Abstract
Obesity is a key risk factor for metabolic and cardiovascular diseases, and although we understand the mechanisms regulating weight and energy balance, the causes of some forms of obesity remain enigmatic. Despite the well-established connections between lymphatics and lipids, and that intestinal lacteals play key roles in dietary fat absorption, the function of the lymphatic vasculature in adipose metabolism has only recently been recognized. It is well established that angiogenesis is tightly associated with the outgrowth of adipose tissue, as expanding adipose tissue requires increased nutrient supply from blood vessels. Results supporting a crosstalk between lymphatics and adipose tissue, and linking lymphatic function with metabolic diseases, obesity and adipose tissue also started to accumulate in the last years. Here we review our current knowledge of the mechanisms by which defective lymphatics contribute to obesity and fat accumulation in mouse models, as well as our understanding of the lymphatics-adipose tissue relationship.
Keywords: lymphatics, obesity, adipose tissue, endothelial cell, inflammation, chyle, lymph, Prox1
Adipose Tissue and Obesity
Obesity is defined as a medical condition characterized by an imbalance between energy intake and expenditure that is associated with increased consumption of high-energy foods and decreased physical activity leading to excessive accumulation and storage of adipose tissue. In the last decades, the prevalence of obesity has increased dramatically and it has been suggested to be the leading cause for the reduced life expectancy of the next generation (Tinahones et al., 2012). The complications of obesity account for much of its health risk, including type 2 diabetes mellitus, hypertension, coronary heart disease, stroke, dyslipidemia, gallbladder disease, hepatic steatosis, sleep apnea, stroke, endometrial disorder, and certain types of cancer (Friedman, 2000; Kopelman, 2000; Roth et al., 2004).
Adipocytes, also known as fat cells, are the main component of the adipose tissue. They are responsible for the storage of the excess of calories as triglycerides, and also produce secreted factors that regulate systemic nutrient and energy homeostasis within the whole-body (Rutkowski et al., 2015; Stern et al., 2016). Additionally, and because of their caloric reservoir capacity, they are able to expand in response to overnutrition and release lipids in response to energy deficit (Rutkowski et al., 2015). A transformation in understanding the biologic function of adipose tissue came with the discovery that this tissue not only stores energy, but also functions as an active endocrine organ, which synthesizes and releases fatty acids, hormones, growth factors and adipokines (such as leptin, adiponectin and resistin) that act as autocrine, paracrine, and endocrine factors to regulate metabolism, either nearby or distal sites, including brain (Sun et al., 2011). These active signaling molecules can regulate central control of energy expenditure and satiety, as well as modifications of glucoregulatory hormone secretion from the endocrine pancreas (Stern et al., 2016).
There are different types of adipocytes identified by their color and mitochondrial content: white, beige and brown adipose tissue. White adipose tissue (WAT), the focus of this review, is the classical fat cell found in visceral and subcutaneous adipose depots with the potential for growth and expansion depending on energy intake and the metabolic need of the host (Cao, 2013; Rutkowski et al., 2015). Obese WAT displays classical signs of chronic inflammation, a key feature of obesity. Obesity leads to a chronic inflammatory state that is initiated and exacerbated in the WAT (Ge et al., 2014; Xu et al., 2003). For example, visceral adipocytes promote systemic inflammation by secreting mediators such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and leptin, among others (Fontana et al., 2007). Previous studies determined that chronic inflammation in obesity is initiated by the infiltration of CD8(+) T cells followed by macrophage accumulation in the adipose tissue (Nishimura et al., 2009) and local proliferation of macrophages which contributes to the obesity-associated adipose tissue inflammation (Amano et al., 2014). Subsequently, macrophage differentiation and phagocytosis of necrotic adipocytes leads to the release of inflammatory cytokines, chemokines, increased inflammatory cell recruitment, and the activation of pathways that regulate progressive inflammation, including JNK and NF-kB pathways (Torrisi et al., 2016; Weisberg et al., 2006). Further, oxygen depletion and induction of the hypoxia inducible factor-1α (HIF-1α), inhibits neoangiogenesis and enhances the inflammatory response in adipocytes (Takikawa et al., 2016).
Functions of the adipose tissue-associated blood vasculature
The adipose tissue is highly vascularized and each adipocyte is nourished by a capillary network (Cao, 2007). Considering that adipose tissue is characterized by its almost unlimited expansion, then this tissue enlargement will require a network of new blood vessels to support delivery of oxygen and nutrients (Cao, 2007). Consequently, the outgrowth of adipose tissue is tightly related with angiogenesis -the growth of new blood vessels-. In order to accomplish this neovascularization process, the adipose tissue expresses angiogenic growth factors such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), placental growth factor (PlGF) and leptin (Cao, 2007; Sung et al., 2013). In growing adipose tissue, the new blood vessels contribute to adipogenesis by performing multiple functions such as: provide nutrients and oxygen to nourish adipocytes, removing waste products from the adipose tissue, carrying monocytes and neutrophils that can affect adipocyte function, and also providing adipose precursors and stem cells that can eventually differentiate into preadipocytes and adipocytes, among others (Cao, 2010, 2013). Adipocyte expansion thus involves dynamic cross talk between adipocytes and the blood vasculature, were adipocytes produce angiogenic factors and in turn, adipokines induce angiogenesis. This has led to the proposal that angiogenesis has an essential role in the modulation of adipogenesis and obesity. Thus, anti-angiogenesis therapy has emerged as a potential treatment for obesity, though such an approach may be limited by inducing ectopic lipid deposition outside of the adipose depot. Previous studies have shown that changes in the size and mass of the adipose tissue are dependent on a parallel modification of its vascular supply (Cao, 2013; Sung et al., 2013); therefore, adipose tissue can be regulated through its embedded vasculature. It is well known that the VEGF system contributes to the angiogenic activity in adipose tissue (Sun et al., 2012). VEGF-A is a major angiogenic factor that stimulates proliferation and migration of endothelial cells (Carmeliet et al., 1996), is highly expressed in adipose tissue (Tinahones et al., 2012), and is responsible for most of the angiogenic capacity of adipose tissue (Zhang et al., 1997). Elias et al. (2012) found that transgenic mice overexpressing VEGF-A in adipose tissue developed more numerous and larger blood vessels, and that they were protected against high-fat diet (HFD, 60% of calories from fat)-induced obesity, with no differences in food intake. Moreover, whole-body insulin sensitivity and glucose tolerance were improved in these animals (Elias et al., 2012) suggesting that overexpression of VEGF-A in adipose tissue is a potential therapeutic strategy for the prevention of obesity and insulin resistance. In the same way, a recent report from Robciuc et al. (Robciuc et al., 2016) found that vascular endothelial growth factor B (VEGFB) binding to its receptor VEGFR1, displaced VEGF-A from VEGFR1 and activated VEGFR2, increasing adipose tissue vascularity, reducing inflammation and reversing metabolic complications induced by HFD and the metabolic syndrome (Robciuc et al., 2016). Therefore, induction of adipose tissue-associated vasculature through treatment with VEGFB could potentially offer a possible therapeutic option for improving obesity-related metabolic complications and promoting weight loss in obesity; however, more studies will be necessary to better understand the biological implications of these results.
In addition, it has been suggested that hypoxia, consequence of deficient vascularization of the adipose tissue, is also an important contributing factor in obesity (Hosogai et al., 2007). For example, an increase in adipose tissue angiogenesis protects against obesity-induced hypoxia (Elias et al., 2012; Sun et al., 2012). Moreover, deletion of adipose-VEGF-A in mice shows reduced adipose vascular density and adipose hypoxia, apoptosis, inflammation, and metabolic defects on a HFD; instead, rescue of VEGF-A levels leads to increased adipose vasculature, reduced hypoxia and fat inflammation, reduction of adipocyte size and reversal of metabolic morbidities caused by HFD (Sung et al., 2013). Although these data suggest that metabolic misbalance could be potentially reverted by an increase in adipose-associated vessel density, other results instead suggest that reduced angiogenesis and vascularization in human fat depots might contribute to impaired adipose tissue perfusion, nutrient exchange and metabolic dysregulation (Gealekman et al., 2011; Karki et al., 2017; Ngo et al., 2014). These apparently contradicting results argue that much more work is still required to better understand the cross-talk and relationship between adipose tissue and vascular beds.
Role of endothelial cells in pathophysiological processes
Endothelial cells synthesize and release various factors that regulate angiogenesis, inflammatory responses, hemostasis, as well as vascular tone and permeability (Feletou and Vanhoutte, 2006). A healthy vasculature requires functional integrity of the endothelial cells that form the vessel wall. In late 1990s, the concept of “endothelial therapy” was introduced as an approach to preserve or restore endothelial cell health (Barton, 2013). Importantly, during the last decade endothelial dysfunction promoting injury to the endothelium has been linked with a number of pathophysiological processes, such as obesity, Type I and Type II diabetes, atherosclerosis and arterial hypertension among others (Barton, 2013; Feletou and Vanhoutte, 2006). The continuous damaging effects of the disease might end in cellular and functional changes commonly known as “endothelial dysfunction” (Barton, 2013; Feletou and Vanhoutte, 2006), which ranges from inflammatory activation and increased release of reactive oxygen species, to changes in hypertension and anticoagulatory properties of the endothelial cell (Barton, 2013; Lobato et al., 2012).
