Abstract
Microbiological and chemical changes in shrimp Acetes vulgaris during production of Kapi (salted shrimp paste of Thailand) including salting, drying and fermentation were monitored. Moisture content of samples decreased rapidly after salting and drying steps. The lower water activity was found in the final product (0.694). The pH decreased within the first 10 days of fermentation and continuously increased as fermentation progressed. Protein underwent degradation throughout Kapi production as indicated by increasing TCA-soluble peptides and degree of hydrolysis. The increases in peroxide value as well as thiobarbituric acid reactive substances value revealed that lipid oxidation occurred throughout all processes. Total viable count, halophilic, proteolytic and lipolytic bacteria counts increased continuously during Kapi production, while lactic acid bacteria count slightly decreased at the final stage of fermentation. Thus, proteolysis and lipolysis took place throughout Kapi production, and contributed to the characteristics of finished product. These changes were governed by both endogenous and microbial enzymes.
Keywords: Salted shrimp paste, Kapi, Fermentation, Acetes vulgaris, Microbial population
Introduction
Kapi, a purplish gray or dark grayish brown salted shrimp paste of Thailand, has been widely used as a condiment in several Thai foods (Pongsetkul et al. 2014). Kapi is known with different names in each region, e.g. Terasi Udang in Indonesia, Belacan in Malaysia, Bagoong-alamang in the Philippines, Mam ruoc in Vietnam, etc. (Hajeb and Jinap 2015). Although those products were different in term of raw material, the ratio of salt used, fermentation process and time, they can provide a salty and umami taste to cuisines. Different shrimp are used for Kapi production in various regions. Stocks of krill (Mesopodopsis orientalis), a traditional raw material for Kapi production, have decreased by 3% per year since 1990 (Meland and Willassen 2007). Other raw materials for Kapi production have been researched. Acetes vulgaris is one of small shrimp found in the southern part of Thailand throughout the years (Pongsetkul et al. 2015). Our previous study revealed that Kapi prepared from A. vulgaris had comparable sensory properties to commercial products (Pongsetkul et al. 2015).
For Kapi production, there are 2 major ingredients including shrimp and salt. The ratio between shrimp and salt varies from 1:6 to 1:2 (w/w) (Hajeb and Jinap 2015). After salting overnight, salted shrimp is dried with sunlight, ground into a fine paste and fermented under anaerobic condition at ambient temperature (28–30 °C) up to 30 days or until the typical aroma is formed (Faithong and Benjakul 2012). Salting and drying are two ancient practices used for food preservation and yield a particular flavor (Rodrigo et al. 1998). During fermentation, microbiological and biochemical change occur. Proteolysis is an essential biochemical reaction occurring during the fermentation of Kapi. It is induced by endogenous proteases in shrimp as well as those produced by halophilic bacteria surviving under high salt condition (Peralta et al. 2008). Proteolysis affects both texture and flavor of fermented products by inducing the formation of low molecular weight compounds, e.g. peptides, amino acids, aldehydes, organic acids and amines (Mizutani et al. 1987). Lipolysis during fermentation is also important since free fatty acids (FFA) released undergo oxidation, responsible for aroma development (Lizaso et al. 1999). Oxidation products include aldehydes and ketones (Takeungwongtrakul and Benjakul 2013) and more likely contribute to the development of typical taste and flavor of fermented product (Lizaso et al. 1999). Furthermore, Amano (1962) revealed that growth of anaerobes in high salt environment contributes to the typical flavor of fermented products. Initial microflora, in both the raw materials and salt, have the influence on the numbers of microorganisms in final product, especially during the early stages of fermentation (Rodrigo et al. 1998). Microaerophilic or anaerobic conditions with high salt during Kapi fermentation could favor the proliferation of some bacteria, leading to different flavor or odor in the finished products. However, a little information concerning chemical and microbiological changes during Kapi processing/fermentation exists. Therefore, this study aimed to monitor the changes in chemical compositions and microbial populations of shrimp A. vulgaris during Kapi production.
