Abstract
Background
Although traditionally-dosed combined oral contraceptives (21 days of active pills, 7 days of inactive pills) have not been demonstrated as superior to placebo for the treatment of premenstrual dysphoria (PMD), some RCTs indicate that oral contraceptives administered with a shortened or eliminated hormone-free-interval are superior to placebo. However, results of such trials are mixed, and no existing studies have directly compared continuous and intermittent dosing schedules of the same oral contraceptive. The present study compared placebo, intermittent dosing of oral contraceptives, and continuous dosing of contraceptives for the treatment of PMD.
Methods
55 women with prospectively-confirmed premenstrual dysphoria completed a three-arm, randomized controlled trial in which they were randomized to three months of placebo (n=22), intermittent drospirenone/ethinyl estradiol dosed on a 21-7 schedule (n=17), or continuous drospirenone/estradiol (n=16) following a baseline assessment month.
Results
All three groups demonstrated similar, robust reductions in premenstrual symptoms over time. A marked placebo response was observed.
Conclusions
The study fails to replicate a uniquely beneficial effect of continuous COC on premenstrual dysphoria. Additional work is needed to understand the psychosocial context bolstering the placebo response in women with PMD.
Clinical Trials Registration
Registration Number NCT00927095.
Keywords: Premenstrual Syndrome, Oral Contraceptives, Randomized Controlled Trial
1. Introduction
Premenstrual dysphoria (PMD) refers to the cyclical emergence of emotional and behavioral symptoms in the luteal phase of the menstrual cycle that remit within 4 days following menstrual onset (ACOG, 1995). It is estimated that 13–18% of women show evidence of PMD characterized by cyclical patterns of distress, treatment-seeking, and life interference (Halbreich et al., 2003). Symptoms of PMD can cause distress and impairment similar to that found in major depressive disorder, panic disorder, and post-traumatic stress disorder (Halbreich, Borenstein, Pearlstein, & Kahn, 2003). Treatment with selective serotonin reuptake inhibitors (SSRIs) resolves PMD in many women; however, nearly 40% do not respond (Halbreich, 2014), signaling the need for alternative treatments.
Many women with PMD demonstrate an abnormal emotional sensitivity to normal fluctuations in the steroid hormones estradiol (E2) and progesterone (P4) (Schmidt et al., 1998). These abnormal responses to normal changes in ovarian steroids among women with PMD have led to the hypothesis that stabilization of hormones may represent a key target for the treatment of PMD. In one study, spontaneous anovulatory cycles, associated with reduced ovarian hormone flux, were asymptomatic among women with PMD (Hammarbäck, Ekholm, & Bäckström, 1991). Suppression of ovarian function using GNRH agonists (e.g., leuprolide) also prevents luteal symptoms (see Wyatt et al., 2004, for meta-analysis), while addback of either E2 of P4 following ovarian suppression results in the re-emergence of symptoms among women with PMD (but not among controls) (Schmidt et al., 1998). Because GNRH agonists are not a feasible long-term treatment due to the need for hormonal addback (which causes a recurrence of symptoms; Schmidt et al., 1998), monophasic COCs have been investigated as an alternative method of stabilizing hormones to reduce premenstrual symptoms.
Accordingly, several RCTs have examined combined oral contraceptives (COCs) containing ethinyl estradiol (EE) and a progestin (e.g., drospirenone or DROS; levonorgestrel or LNG) for the treatment of premenstrual symptoms. Typical 21/7 dosing regimens (21 days active hormone and a 7 day hormone-free interval or HFI) have not demonstrated efficacy compared with placebo (Freeman et al., 2001; Graham & Sherwin, 1992), whereas two shortened-HFI COC trials (24/4) of DROS/EE have shown some benefit relative to placebo (Pearlstein, Bachmann, Zacur, & Yonkers, 2005; Yonkers et al., 2005; Freeman et al., 2012), although a review concluded that the efficacy of DROS/EE for premenstrual dysphoria may have been overestimated in these previous reports (Lopez, Kaptein, & Helmerhorst, 1996). Another study examining continuous LNG/EE showed greater effectiveness in the first month of treatment relative to placebo (of note, the first month of COC treatment is associated with greater ovarian suppression; Sullivan, Furniss, Spona, & Elstein, 1999), but most COC-related improvements were not sustained across follow-up, whereas placebo responses were sustained (Halbreich et al., 2012). Therefore, although studies with shortened or eliminated HFIs have resulted in the most beneficial effects thus far (Marr, Heinemann, Kunz, & Rapkin, 2011a; Marr, Niknian, Shulman, & Lynen, 2011b; Pearlstein et al., 2005; Yonkers et al., 2005), additional work is needed to confirm these findings and to clarify the roles of COC regimens (i.e., the use of a shortened or eliminated HFI) vs. COC formulations (i.e., the inclusion of different progestins) in the effects of COC on premenstrual dysphoria.
Historically, study designs have conflated the roles of COC formulation and regimen. The greater benefit found in DROS/EE 24/4 trials has been attributed to COC formulation with DROS, a progestin with antiandrogen properties; however, the use of the 24/4 regimen, which is associated with greater suppression of ovarian function and steroid fluctuations (Sullivan et al., 1999), may be responsible. Although COC regimens with longer HFIs (e.g., 21/7) are effective for contraception, 21/7 dosing schedules—and, to a much lesser extent, 24/4 dosing schedules—still permit follicular development during the HFl, which results in continued fluctuations of E2 both during active pills and the HFI (Schlaff, Lynch, Hughes, Cedars, & Smith, 2004; Sullivan et al., 1999). Therefore, it is possible that the modestly greater efficacy of DROS-containing COCs, which has generally been attributed to the greater antiandrogen properties of DROS, may be more appropriately attributed to the shorter HFIs in those trials.
The purpose of this study was to delineate the role of regimen, holding formulation constant, in the effects of COCs in PMD. If COCs reduce the symptoms of PMD by minimizing susceptible women’s exposure to steroid changes, and if such stabilization of hormones is inversely proportional to the length of the HFI (Sulak et al., 1997; Sullivan et al., 1999), then a reduced or eliminated HFI regimen of any COC (e.g., DROS/EE) should be more effective than a regimen of the same COC dosed on a regimen with a longer HFI. Further, a long-HFI regimen (e.g., 21-7) may result in greater symptom expression during either the HFI (Pearlstein et al., 2005; Yonkers et al., 2005) (due to the rapid increases in endogenous E2 that begin during the HFI (Sullivan et al., 1999)) or during active pill administration (due to the rapid decreases in endogenous E2 that occur following ovarian suppression (Sullivan et al., 1999)). In contrast, continuous regimens stabilize ovarian steroids more completely. To date, no study has compared continuous COC to placebo or to intermittent COC; these comparisons are the central goals of the present three-arm RCT. To minimize confounds, we utilized the same COC that has been described as efficacious in PMD when administered in a reduced-HFI regimen. We hypothesized that continuous DROS/EE would be associated with greater reductions in mood symptoms compared with placebo and intermittent DROS/EE, whereas intermittent DROS/EE, which allows continued cycling of steroids, would not demonstrate a benefit compared with placebo.