It is well known that obesity impairs vascular homeostasis and leads to endothelial cell dysfunction (Al Suwaidi et al., 2001; Iantorno et al., 2014). Related to this, the treatment of obesity frequently not only alleviates, but also might normalize obesity-associated diabetes or arterial hypertension, thereby also reducing endothelial cell dysfunction (Barton, 2013). Recent findings also support the concept of endothelial and perivascular origins of preadipocytes. Vascular endothelial cells retain multipotent stem cell-like features and can differentiate into a preadipocytes and adipocytes (Medici et al., 2010), as well as into other cell types such as pericytes (Tran et al., 2012). These data suggest that adipogenesis, angiogenesis, and vascular remodeling are tightly and coordinately regulated through the modulation of endothelial cell behavior.
Lymphatic Vascular System
The lymphatic vascular system is a one-direction network of thin-walled capillaries and larger vessels covered by a continuous layer of endothelial cells that provides a unidirectional conduit to return filtered arterial and tissue metabolites towards the venous circulation. Their principal function is to maintain fluid homeostasis by removing the protein-rich lymph from the extracellular space among tissues and returns it to the bloodstream (Escobedo and Oliver, 2016). In addition to this fluid homeostasis function, the lymphatic vasculature is important for transport of antigens-presenting cells to lymph nodes, absorption of dietary fat in the gastrointestinal organs, management of immune cell trafficking and inflammation, regulation of blood pressure and reverse cholesterol transport (Dieterich et al., 2014; Kerjaschki, 2014; Lim et al., 2013; Machnik et al., 2009; Martel et al., 2013; Randolph et al., 2005; Wiig et al., 2013).
Two types of vessels form the lymphatics vasculature: close-ended lymphatic capillaries and larger collecting lymphatic vessels. Lymphatic capillaries are composed of a single layer of lymphatic endothelial cells (LECs) that form discontinuous or “button-like” junctions with a discontinuous basement membrane, which allows entrance of interstitial fluid and cells into the vessel lumen, making these vessels highly permeable (Baluk et al., 2007). The lymphatic capillaries are in direct contact with the tissue thanks to connective filaments that tie the vessels to the surrounding extracellular matrix, thus allowing the absorption of fluid when the external tissue pressure increases (Proulx et al., 2013). The interstitial fluid (called lymph when it enters the lymphatic capillaries) then moves into larger pre-collecting vessels that merge into large collecting vessels, which are characterized by a continuous perivascular sheath of smooth muscle cells, a basement membrane, continuous “zipper-like” inter-endothelial junctions, and the presence of bi-leaflet valves (Tammela and Alitalo, 2010). These characteristics facilitate lymph flow, where the intrinsic contractility of smooth muscle cells, together with the contraction of surrounding skeletal muscles and arterial pulsations provide contractile activity for lymph propulsion, whereas the valves impede lymph backflow (Bazigou et al., 2009). Eventually, the lymph is drained into the thoracic duct and is returned to the blood circulation through the lymphatic-blood vessel connections mediated by two nearby valves located at the junction of the jugular and subclavian veins (Srinivasan and Oliver, 2011).
Defects affecting normal lymphatic vascular structure and function can lead to lymphedema, a clinical condition characterized by localized accumulation of interstitial protein-rich fluid and tissue swelling (Rockson, 2001). This disease affects millions of persons worldwide and most commonly involves swelling of the extremities, tissue fibrosis, susceptibility to infections, and accumulation of subcutaneous fat (Aschen et al., 2012; Brorson, 2016; Rockson, 2001; Witte et al., 2001). Complications of lymphedema include psychosocial morbidity, infection, functional disability, skin changes, and malignant transformation (Greene and Maclellan, 2013). Lymphedema can result from either primary or acquired (secondary) disorders. Primary lymphedema is produced by genetic defects altering the development of the lymphatic vasculature and are typically manifest during infancy, childhood, or adolescence (Greene and Maclellan, 2013; Rockson, 2001). Secondary lymphedema is the most common cause of the disease and is caused by surgery, radiation therapy, infection or trauma (Greene and Maclellan, 2013; Rockson, 2001; Witte et al., 2001).
Lipedema is a chronic vascular disease characterized by bilateral and symmetrical swelling in the legs due to the deposit of subcutaneous adipose tissue (Bilancini et al., 1995; Lohrmann et al., 2009; Wold et al., 1951). Lipedema was first described in 1940 by Allen and Hines and was characterized as a “lipodystrophy” (Wold et al., 1951). They described that the deposition of fat developed frequently after puberty, progressed gradually, and was aggravated by activity and warm temperatures (Lohrmann et al., 2009). Lipedema is found nearly exclusively in females and probably caused by a genetic condition (Bilancini et al., 1995; Okhovat and Alavi, 2015; Shin et al., 2011; Wagner, 2011). Lipedema is often misdiagnosed as obesity or lymphedema (Bilancini et al., 1995; Lohrmann et al., 2009). Nevertheless and contrary to lymphedema, in lipedema no edema is observed in the ankles and feet, a family history is common and occurs bilaterally and symmetrically in the legs (Shin et al., 2011). In addition, the lymph circulation is usually normal in patients with lipedema; however, advanced cases of lipedema led to venous disease (venolipedema), lymphatic abnormalities (lympholipedema), and subsequently ulceration and recurrent infection (Okhovat and Alavi, 2015). Initial studies by Bilancini et al., (Bilancini et al., 1995) described that lipedema is constantly associated with functional alterations of the lymphatic system (Bilancini et al., 1995). By dynamic lymphoscintigraphy, they showed that patients suffering from lipedema have an abnormal lymphoscintigraphic pattern with a slowing of the lymphatic flow similar to the alterations found in the patients suffering from lymphedema (Bilancini et al., 1995). Thus, when the disease has chronically progressed, circulatory disturbance exist due to the pressure of fat cells on lymph collectors at the superficial layers (Shin et al., 2011). Therefore, lipedema is another adipose disorder with lymphatic function contribution [for a further discussion of lipedema see (Okhovat and Alavi, 2015; Shin et al., 2011).
A bi-directional cross-talk between the lymphatic vasculature and adipose tissue
Obesity impairs lymphatic vasculature function
Despite the well-established connections between lymphatics and lipids absorption and transport, the role of the lymphatic vasculature in adipose metabolism and obesity has only recently started to be recognized. In the last few years, data has emerged to indicate that obesity can cause pathological changes in the lymphatic vasculature, such that could eventually impair its function. On the other hand, recent studies supporting a crosstalk between lymphatics and adipose tissue, and linking lymphatic function with metabolic diseases, obesity and adipose tissue started to accumulate only in the last few years. For example, mesenteric lymph nodes and collecting lymph vessels are commonly embedded in subcutaneous or visceral fat, and lymphatic vessels and adipose tissue are also related by the uptake by intestinal lymphatics of dietary lipids as chylomicrons for transfer to the bloodstream (Bernier-Latmani et al., 2015; Tammela et al., 2008). Adipose tissue accumulation is also observed in lymphedema patients around affected regions. Studies in patients also showed that malformation of cutaneous lymphatics causes bilateral fat accumulation in the thigh and buttock (Tavakkolizadeh et al., 2001; Wang and Oliver, 2010), while dermal lipid accumulation occurs in idiopathic lymphedema patients (Pond, 2005; Rosen, 2002). Furthermore, additional work demonstrated that a defective lymphatic vasculature can lead to obesity. Initially, experimental studies using the apolipoprotein E-deficient mouse (apoE−/−), a model of hypercholesterolemia, showed for first time that structural and functional abnormalities of the lymphatic vasculature are associated with hypercholesterolemia. These apoE−/− mice develop progressive lymphatic dysfunction resulting in tissue swelling, lymphatic vessel leakage, and decreased lymphatic transport of fluids and dendritic cells (Lim et al., 2009). Interestingly, Lim et al., (2009) showed that HFD (21% milk fat and 0.15% cholesterol) exacerbated the enlargement of the initial lymphatic vessels observed in apoE−/− mice when compared with apoE−/− mice fed in a regular chow diet (Lim et al., 2009). Then, Weitman et al. (Weitman et al., 2013) observed that HFD-induced obesity resulted in significantly impaired lymphatic fluid transport and lymph node uptake. Also, obese mice exhibit smaller lymph nodes with fewer lymphatic vessels (Weitman et al., 2013). These results are supported by clinical reports that documented the spontaneous development of lymphedema in obese patients. For example, lymphatic drainage of macromolecules in abdominal subcutaneous adipose tissue is significantly reduced in obese patients (Arngrim et al., 2013). Moreover, being overweight is an important risk factor for lymphedema (Swenson et al., 2009), and obesity might be a risk factor for lymphedema in the extremities (Greene et al., 2012; Greene and Maclellan, 2013). Additionally, other results suggested a relationship between obesity and development of postoperative lymphedema. For example, it was initially shown that higher body weight and body mass index (BMI) are risk factors for lymphedema after lymph node dissection; however, the functional cause of this link is yet unknown (McLaughlin et al., 2008). Furthermore, a significant correlation has been found between BMI and lymphedema in patients with breast cancer (Sato et al., 2016), where breast cancer survivors whose BMI was ≥30 at the time of cancer treatment were more likely to develop lymphedema than those with a BMI < 30 (Ridner et al., 2011). Thus, elevated BMI increases the development of breast cancer treatment-related lymphedema.