Materials and methods
Sample collection and preservation
Shrimp (A. vulgaris) were caught off and collected from the Ko-yo area, Songkhla province, Thailand. After capture, shrimp were delivered to the Department of Food Technology, Prince of Songkla University, Hat Yai, Thailand, in ice using a shrimp/ice ratio of 1:2 (w/w) in a polystyrene container, within approximately 2 h.
Preparation of Kapi
Upon arrival, shrimp [R] were mixed with solar salt at the ratio of 5:1 (w/w). The mixture was placed in the basket, covered with a cheese cloth and allowed to salting overnight at room temperature (28–32 °C) [S]. Subsequently, salted shrimp were drained, mashed, followed by spreading out on fiberglass mats to dry with sunlight until the moisture content reached 35–40% [D]. After drying, samples were compacted into earthen jars and covered with plastic bag tightly (in the close system). The samples were allowed to ferment at room temperature (28–32 °C) for 1 month. During fermentation, the samples were taken at day 10 [F1], 20 [F2] and 30 [F3] of fermentation. All samples were subjected to determination of chemical composition and microbial load.
Changes in chemical compositions
Moisture content, water activity (Aw) and pH
Moisture content of all samples was measured as per AOAC method (2000) with the analytical No. of 35.1.13. Aw was determined by a water activity analyzer (Thermoconstanter, Novasina, Switzerland). The pH of samples was analyzed by a pH meter (Sartorius, Gottingen, Germany) following the method of Nirmal and Benjakul (2009).
Free amino acid composition
Free amino acid composition of samples was determined according to the method of Minh-Thuy et al. (2014). Free amino acids were firstly extracted using 6% (v/v) perchloric acid. The extracts were subsequently neutralized and filtrated as per the method of Minh-Thuy et al. (2014). The filtrate was used for amino acid analysis using an amino acid analysis system (Prominence; Shimadzu, Kyoto, Japan) equipped with a column (Shim-pack Amino-Li, 100 mm × 6.0 mm i.d.; column temperature, 39.0 °C; Shimadzu) and pre-column (Shim-pack ISC-30/S0504 Li, 150 mm × 4.0 mm i.d.; Shimadzu). Amino acids were detected using a fluorescence detector (RF-10AXL; Shimadzu). The content was reported in term of mg/g dry weight sample.
TCA-soluble peptide content
TCA-soluble peptide content of samples was determined according to the method of Pongsetkul et al. (2015). Ground sample (3 g) was homogenized with 27 mL of cold 5% TCA using a homogenizer at a speed of 11,000 rpm for 1 min. The homogenate was stored in ice for 30 min, followed by centrifugation at 5000 g for 20 min at 4 °C using a refrigerated centrifuge (Model RC-B Plus centrifuge Newtown, CT). Soluble oligopeptide content in the supernatant was measured according to the Lowry method (Lowry et al. 1951) and expressed as mmol tyrosine equivalent/g dry weight sample.
Degree of hydrolysis (DH)
DH of all samples was determined following the method of Benjakul and Morrissey (1997). DH was calculated and expressed as the percentage as follows:
where L is the amount of free amino group of sample and Lmax is the total free amino group after acid hydrolysis (6 M HCl at 100 °C for 24 h).
Peroxide value (PV)
PV was determined as described by Sae-leaw et al. (2013). Cumene hydroperoxide at a concentration range of 0.5–2 ppm was used for standard preparation. PV was calculated and expressed as mg hydroperoxide/kg dry sample.
Thiobarbituric acid reactive substances (TBARS)
TBARS value was examined following the method of Nirmal and Benjakul (2009). A standard curve was prepared using malondialdehyde bis (dimethyl acetal) (0–2 ppm). TBARS value was calculated and expressed as mg malondialdehyde (MDA)/kg dry sample.