2. Methods
The study, carried out between 2010 and 2015 at a single university-based medical research center in North Carolina, employed a double-blind, randomized, placebo-controlled trial with three conditions: (1) placebo, (2) intermittently dosed DROS/EE 3mg/20μg (21/7), and (3) continuous DROS/EE 3mg/20μg. Following a phone screening, potential participants self-reported emotional symptoms daily for 2–4 menstrual cycles to prospectively confirm cyclical emotional symptoms of PMD; those who demonstrated luteal phase confinement (operationalized in more detail below) of at least one emotional symptom were invited to enroll. Participants reported daily symptoms across a baseline cycle, at which point symptoms were once again reviewed by a clinical consensus group to verify the persistence of cyclical mood symptoms. Following randomization, participants completed up to 3 treatment cycles, including continued symptom ratings. Individuals were also visited in their homes five times per month in the baseline and treatment months to confirm adherence and collect blood samples (for another set of hypotheses that are not the focus of this report). All participants agreed to use barrier contraceptive methods during the trial. The study was approved by the institutional review board in accordance with the standards of the Declaration of Helsinki, and all participants provided verbal and written informed consent after review of study activities and possible side effects. Women were paid $575.
Eligibility criteria were as follows: age 18–40, prospectively-confirmed PMD, no medication use except stable thyroid supplementation, no hormonal preparations for 3 months, no current psychiatric treatment with medication or psychotherapy, regular menstrual cycles (21–35 days), no uncontrolled hypertension or end-organ vascular disease, diabetes, or migraine, an unsuspicious PAP smear in the past year, no current pregnancy or breastfeeding, no family history of premenopausal breast cancer or breast cancer in more than one first degree relative, no medications or conditions that increase potassium, no history of endometriosis, recent, rapid growth uterine fibroid tumors, hepatic disease, breast carcinoma, pulmonary embolism or phlebothrombosis, undiagnosed vaginal bleeding, porphyria, malignant melanoma, cholecystitis or pancreatitis, or hypercholesterolemia, no diagnosis of an ill-defined, obscure pelvic lesion, no obesity (BMI>30) in women over 35, and although women with a history of MDD and other mood disorders were included as long as they were in remission for >= 1 year, women with a history of any other psychiatric disorder were excluded unless they had been in remission for >=2 years.
A prospective diagnosis of PMD was established across 2–5 screening cycles using the Daily Record of Severity of Problems (DRSP; Endicott & Halbreich, 1982). These screening cycles were utilized for diagnostic purposes only. Women mailed in responses weekly to discourage retrospective reporting. The DRSP measures each of the 11 premenstrual dysphoric disorder (PMDD) symptom domains listed in the DSM-5, rated on the following scale: 1=Not at All, 2=Minimal, 3=Mild, 4=Moderate, 5=Severe, and 6=Extreme. Women were considered to demonstrate PMD if, for at least one core emotional symptom (DRSP items 1–8), symptoms reached at least a 4 for at least two days of the premenstrual week, and the mean symptom severity across at least 2 premenstrual phases (defined as days −7 to −1 where menses start day is 0) was at least 30% greater than their mean symptom severity across at least 2 postmenstrual phases (defined as days 4–10 where menses start day is 0). This percent premenstrual elevation above one’s postmenstrual baseline was calculated as follows: premenstrual mean – postmenstrual mean / premenstrual mean. Premenstrual impairment was not required for diagnosis given the evidence that many women experience severe premenstrual distress but do not experience significant changes in impairment (Halbreich, 2004; Schmalenberger, Eisenlohr-Moul, Surana, Rubinow, & Girdler, 2017).
We chose to study PMD rather than PMDD for the following reasons: unlike the method of diagnosis described above, the DSM-IV diagnosis of PMDD fails to operationalize the specific numeric criteria for diagnosis using daily ratings, thus rendering the diagnosis ambiguous and ungeneralizable in practice; there are no data demonstrating that women who meet operational criteria for a single core emotional symptom (i.e., demonstration of confinement of a cyclical symptom to the luteal phase) differ from women with multiple symptoms (PMDD) in course, treatment response characteristics, or biology. Nonetheless, we considered women as having DSM-5 PMDD if they met our operationalized PMD criteria on at least one core emotional item and five items total. After screening, participants meeting PMD criteria underwent a SCID-I interview to rule out exclusionary diagnoses. Therefore, all participants met criteria for PMD; 36% (n=20) of the sample demonstrated symptoms consistent with PMDD.
Participants were randomly assigned to treatment groups by the study’s biostatistician in a 1:1:1 ratio using SAS to generate random values corresponding to group assignment. The university’s investigational drug service dispensed the medications to study coordinators in a double-blind manner, maintaining the blind for both participants and investigators. Participants were enrolled and monitored by study coordinators. Medication was taken on the prescribed schedule regardless of menstrual or intermenstrual/breakthrough bleeding. Therefore, cycle day in the intermittent and continuous COC groups was considered to be standardized to 28 days during treatment. The premenstrual week was defined at post-treatment in the two active treatment groups as pill days 21 to 28. In the placebo condition and in baseline and screening months for all participants, the premenstrual week was defined as days −7 to −1 where day 0 is menstrual onset.
The Daily Record of Severity of Problems (DRSP) was also utilized as the primary outcome measure. Several parameters of the DRSP were considered as outcomes in the present study, including mean levels of one’s worst baseline symptom, one’s worst baseline emotional symptom, physical symptoms (average of items measuring breast tenderness; breast swelling, bloating, or weight gain; and joint or muscle pain), depression (item: “felt depressed, blue”), anxiety (item: “felt anxious, “keyed up”, or “on edge”), mood swings (item: “had mood swings (e.g., suddenly felt sad or tearful)”), anger/irritability (average of items “felt angry, irritable” and “had conflicts or problems with people”), and functional impairment (average of three interference items). Although the total DRSP score was originally considered as an outcome, previous analyses of data from the baseline month of this trial demonstrated poor reliability of change over time for the total DRSP score (i.e., items did not change together over time).