Further support for a cross-talk between lymphatics and obesity was provided by data showing that obese mice also have impaired lymphatic function, characterized by leaky capillary lymphatics and decreased collecting vessel pumping capacity, decreased lymphatic vessel density, decreased lymphatic migration of immune cells, and decreased expression of lymphatic specific markers compared with lean mice (Garcia Nores et al., 2016; Hespe et al., 2016; Nitti et al., 2016; Savetsky et al., 2015a; Torrisi et al., 2016). Savetsky et al. (2014) described that HFD-induced obesity in mice decreases lymphatic function and increases the propensity for inflammation at baseline, and these effects are amplified by lymphatic injury, resulting in increased subcutaneous adipose deposition, inflammation and fibrosis, which finally leads to a more severe lymphedema phenotype (Savetsky et al., 2014). These results imply that obese patients are at higher risk for lymphedema due to reduced baseline lymphatic clearance, and are more prone to inflammation after injury. Similarly, Blum et al. (2014) showed that HFD is associated with impaired collecting lymphatic vessel function, as evidenced by reduced frequency of contractions and diminished response to mechanostimulation in mice. Additionally, they found a negative correlation between collecting lymphatic vessel function and body weight (Blum et al., 2014).
How obesity affects lymphatic function
The obvious question then is how obesity regulates lymphatic function. Is inflammation responsible for the obesity-mediated lymphatic dysfunction? Identifying how obesity regulates lymphatic function is critical since one of the roles of the lymphatic vasculature is the transport of immune cells, and is therefore involved in the inflammatory response. Recent studies suggested that obesity-induced lymphatic dysfunction can intensify the pathological effects of obesity in other organ systems by regulating leukocyte infiltration and expression of inflammatory cytokines (Savetsky et al., 2015a); therefore, obesity-related lymphatic dysfunction worsen the pathological changes associated to obesity. For example, reduced lymphatic function in obese mice favors inflammatory responses in dermatitis, while improving lymphatic function by the injection of recombinant vascular endothelial growth factor-C (VEGF-C), a growth factor that plays a key role in lymphangiogenesis and lymphatic development (Gomez-Ambrosi et al., 2010; Silha et al., 2005; Wada et al., 2011), resulted in considerably decreased dermatitis responses (Savetsky et al., 2015a). These results are also supported by recent studies demonstrating that inhibition of inflammation improves lymphatic function in HFD-induced obesity (Torrisi et al., 2016). Torrisi et al. (2016) found that obese mice had decreased density of capillary lymphatics in their dermis and subcutaneous tissues, and that this effect was reversed by treatment with Tacrolimus, an inhibitor of T cell differentiation (Torrisi et al., 2016). Local T cell inhibition markedly decreases perilymphatic inflammation and restores lymphatic function in obese mice by increasing capillary lymphatic density and augmenting collecting lymphatic contraction frequency (Torrisi et al., 2016). Importantly, these results indicate that that obesity-induced lymphatic dysfunction is reversible. Moreover, mice deficient in CD4+ cells fed with HFD did not exhibit significant lymphatic dysfunction even after prolonged HFD feeding, suggesting that local T cell responses in obesity are necessary for perilymphatic inflammation (Torrisi et al., 2016). This result also suggests that dietary changes alone are not sufficient to induce lymphatic dysfunction, and that this lymphatic malfunction is mainly due to the obesity-promoted inflammation. Importantly, new findings by Garcia-Nores et al. (2016) propose that the inflammation induced by obesity, and not HFD, is responsible for the pathologic changes observed in the lymphatic vasculature (Garcia Nores et al., 2016). Moreover, they propose that these changes are due at least in part, to direct LECs injury by products generated by inflamed adipose tissues, including long-chain free fatty acids (Garcia-Nores et al., 2016).
A new role for leptin in lymphatic endothelial cell tube formation and proliferation has been recently described (Sato et al., 2016). Interestingly, leptin treatment on human lymphatic ducts resulted in disorganization and morphological changes of the lymphatic duct. At the mechanistic level, high dose of leptin inhibited tube formation and cell proliferation in HDLECs. Therefore, it is possible that the increased serum levels of leptin detected in obese patients (Leal-Cerro et al., 1996; Rose et al., 2002), also compromises lymphatic endothelial cell homeostasis, and suggests that leptin has an important direct role in the pathogenesis of lymphedema in obese patients [leptin receptor (Ob-R) is expressed in human lymphatic ducts; (Sato et al., 2016)].
Adiponectin is another abundantly produced adipokine secreted by the adipose tissue and its expression and serum levels are decreased in obese patients (De Rosa et al., 2013; Shibata et al., 2009). It is well known that adiponectin exerts beneficial effects on obesity and related disorders [for a further role of adiponectin in obesity, see review by Nigro et al., 2014]. Interestingly, Shimizu et al., (2013) described a new role of adiponectin in the lymphatic system by showing that adiponectin-knockout mice develop exacerbated lymphedema in damaged tails; a phenotype that was also accompanied by a reduction in lymphangiogenesis (Shimizu et al., 2013). Mechanistically, adiponectin promotes LEC differentiation and viability (Shimizu et al., 2013). Furthermore, systemic administration of adiponectin resulted in improvement of lymphedema and enhancement of LEC formation in WT and obese mice (Shimizu et al., 2013). Thus, adiponectin also functions as a novel modulator of lymphatic vessel function.
Apelin, an endogenous ligand orphan G protein–coupled receptor (APJ), is expressed widely in the vascular endothelium and promotes stabilization of lymphatic vessels in inflamed tissues (Sawane et al., 2011). Initially, it was described that apelin attenuates edema formation and UVB-induced inflammation by promoting lymphatic function in vivo (Sawane et al., 2011). Additional studies proposed a new anti-obesity strategy based on enhancement of lymphatic and blood vessel integrity mediated by apelin treatment (Sawane et al., 2013). Apelin knockout mice fed on a HFD showed an obese phenotype associated with abnormal lymphatic and blood vessel enlargement (Sawane et al., 2013). Moreover, the fatty acids present in the HFD induced hyperpermeability of endothelial cells, causing adipocyte differentiation, whereas apelin/APJ seems to enhance the integrity of lymphatic and blood vessels exposed to dietary fatty acids, resulting in inhibition of HFD-induced obesity (Sawane et al., 2013). These results also suggest that enhancing vessel integrity can regulate HFD-induced obesity.
Long-chain fatty acids (LCFAs) cross the plasma membrane via a protein-mediated mechanism involving one or more LCFA-binding proteins. Among these, CD36 (also known as fatty acid translocase, FAT) has been identified as a key fatty acid transporter. CD36 is a scavenger receptor ubiquitously expressed on a variety of cell types, whose expression in adipocytes, myocytes, monocytes, macrophages, platelets, hepatocytes, vascular endothelial cells and intestinal enterocytes promotes fatty acid uptake (Cai et al., 2012; Chen et al., 2001; Koonen et al., 2005; Love-Gregory and Abumrad, 2011; Silverstein and Febbraio, 2009). CD36 also has a role in macrophage lipid accumulation and inflammatory responses (Silverstein and Febbraio, 2009). CD36 expression associates with obesity and related complications. For example, in subcutaneous adipose tissue CD36 expression is upregulated in obesity and type 2 diabetes and accomplishes important functions in WAT metabolism (Bonen et al., 2006). Also, the CD36 KO mouse is protected from HFD-induced weight gain due to a reduced food intake and elevated production of leptin (Hajri et al., 2007) and to a reduction in macrophage infiltration (Nicholls et al., 2011) with decreased adipocyte cell death, pro-inflammatory cytokine expression and macrophage and T-cell accumulation (Cai et al., 2012). Additionally, adipose tissue from CD36-null mice demonstrated a less inflammatory phenotype and improved insulin signaling at the level of the adipocyte and macrophage (Kennedy et al., 2011). These data suggest that CD36 enhances adipose tissue inflammation and cell death in diet-induced obesity through its expression in adipocytes and macrophages and that a CD36-dependent inflammatory paracrine loop between adipocytes and macrophages ease chronic inflammation and cooperate to insulin resistance in obesity (Kennedy et al., 2011). Furthermore, considering that CD36 is a fatty acid translocase expressed in endothelial cells, and that LECs use fatty acid β-oxidation to proliferate (Wong et al., 2017), it is tempting to speculate that loss of CD36 might affect the fuel supply needed for LECs maintenance, and ultimately their proliferation and migration.
Finally, it has been proposed that behavioral modifications also can reverse the obesity-induced lymphatic endothelial malfunction. Progressive weight gain is directly correlated with impaired lymphatic function; however, weight loss via dietary modification also can effectively reverse these deleterious effects. For example, weight loss resulting from conversion to a normal chow diet after diet-induced obesity, resulted in more than a 25% decrease in body weight, normalized cutaneous lymphatic collecting vessel pumping rate, lymphatic vessel density, lymphatic leakiness, lymphatic macromolecule clearance and decreased perilymphatic accumulation of inflammatory cells (T cells or macrophages) (Nitti et al., 2016). Therefore, diet-induced weight loss by caloric restriction reverses the pathological effects of obesity on the lymphatic vasculature by improving lymphatic function. Additionally, even whereas aerobic exercise could not cause weight loss, it can improve lymphatic function (Hespe et al., 2016). For example, exercise by itself has a significant anti-inflammatory effect, resulting in decreased perilymphatic accumulation of inflammatory cells (Hespe et al., 2016). Also, exercise improves collecting lymphatic vessel pumping and decreases lymphatic leakiness and improves migration of dendritic cells in obesity (Hespe et al., 2016). Therefore, lymphatic malfunction due to obesity is reversible by behavioral modifications (diet and exercise). These findings suggest the utilization of aerobic exercise and/or weight loss by reducing caloric intake, as an auxiliary therapy for obese patients with reduced lymphatic drainage.
