%, TCA). The paper discs were washed three times with TCA (5%), rinsed with ethanol (95%), dried at 80°C for 20 min, and read by scintillation counting. For each sample, blank reaction (containing no enzyme) was set as background. Kinetic parameters were derived from plotting the initial velocity of a series of reactions that contained varied concentrations of tRNATyr and analyzed by nonlinear regression with GraFit software.
In vitro deacetylation assay
The deacetylation assay was modified from previous experiments.[33] The reaction was performed in a mixture (100 μL) containing HEPES·KOH (50 mM, pH 7.0), MgCl2 (5 mM), NAD+ (1 mM), DTT (2 mM), glycerol (10%), TyrRS or its variants (10 μg), and CobB or YcgC (10 μg). Reactions were incubated at 37°C for 1 h.
In vitro acetylation assay
The acetylation assay was modified from previous experiments.[33] The reaction was performed in a mixture (100 μL) containing Tris (50 mM, pH 8.0), EDTA (0.1 mM), DTT (2 mM), glycerol (10%), and sodium butyrate (10 mM). The acetylation was carried out by adding TyrRS or its variants (10 μg), individual candidate GNAT family acetyltransferase (10 μg), and acetyl-CoA (0.2 mM). Reaction mixtures were completely mixed and incubated at 37°C for 1 h.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH, R21 AI119813 to C.F.) and startup funds from the University of Arkansas.
Footnotes
Supporting information and the ORCID identification numbers for the authors of this article can be found under https://doi.org/10.1002/cbic.201700343.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.a) Schimmel PR, Soll D. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]; b) Mirande M. Prog Nucleic Acid Res Mol Biol. 1991;40:95–142. doi: 10.1016/s0079-6603(08)60840-5. [DOI] [PubMed] [Google Scholar]; c) Delarue M, Moras D. Bioessays. 1993;15:675–687. doi: 10.1002/bies.950151007. [DOI] [PubMed] [Google Scholar]; d) Cusack S. Curr Opin Struct Biol. 1997;7:881–889. doi: 10.1016/s0959-440x(97)80161-3. [DOI] [PubMed] [Google Scholar]; e) Ibba M, Soll D. Annu Rev Biochem. 2000;69:617–650. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]; f) Woese CR, Olsen GJ, Ibba M, Soll D. Microbiol Mol Biol Rev. 2000;64:202–236. doi: 10.1128/mmbr.64.1.202-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Francklyn C, Perona JJ, Puetz J, Hou YM. RNA. 2002;8:1363–1372. doi: 10.1017/s1355838202021180. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Ling J, Reynolds N, Ibba M. Annu Rev Microbiol. 2009;63:61–78. doi: 10.1146/annurev.micro.091208.073210. [DOI] [PubMed] [Google Scholar]; i) Pang YL, Poruri K, Martinis SA. Wiley Interdiscip Rev RNA. 2014;5:461–480. doi: 10.1002/wrna.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Francklyn CS. Methods. 2017;113:1–2. doi: 10.1016/j.ymeth.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Martinis SA, Plateau P, Cavarelli J, Florentz C. Biochimie. 1999;81:683–700. doi: 10.1016/s0300-9084(99)80126-6. [DOI] [PubMed] [Google Scholar]; b) Ivanov KA, Moor NA, Lavrik OI. Biochemistry. 2000;65:888–897. [PubMed] [Google Scholar]; c) Szymanski M, Deniziak M, Barciszewski J. Acta Biochim Pol. 2000;47:821–834. [PubMed] [Google Scholar]; d) Smirnova EV, Lakunina VA, Tarassov I, Krasheninnikov IA, Kamenski PA. Biochemistry (Mosc) 2012;77:15–25. doi: 10.1134/S0006297912010026. [DOI] [PubMed] [Google Scholar]; e) Guo M, Schimmel P. Nat Chem Biol. 2013;9:145–153. doi: 10.1038/nchembio.