Abstract
The aim of this study was to evaluate organic acids as potential indicators of tofu freshness. To achieve this, relationships between organic acids concentrations and the growth of microorganisms in fresh tofu were investigated. The levels of microorganisms (total bacterial count) and organic acids (phytic acid, oxalic acid, citric acid, lactic acid, formic acid and acetic acid) were analyzed in tofu (packed and unpacked) every 3 days during 15 days of storage at different temperatures (4, 10, and 25 °C). Organic acids were analyzed by high performance liquid chromatography and microbial analysis was conducted by plate counting method. The levels of oxalic acid, citric acid and formic acid decreased significantly during the storage period, while the levels of lactic acid and acetic acid increased significantly when stored at 10 °C. The acetic, lactic and formic acids showed significant correlation to the levels of microorganisms in packed tofu, suggesting the use of these organic acids as potential freshness quality indicators of tofu. Current study demonstrated the effective way of predicting freshness of tofu by utilizing organic acid analysis, as opposed to traditional method relying on microbial count.
Keywords: Tofu, Soybean curd, Organic acids, Freshness indicator
Introduction
The soybean is one of the most common food ingredients in East Asian countries, due to its high protein content and special bioactive compounds. It has been known as a cholesterol-free source of high quality nutrients (De Oliveira and Perrone 2015), and having health-promoting properties including antioxidant, anticarcinogenic, and antiatherogenic properties (Carroll 1991; Jackson et al. 2002; Liu et al. 2005). As such, food products utilizing soybean as a main source of ingredients are also known to possess similar health-related properties including protective effects on cancer developments such as breast, intestine, liver, bladder, prostate, skin and stomach (Messina and Barnes 1991; Messina et al. 1994; Jackson et al. 2002). Tofu, also called soybean curd, is the most commonly known soybean-processed food item, made by coagulating hot soymilk, followed by molding and pressing the curds (He and Chen 2013). According to the processing method and subsequent textural properties, commercial tofu can be divided into three categories: dry, firm, and soft, and these tofu products can be sold either unpacked and/or packed (Liu and Chang 2004). Unpacked tofu are still distributed in traditional market in Korea, while the market share of unpacked tofu is decreasing every year. Recent form of distribution of tofu in many commercial markets in all around the world-from East Asia to western countries-, are distributed in refrigeration after packaging (packed tofu).
While the high nutritional benefits associated with tofu is widely known, it is perishable in nature due to its high protein and moisture content with a neutral pH (Lee et al. 2014). Traditionally, tofu is known to have relatively short shelf-life, and it is usually consumed within 2–3 days of purchasing from the market (Kim et al. 2004). Given that the freshly made tofu are distributed in traditional market in room temperature, tofu is more vulnerable to microbial safety. Therefore, keeping the quality and safety of tofu during the distribution in traditional market is a major issue (Lee et al. 2014). Considering that more than 40% of tofu is still distributed and sold through the traditional market, meaning that freshly made tofu are kept in room temperature without packaging, it is even crucial to keeping the quality and safety of tofu. Unfortunately, it is challenging for consumers to judge the freshness of tofu by sensorial information at the market (Kuswandi et al. 2011). Therefore, setting a scientific method to ensure the safety measure of tofu is in need. To this date, the only reliable and scientific method known in the field, is measuring the amount of microorganisms. However, these methods are time-consuming; hence inadequate for fast decision-making process.
There were several studies to develop the fast method to determine the freshness of tofu. These studies utilized chemical quality indicators including pHs (Shin et al. 2006), and volatile aromatic compounds during storage (Lee et al. 2014). Lee et al. (2014) in fact, reported the concept of “marker of freshness” in fresh tofu, by selecting three volatile aromatic compounds including hexanal, ethanol, and 1-hexanol, showing high impact on tofu quality. This concept of freshness marker in tofu was derived from previous studies on fish. Fish products were perishable, similar to tofu; hence many studies on quality markers for fish freshness were previously reported. For example, a previous study on cod fillets reported the use of volatile compounds in chilled cod (Gadus morhua) for quality indicators (Olafsdóttir et al. 2005). Their study utilized electronic nose for characterize volatile compounds in cod fillet and identify the quality indicator, that is, carbonyls (3-hydroxy-2-butanone, acetaldehyde, 2-butanone, 3-pentanone, and 6-methyl-5-heptene-2-one). As such, the use of quality indicator for checking the freshness of food product is widely conducted.
This study aimed at determining faster and simpler way of measuring freshness of tofu. Organic acids including lactic, acetic, citric, formic, phytic and oxalic acids are commonly found in tofu products as the storage time increases, and were associated with the deterioration of tofu (Foster et al. 1991; Nielsen and Richelieu 1999; Ogawa et al. 2000; Onyango et al. 2013). To this date, use of organic acids as potential use of freshness indicator in tofu has never been conducted. The objectives of this study include a comprehensive evaluation of organic acids as an indicator of tofu freshness by investigating the relationship between the growth of microorganisms and the concentrations of organic acids in fresh tofu.
