Abstract
Objective
CT-P13 is a biosimilar with comparable pharmacokinetics, efficacy and safety to its reference product (RP), infliximab. Studies have shown that switching from RP to CT-P13 does not reduce the effectiveness or safety of treatment.
Methods
In this retrospective real-world study, patients with inflammatory diseases treated with RP were switched to CT-P13 (n = 7) or continued on RP (n = 6). Clinical outcomes were compared between groups after four treatment cycles.
Results
CT-P13 demonstrated comparable effectiveness to its RP. All patients who switched to the biosimilar maintained or improved their clinical response, including two who remained in remission and three who moved into remission. In the RP group, five patients maintained their clinical response, with one achieving remission. Safety profiles were similar between groups.
Conclusions
CT-P13 was equally effective as infliximab RP in this real-world study. CT-P13 is a valid, lower-cost alternative for patients currently receiving RP.
Key Points
| The effectiveness and safety of switching from an innovator biologic or reference product (RP) to a biosimilar is a hot topic in the fields of rheumatology and gastroenterology. |
| In a retrospective real-world study, we showed that all patients who switched from the RP infliximab to the biosimilar CT-P13 maintained or improved their clinical response. |
| These real-world findings complement observations from recent clinical trials, supporting the effectiveness and safety of switching from the RP infliximab to CT-P13. |
Introduction
Biosimilars are developed to be highly similar to an existing innovator biologic drug (or reference product [RP]) [1]. Although the approval process for biosimilars is highly rigorous, its expedited nature ensures biosimilars are associated with lower developmental costs and thus are generally less expensive than their RPs. In many countries, patient access to RP biologics is restricted due to their high cost [2]. By providing a more cost-effective treatment option, biosimilars can reduce pressure on healthcare budgets, drive competition, and increase patient access to biologic therapy [3].
CT-P13 (Remsima®, CELLTRION, Incheon, Republic of Korea; Inflectra®, Hospira, Maidenhead, UK) is a biosimilar of the infliximab RP (Remicade®, Janssen Biotech, Horsham, PA, USA), an anti-tumor necrosis factor (TNF) agent approved for treatment of certain inflammatory diseases [4, 5]. In clinical trials, CT-P13 demonstrated equivalent pharmacokinetics and efficacy to its RP [6, 7], and comparable safety and immunogenicity up to week 54 [8, 9]. In 2015, almost 13% of patients in hospitals in Madrid, Spain with a prescription for infliximab received a biosimilar. However, for new patients starting infliximab treatment, this figure was 54% [10]. Such data suggest a reluctance by clinicians to switch patients already treated with the RP to the biosimilar, despite clinical studies demonstrating that switching to CT-P13 had no detrimental effects on efficacy or safety [11, 12].
The aim of this retrospective real-world study was to investigate whether there was any meaningful difference in the clinical response of patients with inflammatory diseases who switched from the RP to CT-P13 compared with patients who remained on RP.
Patients and Methods
This study included 13 patients diagnosed with rheumatoid arthritis (RA), psoriatic arthritis (PsA), or ankylosing spondylitis (AS) who had been treated with, and had responded to, infliximab RP. Between June 2015 and January 2016, seven patients were switched to CT-P13 while six remained on RP treatment. Doses of study drug (i.e., CT-P13 or RP) were individually optimized during the study according to clinician judgement. Patients receiving concomitant disease-modifying antirheumatic drugs before the start of the study continued such treatment in line with clinical need.
The study was conducted according to the principles of the Declaration of Helsinki.
Clinical response was assessed before and after four cycles of treatment using measurements of disease activities employed in the study center during routine clinical practice. These were the Disease Activity Score in 28 joints (DAS28) in patients with RA and PsA (remission: <2.6 points; significant response: reduction ≥1.2 points; continued improvement: reduction <1.2 points) [13], and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) in patients with AS (remission: <2.0; significant response: reduction ≥2.0 points; continued improvement: reduction <2.0 points) [14].