Data Preparation and Analytic Plan
Baseline data included two diagnostic screening cycles and one baseline cycle; therefore, women contributed three pre-treatment cycles, from which their baseline values were calculated. Post-treatment values were evaluated based on the single final on-treatment cycle (treament cycle 3 for 95% of participants). The mean of the premenstrual week was always calculated as the 7 days before menses (at baseline and in the placebo condition) or day 21 through 28 of the pill pack (at post-treatment in OC conditions). Symptom mean and variance across the cycle were computed as the mean or variance of a symptom across all days of a cycle. An individual’s worst emotional symptom at baseline (selected from DRSP items 1–8) and worst overall symptom at baseline (selected from DRSP items 1–21) were defined as the symptom showing the greatest difference (elevation) between the premenstrual week average and the postmenstrual week average during pre-treatment cycles. Of note, alternative specification of the “premenstrual” window as the week of HFI-induced withdrawal bleeding in the intermittent group (i.e., days 21–28 of the pill pack) did not substantively change the results presented herein.
Hypotheses were tested by comparing change from baseline cycles to final on-treatment cycle. We analyzed the data only from those compliant subjects who provided at least one complete treatment menstrual cycle. For each outcome, an analysis of covariance model assuming Gaussian errors and with treatment group and baseline value as predictors was fit. Hypothesis tests are reported for whether change from baseline to post-baseline within each treatment group was different from zero and for whether treatment groups differed with respect to this change.
3. Results
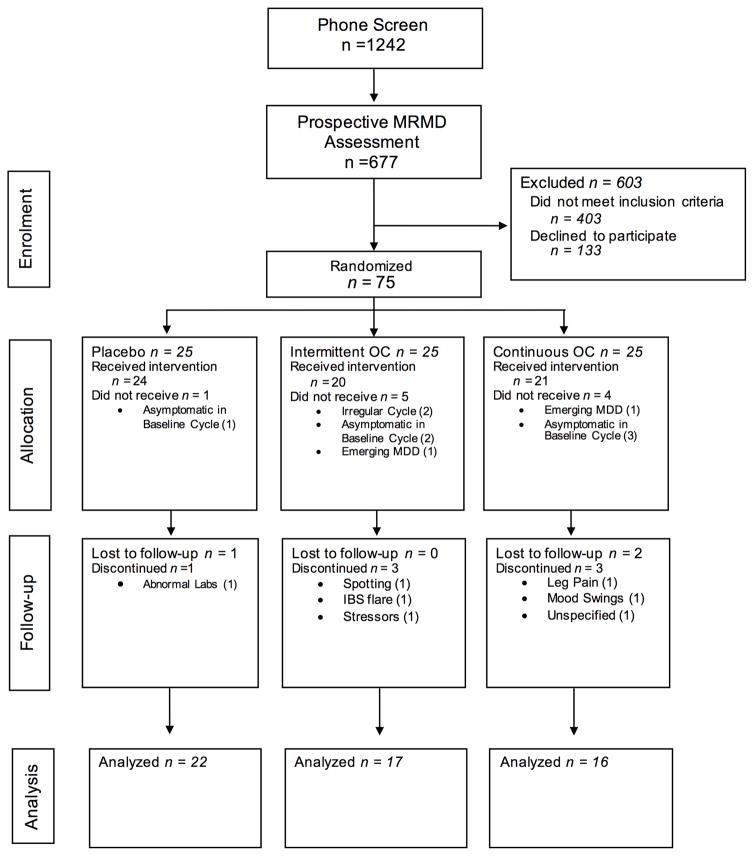
See Figure 1 for CONSORT diagram. Initially, a sample size of 90 participants was selected with the goal of achieving 80% power to detecting conventionally medium (f = .30) differences between treatment groups (with alpha set at .05). Due to the stringency of our prospective screening criteria for PMD and time constraints, 75 women were randomized to a treatment arm (Placebo = 25; Intermittent = 25; Continuous = 25). 10 of those randomized did not receive study medication, and an additional 10 discontinued the study prior to the assessment of first-month premenstrual symptoms (see Figure 1 for details). Therefore, 55 women provided at least one on-treatment premenstrual week and were included in analyses (Final Sample: Placebo = 22; Intermittent = 17; Continuous = 16). A post-hoc power analysis in this final sample indicates that, given alpha set at .05, a sample of 55 women achieves 80% power to detect a conventionally large effect of group on change in premenstrual symptoms over time (smallest detectible effect size f = .38). Therefore, the present study is powered only to detect conventionally large effects of condition, and is not powered to detect conventionally small-to-medium effects.
Figure 1.
CONSORT Diagram.
Demographic characteristics of this sample can be found in Table 1. Preliminary examination of the worst emotional symptom variable at pre-treament revealed that anger/interpersonal conflict was the most common worst symptom (38.4% of women), followed by anxiety (29.09%), mood lability or rejection sensitivity (20%), and finally, depression (12.73%). Worst overall symptoms produced different results; fatigue emerged as the most common worst symptom (21.82%), followed by anger and interpersonal conflict (18.18%), physical symptoms; (12.73%), eating symptoms (10.91%), anxiety (9.09%), depression (7.27%), sleep disturbance (7.27%), mood lability and rejection sensitivity (5.46%), loss of interest (3.64%), overwhelm (1.82%), and feeling out of control (1.82%). These are similar to the worst symptoms reported in previous trials, in which irritability, anxiety, mood lability, and fatigue were the most common worst symptoms (Bloch, Schmidt, & Rubinow, 1997). Mean total DRSP score during the premenstrual week (days −7 to −1 prior to menses onset) was 70.02 (SD = 19.32). In the largest RCT of DROS conducted (Yonkers et al., 2005), the mean premenstrual symptom score for the DRSP Sum was reported as 77.40 (SD = 16.70); therefore, our sample demonstrated a comparable baseline severity to the sample described by Yonkers et al. (2005) as having Premenstrual Dysphoric Disorder (PMDD).
Table 1.
Demographic Characteristics of Study Groups
| Characteristic | Full Sample (N = 55) |
|
||
|---|---|---|---|---|
| Treatment Group | ||||
|
| ||||
| Placebo (N = 21) | Intermittent (21-7) DROS/EE (N = 17) | Continuous DROS/EE (N = 16) | ||
| Age | 32.5 (7.7) | 32.2 (8.6) | 32.1 (6.7) | 33.2 (8.1) |
| Race | ||||
| White | 65.5% | 63.6% | 52.9% | 81.3% |
| Black | 23.6% | 27.3% | 35.3% | 6.3% |
| Latina | 3.6% | 0.0% | 5.9% | 0.0% |
| Asian | 7.3% | 9.1% | 5.9% | 12.5% |
| Mixed or Other | 0.0% | 0.0% | 0.0% | 0.0% |
| Marital Status | ||||
| Single | 52.7% | 52.7% | 54.5% | 52.9% |
| Married | 47.3% | 47.3% | 45.5% | 47.1% |
| Smoking Status | ||||
| Nonsmoker | 96.4% | 95.5% | 100.0% | 93.8% |
| Smoker | 3.6% | 4.5% | 0.0% | 6.3% |
Results are presented in Table 2 and mean total DRSP across the baseline and treatment cycles by group are presented in Figure 2. Across outcomes, premenstrual symptoms declined significantly in all groups, and these marked declines over time did not significantly differ by treatment group. To address the impact of the three treatments on global burden and total variance in symptoms across a given cycle, we also compared the impact of treatment on variances in symptoms across the entirety of the baseline and last on-treatment cycles. Across all three groups and all symptoms, there were significant reductions in mean symptom levels and as well as significant reductions in the variance of symptoms across the entire cycle (all p’s < .01). Results generally did not reveal any significant differences in the reductions of means or variances over time between groups, with one exception: reduction in the variance in one’s worst overall symptom across the entire cycle was significantly less robust in the intermittent group compared with the continuous group (d = −.91, p = .03) and showed a trend toward being less robust in the intermittent group than in the placebo group (d = −.60, p = .09). Therefore, although variance in one’s worst symptom did decline significantly in the intermittent treatment group, these reductions were less robust than those in the continuous and placebo groups.