Lymphatic malfunction also contributes to obesity
The relationship between obesity and the lymphatic system are bidirectional, such as defects in the lymphatic function also contribute to the development of obesity. Data linking lymphatic dysfunction with obesity were initially provided by mouse models with lymphatic defect, and from patients with secondary lymphedema where adipose tissue accumulation has been described after lymphatic vascular disruption. For example, Chy mice, a mouse model of lymphedema due to heterozygous inactivating mutations in VEGFR-3 exhibit: (i) abnormal subcutaneous fat deposition predominantly in the edematous subcutaneous adipose layer adjacent to the dysfunctional hypoplastic lymphatic vessels (Karkkainen et al., 2001); (ii) lipid accumulation in the edematous tail skin (Rutkowski et al., 2010) and (iii) the skin of Chy mice exhibit high levels of collagen and fat (Rutkowski et al., 2010).
While the mechanism of adipose tissue accumulation due to lymphatic malfunction has not yet been determined, insights into a possible mechanism have been postulated when using the Prox1 heterozygous (Prox1+/−) mouse model of lymphatic malfunction. Prox1 is a homeobox transcription factor crucial for the specification of lymphatic endothelial cell fate and the formation of the entire lymphatic vasculature (Wigle et al., 2002; Wigle and Oliver, 1999). On the other hand, removal of a single copy of Prox1 in mice was shown to be sufficient to promote lymphatic malfunction with an associated adult onset obesity phenotype (Harvey 2005). Prox1 null mice die at around embryonic (E) stage E14.5 (Wigle and Oliver, 1999) as they display pronounced edema due to the lack of the lymphatic vasculature. Also, Prox1 gene dosage is crucial for postnatal survival. While most Prox1+/− pups die soon after birth due to chylous ascites in the peritoneal cavity (Harvey et al., 2005), the few surviving pups that reach adulthood develop adult-onset obesity (Harvey et al., 2005). Thus, it was proposed that at least in this mouse model, excessive accumulation of fat is closely associated with lymphatic dysfunction. We have extensively characterized various metabolic parameters such as food consumption, energy expenditure, exercise and water intake in Prox1+/− before and after the onset of obesity (around 4 months of age) and concluded that obesity in adult Prox1+/− mice is a direct consequence of a primary defect in the lymphatic vasculature, rather than the result of changes in caloric intake or energy expenditure (Escobedo and Oliver, unpublished results). Consistent with this, and considering that Prox1 is also expressed in others tissues such as pancreas and hypothalamus (Lavado and Oliver, 2007; Oliver et al., 1993) that can control metabolism and appetite, using a conditional gain of function approach we recently conclusively demonstrated that in vivo restoration of Prox1 levels specifically in LECs was sufficient to rescue not only the lymphatic defects seen in Prox1+/− mice, but also their survival rate associated with an improvement in their obesity phenotype (Escobedo et al., 2016). Additionally, the lymphatic vasculature defects identified in Prox1+/− mice are observed long before these animals become obese (Escobedo et al., 2016). Moreover, we determined that there was a tight correlation between the degree of lymphatic vascular dysfunction and the amount of body weight in Prox1+/− mice (Escobedo et al., 2016). Therefore, these results provided one of the first conclusive results linking lymphatic vasculature malfunction to obesity.
How a defective lymphatic vasculature leads to adipose tissue accumulation and obesity
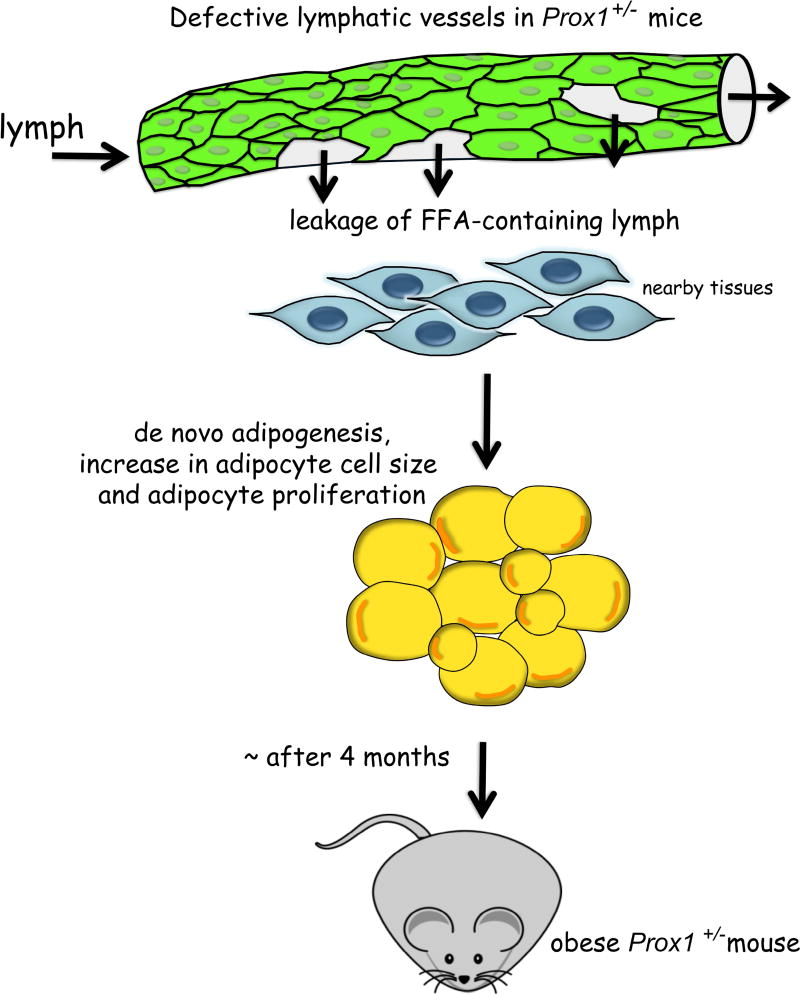
The lymphatic vessels that were most severely affected in Prox1+/− mice were those of the viscera, particularly of the mesentery and intestine, and ingestion of a fluorescent lipid allowed us to conclude that these mesenteric vessels were leaky (Harvey et al., 2005). In Prox1+/− pups these leaky lymphatics promote the accumulation of chyle (the lipid-rich fluid transported by lymphatic vessels of the small intestine) into the abdominal cavity and accumulation of lipids in the intestinal wall [chylus ascites (Harvey et al., 2005)], therefore most of the Prox1+/− pups die soon after birth, whilst the few surviving offspring (without evident chyle accumulation) start gaining weight at around 4 months of age (Escobedo et al., 2016). These results, together with the fact that there is fat accumulation near of the mesenteric lymphatic vessels in Prox1+/− pups, led us to propose that lymphatic leakage has a role in adipocyte hypertrophy and/or ectopic adipogenesis. Initial studies, indicated that chyle collected form the thoracic cavity of newborn Prox1+/− pups was able to promote adipogenesis in vitro (Harvey et al., 2005). Later on we showed that the adipogenic factor within the chyle is a lipid (Escobedo et al., 2016). Interestingly, addition of lymph fluid (either from WT or Prox1+/− mice) to cultured preadipocytes also promoted their differentiation into mature adipocytes (Escobedo et al., 2016). Moreover, no significant differences in the composition of WT and Prox1+/− lymph were determined (Escobedo et al., 2016). Therefore, we can conclude that lymph by itself is highly enriched in lipids and is adipogenic. These results agree with earlier work in which mesenteric lymph or the chylomicron fraction of lymph added to the culture medium, promoted the differentiation of adipocyte precursors isolated from the stromal-vascular fraction of embryonic rabbit adipose tissue (Nougues et al., 1988). Furthermore, we found that lymph is highly abundant in free fatty acids (FFAs), and among those, oleic acid, α-linoleic acid, palmitoleic acid, and palmitic acid were capable to induce adipogenesis in vitro (Escobedo et al., 2016). These results led us to conclude that the fatty acid fraction of the lymph that leaks from the ruptured lymphatic vessels of Prox1+/− mice promotes de novo adipogenesis in the surrounding tissue, leading eventually to obesity (Figure 1).
Figure 1. Lymphatic vascular defects contribute to adult-onset obesity.
In Prox1+/− mice, compromised lymphatic vascular integrity causes subtle leakage of lymph. FFA-containing lymph accumulates in the nearby tissues, particularly in the visceral area where it induces de novo differentiation of fat cell precursors, fat cell hypertrophy and eventually adipocyte proliferation. With age, Prox1+/− mice became progressively obese.