Materials and methods
Materials
Two types of tofu, packed and unpacked, were purchased from a commercial market in Seoul, Korea. For unpacked tofu, it was purchased through the traditional street market in Korea, and packed tofu was purchased through the commercial super market located in Seoul, Korea. All tofu was purchased upon manufactured, therefore the purchase date of tofu in both packed and unpacked tofu was day 0 of manufacturing tofu. All testing on tofu was conducted on the day of purchase. For microbiological analysis, Petri film was used (3M, St. Paul, MN, USA). Organic acid standards, such as phytic acid, oxalic acid, citric acid, lactic acid, formic acid and acetic acid, were purchased from Aldrich Chemical Co. (Sigma-Aldrich Co., St. Louis, MO, USA). Water and methanol were used as solvents for high performance liquid chromatography (HPLC) analysis (J.T. Baker, Philipsburg, NJ, USA).
Sample preparation
The samples were prepared following the previous study (Lee et al. 2014). The packed and unpacked tofu were cut into pieces (5 cm × 5 cm × 3 cm) and were individually placed in the glass containers with 160 mL of purified water. This purified water was sterilized prior to use by autoclaving at 121 °C for 30 min. All processes for the sample preparation were performed in a clean bench. The tofu containers were divided into three groups according to the selected storage temperatures (4, 10 and 25 °C). Samples were stored in a refrigerator (R-B521+M, LG, Seoul, Korea) at 4 °C, and the others were stored in a thermo-hygrostat (TH-81, HYSC, Seoul, Korea) at 10 and 25 °C, respectively. Samples kept at each temperature were stored for 15 days, with the exception of tofu stored at 25 °C, which was only kept for 5 days. The relative humidity was maintained at 90% for all samples during the storage period.
Determination of organic acids using HPLC
Twenty grams of tofu sample was mashed using a mortar and pestle. The mashed sample was transferred to a 50 mL falcon tube (BD Biosciences, Franklin Lakes, NJ, USA). 10 mL water–methanol mixture (75:25 v/v) was added and homogenized with a vortex mixer (Scientific Industries, Bohemia, NY, USA) for 1 min. The homogenized mixture was centrifuged (Combi-514R, Hanil, Seoul, South Korea) with 3500 rpm/min for 30 min at 25 °C. The supernatant was filtered by a syringe filter (0.45 μm nylon syringe filter, Henke Sass Wolf, Tuttlingen, Germany) and the filtrates were collected. The sample solution was adjusted to exactly 10.0 mL in volume with a water–methanol mixture.
For the analysis of organic acids, HPLC coupled with UV detector (1200 series, Agilent, Santa Clara, CA, USA) was used. The Aminex 87 H column (300 × 7.8 mm, Bio-RAD, Hercules, CA, USA) was selected. Operational conditions were as follows: mobile phase, 0.1% phosphoric acid (w/v); flow rate, 0.5 mL/min; column temperature, 65 °C; detector wavelength, 215 nm; injection volume, 10 µL; and reference standards of organic acids included phytic acid, oxalic acid, citric acid, lactic acid, formic acid and acetic acid. A standard calibration curve for the six organic acids was prepared using various concentrations (5, 10, 20, 50 and 100 µg/mL). Stock solutions of all organic acids were diluted with HPLC grade water. The experiments were repeated three times. The analytical method of organic acids was validated in terms of recovery efficiency, linearity value, limit of detection (LOD) and limit of quantification (LOQ). The linearity of the method was calculated and expressed as the linearity value (R2). Recovery efficiencies for the six organic acids were completed. The water–methanol mixture (10 mL) was spiked with 10 µL of standard solution (5000 µg/mL). The testing samples were analyzed with HPLC according to the method described above. LOD and LOQ were respectively calculated from an equation of 3.3 σ/S and 10 σ/S, where σ is the standard deviation of response, and S is the slope of calibration curve.
Determination of total bacterial count
Microbial analysis was measured according to the standard methods of analysis listed in the Korean Food Standard Codex (MFDS 2012). Ten grams of tofu and 90 mL of buffered peptone water (BPW, Difco, Franklin Lakes, NJ, USA) were homogenized in a sterile stomacher bag (Labplas, Quebec, Canada) for one min using a stomacher (bag mixer model, Inter Science, St. Nom, France). The number of CFU (colony forming units) per 1-mL was determined by petri film (3M, St. Paul, MN, USA). The petri film was incubated (IL-11, Medline, Oxfordshire, UK) at 37 °C for 48 h and the total bacterial count was carried out. In addition, the pH values of surface and soaking water were determined using a pH meter (Mettler-Toledo, Greifensee, Switzerland).