Table 2.
Outcomes Within and Between Treatment Groups
| Symptom | Outcome | N | Treatment | Mean Across Baseline Cycles | Mean of Last On-Treatment Cycle | Within-Group Least Squares Change from Baseline to Post-Treatment | Within-Group p1 | Group Difference2 | Effect Size3 | Between-Group p4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Worst Emotional Symptom (DRSP1–DRSP8) | Pre-menstrual Mean | 16 | Continuous | 3.67 (0.69) | 1.65 (0.64) | 1.94 (0.22) | 0.000 | 0.27 | 0.30 | NS |
| 16 | Intermittent | 3.27 (0.67) | 1.87 (1.01) | 1.67 (0.23) | 0.000 | 0.31 | 0.34 | NS | ||
| 21 | Placebo | 3.76 (0.92) | 1.97 (0.93) | 1.64 (0.19) | 0.000 | 0.04 | 0.04 | NS | ||
| Cycle Mean | 16 | Continuous | 2.28 (0.34) | 1.77 (0.62) | 0.51 (0.15) | 0.001 | 0.09 | 0.16 | NS | |
| 17 | Intermittent | 2.08 (0.38) | 1.79 (0.61) | 0.42 (0.15) | 0.007 | −0.01 | −0.02 | NS | ||
| 21 | Placebo | 2.43 (0.56) | 1.81 (0.60) | 0.52 (0.13) | 0.000 | −0.10 | −0.17 | NS | ||
| Cycle Variance | 16 | Continuous | 1.91 (0.58) | 0.68 (0.55) | 1.39 (0.15) | 0.000 | 0.25 | 0.43 | NS | |
| 16 | Intermittent | 1.98 (1.00) | 0.94 (0.70) | 1.13 (0.15) | 0.000 | 0.03 | 0.06 | NS | ||
| 21 | Placebo | 2.28 (1.19) | 0.74 (0.49) | 1.35 (0.13) | 0.000 | −0.22 | −0.38 | NS | ||
|
| ||||||||||
| Worst Symptom (DRSP1–DRSP21) | Pre-menstrual Mean | 16 | Continuous | 3.88 (0.61) | 1.79 (0.79) | 2.05 (0.26) | 0.000 | 0.38 | 0.36 | NS |
| 17 | Intermittent | 3.59 (0.75) | 2.09 (1.17) | 1.68 (0.26) | 0.000 | 0.59 | 0.56 | NS | ||
| 21 | Placebo | 3.97 (0.96) | 2.40 (1.12) | 1.46 (0.23) | 0.000 | 0.21 | 0.20 | NS | ||
| Cycle Mean | 16 | Continuous | 2.64 (0.40) | 2.22 (0.69) | 0.38 (0.19) | 0.055 | −0.07 | −0.10 | NS | |
| 17 | Intermittent | 2.32 (0.47) | 1.94 (0.80) | 0.46 (0.19) | 0.022 | −0.06 | −0.08 | NS | ||
| 21 | Placebo | 2.63 (0.64) | 2.15 (0.97) | 0.45 (0.17) | 0.012 | 0.01 | 0.01 | NS | ||
| Cycle Variance | 16 | Continuous | 2.24 (0.71) | 0.63 (0.45) | 1.74 (0.17) | 0.000 | 0.61 | 0.91 | 0.031 | |
| 17 | Intermittent | 2.23 (0.93) | 1.23 (0.93) | 1.13 (0.16) | 0.000 | 0.20 | 0.31 | NS | ||
| 21 | Placebo | 2.54 (1.21) | 0.81 (0.52) | 1.53 (0.15) | 0.000 | −0.40 | −0.60 | NS | ||
|
| ||||||||||
| Physical Symptoms | Pre-menstrual Mean | 16 | Continuous | 2.20 (0.82) | 1.69 (0.73) | 0.67 (0.16) | 0.000 | −0.21 | −0.32 | NS |
| 16 | Intermittent | 2.41 (0.78) | 1.56 (0.59) | 0.88 (0.16) | 0.000 | 0.03 | 0.05 | NS | ||
| 21 | Placebo | 2.71 (0.98) | 1.92 (0.82) | 0.64 (0.14) | 0.000 | 0.25 | 0.38 | NS | ||
| Cycle Mean | 16 | Continuous | 1.55 (0.34) | 1.74 (0.68) | −0.15 (0.14) | NS | −0.07 | −0.11 | NS | |
| 17 | Intermittent | 1.59 (0.29) | 1.70 (0.62) | −0.08 (0.14) | NS | −0.28 | −0.49 | NS | ||
| 21 | Placebo | 1.84 (0.54) | 1.66 (0.62) | 0.13 (0.13) | NS | −0.22 | −0.37 | NS | ||
| Cycle Variance | 16 | Continuous | 0.80 (0.53) | 0.37 (0.26) | 0.68 (0.13) | 0.000 | 0.32 | 0.65 | NS | |
| 16 | Intermittent | 1.18 (0.74) | 0.77 (0.70) | 0.35 (0.12) | 0.005 | 0.13 | 0.26 | NS | ||
| 21 | Placebo | 1.28 (1.02) | 0.59 (0.47) | 0.55 (0.11) | 0.000 | −0.20 | −0.40 | NS | ||
|
| ||||||||||
| Depression (DRSP1) | Pre-menstrual Mean | 16 | Continuous | 2.63 (0.93) | 1.45 (0.47) | 1.28 (0.19) | 0.000 | 0.09 | 0.12 | NS |
| 17 | Intermittent | 2.57 (0.88) | 1.53 (0.88) | 1.19 (0.18) | 0.000 | 0.25 | 0.33 | NS | ||
| 21 | Placebo | 2.99 (1.06) | 1.76 (0.83) | 1.03 (0.17) | 0.000 | 0.15 | 0.20 | NS | ||
| Cycle Mean | 16 | Continuous | 1.87 (0.53) | 1.57 (0.57) | 0.29 (0.13) | 0.026 | 0.06 | 0.12 | NS | |
| 17 | Intermittent | 1.71 (0.45) | 1.57 (0.54) | 0.23 (0.13) | 0.072 | −0.01 | −0.02 | NS | ||
| 21 | Placebo | 1.95 (0.55) | 1.59 (0.53) | 0.30 (0.11) | 0.010 | −0.07 | −0.14 | NS | ||
| Cycle Variance | 16 | Continuous | 1.12 (0.54) | 0.58 (0.76) | 0.65 (0.16) | 0.000 | 0.07 | 0.11 | NS | |
| 17 | Intermittent | 1.12 (0.82) | 0.65 (0.77) | 0.58 (0.16) | 0.001 | −0.03 | −0.04 | NS | ||