Fatty acids are involved in multiple cellular processes, such as inflammation and cellular viability. Additionally, plasma FFA levels are elevated in most obese subjects (Boden, 1997). For example, compared to lean mice, obese mice had significant increased levels of palmitic acid and stearic acid, which in turn are stimulators of inflammation in adipose tissue (Li et al., 2010). Moreover, stearic acid and palmitic acid also significantly impact growth and viability of endothelial cells (Harvey et al., 2010). Additionally, we have observed that high doses of FFAs and chyle are toxic to cultured LECs due to cellular viability (Escobedo & Oliver, unpublished results). Other in vitro results have shown that exposure of LECs to stearic acid is highly toxic, resulting in a dose dependent increase in cellular apoptosis, as reflected by increasing activity of caspase-3 and Annexin V, as well as a significant decrease in Prox1 and VEGFR-3 expression levels (Garcia Nores et al., 2016). Furthermore, previous results by Sawane and collaborators revealed that elevated fatty acids in plasma of HFD-fed mice induced leakiness in the lymphatic and vascular structures via apelin depletion, producing adipocyte hypertrophy and thereby promoting obesity (Sawane et al., 2013). Thus, leakage of dietary free fatty acids, possibly from leaky lymphatic vessels, could trigger adipocyte differentiation. Beside this, adipose tissue from obese subjects contains more saturated fatty acids that not only contribute to inflammation, but also might be responsible to the lymphatic vasculature rupture that at least in Prox1+/− mice lead to obesity-induced lymphatic dysfunction.
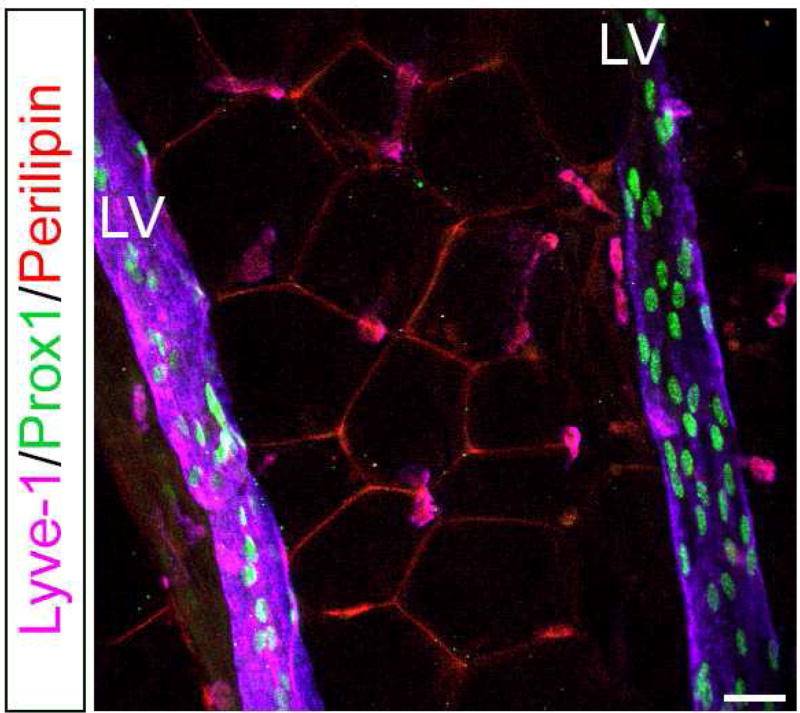
At the beginning of the onset of obesity, the expanding fat tissue requires an increased nutrient supply from new blood vessels to overcome their physiological needs. Usually, lymphatic vessels run parallel and close to the blood vessels, and since the development of obesity is accompanied by neovascularization, it is possible that lymphatic vessels also play a role in the onset of obesity. Additionally, it has been previously described that circulating levels of VEGF-A and VEGF-C are elevated in sera from obese subjects (Silha et al., 2005; Wada et al., 2011). Then, considering that VEGF-A plays a pivotal role in angiogenesis and VEGF-C in lymphangiogenesis, it is plausible that obesity expansion also requires lymphatic network growth. Interestingly, in mice we have observed lymphatic vessels running deeply inside the WAT in visceral fat depots and close to a blood vessel (Figure 2). On the other hand, a large number of studies have shown that inflammation increases lymphangiogenesis (Kataru et al., 2009; Savetsky et al., 2015b); therefore, it is possible that once a certain "threshold" of obesity is overcome, this obesity (through inflammation) compromises the function of the lymphatic vasculature. This would explain why only certain obese patients have defective lymphatic drainage in which severely obese individuals with a BMI greater than 59 have lower extremity lymphedema, whereas obese patients with a BMI between 30 and 53 had normal lower extremity lymphatic function (Greene et al., 2012). At the same time, excess FFAs in the enlarged adipocytes would facilitate endothelial damage, which also contributes to lymphatic function defects. At least in mice, any minor injury to the lymphatic system activates adipose differentiation genes and leads to adipose tissue hypertrophy and proliferation (Aschen et al., 2012). Finally, and as the lymphatic system is a physiologic regulator of inflammation and immune responses, obesity-induced lymphatic dysfunction may act in a feed-forward manner to amplify the pathological consequences of obesity.
Figure 2. Presence of lymphatic vessels in fat depots of adult mice.
Visceral fat depots were dissected and subject to whole mount immunostaining with antibodies against lymphatic endothelial cells (Lyve1, Prox1) and adipocytes (Perilipin). Lymphatic vessels are seen deep inside the WAT and nearby blood vessels (not shown). LV: lymphatic vessel. Scale bar 30 uM.
Intestinal lymphatics, lipid absorption and VEGFR-3/VEGF-C signaling
In the intestine, lymphatic capillaries (lacteals) are necessary to absorb dietary fats in the villi of the small intestine. Lacteals are important for lipid transport from the small intestinal epithelium to the lymphatic vasculature and blood circulation (Dixon, 2010). Defects in lacteals formation (e.g., VEGFR3, VEGFC, Prox1 heterozygous mice and Chy mice) lead to chylus ascites (abnormal accumulation of chyle in the abdominal cavity). Recent work showed that VEGF-C is required for lipid absorption, and VEGF-C gene deletion in mice affects the intestinal lymphatic vasculature and protects against HFD-induced obesity and glucose metabolism (Nurmi et al., 2015). In fact, this group determined that VEGFC is normally expressed by a subset of smooth muscle cells next to the lymphatic lacteals in the intestinal villus and intestinal wall (Nurmi et al., 2015). By genetic deletion of VEGFC they found that this growth factor plays a fundamental role in the maintenance of adult intestinal lymphatics. Furthermore, they determined that those mutant mice have defective lipid absorption and increased fecal excretion of dietary cholesterol and fatty acids, results that argue that VEGFC activity is critical for the intestinal lymphatic vasculature and for lipid absorption.
Also related to VEGF-C, as mentioned before obese subjects show significantly increased circulating levels of VEGF-C. Those levels are closely correlated with metabolic and lipid parameters (Wada et al., 2011), dyslipidemia and atherosclerosis (Wada et al., 2011). In mice, VEGF-C mRNA levels are also increased in the adipose tissues of both, genetic and diet-induced obesity models, suggesting that adipose tissue might be one of the sources of elevated VEGF-C levels in obesity (Karaman et al., 2014). A recent study shows that overexpression of VEGF-C induces weight gain and insulin resistance in mice (Karaman et al., 2016). K14-VEGF-C mice overexpressing human VEGF-C in the skin have increased weight gain and subcutaneous adipose tissue accumulation when compared with wild type (WT) littermates, and they also became insulin resistant and have increased ectopic lipid accumulation (Karaman et al., 2016).
Moreover, K14-VEGFR-3-Ig mice that constitutively express soluble-VEGFR-3-Ig in the skin, inhibiting VEGF-C and –D signaling, are protected from obesity-induced insulin resistance and hepatic lipid accumulation by favoring a higher anti-inflammatory/pro-inflammatory macrophage ratio in subcutaneous WAT, which supported adipocyte differentiation (Karaman et al., 2014).
Lymphatic vasculature and obesity in humans
Our finding that lymph itself can induce adipogenesis in vitro is partially supported by clinical studies demonstrating that secondary lymphedema induces localized fat accumulation in the nearby tissue. For example, secondary lymphedema consequence of surgical excision of regional axillary lymph nodes in breast cancer patients leads to arm lymph accumulation, followed by subcutaneous adipose tissue accumulation nearby, and in these cases, liposuction is an effective treatment for lymphedema (Boyages et al., 2015; Brorson, 2003, 2010, 2016).
Additional data in humans associating a genetic lymphatic vasculature dysfunction with obesity is provided by the transcription factor Forkhead box protein C2 (FOXC2), which is required for the formation and maturation of collecting vessels and lymphatic valves (Norrmen et al., 2009). Loss of Foxc2 in mice leads to defects in lymphatic remodeling, failure to form lymphatic valves, and increased pericyte coverage of lymphatic vessels (Petrova et al., 2004). In humans, inactivating mutations in FOXC2 produce the lymphedema–distichiasis syndrome, a major form of hereditary lymphedema (Dagenais et al., 2004; Fang et al., 2000). It has been described that a single nucleotide polymorphism (SNP) found in the putative promoter region of FOXC2 is associated with BMI and percentage of body fat in a group of native americans (Kovacs et al., 2003). Later on, this FOXC2 polymorphism was fund to be associated with obesity, and features of the dysmetabolic syndrome in Scandinavian subjects (Carlsson et al., 2005). Additionally, another study demonstrated that transgenic mice overexpressing FOXC2 in adipocytes, display a reduction in the intra-abdominal white adipose tissue under normal or HFD; and have a relative resistance to diet-induced weight gain as well as diet-induced insulin resistance (Cederberg et al., 2001), a result suggesting that gain of function of FOXC2 is consistent with protection against obesity.