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Mirando AC, Francklyn CS, Lounsbury KM. Int J Mol Sci. 2014;15:23725–23748. doi: 10.3390/ijms151223725. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Son SH, Park MC, Kim S. Top Curr Chem. 2013;344:145–166. doi: 10.1007/128_2013_476. [DOI] [PubMed] [Google Scholar]; h) Yao P, Poruri K, Martinis SA, Fox PL. Top Curr Chem. 2013;344:167–187. doi: 10.1007/128_2013_422. [DOI] [PubMed] [Google Scholar]
- 3.a) Antonellis A, Green ED. Annu Rev Genomics Hum Genet. 2008;9:87–107. doi: 10.1146/annurev.genom.9.081307.164204. [DOI] [PubMed] [Google Scholar]; b) Kim S, You S, Hwang D. Nat Rev Cancer. 2011;11:708–718. doi: 10.1038/nrc3124. [DOI] [PubMed] [Google Scholar]; c) Konovalova S, Tyynismaa H. Mol Genet Metab. 2013;108:206–211. doi: 10.1016/j.ymgme.2013.01.010. [DOI] [PubMed] [Google Scholar]; d) Motzik A, Nechushtan H, Foo SY, Razin E. Trends Mol Med. 2013;19:726–731. doi: 10.1016/j.molmed.2013.07.011. [DOI] [PubMed] [Google Scholar]; e) Yao P, Fox PL. EMBO Mol Med. 2013;5:332–343. doi: 10.1002/emmm.201100626. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Dewan V, Reader J, Forsyth KM. Top Curr Chem. 2013;344:293–329. doi: 10.1007/128_2013_425. [DOI] [PubMed] [Google Scholar]; g) Kim D, Kwon NH, Kim S. Top Curr Chem. 2013;344:207–245. doi: 10.1007/128_2013_455. [DOI] [PubMed] [Google Scholar]; h) Schwenzer H, Zoll J, Florentz C, Sissler M. Top Curr Chem. 2013;344:247–292. doi: 10.1007/128_2013_457. [DOI] [PubMed] [Google Scholar]; i) Oprescu SN, Griffin LB, Beg AA, Antonellis A. Methods. 2017;113:139–151. doi: 10.1016/j.ymeth.2016.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Wang Q, Parrish AR, Wang L. Chem Biol. 2009;16:323–336. doi: 10.1016/j.chembiol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu CC, Schultz PG. Annu Rev Biochem. 2010;79:413–444. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]; c) Hoesl MG, Budisa N. Curr Opin Biotechnol. 2012;23:751–757. doi: 10.1016/j.copbio.2011.12.027. [DOI] [PubMed] [Google Scholar]; d) Neumann H. FEBS Lett. 2012;586:2057–2064. doi: 10.1016/j.febslet.2012.02.002. [DOI] [PubMed] [Google Scholar]; e) O’Donoghue P, Ling J, Wang YS, Soll D. Nat Chem Biol. 2013;9:594–598. doi: 10.1038/nchembio.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Chin JW. Annu Rev Biochem. 2014;83:379–408. doi: 10.1146/annurev-biochem-060713-035737. [DOI] [PubMed] [Google Scholar]; g) Lemke EA. ChemBioChem. 2014;15:1691–1694. doi: 10.1002/cbic.201402362. [DOI] [PubMed] [Google Scholar]
- 5.a) Krishna RG, Wold F. Adv Enzymol Relat Areas Mol Biol. 1993;67:265–298. doi: 10.1002/9780470123133.ch3. [DOI] [PubMed] [Google Scholar]; b) Lothrop AP, Torres MP, Fuchs SM. FEBS Lett. 2013;587:1247–1257. doi: 10.1016/j.febslet.2013.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Walsh CT. Posttranslational Modification of Proteins: Expanding Nature’s Inventory. Roberts and Company; Englewood: 2006. [Google Scholar]; d) Parekh RB, Rohlff C. Curr Opin Biotechnol. 