Statistical analysis
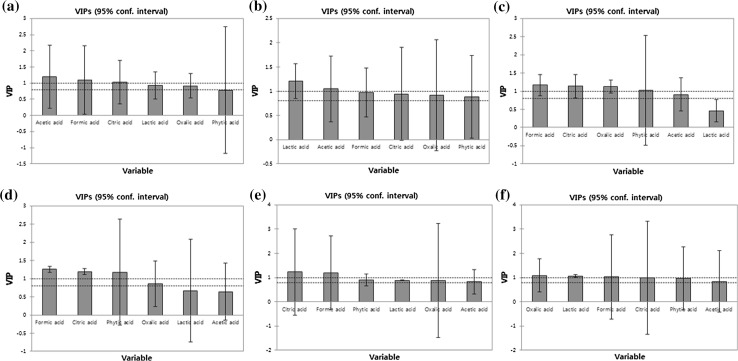
Results are expressed as mean ± standard deviation (SD), and the mean values of samples represent the triplicate measurements from each analysis. The analysis of variance (ANOVA) was followed by Duncan’s multiple range test performed at α = 0.05 level. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS; v.16.0, IBM, Chicago, IL, USA). To identify the relative importance of the indicators (organic acids) in evaluating the freshness of tofu, partial least square regression followed by variable importance in projection (PLSR-VIP) were carried out using XLSTAT (version 2012, Addinsoft Inc., Paris, France). The predictor variables were set as organic acids including phytic, oxalic, citric, lactic, formic and acetic acids, and the threshold for VIP chart was set at 0.8, which serves as a borderline for important indicator (organic acids) in evaluating the freshness of tofu in this study.
Results and discussion
Total bacterial count during storage of packed and unpacked tofu
Figure 1 shows the microbial population and pH levels in packed and unpacked tofu during the storage period at 4, 10 and 25 °C. The initial count of aerobic mesophilic microorganisms (total bacterial count) and pH showed different trends between packed tofu and unpacked tofu. Storage 4 and 10 °C led to end of incubation on day 15. During storage at 25 °C the incubation was complete on day five since 8.0 log CFU/g was reached on day two. No microorganisms in the packed tofu stored at 4 and 10 °C were detected until storage days nine and three, respectively. Then, their level reached 7.3 log CFU/g on day 15 for tofu stored at 4 °C, 8.9 log CFU/g on day 12 for stored at 10 °C, and 8.6 log CFU/g on day two for stored at 25 °C. As shown Fig. 1, storage temperature is one of the most important factors impacting the safe storage of tofu. Bacteria grow most rapidly in the range of temperatures between 5 and 57 °C. This is called the “temperature danger zone” and it was within that range that some bacteria can double in number in as little as 20 min (Almanza et al. 2007).
Fig. 1.
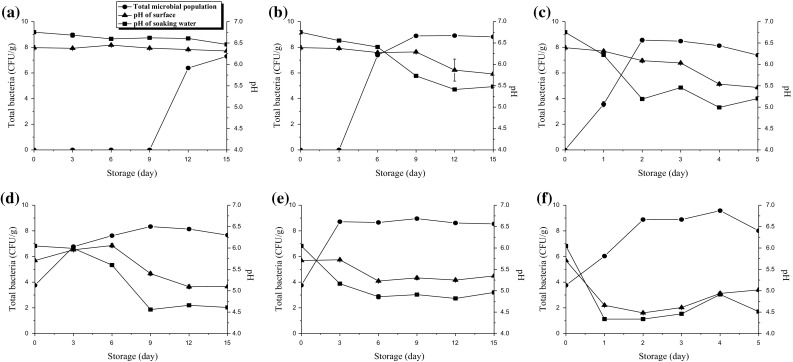
Relationship of microbial growth (total bacterial count) and pH in packed tofu during storage at 4 °C (a), 10 °C (b), 25 °C (c), unpacked tofu during storage at 4 °C (d), 10 °C (e), 25 °C (f)
The unpacked tofu had rapidly growing microbial flora regardless of storage temperature compared to the product in the packed tofu because it was exposed to air continuously. The initial CFUs of total bacteria in unpacked tofu were 3.8 log CFU/g, which was much higher than that of packed tofu. The number of total bacteria in unpacked tofu for all storage days ranged from 3.8 to 7.7 log CFU/g for stored at 4 °C, from 3.8 to 8.5 log CFU/g for stored at 10 °C, and 8.0 log CFU/g for stored at 25 °C. The maximum log CFU/g in unpacked tofu stored at 4, 10 and 25 °C were found on nine, 9 and 4 days of storage, respectively. Once the total bacteria increased to a certain level, the pH of packed tofu and unpacked tofu decreased significantly regardless of storage temperature (p < 0.05). The change in pH in the packed and unpacked tofu matches the formation and degradation of various organic acids by aerobic mesophilic microorganisms (Piard and Desmzeaud 1991; Hurrell 2004; Onyango et al. 2013). In Korea the maximum allowable microbial (aerobic mesophilic microorganisms) count for safe consumption of tofu was set at 6–7 log CFU/g (Shin et al. 2006). Besides the food safety concern, it was reported that the tofu a total bacteria count over 6.0 log CFU/g also received low consumer acceptability for sensorial perspectives such as taste (Shin et al. 2006). Although there is no specific regulation for monitoring general total bacteria, coli form should be <1 log CFU/g in tofu (Lee et al. 2014). In this study, the concentration of total bacteria of unpacked tofu reached the value of 6.0 log CFU/g after three storage days at all three temperatures. Therefore, we assumed that the unpacked tofu was deteriorated after three days of storage in the present study.