Regarding Prox1, recent findings in family-based genome-wide association studies (GWAS) combined with a genome-wide linkage study in Asian populations, identified one SNP (rs1704198) in the proximity of the PROX1 gene, which was associated with a larger waist circumference (Kim et al., 2013). Additional large meta-analyses of GWAS confirmed a SNP (rs340874) in the 5’UTR region of PROX1 that is associated with fasting glycemia and type 2 diabetes mellitus (Dupuis et al., 2010; Lecompte et al., 2013). More recently, a new study with subjects of Polish origin evaluated the functional/phenotypic differences related to rs340874 PROX1 variants (different allelic variants, C or T allele) analyzing behavioral patterns, body fat distribution and glucose/fat metabolism after standardized meals and during the oral glucose tolerance test (Kretowski et al., 2015). Interestingly they found that subjects with the PROX1 CC variant, had higher non-esterified fatty acids levels after high-fat meal, higher accumulation of visceral fat, but surprisingly lower daily food consumption (Kretowski et al., 2015). These results suggest that there is a percentage of obese individuals whose accumulation of abdominal fat is not due to an excess of caloric intake, but rather to a SNP PROX1 allelic variant that predisposes them to accumulate abdominal fat.
Future directions
The data summarized in this review describe some of the observations supporting a reciprocal regulation between lymphatic vascular function and obesity, where lymphatic defects lead to enhanced adipose tissue accumulation and obesity.
It is important to consider that those individuals who suffer from obesity are more likely to also experience defects in the lymphatic vasculature. Additionally, obesity-mediated lymphatic dysfunction predisposes the subjects to more acute and chronic inflammatory responses that in turn, further impair lymphatic function and increases the risk of developing some form of associated lymphedema.
The possibility that subtle defects in the lymphatic vasculature might be responsible among others, for certain types of metabolic disorders and obesity in humans needs to be further evaluated. “The idea that lymph leakage due to inherited subtle defects in the lymphatic circulation might contribute to obesity represents a major paradigm shift and the implication that obesity might be regulated by the local accumulation of factors released from the lymphatic circulation is of enormous relevance. If it is true, considerable efforts could be exerted to identify effective therapeutic strategies, which might include promoting lymphatic endothelial integrity, preventing release of the adipogenic factors from the lymphatics or interfering functionally with the adipogenic activity” (Schneider et al., 2005). Therefore, the use of available mouse models to perform a detailed characterization of the mechanisms leading to a defective lymphatic vasculature (particularly intestinal lymphatics), the understanding of how defective lymphatics contribute to obesity and fat accumulation, and the characterization of the lymphatics-adipose tissue relationship are highly significant. This knowledge should help to eventually translate these findings into human patients affected by metabolic disorders and obesity.
Finally, it is critical to emphasize that blood vessels, adipocytes, lymphatic vessels, macrophages and immune cells all coexist and interact extensively within the adipose tissue, therefore the future treatment of obesity and associated disorders should be focused on multiple therapies considering all these factors and its possible consequences. Additionally, those findings of lymphedema-induced obesity call for translation into clinical applications, and suggest that ‘lymphatic endothelial therapy’ can also be applied for obesity treatment. For example, specific inhibitors of the VEGF-C/VEGFR-3 signaling pathway could provide benefits for the treatment of obesity by reducing the absorption of excess dietary lipids and regulating insulin resistance.
Acknowledgments
N.E is supported by Fondecyt 11160592 and G.O. by the Leducq Foundation Transatlantic Network of Excellence grant Lymph Vessels in Obesity and Cardiovascular Disease (11CVD03). We want to thank Joseph Bass and Navdeep Chandel for helpful comments and suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al Suwaidi J, Higano ST, Holmes DR, Jr, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37:1523–1528. doi: 10.1016/s0735-1097(01)01212-8. [DOI] [PubMed] [Google Scholar]
- Amano SU, Cohen JL, Vangala P, Tencerova M, Nicoloro SM, Yawe JC, Shen Y, Czech MP, Aouadi M. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell Metab. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arngrim N, Simonsen L, Holst JJ, Bulow J. Reduced adipose tissue lymphatic drainage of macromolecules in obese subjects: a possible link between obesity and local tissue inflammation? Int J Obes. 2013;37:748–750. doi: 10.1038/ijo.2012.98. (Lond) [DOI] [PubMed] [Google Scholar]
- Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot M, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis: part II. Expression of adipose differentiation genes. Plast Reconstr Surg. 2012;129:838–847. doi: 10.1097/PRS.0b013e3182450b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M. Prevention and endothelial therapy of coronary artery disease. Curr Opin Pharmacol. 2013;13:226–241. doi: 10.1016/j.coph.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier-Latmani J, Cisarovsky C, Demir CS, Bruand M, Jaquet M, Davanture S, Ragusa S, Siegert S, Dormond O, Benedito R, et al. DLL4 promotes continuous adult intestinal lacteal regeneration and dietary fat transport. J Clin Invest. 2015;125:4572–4586. doi: 10.1172/JCI82045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilancini S, Lucchi M, Tucci S, Eleuteri P. Functional lymphatic alterations in patients suffering from lipedema. Angiology. 1995;46:333–339. doi: 10.1177/000331979504600408. [DOI] [PubMed] [Google Scholar]
- Blum KS, Karaman S, Proulx ST, Ochsenbein AM, Luciani P, Leroux JC, Wolfrum C, Detmar M. Chronic high-fat diet impairs collecting lymphatic vessel function in mice. PLoS One. 2014;9:e94713. doi: 10.1371/journal.pone.0094713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- Bonen A, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes. 2006;30:877–883. doi: 10.1038/sj.ijo.0803212. (Lond) [DOI] [PubMed] [Google Scholar]
- Boyages J, Kastanias K, Koelmeyer LA, Winch CJ, Lam TC, Sherman KA, Munnoch DA, Brorson H, Ngo QD, Heydon-White A, et al. Liposuction for Advanced Lymphedema: A Multidisciplinary Approach for Complete Reduction of Arm and Leg Swelling. Ann Surg Oncol. 2015;22(Suppl 3):S1263–1270. doi: 10.1245/s10434-015-4700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson H. Liposuction in arm lymphedema treatment. Scand J Surg. 2003;92:287–295. doi: 10.1177/145749690309200409. [DOI] [PubMed] [Google Scholar]
- Brorson H. From lymph to fat: complete reduction of lymphoedema. Phlebology. 2010;25(Suppl 1):52–63. doi: 10.1258/phleb.2010.010s08. [DOI] [PubMed] [Google Scholar]
- Brorson H. Liposuction in Lymphedema Treatment. J Reconstr Microsurg. 2016;32:56–65. doi: 10.1055/s-0035-1549158. [DOI] [PubMed] [Google Scholar]
- Cai L, Wang Z, Ji A, Meyer JM, van der Westhuyzen DR. Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS One. 2012;7:e36785. doi: 10.1371/journal.pone.0036785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y. Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov. 2010;9:107–115. doi: 10.1038/nrd3055. [DOI] [PubMed] [Google Scholar]
- Cao Y. Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 2013;18:478–489. doi: 10.1016/j.cmet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Carlsson E, Groop L, Ridderstrale M. Role of the FOXC2 −512C>T polymorphism in type 2 diabetes: possible association with the dysmetabolic syndrome. Int J Obes. 2005;29:268–274. doi: 10.1038/sj.ijo.0802876. (Lond) [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- Chen M, Yang Y, Braunstein E, Georgeson KE, Harmon CM. Gut expression and regulation of FAT/CD36: possible role in fatty acid transport in rat enterocytes. Am J Physiol Endocrinol Metab. 2001;281:E916–923. doi: 10.1152/ajpendo.2001.281.5.E916. [DOI] [PubMed] [Google Scholar]
- Dagenais SL, Hartsough RL, Erickson RP, Witte MH, Butler MG, Glover TW. Foxc2 is expressed in developing lymphatic vessels and other tissues associated with lymphedema-distichiasis syndrome. Gene Expr Patterns. 2004;4:611–619. doi: 10.1016/j.modgep.2004.07.004. [DOI] [PubMed] [Google Scholar]
- De Rosa A, Monaco ML, Capasso M, Forestieri P, Pilone V, Nardelli C, Buono P, Daniele A. Adiponectin oligomers as potential indicators of adipose tissue improvement in obese subjects. Eur J Endocrinol. 2013;169:37–43. doi: 10.1530/EJE-12-1039. [DOI] [PubMed] [Google Scholar]
- Dieterich LC, Seidel CD, Detmar M. Lymphatic vessels: new targets for the treatment of inflammatory diseases. Angiogenesis. 2014;17:359–371. doi: 10.1007/s10456-013-9406-1. [DOI] [PubMed] [Google Scholar]