1997;8:718–723. doi: 10.1016/s0958-1669(97)80126-7. [DOI] [PubMed] [Google Scholar]; e) Wang X, Pattison JS, Su H. Circ Res. 2013;112:367–381. doi: 10.1161/CIRCRESAHA.112.268706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Cohen T, Yao TP. Sci Signaling. 2004;2004 pe42. [Google Scholar]; b) Arif M, Selvi BR, Kundu TK. ChemBioChem. 2010;11:1501–1504. doi: 10.1002/cbic.201000292. [DOI] [PubMed] [Google Scholar]; c) Choudhary C, Weinert BT, Nishida Y, Verdin E, Mann M. Nat Rev Mol Cell Biol. 2014;15:536–550. doi: 10.1038/nrm3841. [DOI] [PubMed] [Google Scholar]; d) Verdin E, Ott M. Nat Rev Mol Cell Biol. 2015;16:258–264. doi: 10.1038/nrm3931. [DOI] [PubMed] [Google Scholar]; e) Drazic A, Myklebust LM, Ree R, Arnesen T. Biochim Biophys Acta. 2016;1864:1372–1401. doi: 10.1016/j.bbapap.2016.06.007. [DOI] [PubMed] [Google Scholar]; f) Escalante-Semerena JC. Microbe. 2010;5:340–344. doi: 10.1128/microbe.5.340.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 8.a) Baeza J, Dowell JA, Smallegan MJ, Fan J, Amador-Noguez D, Khan Z, Denu JM. J Biol Chem. 2014;289:21326–21338. doi: 10.1074/jbc.M114.581843. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kuhn ML, Zemaitaitis B, Hu LI, Sahu A, Sorensen D, Minasov G, Lima BP, Scholle M, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ. PLoS One. 2014;9:e94816. doi: 10.1371/journal.pone.0094816. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Schilling B, Christensen D, Davis R, Sahu AK, Hu LI, Walker-Peddakotla A, Sorensen DJ, Zemaitaitis B, Gibson BW, Wolfe AJ. Mol Microbiol. 2015;98:847–863. doi: 10.1111/mmi.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Weinert BT, Iesmantavicius V, Wagner SA, Scholz C, Gummesson B, Beli P, Nystrom T, Choudhary C. Mol Cell. 2013;51:265–272. doi: 10.1016/j.molcel.2013.06.003. [DOI] [PubMed] [Google Scholar]; e) Yu BJ, Kim JA, Moon JH, Ryu SE, Pan JG. J Microbiol Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]; f) Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, Liu CF, Grishin NV, Zhao Y. Mol Cell Proteomics. 2009;8:215–225. doi: 10.1074/mcp.M800187-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Zhang K, Zheng S, Yang JS, Chen Y, Cheng Z. J Proteome Res. 2013;12:844–851. doi: 10.1021/pr300912q. [DOI] [PubMed] [Google Scholar]
- 9.Cao X, Li C, Xiao S, Tang Y, Huang J, Zhao S, Li X, Li J, Zhang R, Yu W. Proc Natl Acad Sci USA. 2017;114:687–692. doi: 10.1073/pnas.1608488114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Mukai T, Kobayashi T, Hino N, Yanagisawa T, Sakamoto K, Yokoyama S. Biochem Biophys Res Commun. 2008;371:818–822. doi: 10.1016/j.bbrc.2008.04.164. [DOI] [PubMed] [Google Scholar]; b) Neumann H, Peak-Chew SY, Chin JW. Nat Chem Biol. 2008;4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]; c) Umehara T, Kim J, Lee S, Guo LT, Sçll D, Park HS. FEBS Lett. 2012;586:729–733. doi: 10.1016/j.febslet.2012.01.029. [DOI] [PubMed] [Google Scholar]; d) Fan C, Xiong H, Reynolds NM, Soll D. Nucleic Acids Res. 2015;43:e156. doi: 10.1093/nar/gkv800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye Q, Ji QQ, Yan W, Yang F, Wang ED. J Biol Chem. 2017;292:10709–10722. doi: 10.1074/jbc.M116.770826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbaum JJ, Schimmel P. J Biol Chem. 1991;266:16965–16968. [PubMed] [Google Scholar]