Changes in microbial load
Total viable count
Total viable count was determined using a standard plate count agar containing 10% NaCl (pH 7.5) and the incubation was performed for 3–5 days at 30 °C (BAM 2001). Samples (25 g) were mixed with 225 mL of peptone water containing 10% (w/v) NaCl in a Stomacher 400 Lab Blender (Seward Ltd., Worthing, UK) at high speed for 3 min. Peptone water containing 10% (w/v) NaCl was used for sample dilution. The sample appropriately diluted in serial tenfold steps was used for analysis by the spread plate technique. Microbial load was reported as colony forming units/g dry sample (CFU/g dry sample).
Halophilic bacteria count
Halophilic bacteria content was measured using JCM media (Namwong et al. 2009). Diluted sample (0.1 mL) was applied on the surface of media, spread and incubated for 5–7 days at 30 °C.
Proteolytic bacteria count
Proteolytic bacteria count was determined using a standard plate count agar having 10% NaCl and 1% (w/v) sodium caseinate (pH 7.5) with the incubation for 3–5 days at 30 °C (Tanasupawat et al. 2011). Proteolytic bacteria, which showed clear zone around colonies on plate, were counted.
Lipolytic bacteria count
Lipolytic bacteria count was determined using a standard plate count agar including 10% NaCl and 1% (w/v) tributyrin (pH 7.5). The incubation for 3–5 days at 30 °C was performed (Chappe et al. 1994). Lipolytic bacteria showing clear zone around colonies on plate were counted.
Lactic acid bacteria (LAB) count
LAB count was determined using De Man, Rogosa, and Sharpe (MRS) agar containing 10% NaCl following the method of Tanasupawat et al. (2011). Aliquot of 0.1 ml of appropriately decimally diluted samples was introduced on MRS agar containing CaCO3 (1%) and 10% NaCl (pH 7.5) and incubated for 3–5 days at 30 °C.
Statistical analysis
All experiments were conducted in triplicate using three lots of samples. Statistical analysis was done by using one-way analysis of variance (ANOVA). Mean comparison was conducted using the Duncan’s multiple range test (Steel et al. 1980). Analysis was performed using the SPSS package (Version 10.0) (SPSS for windows, SPSS Inc., Chicago, IL, USA).
Result and discussion
Changes in chemical compositions of shrimp during Kapi production
Changes in moisture content, water activity (Aw) and pH
Moisture content of shrimp during Kapi production was monitored (Fig. 1a). Fresh shrimp had the moisture content of 83.53%. Moisture content of shrimp after being salted overnight was rapidly lowered to 66.03% (P < 0.05). During salting, salt was able to penetrate into the shrimp meat, whereas water was removed from shrimp via the osmotic pressure. This led to the decrease in moisture content (Chaijan 2011). During the first 10 h of fish flesh salting, water content decreased more rapidly during dry salting and the amount of exudate was related with lowered moisture content (Bellagha et al. 2007). Moisture content of salted shrimp sharply decreased after drying with the sunlight and low moisture content (32.43%) was obtained after this process. Moisture content of samples was slightly increased after the first 10 days of fermentation (P < 0.05). Partially dried sample more likely absorbed the water vapor from the environment to some degree. However, no difference was observed among samples as fermentation time was extended up to 30 days (P > 0.05). Moisture content directly affected the consistency of Kapi. In general, consistency of commercial Kapi was varied from soft and pasty to dry and hard, depending upon the processes used (Faithong et al. 2010). Moisture content was within the moisture range of commercial Kapi produced in Thailand (33.79–52.54%) (Pongsetkul et al. 2014). After 30 days of fermentation, our product had the moisture content of 35.07%. This was associated with texture, because it became slightly dry and hard. Moreover, the result also suggested that salting and drying step for Kapi production were the main steps to reduce the moisture content.
Fig. 1.