Results
Baseline patient and treatment characteristics were generally similar between groups (Table 1). Median doses of infliximab RP (before the study) and study drug (CT-P13 or RP [during the study]) were lower in the RP group than in the CT-P13 group (Table 1). Differences in median doses between the two groups of patients reflected the fact that more patients in the RP group than in the CT-P13 group had their treatment doses individually optimized (reduced) (before the start of the study and as part of routine clinical practice).
Table 1.
Patient demographics, treatment characteristics, and clinical outcomes after four cycles of treatment in patients who switched from infliximab (reference product) to the biosimilar CT-P13 versus those who continued on reference product
| Switched to CT-P13 (n = 7) | Continued on RP (n = 6) | |
|---|---|---|
| Baseline characteristics | ||
| Indication | 2 RA, 2 PsA, 3 AS | 3 PsA, 3 AS |
| Age (years) | 49 (13) | 53 (15) |
| Gender (male:female) | 5:2 | 6:0 |
| Treatment prior to study | ||
| Infliximab RP dose (mg/kg) | 4.8 (0.7)a | 3.3 (0.5) |
| Infliximab RP dose interval (days) | 56 (4) | 56 (5) |
| Duration of infliximab RP treatment (cycles) | 76 (26) | 75 (18) |
| Treatment during study | ||
| Study drug | CT-P13 | Infliximab RP |
| Dose (mg/kg) | 5.0 (0.4)a | 3.3 (0.5) |
| Dose interval (days) | 56 (7) | 56 (5) |
| Concomitant DMARDs [n (%)] | 5 (71.4) | 5 (83.3) |
| Clinical statusb | ||
| At start of study | ||
| Remission [n (%)] | 2 (28.6) | 4 (66.7) |
| At end of study | ||
| Remission [n (%)] | 5 (71.4) | 5 (83.3) |
| Significant response [n (%)] | 1 (14.3) | 0 |
| Continued improvement [n (%)] | 1 (14.3) | 1 (16.7) |
| Safety results | ||
| Patients experiencing ≥1 AE [n (%)] | 4 (57.1)c | 3 (50.0) |
| Upper airway infection [n (severity)] | 3 (mild) | 2 (mild) |
| Gastrointestinal infection [n (severity)] | 1 (mild) | 1 (mild) |
| Cutaneous AE [n (severity)] | 1 (severe)d | 0 |
| Discontinuation due to AE [n (%)] | 1 (14.3)d | 0 |
Data are median (interquartile range) unless otherwise stated
AE adverse event, AS ankylosing spondylitis, BASDAI, Bath Ankylosing Spondylitis Disease Activity Index, DAS28 Disease Activity Score in 28 joints, DMARD disease-modifying antirheumatic drug, PsA psoriatic arthritis, RA rheumatoid arthritis, RP reference product
a P < 0.005 (Mann–Whitney U test)
bRemission defined as DAS28 <2.6 in RA/PsA patients or BASDAI <2.0 in AS patients. Significant response defined as a reduction in DAS28 of ≥1.2 points in RA/PsA patients or a reduction in BASDAI of ≥2.0 points in AS patients. Continued improvement defined as a reduction in DAS28 <1.2 points in RA/PsA patients or a reduction in BASDAI <2.0 points in AS patients. All responses were assessed in relation to the start of the study
cOne patient experienced two AEs
dDermatitis
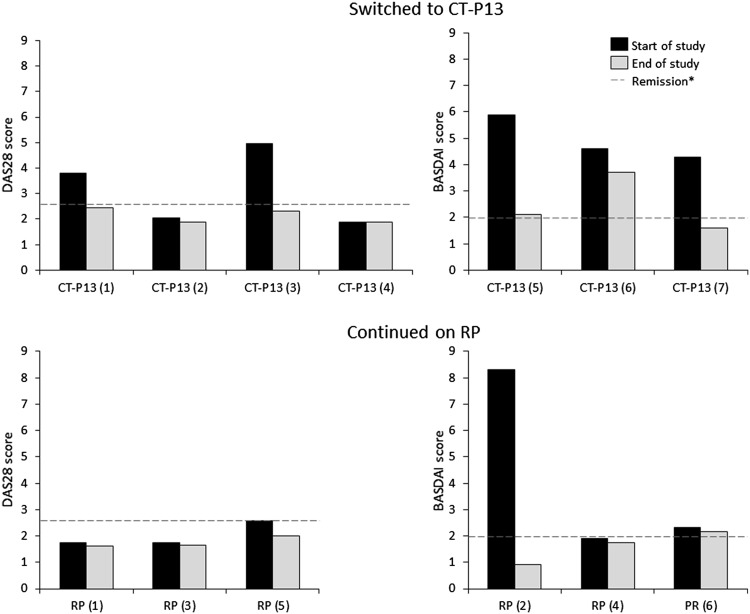
In the CT-P13 group, the two patients in remission at the start of the study maintained their clinical response, while an additional three patients moved into remission after switching from RP to CT-P13 (Table 1; Fig. 1). The remaining two patients in this group continued to show a response to treatment. In the RP group, five patients maintained their previous clinical response (four in remission) and one patient, who had previously received 80 cycles of RP and in whom the RP dose was not escalated, achieved remission by the end of follow-up. Adverse events were mainly infectious in nature and mild in severity, with the exception of one case of severe dermatitis in the CT-P13 group, which led to discontinuation of treatment (Table 1).