- 13.Kiga D, Sakamoto K, Kodama K, Kigawa T, Matsuda T, Yabuki T, Shirouzu M, Harada Y, Nakayama H, Takio K, Hasegawa Y, Endo Y, Hirao I, Yokoyama S. Proc Natl Acad Sci USA. 2002;99:9715–9720. doi: 10.1073/pnas.142220099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamano-Takaku F, Iwama T, Saito-Yano S, Takaku K, Monden Y, Kitabatake M, Soll D, Nishimura S. J Biol Chem. 2000;275:40324–40328. doi: 10.1074/jbc.M003696200. [DOI] [PubMed] [Google Scholar]
- 15.a) Jakes R, Fersht AR. Biochemistry. 1975;14:3344–3350. doi: 10.1021/bi00686a009. [DOI] [PubMed] [Google Scholar]; b) Buonocore V, Harris MH, Schlesinger S. J Biol Chem. 1972;247:4843–4849. [PubMed] [Google Scholar]
- 16.a) Wolffe AP. Science. 1996;272:371–372. doi: 10.1126/science.272.5260.371. [DOI] [PubMed] [Google Scholar]; b) Seto E, Yoshida M. Cold Spring Harbor Perspect Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tong JK. Chem Biol. 2002;9:668–670. doi: 10.1016/s1074-5521(02)00158-8. [DOI] [PubMed] [Google Scholar]
- 17.a) Zhao K, Chai X, Marmorstein R. J Mol Biol. 2004;337:731–741. doi: 10.1016/j.jmb.2004.01.060. [DOI] [PubMed] [Google Scholar]; b) Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]; c) Tu S, Guo SJ, Chen CS, Liu CX, Jiang HW, Ge F, Deng JY, Zhou YM, Czajkowsky DM, Li Y, Qi BR, Ahn YH, Cole PA, Zhu H, Tao SC. eLife. 2015;4:e05322. doi: 10.7554/eLife.05322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AbouElfetouh A, Kuhn ML, Hu LI, Scholle MD, Sorensen DJ, Sahu AK, Becher D, Antelmann H, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ. Microbiologyopen. 2015;4:66–83. doi: 10.1002/mbo3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a) Paulsen IT, Reizer J, Jin RZ, Lin EC, Saier MH., Jr Microbiology. 2000;146:2343–2344. doi: 10.1099/00221287-146-10-2343. [DOI] [PubMed] [Google Scholar]; b) Gutknecht R, Beutler R, Garcia-Alles LF, Baumann U, Erni B. EMBO J. 2001;20:2480–2486. doi: 10.1093/emboj/20.10.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a) Hu LI, Lima BP, Wolfe AJ. Mol Microbiol. 2010;77:15–21. doi: 10.1111/j.1365-2958.2010.07204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Jones JD, O’Connor CD. Proteomics. 2011;11:3012–3022. doi: 10.1002/pmic.201000812. [DOI] [PubMed] [Google Scholar]; c) Kim GW, Yang XJ. Trends Biochem Sci. 2011;36:211–220. doi: 10.1016/j.tibs.2010.10.001. [DOI] [PubMed] [Google Scholar]; d) Thao S, Escalante-Semerena JC. Curr Opin Microbiol. 2011;14:200–204. doi: 10.1016/j.mib.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Bernal V, Castano-Cerezo S, Gallego-Jara J, Ecija-Conesa A, de Diego T, Iborra JL, Canovas M. New Biotechnol. 2014;31:586–595. doi: 10.1016/j.nbt.2014.03.002. [DOI] [PubMed] [Google Scholar]; f) Hentchel KL, Escalante-Semerena JC. Microbiol Mol Biol Rev. 2015;79:321–346. doi: 10.1128/MMBR.00020-15. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Wolfe AJ. Curr Genet. 2016;62:335–341. doi: 10.1007/s00294-015-0552-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.a) Sterner DE, Berger SL. Microbiol Mol Biol Rev. 2000;64:435–459. doi: 10.1128/mmbr.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Marmorstein R, Roth SY. Curr Opini Genet Devel. 