Validation of analytical method of organic acids using HPLC
Recovery efficiency, linearity value, limit of detection (LOD) and limit of quantification (LOQ) of six organic acids are shown in Table 1. All organic acids were eluted within 20 min and the recovery efficiency of organic acids was 63.3% for phytic acid, 101.9% for oxalic acid, 45.3% for citric acid, 100.1% for lactic acid, 94.9% for formic acid and 103.8% for acetic acid. The linearity value (R2) of a standard curve for quantitative analysis of each organic acid was within the international standard (Tašev et al. 2016), as it was 0.999 for phytic acid, 0.999 for oxalic acid, 0.999 for citric acid, 0.999 for lactic acid, 0.988 for formic acid and 0.999 for acetic acid. The LOD for organic acids were 0.05 mg/L for phytic acid, 2.25 mg/L for oxalic acid, 0.0006 mg/L for citric acid, 1.13 mg/L for lactic acid, 0.10 mg/L for formic acid, and 0.54 mg/L for acetic acid. The LOQ ranged from 0.0019 to 7.49 mg/L with citric acid being the lowest and oxalic acid being the highest. The LOQ for phytic acid was 0.17, 3.78 mg/L for lactic acid, 3.33 mg/L for formic acid, and 1.80 mg/L for acetic acid. The LOD and LOQ for lactic acid was reported as 13.6 and 44.9, 12.8 and 42.3 mg/L for citric acid in previous study utilizing HPLC followed by solid phase extraction for organic acid analysis in wine samples (Tašev et al. 2016). The method utilizing for analyzing organic acids in this study was validated and showed higher sensitivity than previous study.
Table 1.
Validation data for analysis of organic acids using HPLC
| Compound | Retention time (min) | Recovery efficiency (%) | R2 | LOD (mg/L)a | LOQ (mg/L)b |
|---|---|---|---|---|---|
| Phytic acid | 7.73 | 63.3 ± 8.9 | 0.999 | 0.05 | 0.17 |
| Oxalic acid | 8.52 | 101.9 ± 5.81 | 0.999 | 2.25 | 7.49 |
| Citric acid | 9.59 | 45.3 ± 3.87 | 0.999 | 0.0006 | 0.0019 |
| Lactic acid | 14.52 | 100.1 ± 1.99 | 0.999 | 1.13 | 3.78 |
| Formic acid | 15.96 | 94.9 ± 6.39 | 0.988 | 0.10 | 3.33 |
| Acetic acid | 17.42 | 103.8 ± 6.45 | 0.999 | 0.54 | 1.80 |
aLimit of detection
bLimit of quantification
Changes of organic acids profile during storage of tofu
The concentrations of organic acids in the tofu were analyzed by HPLC coupled with an ultraviolet (UV) detector to measure freshness and quality changes of the tofu. As shown in Tables 2 and 3, packaging and storage temperature influenced the organic acids profile differently. Levels of lactic acid in the packed tofu stored at 4, 10 and 25 °C increased significantly (p < 0.05). The concentration of lactic acid increased significantly from ND to 20.74 µg/g (4 °C), 123.89 µg/g (10 °C) after 15 days of storage, and 20.95 µg/g after 5 days of storage at 25 °C in packed tofu (p < 0.05). In the case of unpacked tofu, lactic and acetic acids were detected on the first day up to 67.95 and 71.59 µg/g. Lactic acid in the unpacked tofu stored at 10 °C increased steadily from 67.95 to 285.07 µg/g during 15 days of storage. Acetic acid in the unpacked tofu stored at 4 °C decreased after 15 days of storage from 71.59 to 24.67 µg/g, except that higher concentration was observed on day 3 with 115.12 µg/g (p < 0.05). The concentration of acetic acid increased significantly after 15 days of storage at 10 °C with 187.61 °C, and 5 days of storage at 25 °C with 168.99 µg/g, respectively (p < 0.05). Lactic and acetic acids are produced as by-products of glucose metabolism by lactic acid bacteria (Piard and Desmzeaud 1991), therefore the higher concentrations of these organic acids after 15 days of storage in tofu may indicate the potential bacterial growth.
Table 2.