Fig. 1.
Clinical response after four cycles of treatment in patients who switched from infliximab (RP) to the biosimilar CT-P13 versus those who continued on RP. *Defined as DAS28 <2.6 in RA/PsA patients or BASDAI <2.0 in AS patients. AS ankylosing spondylitis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, DAS28 Disease Activity Score in 28 joints, PsA psoriatic arthritis, RA rheumatoid arthritis, RP reference product
Discussion
CT-P13 became the first monoclonal antibody biosimilar to receive marketing authorization from the European Medicines Agency (EMA) in 2013 and the US Food and Drug Administration (FDA) in 2016. Extrapolation to all eight indications of the RP (RA, AS, PsA, psoriasis, ulcerative colitis, pediatric ulcerative colitis, Crohn’s disease, and pediatric Crohn’s disease) was allowed based on two clinical trials of CT-P13 in patients with RA and AS, and other clinical and non-clinical evidence collected during the development of CT-P13 [4, 5]. The biosimilar has an identical primary amino acid sequence to the RP and an indistinguishable higher-order protein structure [15]. It is highly similar in terms of in vitro biological activity [4] and has demonstrated comparable efficacy and safety up to week 54 in both RA and AS patient populations [6–9]. In addition, trial extension studies have shown that CT-P13 is well-tolerated and effective for up to 2 years, and that switching from the RP to CT-P13 does not negatively affect the efficacy and safety of treatment [11, 12].
In this real-world study, patients switching from the RP to CT-P13 maintained or even improved their clinical response. CT-P13 was well-tolerated with adverse events similar to those previously reported [6–9, 11, 12]. There were no apparent negative effects on safety as a result of switching to CT-P13 in this study. These findings are consistent with the outcomes of prospective clinical studies that demonstrated that switching to CT-P13 after a year of treatment with the RP did not impact the efficacy or safety of treatment [11, 12]. Furthermore, recent results from the 52-week, multicenter, randomized, double-blind NOR-SWITCH trial have demonstrated that switching from infliximab RP to CT-P13 is not inferior to continued treatment with the RP [16]. Our findings, therefore, further support the validity of switching from the RP to this biosimilar.
There are limitations to the present study, including its non-randomized design, the small number of participants, and the short observational period. The low number of infliximab-treated RA patients at the study center also prevented representation of these patients in the RP group. However, reporting of the use of biosimilars in clinical practice as we have done here is important as it can provide additional confidence in biosimilars for both clinicians and payers. Such real-world evidence complements that obtained from clinical trials and verifies that what is observed in these trials also occurs during normal clinical usage. It is anticipated that the accumulation of additional switching data, from clinical trials and post-marketing studies and registries [17], will lead to increased confidence in switching from the RP to CT-P13 and widespread adoption of this practice.