- Dixon JB. Mechanisms of chylomicron uptake into lacteals. Ann N Y Acad Sci. 2010;1207(Suppl 1):E52–57. doi: 10.1111/j.1749-6632.2010.05716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias I, Franckhauser S, Ferre T, Vila L, Tafuro S, Munoz S, Roca C, Ramos D, Pujol A, Riu E, et al. Adipose tissue overexpression of vascular endothelial growth factor protects against diet-induced obesity and insulin resistance. Diabetes. 2012;61:1801–1813. doi: 10.2337/db11-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo N, Oliver G. Lymphangiogenesis: Origin, Specification, and Cell Fate Determination. Annu Rev Cell Dev Biol. 2016;32:677–691. doi: 10.1146/annurev-cellbio-111315-124944. [DOI] [PubMed] [Google Scholar]
- Escobedo N, Proulx ST, Karaman S, Dillard ME, Johnson N, Detmar M, Oliver G. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Dagenais SL, Erickson RP, Arlt MF, Glynn MW, Gorski JL, Seaver LH, Glover TW. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet. 2000;67:1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- Friedman JM. Obesity in the new millennium. Nature. 2000;404:632–634. doi: 10.1038/35007504. [DOI] [PubMed] [Google Scholar]
- Garcia Nores GD, Cuzzone DA, Albano NJ, Hespe GE, Kataru RP, Torrisi JS, Gardenier JC, Savetsky IL, Aschen SZ, Nitti MD, et al. Obesity but not high-fat diet impairs lymphatic function. Int J Obes (Lond) 2016;40:1582–1590. doi: 10.1038/ijo.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q, Brichard S, Yi X, Li Q. microRNAs as a new mechanism regulating adipose tissue inflammation in obesity and as a novel therapeutic strategy in the metabolic syndrome. J Immunol Res. 2014;2014:987285. doi: 10.1155/2014/987285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 18. 2011;123(2):186–94. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Ambrosi J, Catalan V, Rodriguez A, Ramirez B, Silva C, Gil MJ, Salvador J, Fruhbeck G. Involvement of serum vascular endothelial growth factor family members in the development of obesity in mice and humans. J Nutr Biochem. 2010;21:774–780. doi: 10.1016/j.jnutbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Greene AK, Grant FD, Slavin SA. Lower-extremity lymphedema and elevated body-mass index. N Engl J Med. 2012;366:2136–2137. doi: 10.1056/NEJMc1201684. [DOI] [PubMed] [Google Scholar]
- Greene AK, Maclellan RA. Obesity-induced Upper Extremity Lymphedema. Plast Reconstr Surg Glob Open. 2013;1:e59. doi: 10.1097/GOX.0b013e3182a96359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes. 2007;56:1872–1880. doi: 10.2337/db06-1699. [DOI] [PubMed] [Google Scholar]
- Harvey KA, Walker CL, Pavlina TM, Xu Z, Zaloga GP, Siddiqui RA. Long-chain saturated fatty acids induce pro-inflammatory responses and impact endothelial cell growth. Clin Nutr. 2010;29:492–500. doi: 10.1016/j.clnu.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- Hespe GE, Kataru RP, Savetsky IL, Garcia Nores GD, Torrisi JS, Nitti MD, Gardenier JC, Zhou J, Yu JZ, Jones LW, et al. Exercise training improves obesity-related lymphatic dysfunction. J Physiol. 2016;594:4267–4282. doi: 10.1113/JP271757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- Iantorno M, Campia U, Di Daniele N, Nistico S, Forleo GB, Cardillo C, Tesauro M. Obesity, inflammation and endothelial dysfunction. J Biol Regul Homeost Agents. 2014;28:169–176. [PubMed] [Google Scholar]
- Karaman S, Hollmen M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, et al. Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol Metab. 2014;4:93–105. doi: 10.1016/j.molmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman S, Hollmen M, Yoon SY, Alkan HF, Alitalo K, Wolfrum C, Detmar M. Transgenic overexpression of VEGF-C induces weight gain and insulin resistance in mice. Sci Rep. 2016;6:31566. doi: 10.1038/srep31566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki S, Ngo D, Farb MG, Park SY, Saggese SM, Hamburg NM, Carmine B, Hess DT, Walsh K, Gokce N. WNT5A Regulates Adipose Tissue Angiogenesis via Anti-Angiogenic VEGFA165b in Obese Humans. Am J Physiol Heart Circ Physiol. 2017 Apr 14; doi: 10.1152/ajpheart.00776.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci U S A. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- Kennedy DJ, Kuchibhotla S, Westfall KM, Silverstein RL, Morton RE, Febbraio M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res. 2011;89:604–613. doi: 10.1093/cvr/cvq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D. The lymphatic vasculature revisited. J Clin Invest. 2014;124:874–877. doi: 10.1172/JCI74854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Yoo YJ, Ju YS, Lee S, Cho SI, Sung J, Kim JI, Seo JS. Combined linkage and association analyses identify a novel locus for obesity near PROX1 in Asians. Obesity (Silver Spring) 2013;21:2405–2412. doi: 10.1002/oby.20153. [DOI] [PubMed] [Google Scholar]
- Koonen DP, Glatz JF, Bonen A, Luiken JJ. Long-chain fatty acid uptake and FAT/CD36 translocation in heart and skeletal muscle. Biochim Biophys Acta. 2005;1736:163–180. doi: 10.1016/j.bbalip.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Lehn-Stefan A, Stumvoll M, Bogardus C, Baier LJ. Genetic variation in the human winged helix/forkhead transcription factor gene FOXC2 in Pima Indians. Diabetes. 2003;52:1292–1295. doi: 10.2337/diabetes.52.5.1292. [DOI] [PubMed] [Google Scholar]
- Kretowski A, Adamska E, Maliszewska K, Wawrusiewicz-Kurylonek N, Citko A, Goscik J, Bauer W, Wilk J, Golonko A, Waszczeniuk M, et al. The rs340874 PROX1 type 2 diabetes mellitus risk variant is associated with visceral fat accumulation and alterations in postprandial glucose and lipid metabolism. Genes Nutr. 2015;10:454. doi: 10.1007/s12263-015-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Oliver G. Prox1 expression patterns in the developing and adult murine brain. Dev Dyn. 2007;236:518–524. doi: 10.1002/dvdy.21024. [DOI] [PubMed] [Google Scholar]
- Leal-Cerro A, Considine RV, Peino R, Venegas E, Astorga R, Casanueva FF, Dieguez C. Serum immunoreactive-leptin levels are increased in patients with Cushing’s syndrome. Horm Metab Res. 1996;28:711–713. doi: 10.1055/s-2007-979884. [DOI] [PubMed] [Google Scholar]
- Lecompte S, Pasquetti G, Hermant X, Grenier-Boley B, Gonzalez-Gross M, De Henauw S, Molnar D, Stehle P, Beghin L, Moreno LA, et al. Genetic and molecular insights into the role of PROX1 in glucose metabolism. Diabetes. 2013;62:1738–1745. doi: 10.2337/db12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fu W, Li XA. Differential fatty acid profile in adipose and non-adipose tissues in obese mice. Int J Clin Exp Med. 2010;3:303–307. [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Rutkowski JM, Helft J, Reddy ST, Swartz MA, Randolph GJ, Angeli V. Hypercholesterolemic mice exhibit lymphatic vessel dysfunction and degeneration. Am J Pathol. 2009;175:1328–1337. doi: 10.2353/ajpath.2009.080963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17:671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Lobato NS, Filgueira FP, Akamine EH, Tostes RC, Carvalho MH, Fortes ZB. Mechanisms of endothelial dysfunction in obesity-associated hypertension. Braz J Med Biol Res. 2012;45:392–400. doi: 10.1590/S0100-879X2012007500058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohrmann C, Foeldi E, Langer M. MR imaging of the lymphatic system in patients with lipedema and lipo-lymphedema. Microvasc Res. 2009;77:335–339. doi: 10.1016/j.mvr.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Love-Gregory L, Abumrad NA. CD36 genetics and the metabolic complications of obesity. Curr Opin Clin Nutr Metab Care. 2011;14:527–534. doi: 10.1097/MCO.0b013e32834bbac9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen SH, Thomas MJ, et al. Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Invest. 2013;123:1571–1579. doi: 10.1172/JCI63685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, Hurley KE, Riedel ER, Van Zee KJ. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26:5213–5219. doi: 10.1200/JCO.2008.16.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–1406. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo DT, Farb MG, Kikuchi R, Karki S, Tiwari S, Bigornia SJ, Bates DO, LaValley MP, Hamburg NM, Vita JA, Hess DT, Walsh K, Gokce N. Antiangiogenic actions of vascular endothelial growth factor-A165b, an inhibitory isoform of vascular endothelial growth factor-A, in human obesity. Circulation 23. 2014;130(13):1072–80. doi: 10.1161/CIRCULATIONAHA.113.008171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls HT, Kowalski G, Kennedy DJ, Risis S, Zaffino LA, Watson N, Kanellakis P, Watt MJ, Bobik A, Bonen A, et al. Hematopoietic cell-restricted deletion of CD36 reduces high-fat diet-induced macrophage infiltration and improves insulin signaling in adipose tissue. Diabetes. 2011;60:1100–1110. doi: 10.2337/db10-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nitti MD, Hespe GE, Kataru RP, Garcia Nores GD, Savetsky IL, Torrisi JS, Gardenier JC, Dannenberg AJ, Mehrara BJ. Obesity-induced lymphatic dysfunction is reversible with weight loss. J Physiol. 2016;594:7073–7087. doi: 10.1113/JP273061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nougues J, Reyne Y, Dulor JP. Differentiation of rabbit adipocyte precursors in primary culture. Int J Obes (Lond) 1988;12:321–333. [PubMed] [Google Scholar]