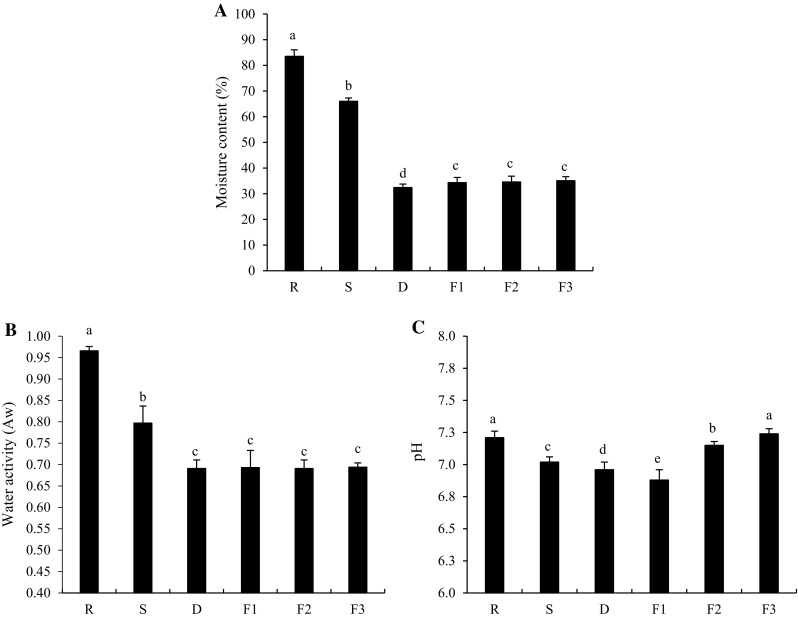
Changes in moisture content (a), water activity (Aw) (b) and pH (c) of shrimp during Kapi production. Bars represent the standard deviation (n = 3). R raw material, S after salting, D after drying, F1, F2 and F3 after fermentation for 10, 20 and 30 days. Different letters on the bars indicate the significant differences (P < 0.05)
Changes in water activity (Aw) of all samples exhibited the similar trends with those of moisture content throughout Kapi production processes as depicted in Fig. 1b. Decreases in Aw of shrimp during salting and drying were observed (P < 0.05) and no changes in Aw were obtained during 30 days of fermentation (P > 0.05). Aw of final product (after 30 days of fermentation) was 0.694, corresponding to low moisture content. Food having Aw range of 0.6–0.7 could be classified as an intermediate moisture food (Fennema 1996). Since Kapi samples had Aw in this range, the growth of food pathogens and spoilage microorganisms could be retarded.
Changes in the pH of shrimp throughout all processes were found. Fresh shrimp had the pH of 7.21. pH of sample was slightly dropped during salting (7.02) and drying (6.96) (P < 0.05) as shown in Fig. 1c. Continuous decrease in pH within the first 10 days of fermentation was observed (P < 0.05). Some microorganisms, particularly LAB, were able to produce some acids, which could result in pH lowering. These LAB produced lactic acid, leading to the decrease in pH of samples. However, pH turned to increase when fermentation time was more than 20 days (P < 0.05). This was related with the decrease in LAB. Furthermore, the increase of pH during fermentation was plausibly associated with the formation of degradation products or volatile base compounds such as ammonia produced by endogenous or microbial enzymes (Pongsetkul et al. 2015). After 30 days of fermentation, the pH of final product was 7.24.
Changes in free amino acid composition
Free amino acid composition of shrimp throughout all processes is given in Table 1. Total free amino acid (TFAA) of fresh shrimp was 44.75 mg/g dry sample. There was no change in TFAA when fresh shrimp were subjected to salting and drying (P > 0.05). However, TFAA was continuously increased during fermentation and reached 64.66 mg/g dry sample after the fermentation was performed for 30 days (P < 0.05). Change in total essential amino acid (TEAA) was fluctuated during Kapi production, but there were no differences between fresh shrimp and final product (P > 0.05). On the other hand, total non-essential amino acid (TNEAA) contents were different among samples (P < 0.05). The increases in TNEAA during all processes were in agreement with the increases in TFAA. TNEAA reached 49.17 mg/g dry sample after 30 days of fermentation.
Table 1.