Conclusions
Switching from the RP to CT-P13 did not affect the safety or effectiveness of treatment in patients with different inflammatory diseases in this study. These findings support other data that show that CT-P13 is a valid alternative to infliximab RP in patients requiring anti-TNF therapy. Replacing innovator biologics with more cost-effective biosimilars will likely expand access to these effective treatments and ensure that increased numbers of patients can benefit.
Acknowledgements
Medical writing support was provided by Sang Wook Yoon, PhD and Jina Kim (employees of CELLTRION Healthcare Co., Ltd, Incheon, Republic of Korea) and Alice Wareham, PhD (Aspire Scientific Ltd, Bollington, UK).
Compliance with Ethical Standards
Funding
Medical writing support was funded by CELLTRION Healthcare Co., Ltd. No other funding was provided for this study.
Conflicts of interest
CV-D, MSB, MCM, PLS, and JJA-S declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Data availability
The data generated or analyzed during this current study are available from the corresponding author on reasonable request.
References
- 1.Feldman SR. Inflammatory diseases: integrating biosimilars into clinical practice. Semin Arthritis Rheum. 2015;44:S16–S21. doi: 10.1016/j.semarthrit.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Putrik P, Ramiro S, Kvien TK, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73:198–206. doi: 10.1136/annrheumdis-2012-202603. [DOI] [PubMed] [Google Scholar]
- 3.Rinaudo-Gaujous M, Paul S, Tedesco ED, Genin C, Roblin X, Peyrin-Biroulet L. Review article: biosimilars are the next generation of drugs for liver and gastrointestinal diseases. Aliment Pharmacol Ther. 2013;38:914–924. doi: 10.1111/apt.12477. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency. Assessment report: Remsima. 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002576/WC500151486.pdf. Accessed 16 Feb 2017.
- 5.US Food and Drug Administration. Inflectra (infliximab-dyyb) prescribing information. 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125544s000lbl.pdf. Accessed 16 Feb 2017.
- 6.Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605–1612. doi: 10.1136/annrheumdis-2012-203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613–1620. doi: 10.1136/annrheumdis-2012-203090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25. doi: 10.1186/s13075-016-0930-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2016;18:82. doi: 10.1186/s13075-016-0981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Generics and Biosimilar Initiative Improving biosimilars uptake: experience gained in Madrid, Spain. GaBI J. 2016;5:89–91. doi: 10.5639/gabij.2016.0502.021. [DOI] [Google Scholar]
- 11.Park W, Yoo DH, Miranda P, et al. Efficacy and safety of switching from reference infliximab to CT-P13 compared with maintenance of CT-P13 in ankylosing spondylitis: 102-week data from the PLANETAS extension study. Ann Rheum Dis. 2016;76:346–354. doi: 10.1136/annrheumdis-2015-208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoo DH, Prodanovic N, Jaworski J, et al. Efficacy and safety of CT-P13 (biosimilar infliximab) in patients with rheumatoid arthritis: comparison between switching from reference infliximab to CT-P13 and continuing CT-P13 in the PLANETRA extension study. Ann Rheum Dis. 2016;76:355–363. doi: 10.1136/annrheumdis-2015-208786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–1850. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Braun J, Pham T, Sieper J, et al. International ASAS consensus statement for the use of anti-tumour necrosis factor agents in patients with ankylosing spondylitis. Ann Rheum Dis. 2003;62:817–824. doi: 10.1136/ard.62.9.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung SK, Lee KH, Jeon JW, et al. Physicochemical characterization of Remsima®. MAbs. 2014;6:1163–1177. doi: 10.4161/mabs.32221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goll G, Olsen I, Jørgensen K, et al. Biosimilar infliximab (CT-P13) is not inferior to originator infliximab: results from a 52-week randomized switch trial in Norway [abstract] Arthritis Rheumatol. 2016;68(suppl 10):19L. [Google Scholar]
- 17.Braun J, Kudrin A. Switching to biosimilar infliximab (CT-P13): evidence of clinical safety, effectiveness and impact on public health. Biologicals. 2016;44:257–266. doi: 10.1016/j.biologicals.2016.03.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated or analyzed during this current study are available from the corresponding author on reasonable request.