| 21 | Placebo | 1.48 (1.12) | 0.62 (0.49) | 0.68 (0.15) | 0.000 | −0.10 | −0.15 | NS | ||
|
| ||||||||||
| Anxiety (DRSP4) | Pre-menstrual Mean | 16 | Continuous | 3.19 (0.74) | 1.72 (0.62) | 1.43 (0.20) | 0.000 | 0.11 | 0.14 | NS |
| 16 | Intermittent | 2.65 (0.73) | 1.61 (0.96) | 1.32 (0.21) | 0.000 | 0.16 | 0.19 | NS | ||
| 21 | Placebo | 3.46 (1.01) | 1.99 (0.98) | 1.27 (0.18) | 0.000 | 0.04 | 0.05 | NS | ||
| Cycle Mean | 16 | Continuous | 2.03 (0.45) | 1.76 (0.67) | 0.28 (0.14) | 0.058 | −0.02 | −0.03 | NS | |
| 17 | Intermittent | 1.83 (0.45) | 1.62 (0.65) | 0.30 (0.14) | 0.044 | −0.13 | −0.21 | NS | ||
| 21 | Placebo | 2.27 (0.58) | 1.78 (0.63) | 0.40 (0.13) | 0.003 | −0.11 | −0.18 | NS | ||
| Cycle Variance | 16 | Continuous | 1.56 (0.74) | 0.71 (0.60) | 0.87 (0.15) | 0.000 | 0.05 | 0.08 | NS | |
| 16 | Intermittent | 1.20 (0.64) | 0.69 (0.72) | 0.82 (0.15) | 0.000 | −0.00 | −0.01 | NS | ||
| 21 | Placebo | 1.91 (1.18) | 0.78 (0.51) | 0.87 (0.13) | 0.000 | −0.05 | −0.09 | NS | ||
|
| ||||||||||
| Mood Swings (DRSP5) | Pre-menstrual Mean | 16 | Continuous | 2.89 (0.84) | 1.40 (0.50) | 1.56 (0.19) | 0.000 | 0.33 | 0.44 | NS |
| 17 | Intermittent | 2.80 (0.84) | 1.71 (0.88) | 1.23 (0.19) | 0.000 | 0.22 | 0.29 | NS | ||
| 21 | Placebo | 3.18 (1.13) | 1.68 (0.85) | 1.34 (0.16) | 0.000 | −0.11 | −0.15 | NS | ||
| Cycle Mean | 16 | Continuous | 1.88 (0.40) | 1.61 (0.46) | 0.30 (0.13) | 0.026 | 0.17 | 0.32 | NS | |
| 17 | Intermittent | 1.79 (0.39) | 1.75 (0.63) | 0.13 (0.13) | NS | −0.10 | −0.20 | NS | ||
| 21 | Placebo | 2.05 (0.63) | 1.56 (0.52) | 0.40 (0.11) | 0.001 | −0.27 | −0.52 | NS | ||
| Cycle Variance | 16 | Continuous | 1.39 (0.53) | 0.95 (0.95) | 0.59 (0.19) | 0.003 | −0.11 | −0.15 | NS | |
| 17 | Intermittent | 1.45 (0.77) | 0.84 (0.78) | 0.70 (0.19) | 0.000 | −0.31 | −0.42 | NS | ||
| 21 | Placebo | 1.76 (1.20) | 0.68 (0.49) | 0.90 (0.16) | 0.000 | −0.20 | −0.26 | NS | ||
|
| ||||||||||
| Anger/Irritability (DRSP7) | Pre-menstrual Mean | 16 | Continuous | 3.45 (0.82) | 1.70 (0.65) | 1.68 (0.21) | 0.000 | 0.21 | 0.24 | NS |
| 16 | Intermittent | 3.06 (0.57) | 1.81 (0.97) | 1.48 (0.22) | 0.000 | 0.19 | 0.22 | NS | ||
| 21 | Placebo | 3.53 (0.98) | 1.91 (0.94) | 1.50 (0.19) | 0.000 | −0.02 | −0.02 | NS | ||
| Cycle Mean | 16 | Continuous | 2.15 (0.43) | 1.78 (0.66) | 0.37 (0.14) | 0.014 | 0.08 | 0.14 | NS | |
| 17 | Intermittent | 1.97 (0.40) | 1.79 (0.57) | 0.29 (0.15) | 0.056 | −0.08 | −0.14 | NS | ||
| 21 | Placebo | 2.29 (0.59) | 1.76 (0.58) | 0.45 (0.13) | 0.001 | −0.16 | −0.27 | NS | ||
| Cycle Variance | 16 | Continuous | 1.66 (0.67) | 0.74 (0.66) | 1.07 (0.16) | 0.000 | 0.18 | 0.28 | NS | |
| 16 | Intermittent | 1.73 (0.70) | 0.93 (0.70) | 0.90 (0.16) | 0.000 | 0.01 | 0.02 | NS | ||
| 21 | Placebo | 2.05 (1.18) | 0.81 (0.57) | 1.06 (0.14) | 0.000 | −0.17 | −0.26 | NS | ||
|
| ||||||||||
| Functional Impairment (Avg of DRSP22–DRSP24) | Pre-menstrual Mean | 16 | Continuous | 2.85 (0.86) | 1.48 (0.53) | 1.25 (0.15) | 0.000 | 0.10 | 0.17 | NS |
| 17 | Intermittent | 2.37 (0.74) | 1.46 (0.56) | 1.15 (0.14) | 0.000 | 0.21 | 0.36 | NS | ||
| 21 | Placebo | 2.83 (1.01) | 1.69 (0.72) | 1.04 (0.13) | 0.000 | 0.11 | 0.19 | NS | ||
| Cycle Mean | 16 | Continuous | 1.84 (0.45) | 1.54 (0.49) | 0.27 (0.12) | 0.026 | 0.03 | 0.06 | NS | |
| 17 | Intermittent | 1.59 (0.35) | 1.46 (0.48) | 0.24 (0.12) | 0.043 | 0.00 | 0.00 | NS | ||
| 21 | Placebo | 1.92 (0.57) | 1.58 (0.56) | 0.27 (0.11) | 0.015 | −0.03 | −0.06 | NS | ||
| Cycle Variance | 16 | Continuous | 1.37 (0.71) | 0.55 (0.39) | 0.80 (0.13) | 0.000 | 0.07 | 0.13 | NS | |
| 17 | Intermittent | 1.12 (0.73) | 0.58 (0.63) | 0.74 (0.13) | 0.000 | 0.02 | 0.04 | NS | ||
| 21 | Placebo | 1.54 (0.98) | 0.61 (0.54) | 0.78 (0.12) | 0.000 | −0.04 | −0.09 | NS | ||
Note. Premenstrual Mean: Days −7 to −1 where day 0 represents either the onset of menses (or the first pill day in last on-treatment cycle for Continuous and Intermittent groups).