Changes in free amino acid composition of shrimp during Kapi production
| Free amino acid composition (mg/g dry weight sample) | Sample | |||||
|---|---|---|---|---|---|---|
| R | S | D | F1 | F2 | F3 | |
| Essential amino acid | ||||||
| Histidine (His)a | 0.89 ± 0.01ab*,** | 0.93 ± 0.02a | 0.85 ± 0.01b | 0.54 ± 0.01c | 0.55 ± 0.07c | 0.59 ± 0.01c |
| Isoleucine (Ile) | 1.56 ± 0.03a | 1.30 ± 0.01b | 1.32 ± 0.01b | 1.21 ± 0.02c | 1.18 ± 0.01c | 1.18 ± 0.01c |
| Leucine (Leu) | 2.71 ± 0.01c | 2.81 ± 0.01c | 3.25 ± 0.01b | 3.99 ± 0.01a | 3.99 ± 0.03a | 4.05 ± 0.01a |
| Lysine (Lys) | 2.88 ± 0.03 cd | 3.01 ± 0.01c | 3.03 ± 0.01c | 3.95 ± 0.05b | 4.26 ± 0.02a | 4.38 ± 0.03a |
| Methionine (Met) | 0.72 ± 0.01b | 0.75 ± 0.02b | 0.72 ± 0.00b | 1.12 ± 0.01a | 1.09 ± 0.01a | 1.16 ± 0.01a |
| Phenylalanine (Phe) | 2.10 ± 0.06a | 2.09 ± 0.04a | 2.24 ± 0.01a | 2.05 ± 0.01a | 1.66 ± 0.01b | 1.43 ± 0.01b |
| Threonine (Thr) | 1.98 ± 0.02a | 1.72 ± 0.04a | 1.33 ± 0.01a | 0.05 ± 0.00b | 0.08 ± 0.01b | 0.03 ± 0.00b |
| Tryptophan (Trp) | ND | ND | ND | ND | 0.03 ± 0.00a | 0.03 ± 0.01a |
| Valine (Val) | 2.34 ± 0.01b | 2.32 ± 0.01b | 2.36 ± 0.04b | 2.55 ± 0.03a | 2.39 ± 0.02ab | 2.64 ± 0.03a |
| Total EAA | 15.18 ± 0.06a | 14.93 ± 0.04b | 15.10 ± 0.03b | 15.46 ± 0.05a | 15.23 ± 0.06a | 15.49 ± 0.03a |
| Non-essential amino acid | ||||||
| Alanine (Ala) | 3.57 ± 0.02c | 3.58 ± 0.04c | 4.1 ± 0.04b | 5.23 ± 0.06a | 5.62 ± 0.04a | 5.66 ± 0.02a |
| Anserine (Ans) | ND | ND | ND | ND | 0.03 ± 0.00b | 0.06 ± 0.00a |
| Arginine (Arg) | 5.16 ± 0.06a | 5.02 ± 0.03a | 4.55 ± 0.01b | 4.05 ± 0.01c | 4.05 ± 0.04c | 3.14 ± 0.01d |
| Aspartic acid/Asparagine (Asp/Asn) | 4.13 ± 0.04c | 4.55 ± 0.01c | 4.53 ± 0.04c | 6.69 ± 0.08b | 8.04 ± 0.09a | 8.51 ± 0.04a |
| Carnosine (Car) | ND | ND | ND | ND | ND | 0.03 ± 0.00 |
| Citrulline (Cit) | ND | ND | ND | ND | 0.07 ± 0.00a | 0.09 ± 0.01a |
| Cysteine (Cys) | ND | ND | 0.04 ± 0.01c | 0.06 ± 0.01b | 0.06 ± 0.00b | 0.12 ± 0.01a |
| Glycine (Gly) | 3.31 ± 0.02d | 3.54 ± 0.01c | 3.91 ± 0.05a | 3.55 ± 0.01c | 3.75 ± 0.03b | 3.66 ± 0.02bc |
| Glutamic acid/Glutamine (Glu/Gln) | 5.42 ± 0.10e | 5.35 ± 0.04e | 6.02 ± 0.04d | 9.11 ± 0.03c | 14.15 ± 0.07b | 18.18 ± 0.05a |
| Hydroxylysine (Hyl) | ND | ND | ND | ND | ND | 0.02 ± 0.00 |
| Hydroxyproline (Hyp) | ND | ND | ND | ND | ND | ND |
| Ornithine | ND | ND | ND | 1.04 ± 0.01b | 1.15 ± 0.01b | 2.02 ± 0.02a |
| Proline (Pro) | 3.48 ± 0.02c | 3.55 ± 0.01c | 3.28 ± 0.02c | 4.22 ± 0.01b | 4.59 ± 0.02ab | 5.01 ± 0.01a |
| Serine (Ser) | 2.94 ± 0.05a | 2.05 ± 0.02b | 2.15 ± 0.01b | 1.64 ± 0.02c | 0.92 ± 0.00d | 0.93 ± 0.01d |