The concentration (µg/g) of organic acids in packed tofu during storage at 4, 10 and 25 °C
| Storage temp. (°C) | Storage days | Phytic acid | Oxalic acid | Citric acid | Lactic acid | Formic acid | Acetic acid |
|---|---|---|---|---|---|---|---|
| Packed tofu | |||||||
| 4 | 0 | 17.25 ± 0.87a | 2480.86 ± 258.51a | 9.98 ± 0.85a | N.D. | 60.26 ± 3.46a | N.D. |
| 3 | 6.32 ± 0.52b | 973.18 ± 19.42b | 5.51 ± 0.06b | 30.00 ± 2.78ab | 44.74 ± 1.18b | 74.96 ± 1.24a | |
| 6 | 4.51 ± 0.12d | 740.45 ± 40.85c | 3.27 ± 0.12c | 23.45 ± 0.94bc | 34.68 ± 0.41c | 26.85 ± 2.86c | |
| 9 | 5.07 ± 0.07cd | 724.50 ± 9.93c | 3.18 ± 0.15c | 35.85 ± 1.01a | 34.09 ± 0.69c | 55.46 ± 2.77b | |
| 12 | 4.91 ± 1.02cd | 590.78 ± 111.87c | 2.63 ± 0.52cd | 16.99 ± 9.38c | 26.43 ± 4.77d | 19.73 ± 9.07c | |
| 15 | 6.00 ± 0.28bc | 569.51 ± 1.83c | 2.32 ± 0.13d | 20.74 ± 0.64c | 32.96 ± 0.46c | 11.90 ± 0.74d | |
| 10 | 0 | 17.25 ± 0.87a | 2480.86 ± 258.51a | 9.98 ± 0.85a | N.D. | 60.26 ± 3.46a | N.D. |
| 3 | 6.05 ± 0.49b | 679.76 ± 27.96b | 3.18 ± 0.08b | 17.71 ± 0.64d | 31.95 ± 1.95b | 12.73 ± 3.99e | |
| 6 | 3.97 ± 0.16c | 80.15 ± 3.65c | 1.53 ± 0.05c | 82.08 ± 0.37c | 13.96 ± 0.60e | 52.21 ± 15.2d | |
| 9 | 4.34 ± 0.02c | 99.06 ± 3.57c | 0.68 ± 0.02d | 148.47 ± 0.94a | 17.72 ± 0.80d | 135.69 ± 0.54b | |
| 12 | 5.35 ± 0.75b | 72.86 ± 6.37c | 0.61 ± 0.05d | 147.67 ± 17.27a | 20.93 ± 1.62d | 106.11 ± 8.49c | |
| 15 | 5.46 ± 0.34b | N.D. | 0.36 ± 0.02d | 123.89 ± 0.46b | 25.38 ± 2.09c | 214.83 ± 11.81a | |
| 25 | 0 | 17.25 ± 0.87a | 2480.86 ± 258.51a | 9.98 ± 0.85a | N.D. | 60.26 ± 3.46a | N.D. |
| 1 | 6.27 ± 0.12bc | 714.85 ± 28.67b | 3.00 ± 0.11b | 103.99 ± 2.56b | 35.13 ± 1.39b | 142.75 ± 8.21b | |
| 2 | 5.51 ± 0.96c | 387.38 ± 56.02c | 1.55 ± 0.18c | 331.91 ± 51.98a | 14.67 ± 3.95d | 76.79 ± 17.65c | |
| 3 | 7.17 ± 0.41b | 104.65 ± 79.87d | 0.09 ± 0.00d | 80.99 ± 5.21b | 23.82 ± 0.50c | 70.38 ± 10.56c | |
| 4 | 6.66 ± 0.29bc | 28.83 ± 5.83d | 0.37 ± 0.01d | 3.89 ± 0.11c | 26.68 ± 1.90c | 73.60 ± 2.93c | |
| 5 | 7.27 ± 0.76b | 12.38 ± 1.58d | 0.43 ± 0.19d | 20.95 ± 1.63c | 26.72 ± 1.02c | 171.18 ± 14.50a | |
Table 3.
The concentration (µg/g) of organic acids in unpacked tofu during storage at 4, 10 and 25 °C
| Storage temp. (°C) | Storage days | Phytic acid | Oxalic acid | Citric acid | Lactic acid | Formic acid | Acetic acid |
|---|---|---|---|---|---|---|---|
| Unpacked tofu | |||||||
| 4 | 0 | 4.67 ± 0.62a | 1188.04 ± 137.88a | 11.92 ± 0.71a | 67.95 ± 3.59d | 201.10 ± 46.04a | 71.59 ± 4.28c |
| 3 | 3.65 ± 0.17b | 1083.33 ± 13.39ab | 9.59 ± 0.04b | 92.29 ± 15.14c | 124.37 ± 6.25b | 115.12 ± 6.46a | |
| 6 | 2.28 ± 0.07d | 854.05 ± 9.09c | 5.51 ± 0.08c | 38.72 ± 1.06e | 94.24 ± 3.18b | 73.63 ± 1.25c | |
| 9 | 2.73 ± 0.07cd | 1075.21 ± 36.59b | 5.44 ± 0.15c | 153.87 ± 1.80a | 56.27 ± 4.48c | 91.95 ± 4.00b | |
| 12 | 3.02 ± 0.14c | 938.86 ± 21.55c | 4.41 ± 0.20d | 116.51 ± 3.48b | 42.16 ± 3.46c | 28.60 ± 2.93d | |
| 15 | 2.56 ± 0.12cd | 745.88 ± 30.50d | 4.13 ± 0.20d | 50.11 ± 2.51e | 47.52 ± 2.51c | 24.67 ± 0.58d | |
| 10 | 0 | 4.67 ± 0.62a | 1188.04 ± 137.88a | 11.92 ± 0.71a | 67.95 ± 3.59f | 201.10 ± 46.04a | 71.59 ± 4.28e |
| 3 | 2.99 ± 0.22b | 1090.67 ± 52.09a | 3.74 ± 0.21b | 117.25 ± 2.86e | 63.40 ± 2.48b | 89.29 ± 7.24d | |
| 6 | 4.22 ± 1.44a | 849.39 ± 10.86b | 1.76 ± 0.05c | 304.23 ± 10.48c | 50.72 ± 2.18bc | 193.16 ± 5.25ab | |
| 9 | 2.78 ± 0.05b | 912.22 ± 2.47b | 1.33 ± 0.02c | 422.82 ± 1.21b | 26.15 ± 0.08cd | 202.32 ± 4.24a | |
| 12 | 2.11 ± 0.21b | 530.42 ± 43.35c | 0.69 ± 0.05d | 280.67 ± 19.72d | 10.77 ± 1.37d | 104.84 ± 9.98c | |