P-values for the within treatment LS change from baseline to post.
Differences between treatments in LS change
Effect sizes for differences in LS change between treatments.
P-values for a test of whether LS change differs between treatments.
For superscript numbers 2–4, treatment comparisons are in the order Continuous-Intermittent, Continuous-Placebo, and Intermittent-Placebo.
Figure 2.
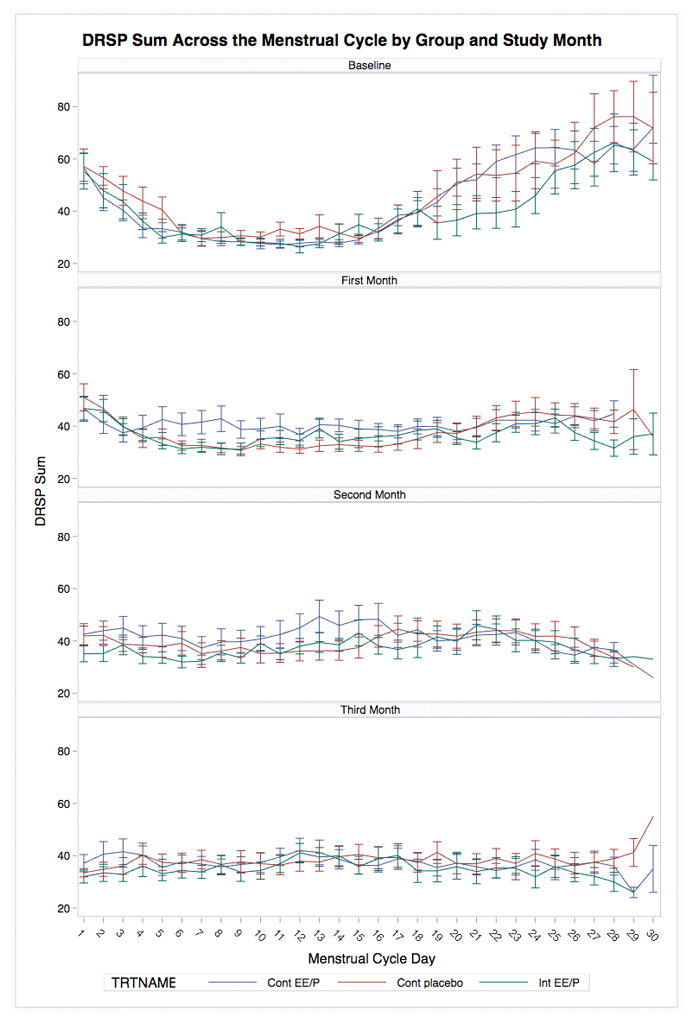
Daily total symptoms (Mean DRSP summed score) across the menstrual cycle by treatment group and study month.
4. Discussion
Results of this double-blind, three-arm, randomized, placebo-controlled trial indicated no significant differences in the effects on symptoms of continuous DROS/EE and intermittent (21/7) DROS/EE (neither of which differed from placebo). Just one significant group effect emerged. Intermittent DROS/EE was associated with a less robust reduction in the variance of one’s worst baseline symptom when compared to placebo or the continuous DROS/EE.
The symptom reductions observed in the placebo group despite standardized prospective diagnostic screening and the extended nature of the trial (3 months) were remarkable. The strength of this placebo effect is consistent with previous work demonstrating high placebo response rates in psychiatric disorders generally (Fava, Evins, Dorer, & Schoenfeld, 2003) and premenstrual symptoms specifically (Freeman & Rickels, 1999; Halbreich, 2014; Halbreich et al., 2012). Many factors known to be associated with high placebo response rates may be relevant to the outcome of the present trial (Fava et al., 2003; Price, Finniss, & Benedetti, 2008), including the following: the high face validity of a hormonal treatment for premenstrual symptoms, which may enhance expectation of benefit; intensive contact with a single, supportive study coordinator; and use of two active conditions (as the likelihood that any one individual is randomized to an active treatment is positively associated with the size of the placebo effect; Lidstone, Schulzer, & Dinelle, 2010; Rutherford, Sneed, & Roose, 2009; Schatzberg & Kraemer, 2000; Sinyor, Levitt, Cheung, & Schaffer, 2010). On the other hand, the present trial had notable strengths with regard to factors known to reduce placebo response, including the use of standardized prospective diagnostic practices, use of outcome measures that are sensitive to change, and a single site design. Additional work is needed to clarify factors that drive placebo response in PMD, especially the roles of expectation of benefit, validation and social support (Price et al., 2008), and somatic symptom focus (Geers, Helfer, Weiland, & Kosbab, 2005).
Although the sample in the present study was not recruited explicitly to meet prospective DSM-IV or DSM-5 criteria for premenstrual dysphoric disorder (PMDD), which requires at least five symptoms to demonstrate luteal phase elevation and confinement (see Eisenlohr-Moul et al., 2016), we did recruit women with prospectively-documented luteal phase confinement of at least one emotional symptom. Furthermore, mean baseline premenstrual symptom levels in the present study (quantified by DRSP sum score in the baseline premenstrual week) were comparable (70 vs. 77) to that reported in the largest RCT of DROS for PMDD (Yonkers et al., 2005). Therefore, despite the fact that this sample was not recruited for PMDD, it is likely that the results presented herein are generalizable to most women with PMDD.