2001;11:155–161. doi: 10.1016/s0959-437x(00)00173-8. [DOI] [PubMed] [Google Scholar]; c) Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]; d) Lee KK, Workman JL. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- 22.Starai VJ, Escalante-Semerena JC. J Mol Biol. 2004;340:1005–1012. doi: 10.1016/j.jmb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 23.a) Hu LI, Chi BK, Kuhn ML, Filippova EV, Walker-Peddakotla AJ, Basell K, Becher D, Anderson WF, Antelmann H, Wolfe AJ. J Bacteriol. 2013;195:4174–4186. doi: 10.1128/JB.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Thao S, Chen CS, Zhu H, Escalante-Semerena JC. PLoS One. 2010;5:e15123. doi: 10.1371/journal.pone.0015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crosby HA, Escalante-Semerena JC. J Bacteriol. 2014;196:1496–1504. doi: 10.1128/JB.00004-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Zhou J, Lin S, Deng W, Zhang Y, Xue Y. J Genet Genomics. 2017;44:243–250. doi: 10.1016/j.jgg.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Eriani G, Delarue M, Poch O, Gangloff J, Moras D. Nature. 1990;347:203–206. doi: 10.1038/347203a0. [DOI] [PubMed] [Google Scholar]
- 27.Arnez JG, Moras D. Trends Biochem Sci. 1997;22:211–216. doi: 10.1016/s0968-0004(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 28.a) Fersht AR, Knill-Jones JW, Bedouelle H, Winter G. Biochemistry. 1988;27:1581–1587. doi: 10.1021/bi00405a028. [DOI] [PubMed] [Google Scholar]; b) Xin Y, Li W, First EA. Biochemistry. 2000;39:340–347. doi: 10.1021/bi991675l. [DOI] [PubMed] [Google Scholar]; c) Kobayashi T, Takimura T, Sekine R, Kelly VP, Kamata K, Sakamoto K, Nishimura S, Yokoyama S. J Mol Biol. 2005;346:105–117. doi: 10.1016/j.jmb.2004.11.034. [DOI] [PubMed] [Google Scholar]; d) Brick P, Blow DM. J Mol Biol. 1987;194:287–297. doi: 10.1016/0022-2836(87)90376-7. [DOI] [PubMed] [Google Scholar]; e) Brick P, Bhat TN, Blow DM. J Mol Biol. 1989;208:83–98. doi: 10.1016/0022-2836(89)90090-9. [DOI] [PubMed] [Google Scholar]; f) Winter G, Fersht AR, Wilkinson AJ, Zoller M, Smith M. Nature. 1982;299:756–758. doi: 10.1038/299756a0. [DOI] [PubMed] [Google Scholar]; g) First EA, Fersht AR. Biochemistry. 1993;32:13658–13663. doi: 10.1021/bi00212a034. [DOI] [PubMed] [Google Scholar]; h) First EA, Fersht AR. Biochemistry. 1995;34:5030–5043. doi: 10.1021/bi00015a014. [DOI] [PubMed] [Google Scholar]
- 29.Mertins P, Qiao JW, Patel J, Udeshi ND, Clauser KR, Mani DR, Burgess MW, Gillette MA, Jaffe JD, Carr SA. Nat Methods. 2013;10:634–637. doi: 10.1038/nmeth.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H. DNA Res. 2005;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 31.Amiram M, Haimovich AD, Fan C, Wang YS, Aerni HR, Ntai I, Moonan DW, Ma NJ, Rovner AJ, Hong SH, Kelleher NL, Goodman AL, Jewett MC, Soll D, Rinehart J, Isaacs FJ. Nat Biotechnol. 2015;33:1272–1279. doi: 10.1038/nbt.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salazar JC, Ahel I, Orellana O, Tumbula-Hansen D, Krieger R, Daniels L, Soll D. Proc Natl Acad Sci USA. 2003;100:13863–13868. doi: 10.1073/pnas.1936123100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkat S, Gregory C, Sturges J, Gan Q, Fan C. J Mol Biol. 2017;429:1396–1405. doi: 10.1016/j.jmb.2017.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