| 15 | 4.32 ± 0.29a | 475.60 ± 15.30c | 1.29 ± 0.03c | 485.07 ± 17.86a | 13.00 ± 1.31d | 187.61 ± 3.35b | |
| 25 | 0 | 4.67 ± 0.62c | 1188.04 ± 137.88a | 11.92 ± 0.71a | 67.95 ± 3.59c | 201.10 ± 46.04a | 71.59 ± 4.28e |
| 1 | 6.35 ± 0.50b | 745.99 ± 30.31b | 1.60 ± 0.06bc | 546.66 ± 22.87b | 34.91 ± 1.08b | 264.80 ± 6.04a | |
| 2 | 8.40 ± 0.30a | 676.36 ± 86.67b | 1.22 ± 0.03c | 702.89 ± 20.15a | 12.57 ± 0.29b | 227.15 ± 2.35b | |
| 3 | 6.94 ± 0.86b | 44.02 ± 3.52c | 2.02 ± 0.23b | 594.13 ± 108.40ab | 15.46 ± 1.53b | 135.45 ± 22.04d | |
| 4 | 6.64 ± 0.21b | 18.85 ± 0.44c | 2.12 ± 0.10b | 640.52 ± 155.42ab | 17.78 ± 1.76b | 170.20 ± 7.81c | |
| 5 | 6.21 ± 0.21b | 13.32 ± 11.47c | 0.57 ± 0.01d | 536.73 ± 23.34b | 21.03 ± 0.19b | 168.99 ± 3.91c | |
Oxalic acid and citric acid in the packed tofu decreased significantly during storage days 4, 10 and 25 °C (p < 0.05). The initial concentration of oxalic acid in packed tofu was 2480.86 µg/g, and were measured as 569.51 µg/g after 15 days of storage at 4 °C, non-detectable after 15 days of storage at 10 °C, and 12.38 µg/g after 5 days of storage at 25 °C. Similarly, the concentration of phytic acid at day 0 was at 7.25 µg/g, and decreased significantly after 15 days of storage, with 6.00 µg/g at 4 °C, 5.46 µg/g at 10 °C and 7.27 µg/g after 5 days of storage at 25 °C (p < 0.05). The concentrations of formic acid also decreased over time, in that the concentration of formic acid at day 0 was at 60.26 µg/g, and then decreased to 32.96 µg/g after 15 days of storage at 4 °C, 25.38 µg/g after 15 days of storage at 10 °C, and 26.72 µg/g after 5 days of storage at 25 °C (p < 0.05). Overall, the concentrations of lactic and acetic acid increased significantly over storage time, regardless of storage temperature, while concentrations of other organic acids such as phytic, oxalic, citric and formic acids decreased significantly over time in packed tofu.
The initial concentration of phytic acid, citric acid, formic acid and oxalic acid in the unpacked tofu were significantly different from that of the packed tofu (p < 0.05). As shown Table 3, the levels of oxalic acid, citric acid and formic acid decreased significantly in the unpacked tofu regardless of storage temperature (p < 0.05). The concentration of phytic acid at day 0 was at 4.67 µg/g, and then decreased to 2.56 µg/g at 15 days of storage at 4 °C, and 4.32 µg/g at 10 °C, and 6.21 µg/g after 5 days of storage at 25 °C (p < 0.05). Relatively high concentrations of oxalic acid was detected in unpacked tofu, in comparison to other organic acids included. The concentrations of oxalic acid in unpacked tofu in day 0 was 1188.04, and were found at 745.88 µg/g and 475.60 µg/g after 15 days of storage at 4 and 10 °C, and 13.32 µg/g after 5 days of storage at 25 °C. During the storage of tofu, the microflora originated from the surface of soybean, may produce the microbial phytase enzyme, inducing the phytate hydrolysis, which in turn, may influence the decrease of phytic acids in tofu (Hurrell 2004; Onyango et al. 2013). Citric acid degradation cycle may during glucose metabolism by microorganisms typically produce diacetyl and acetone (Nielsen and Richelieu 1999), and this may be the cause of decrease in citric acid in tofu during storage. In addition, oxalic acid is reduced by decarboxylase enzymes of oxalate-degrading bacteria to form CO2 and formate (Foster 1991; Ogawa et al. 2000). This may have been the reason for the reduction of phytic acid, citric acid and oxalic acid.