An admittedly speculative explanation for the high placebo response in this (and other) studies is the possible relevance of placebo mechanisms to the pathophysiology of PMD. For example: 1) placebo activates the prefrontal cortex and ACC, effects that are critical for efficacy (Petrovic et al., 2005); 2) placebo increases theta coupling, suggestive of recruitment of frontally based cognitive control systems (Meyer et al., 2015); 3) placebo efficacy in Parkinson’s disease leads to release of dopamine in the striatum, the hub of the reward system and a critical player in affective state regulation and valence (De la Fuente-Fernández, 2001; 2002); 4) placebo produces attenuated reactivity to negative salient cues and a neural signature associated with cognitive control (Meyer et al., 2015; Petrovic et al., 2005); 5) uncertainty – the expectation of clinical benefit—increases the placebo response (Benedetti, 2012; de la Fuente-Fernández & Stoessl, 2004). Just as stabilization of hormone levels may provide insights into the triggering of PMD, so the success of placebo in some may suggest the engagement of top-down (prefrontal) cognitive control systems as potentially relevant substrates of affective regulation in PMD.
We failed to replicate previous reports that shortened- or eliminated-HFI treatment with oral contraceptives is superior to placebo or a standard 21/7 regimen. Although the present study suffered from modest statistical power, there were no trends to suggest that better power would have resulted in significant effects. The lack of significant differences between the two DROS conditions might be in part due to the relatively long half-life of DROS, which may have resulted in a somewhat shortened HFI even in the 21/7 condition. On the other hand, it is also quite possible that the effect of DROS-containing oral contraceptives on premenstrual symptoms is not robust; the beneficial effects of shortenened-HFI oral contraceptives relative to placebo on premenstrual symptoms are inconsistent across studies (Freeman et al., 2012), relatively small in size when they are found (Halbreich, 2014), and often do not persist over follow-up (Halbreich et al., 2012). Further, a recent study observed that the beneficial premenstrual effects of COCs in some women may be undermined by a shifting of premenstrual symptoms into other chronological “phases” of the cycle (Lundin et al., 2017); on the other hand, various post-hoc alternative specification of windows in the present study did not reveal consistent patterns of similar symptom shifting. In addition, although the robust placebo effect may have interfered with the detection of a potentially real therapeutic benefit of COC, this does not necessarily explain the failure to find a difference between the two active treatments (continuous vs. intermittent dosing). Our hypothesis that greater stabilization of ovarian steroids in the continuous (vs. intermittent) condition would lead to greater reduction of symptoms was not supported.
The present study adds to a body of RCTs in which oral contraceptive treatment effects in PMD are nonsignificant, small, or short-lived. Given that COCs are among the most commonly-prescribed treatments for PMD, this lack of consistent evidence warrants further investigation. Additional work is needed to understand and directly target the pathophysiology of PMD, and to understand the manner in which psychosocial contexts (e.g., treatment with placebo) influence PMD symptoms.
Acknowledgments
This work was supported by grants from the United States National Institute of Mental Health (R01-MH099076; T32-MH093315; K99-MH109667).
Footnotes
Sponsors had no role in the design, analysis, interpretation, or publication of this study.
The authors have no conflicts of interest to report.
References
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion: premenstrual syndrome. International Journal of Gynaecology and Obstetrics. 1995:5080–5084. [PubMed] [Google Scholar]
- Benedetti F. The placebo response: science versus ethics and the vulnerability of the patient. World Psychiatry. 2012;11(2):70–72. doi: 10.1016/j.wpsyc.2012.05.003. http://doi.org/10.1016/j.wpsyc.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Rubinow DR. Premenstrual Syndrome: Evidence for Symptom Stability Across Cycles. American Journal of Psychiatry. 1997;154(12):1741–1746. doi: 10.1176/ajp.154.12.1741. http://doi.org/10.1176/ajp.154.12.1741. [DOI] [PubMed] [Google Scholar]
- De la Fuente-Fernández R. Expectation and Dopamine Release: Mechanism of the Placebo Effect in Parkinson’s Disease. Science. 2001;293(5532):1164–1166. doi: 10.1126/science.1060937. http://doi.org/10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- De la Fuente-Fernández R. The placebo effect in Parkinson’s disease. Trends in Neurosciences. 2002;25(6):302–306. doi: 10.1016/s0166-2236(02)02181-1. http://doi.org/10.1016/S0166-2236(02)02181-1. [DOI] [PubMed] [Google Scholar]
- De la Fuente-Fernández R, Stoessl AJ. The biochemical bases of the placebo effect. Science and Engineering Ethics. 2004;10(1):143–150. doi: 10.1007/s11948-004-0071-z. http://doi.org/10.1007/s11948-004-0071-z. [DOI] [PubMed] [Google Scholar]
- Eisenlohr-Moul TA, Girdler SS, Schmalenberger KM, Dawson DN, Surana P, Johnson JL, Rubinow DR. Toward the reliable diagnosis of DSM-5 premenstrual dysphoric disorder: the Carolina Premenstrual Assessment Scoring System (C-PASS) American Journal of Psychiatry. 2016;174(1):51–59. doi: 10.1176/appi.ajp.2016.15121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Halbreich U. Retrospective report of premenstrual depressive changes: factors affecting confirmation by daily ratings. Psychopharmacol Bull 1982 [Google Scholar]
- Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The Problem of the Placebo Response in Clinical Trials for Psychiatric Disorders: Culprits, Possible Remedies, and a Novel Study Design Approach. Psychotherapy and Psychosomatics. 2003;72(3):115–127. doi: 10.1159/000069738. http://doi.org/10.1159/000069738. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Rickels K. Characteristics of placebo responses in medical treatment of premenstrual syndrome. American Journal of Psychiatry. 1999;156(9):1403–1408. doi: 10.1176/ajp.156.9.1403. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Halbreich U, Grubb GS, Rapkin AJ, Skouby SO, Smith L, et al. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. 2012;85(5):437–445. doi: 10.1016/j.contraception.2011.09.010. http://doi.org/10.1016/j.contraception.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Kroll R, Rapkin A, Pearlstein T, Brown C, Parsey K, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. Journal of Women’s Health & Gender-Based Medicine. 2001;10(6):561–569. doi: 10.1089/15246090152543148. http://doi.org/10.1089/15246090152543148. [DOI] [PubMed] [Google Scholar]