- Nurmi H, Saharinen P, Zarkada G, Zheng W, Robciuc MR, Alitalo K. VEGF-C is required for intestinal lymphatic vessel maintenance and lipid absorption. EMBO Mol Med. 2015;7:1418–1425. doi: 10.15252/emmm.201505731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okhovat JP, Alavi A. Lipedema: A Review of the Literature. Int J Low Extrem Wounds. 2015;14:262–267. doi: 10.1177/1534734614554284. [DOI] [PubMed] [Google Scholar]
- Oliver G, Sosa-Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox 1, a prospero-related homeobox gene expressed during mouse development. Mech Dev. 1993;44:3–16. doi: 10.1016/0925-4773(93)90012-m. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Pond CM. Adipose tissue and the immune system. Prostaglandins Leukot Essent Fatty Acids. 2005;73:17–30. doi: 10.1016/j.plefa.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Proulx ST, Luciani P, Dieterich LC, Karaman S, Leroux JC, Detmar M. Expansion of the lymphatic vasculature in cancer and inflammation: new opportunities for in vivo imaging and drug delivery. J Control Release. 2013;172:550–557. doi: 10.1016/j.jconrel.2013.04.027. [DOI] [PubMed] [Google Scholar]
- Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- Ridner SH, Dietrich MS, Stewart BR, Armer JM. Body mass index and breast cancer treatment-related lymphedema. Support Care Cancer. 2011;19:853–857. doi: 10.1007/s00520-011-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robciuc MR, Kivela R, Williams IM, de Boer JF, van Dijk TH, Elamaa H, Tigistu-Sahle F, Molotkov D, Leppanen VM, Kakela R, et al. VEGFB/VEGFR1-Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell Metab. 2016;23:712–724. doi: 10.1016/j.cmet.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- Rose DP, Gilhooly EM, Nixon DW. Adverse effects of obesity on breast cancer prognosis, and the biological actions of leptin (review) Int J Oncol. 2002;21:1285–1292. [PubMed] [Google Scholar]
- Rosen ED. The molecular control of adipogenesis, with special reference to lymphatic pathology. Ann N Y Acad Sci. 2002;979:143–158. doi: 10.1111/j.1749-6632.2002.tb04875.x. discussion 188–196. [DOI] [PubMed] [Google Scholar]
- Roth J, Qiang X, Marban SL, Redelt H, Lowell BC. The obesity pandemic: where have we been and where are we going? Obes Res. 2004;12(Suppl 2):88S–101S. doi: 10.1038/oby.2004.273. [DOI] [PubMed] [Google Scholar]
- Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol. 2010;176:1122–1129. doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski JM, Stern JH, Scherer PE. The cell biology of fat expansion. J Cell Biol. 2015;208:501–512. doi: 10.1083/jcb.201409063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Kamekura R, Kawata K, Kawada M, Jitsukawa S, Yamashita K, Sato N, Himi T, Ichimiya S. Novel Mechanisms of Compromised Lymphatic Endothelial Cell Homeostasis in Obesity: The Role of Leptin in Lymphatic Endothelial Cell Tube Formation and Proliferation. PLoS One. 2016;11:e0158408. doi: 10.1371/journal.pone.0158408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savetsky IL, Albano NJ, Cuzzone DA, Gardenier JC, Torrisi JS, Garcia Nores GD, Nitti MD, Hespe GE, Nelson TS, Kataru RP, et al. Lymphatic Function Regulates Contact Hypersensitivity Dermatitis in Obesity. J Invest Dermatol. 2015a;135:2742–2752. doi: 10.1038/jid.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savetsky IL, Ghanta S, Gardenier JC, Torrisi JS, Garcia Nores GD, Hespe GE, Nitti MD, Kataru RP, Mehrara BJ. Th2 cytokines inhibit lymphangiogenesis. PLoS One. 2015b;10:e0126908. doi: 10.1371/journal.pone.0126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savetsky IL, Torrisi JS, Cuzzone DA, Ghanta S, Albano NJ, Gardenier JC, Joseph WJ, Mehrara BJ. Obesity increases inflammation and impairs lymphatic function in a mouse model of lymphedema. Am J Physiol Heart Circ Physiol. 2014;307:H165–172. doi: 10.1152/ajpheart.00244.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawane M, Kajiya K, Kidoya H, Takagi M, Muramatsu F, Takakura N. Apelin inhibits diet-induced obesity by enhancing lymphatic and blood vessel integrity. Diabetes. 2013;62:1970–1980. doi: 10.2337/db12-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawane M, Kidoya H, Muramatsu F, Takakura N, Kajiya K. Apelin attenuates UVB-induced edema and inflammation by promoting vessel function. Am J Pathol. 2011;179:2691–2697. doi: 10.1016/j.ajpath.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Conway EM, Carmeliet P. Lymph makes you fat. Nat Genet. 2005;37:1023–1024. doi: 10.1038/ng1005-1023. [DOI] [PubMed] [Google Scholar]
- Shibata R, Ouchi N, Murohara T. Adiponectin and cardiovascular disease. Circ J. 2009;73:608–14. doi: 10.1253/circj.cj-09-0057. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Shibata R, Ishii M, Ohashi K, Kambara T, Uemura Y, Yuasa D, Kataoka Y, Kihara S, Murohara T, Ouchi N. Adiponectin-mediated modulation of lymphatic vessel formation and lymphedema. J Am Heart Assoc. 19. 2013;2(5):e000438. doi: 10.1161/JAHA.113.000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin BW, Sim YJ, Jeong HJ, Kim GC. Lipedema, a rare disease. Ann Rehabil Med. 2011;35:922–927. doi: 10.5535/arm.2011.35.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. Int J Obes (Lond) 2005;29:1308–1314. doi: 10.1038/sj.ijo.0802987. [DOI] [PubMed] [Google Scholar]
- Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011;25:2187–2197. doi: 10.1101/gad.16974811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JH, Rutkowski JM, Scherer PE. Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell Metab. 2016;23:770–784. doi: 10.1016/j.cmet.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109(15):5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, et al. Adipose vascular endothelial growth factor regulates metabolic homeostasis through angiogenesis. Cell Metab. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Swenson KK, Nissen MJ, Leach JW, Post-White J. Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum. 2009;36:185–193. doi: 10.1188/09.ONF.185-193. [DOI] [PubMed] [Google Scholar]
- Takikawa A, Mahmood A, Nawaz A, Kado T, Okabe K, Yamamoto S, Aminuddin A, Senda S, Tsuneyama K, Ikutani M, Watanabe Y, Igarashi Y, Nagai Y, Takatsu K, Koizumi K, Imura J, Goda N, Sasahara M, Matsumoto M, Saeki K, Nakagawa T, Fujisaka S, Usui I, Tobe K. HIF-1α in Myeloid Cells Promotes Adipose Tissue Remodeling Toward Insulin Resistance. Diabetes. 2016;65:3649–3659. doi: 10.2337/db16-0012. [DOI] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- Tavakkolizadeh A, Wolfe KQ, Kangesu L. Cutaneous lymphatic malformation with secondary fat hypertrophy. Br J Plast Surg. 2001;54:367–369. doi: 10.1054/bjps.2001.3572. [DOI] [PubMed] [Google Scholar]
- Tinahones FJ, Coin-Araguez L, Mayas MD, Garcia-Fuentes E, Hurtado-Del-Pozo C, Vendrell J, Cardona F, Calvo RM, Obregon MJ, El Bekay R. Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol. 2012;12:4. doi: 10.1186/1472-6793-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi JS, Hespe GE, Cuzzone DA, Savetsky IL, Nitti MD, Gardenier JC, Garcia Nores GD, Jowhar D, Kataru RP, Mehrara BJ. Inhibition of Inflammation and iNOS Improves Lymphatic Function in Obesity. Sci Rep. 2016;6:19817. doi: 10.1038/srep19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Ura S, Kitaoka S, Satoh-Asahara N, Horie T, Ono K, Takaya T, Takanabe-Mori R, Akao M, Abe M, et al. Distinct characteristics of circulating vascular endothelial growth factor-a and C levels in human subjects. PLoS One. 2011;6:e29351. doi: 10.1371/journal.pone.0029351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S. Lymphedema and lipedema - an overview of conservative treatment. Vasa. 2011;40:271–279. doi: 10.1024/0301-1526/a000115. [DOI] [PubMed] [Google Scholar]
- Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24:2115–2126. doi: 10.1101/gad.1955910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitman ES, Aschen SZ, Farias-Eisner G, Albano N, Cuzzone DA, Ghanta S, Zampell JC, Thorek D, Mehrara BJ. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 2013;8:e70703. doi: 10.1371/journal.pone.0070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. Embo J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- Wold LE, Hines EA, Jr, Allen EV. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann Intern Med. 1951;34:1243–1250. doi: 10.7326/0003-4819-34-5-1243. [DOI] [PubMed] [Google Scholar]
- Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, García-Caballero M, Missiaen R, Huang H, Brüning U, Blacher S, Vinckier S, Goveia J, Knobloch M, Zhao H, Dierkes C, Shi C, Hägerling R, Moral-Dardé V, Wyns S, Lippens M, Jessberger S, Fendt SM, Luttun A, Noel A, Kiefer F, Ghesquière B, Moons L, Schoonjans L, Dewerchin M, Eelen G, Lambrechts D, Carmeliet P. The role of fatty acid β-oxidation in lymphangiogenesis Nature. 2017;542:49–54. doi: 10.1038/nature21028. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]