To determine indicator sufficiency and relative importance of the indicators, a partial least square-variable importance in projection (PLS-VIP) analysis was carried out. Results from the analysis are shown in Fig. 2. The VIP chart consists of one bar chart per component (variable), and each VIP chart has a border line plotted to identify the VIPs that are greater than 0.8. These thresholds allow identification of the variables that are moderate (0.8 < VIP < 1) or highly influential (VIP > 1) (Mukherjee et al. 2013). The most important of the chosen indicators in the packed tofu stored at 4, 10 and 25 °C was acetic acid, lactic acid and formic acid, in that order, to determine the level of microorganisms, respectively. The commercial packed tofu in the market is refrigerated during storage and display at 4 °C (Khatib et al. 2002). Keeping tofu at the proper temperature in the refrigerator during normal operation (the doors of the refrigerator are not opened) is relatively straightforward. However, when the consumer opens the doors of the refrigerator, the task of maintaining refrigerated temperatures is not so simple. When the door of the refrigerator is opened, cold air rushes out and hot air moves into replace it. Thus, the actual temperatures of the tofu surface may range from 6.1–10.6 °C (Lee et al. 2008). For this reason, lactic acid is the most important of the chosen indicators for commercial packed tofu. In addition, lactic acid had the highest correlation (R2 = 0.96) with the concentration of microorganism among measured organic acids for the packed tofu.
Fig. 2.
VIPs from partial least square regression analysis (PLSR-VIPs) in packed tofu during storage at AT 4 °C (a), 10 °C (b), 25 °C (c), unpacked tofu during storage at 4 °C (d), 10 °C (e), 25 °C (f)
The indicator in the unpacked tofu stored at 4, 10 and 25 °C was formic acid, citric acid and oxalic acid, respectively. Since the unpacked tofu is exposed to the external environment at all times, it could be influenced by various vectors such as food handlers (in particular hands), insects and airborne microorganisms (Reij et al. 2004). For this reason, the initial CFUs of total bacteria in unpacked tofu were much higher than that found in the packed tofu. In addition, the contents of organic acid could be affected by types of microorganisms. Thus, it is difficult to select one organic acid to be a freshness indicator for unpacked tofu.
Conclusion
In the present study, the relationship between levels of microorganisms and the concentrations of organic acids in tofu was investigated. Three of the chosen indicators for packed tofu, stored at 4, 10 and 25 °C, were acetic acid, lactic acid and formic acid and they determined the level of microorganisms, respectively. The best indicators for the unpacked tofu, stored at 4, 10 and 25 °C, were formic acid, citric acid and oxalic acid, respectively. These results suggested that the most suitable indicator for the degree of freshness depend on storage and transport conditions as well as the packaging type of tofu. Thus, it is important to select the right organic acid as a freshness indicator according to the sales system of tofu. For the packed tofu, this study determined that lactic acid is the most suitable organic acid indicator.
Acknowledgements
This research was supported by R&D Convergence Center Support Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea. Further, this research was in part, supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1A2A2A01005772).
References
- Almanza BA, Namkung Y, Ismail JA, Nelson DC. Clients’ safe food-handling knowledge and risk behavior in a home-delivered meal program. J Am Diet Assoc. 2007;107:816–821. doi: 10.1016/j.jada.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Carroll K. Review of clinical studies on cholesterol-lowering response to soy protein. J Am Diet Assoc. 1991;91:820–827. [PubMed] [Google Scholar]
- De Oliveira Silva F, Perrone D. Characterization and stability of bioactive compounds from soybean meal. LWT Food Sci Technol. 2015;63:992–1000. doi: 10.1016/j.lwt.2015.04.032. [DOI] [Google Scholar]
- Foster JW. Salmonella acid shock proteins are required for the adaptive acid tolerance response. J Bacteriol. 1991;173:896–6902. doi: 10.1128/jb.173.2.896-899.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He FJ, Chen JQ. Consumption of soybean, soy foods, soy isoflavones and breast cancer incidence: differences between Chinese women and women in Western countries and possible mechanisms. Food Sci Hum Wellness. 2013;2:146–161. doi: 10.1016/j.fshw.2013.08.002. [DOI] [Google Scholar]
- Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitamese Nutr Res. 2004;74:45–452. doi: 10.1024/0300-9831.74.6.445. [DOI] [PubMed] [Google Scholar]
- Jackson CJC, Dini JP, Lavandier C, Rupasinghe HPV, Faulkner H, Poysa V, Buzzell D, De Grandis S. Effects of processing on the content and composition of isoflavones during manufacturing of soy beverage and tofu. Process Biochem. 2002;37:1117–1123. doi: 10.1016/S0032-9592(01)00323-5. [DOI] [Google Scholar]
- Khatib KA, Aramouni FM, Herald TJ, Boyer JE. Physicochemical characteristics of soft tofu formulated from selected soybean varieties. J Food Qual. 2002;25:289–303. doi: 10.1111/j.1745-4557.2002.tb01026.x. [DOI] [Google Scholar]
- Kim M, Son SI, Han JS. Evaluation of microbiological, physicochemical and sensory qualities of chitosan tofu during storage. J Food Qual. 2004;27:27–40. doi: 10.1111/j.1745-4557.2004.tb00635.x. [DOI] [Google Scholar]
- Kuswandi B, Wicaksono Y, Abdullah A, Heng LY, Ahmad M. Smart packaging: sensors for monitoring of food quality and safety. Sens Instrum Food Qual. 2011;5:137–146. doi: 10.1007/s11694-011-9120-x. [DOI] [Google Scholar]
- Lee YS, Ha JH, Park KH, Lee SY, Choi YJ, Lee DH, Park SH, Moon ES, Ryu K, Shin HS, Han SD. Survey on storage temperature of domestic major chilled foods in refrigerator. J Food Hyg Saf. 2008;23:304–308. [Google Scholar]
- Lee S, Cho H, Lee KG. Volatile compounds as markers of tofu (soybean curd) freshness during storage. J Agric Food Chem. 2014;62:772–779. doi: 10.1021/jf404847g. [DOI] [PubMed] [Google Scholar]
- Liu ZS, Chang SKC. Effect of soy milk characteristics and cooking conditions on coagulant requirement for making filled tofu. J Agric Food Chem. 2004;52:3405–3411. doi: 10.1021/jf035139i. [DOI] [PubMed] [Google Scholar]
- Liu J, Chang SK, Wienborn D, Esenborn D. Antioxidant properties of soybean isoflavone extract and tofu in vitro and in vivo. J Agric Food Chem. 2005;53:2333–2340. doi: 10.1021/jf048552e. [DOI] [PubMed] [Google Scholar]
- Messina M, Barnes S. The role of soybean products in reducing cancer risks. J Nat’l Cancer Inst’t. 1991;83:541–546. doi: 10.1093/jnci/83.8.541. [DOI] [PubMed] [Google Scholar]
- Messina M, Persky V, Setchell K, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- Ministry of Food and Drug Safety (MFDS) Korean Food Standard Codex (2012) http://fse.foodnara.go.kr/residue/mobile/menu_01_03.jsp?idx=317. Accessed on April 2016
- Mukherjee R, Sengupta D, Sikdar SK. Parsimonious use of indicators for evaluating sustainabilitysystems with multivariate statistical analyses. Clean Technol Environ Policy. 2013;15:699–706. doi: 10.1007/s10098-013-0614-6. [DOI] [Google Scholar]
- Nielsen JC, Richelieu M. Control of flavor development in wine during and after malolactic fermentation by Oenococcus oeni. Appl Environ Microbiol. 1999;65:740–745. doi: 10.1128/aem.65.2.740-745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Miyazato T, Hatano T. Oxalate and urinary stones. World J Surg. 2000;24:1154–1159. doi: 10.1007/s002680010193. [DOI] [PubMed] [Google Scholar]
- Olafsdóttir G, Jonsdottir R, Lauzon HL, Luten J, Kristbergsson K. Characterization of volatile compound sin chilled cod (Gadus morhua) fillets by gas chromatography and detection of quality indicators by an electronic nose. J Agric Food Chem. 2005;53:10140–10147. doi: 10.1021/jf0517804. [DOI] [PubMed] [Google Scholar]
- Onyango CA, Ochanda SO, Mwasaru MA, Ochieng JK, Mathooko FM, Kinyuru JN. Effects of malting and fermentation on anti-nutrient reduction and protein digestibility of red sorghum, white sorghum and pearl millet. J Food Res. 2013;2:41–49. doi: 10.5539/jfr.v2n1p41. [DOI] [Google Scholar]
- Piard JC, Desmzeaud M. Inhibiting factors produced by lactic acid bacteria. 1. Oxygen metabolites and catabolism end-products. Le Lait. 1991;71:525–541. doi: 10.1051/lait:1991541. [DOI] [Google Scholar]
- Reij MW, Den Antrekker ED, Ilsi Europe Risk Analysis in Microbiology Task Force Recontamination as a source of pathogens in processed foods. Int J Food Microbiol. 2004;91:1–11. doi: 10.1016/S0168-1605(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Shin HY, Ku KJ, Park SK, Song KB. Use of freshness indicator for determination of freshness and quality change of tofu during storage. J Korean Soc for Appl Biol Chem. 2006;49:158–162. [Google Scholar]
- Tašev K, Stefova M, Ivanova-Petropulos V. HPLC method valdation and application for organic acid analysis in wine after solid phase extraction. Maced J Chem Chem Eng. 2016;35(2):225–233. doi: 10.20450/mjcce.2016.1073. [DOI] [Google Scholar]