- Geers AL, Helfer SG, Weiland PE, Kosbab K. Expectations and Placebo Response: A Laboratory Investigation into the Role of Somatic Focus. Journal of Behavioral Medicine. 2005;29(2):171–178. doi: 10.1007/s10865-005-9040-5. [DOI] [PubMed] [Google Scholar]
- Graham CA, Sherwin BB. A prospective treatment study of premenstrual symptoms using a triphasic oral contraceptive. Journal of Psychosomatic Research. 1992;36(3):257–266. doi: 10.1016/0022-3999(92)90090-o. [DOI] [PubMed] [Google Scholar]
- Halbreich U. The diagnosis of premenstrual syndromes and premenstrual dysphoric disorder - clinical procedures and research perspectives. Gynecological Endocrinology. 2004;19(6):320–334. doi: 10.1080/0951590400018215. http://doi.org/10.1080/0951590400018215. [DOI] [PubMed] [Google Scholar]
- Halbreich U. Selective Serotonin Reuptake Inhibitors and Initial Oral Contraceptives for the Treatment of PMDD: Effective But Not Enough. CNS Spectrums. 2014;13(07):566–572. doi: 10.1017/s1092852900016849. http://doi.org/10.1017/S1092852900016849. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28:1–23. doi: 10.1016/s0306-4530(03)00098-2. http://doi.org/10.1016/S0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Freeman EW, Rapkin AJ, Cohen LS, Grubb GS, Bergeron R, et al. Continuous oral levonorgestrel/ethinyl estradiol for treating premenstrual dysphoric disorder. Contraception. 2012;85(1):19–27. doi: 10.1016/j.contraception.2011.05.008. http://doi.org/10.1016/j.contraception.2011.05.008. [DOI] [PubMed] [Google Scholar]
- Hammarbäck S, Ekholm UB, Bäckström T. Spontaneous anovulation causing disappearance of cyclical symptoms in women with the premenstrual syndrome. Acta Endocrinologica. 1991;125(2):132–137. doi: 10.1530/acta.0.1250132. [DOI] [PubMed] [Google Scholar]
- Lidstone SC, Schulzer M, Dinelle K. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Archives of General Psychiatry. 2010;67(8):857–865. doi: 10.1001/archgenpsychiatry.2010.88. [DOI] [PubMed] [Google Scholar]
- Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. The Cochrane database of systematic reviews. 2012;2:CD006586. doi: 10.1002/14651858.CD006586.pub4. [DOI] [PubMed] [Google Scholar]
- Lundin C, Danielsson KG, Bixo M, Moby L, Bengtsdotter H, Jawad I, … Poromaa IS. Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle—A double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology. 2017;76:135–143. doi: 10.1016/j.psyneuen.2016.11.033. [DOI] [PubMed] [Google Scholar]
- Marr J, Heinemann K, Kunz M, Rapkin A. Ethinyl estradiol 20μg/drospirenone 3mg 24/4 oral contraceptive for the treatment of functional impairment in women with premenstrual dysphoric disorder. International Journal of Gynaecology and Obstetrics. 2011a;113(2):103–107. doi: 10.1016/j.ijgo.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Marr J, Niknian M, Shulman LP, Lynen R. Premenstrual dysphoric disorder symptom cluster improvement by cycle with the combined oral contraceptive ethinylestradiol 20 mcg plus drospirenone 3 mg administered in a 24/4 regimen. Contraception. 2011b;84(1):81–86. doi: 10.1016/j.contraception.2010.10.010. http://doi.org/10.1016/j.contraception.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Meyer B, Yuen KSL, Ertl M, Polomac N, Mulert C, Buchel C, Kalisch R. Neural Mechanisms of Placebo Anxiolysis. Journal of Neuroscience. 2015;35(19):7365–7373. doi: 10.1523/JNEUROSCI.4793-14.2015. http://doi.org/10.1523/JNEUROSCI.4793-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72(6):414–421. doi: 10.1016/j.contraception.2005.08.021. http://doi.org/10.1016/j.contraception.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in Emotional Processing— Induced Expectations of Anxiety Relief Activate a Generalized Modulatory Network. Neuron. 2005;46(6):957–969. doi: 10.1016/j.neuron.2005.05.023. http://doi.org/10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Price DD, Finniss DG, Benedetti F. A Comprehensive Review of the Placebo Effect: Recent Advances and Current Thought. Annual Review of Psychology. 2008;59(1):565–590. doi: 10.1146/annurev.psych.59.113006.095941. http://doi.org/10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome? Psychotherapy and Psychosomatics. 2009;78(3):172–181. doi: 10.1159/000209348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg AF, Kraemer HC. Use of placebo control groups in evaluating efficacy of treatment of unipolar major depression. Biological Psychiatry. 2000;47(8):736–744. doi: 10.1016/s0006-3223(00)00846-5. [DOI] [PubMed] [Google Scholar]
- Schlaff WD, Lynch AM, Hughes HD, Cedars MI, Smith DL. Manipulation of the pill-free interval in oral contraceptive pill users: the effect on follicular suppression. American Journal of Obstetrics and Gynecology. 2004;190(4):943–951. doi: 10.1016/j.ajog.2004.02.012. http://doi.org/10.1016/j.ajog.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Schmalenberger KM, Eisenlohr-Moul TA, Surana P, Rubinow DR, Girdler SS. Predictors of premenstrual impairment among women undergoing prospective assessment for premenstrual dysphoric disorder: a cycle-level analysis. Psychological Medicine. 2017:1–12. doi: 10.1017/S0033291716003524. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. New England Journal of Medicine. 1998;338(4):209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Grover GN, Muller KL, Merriam GR, Rubinow DR. Lack of Effect of Induced Menses on Symptoms in Women with Premenstrual Syndrome. New England Journal of Medicine. 1991;324(17):1174–1179. doi: 10.1056/NEJM199104253241705. http://doi.org/10.1056/NEJM199104253241705. [DOI] [PubMed] [Google Scholar]
- Sinyor M, Levitt AJ, Cheung AH, Schaffer A. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta-analyses. The Journal of Clinical Psychiatry. 2010;71(3):270–279. doi: 10.4088/JCP.08r04516blu. [DOI] [PubMed] [Google Scholar]
- Sulak PJ, Cressman BE, Waldrop E, Holleman S, Kuehl TJ. Extending the duration of active oral contraceptive pills to manage hormone withdrawal symptoms. Obstetrics & Gynecology. 1997;89(2):179–183. doi: 10.1016/S0029-7844(96)00488-7. http://doi.org/10.1016/S0029-7844(96)00488-7. [DOI] [PubMed] [Google Scholar]
- Sullivan H, Furniss H, Spona J, Elstein M. Effect of 21-day and 24-day oral contraceptive regimens containing gestodene (60 microg) and ethinyl estradiol (15 microg) on ovarian activity. Fertility and Sterility. 1999;72(1):115–120. doi: 10.1016/s0015-0282(99)00205-8. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Brown C, Pearlstein TB, Foegh M, Sampson-Landers C, Rapkin A. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstetrics & Gynecology. 2005;106(3):492–501. doi: 10.1097/01.AOG.0000175834.77215.2e. http://doi.org/10.1097/01.AOG.0000175834.77215.2e. [DOI] [PubMed] [Google Scholar]
- Wyatt KM, Dimmock PW, Ismail KM, Jones PW, O’brien PM. The effectiveness of GnRHa with and without ‘add-back’therapy in treating premenstrual syndrome: a meta analysis. BJOG: An International Journal of Obstetrics & Gynaecology. 2004;111(6):585–593. doi: 10.1111/j.1471-0528.2004.00135.x. [DOI] [PubMed] [Google Scholar]