| Taurine (Tau) | 0.02 ± 0.00c | 0.02 ± 0.00c | 0.02 ± 0.00c | 0.07 ± 0.01a | 0.05 ± 0.00b | 0.05 ± 0.00b |
| Tyrosine (Tyr) | 1.54 ± 0.01d | 1.88 ± 0.01b | 1.95 ± 0.01ab | 2.11 ± 0.01a | 1.82 ± 0.01b | 1.69 ± 0.03c |
| Total NEAA | 29.57 ± 0.08d | 29.54 ± 0.03d | 30.58 ± 0.04d | 37.77 ± 0.09c | 44.3 ± 0.09b | 49.17 ± 0.04a |
| Total free amino acid (TAA) | 44.75 ± 0.08d | 44.47 ± 0.06d | 45.68 ± 0.05d | 53.23 ± 0.09c | 59.53 ± 0.10b | 64.66 ± 0.05a |
*Essential amino acid in children
R raw material, S after salting, D after drying, F1, F2 and F3 after fermentation for 10, 20 and 30 days
*Mean ± SD (n=3)
**Different letters in the same row indicate the significant difference (P < 0.05)
Fresh shrimp A. vulgaris contained Glu/Gln, Arg, Asp/Asn, Ala, Pro and Gly as the major free amino acids and their total amount was more than 50% of total free amino acids. The result was in agreement with the other shrimp species including Acetes chinesis (Chung and Lee, 1976), Penaeus notialis (Akintola, 2015), Penaeus kerathurus (Zlatanos et al. 2009), etc. It was noted that changes in amount of each amino acid varied during different processes. As processing proceeded, some amino acids, especially Glu/Gln, Asp/Asn increased, while other amino acids, particularly Ile, Arg and Ser decreased. Some amino acids, which were not found in fresh sample, were generated during Kapi processing. Those included Trp, Ans, Car, Cit, Cys, etc. These changes in free amino acid during processing might be influenced by endogenous proteases in raw material and microbial proteases proliferated during processes. Our previous study revealed that proteolytic activity of fresh shrimp was decreased after being salted and dried, but continuously increased during fermentation up to 30 days (Pongsetkul et al. 2016). Those proteases more likely played a profound role in protein degradation, thus contributing to the liberation of free amino acids throughout Kapi production. The decrease or fluctuation of amino acids might be from the utilization of those amino acids by microorganisms for their growth.
Kapi fermented for 30 days contained Glu/Gln and Asp/Asn as the dominant free amino acids. The sharp increases in Glu/Gln and Asp/Asn were observed during fermentation and reached 18.18 and 8.51 mg/g dry sample, respectively (P < 0.05) at day 30 of fermentation. This result was in accordance with the high amount of Glu/Gln and Asp/Asn in belacan (Hajeb and Jinap 2015), terasi (Mizutani et al. 1987), seinsanga-pi (Tyn 1983) and bagoong-alamang (Peralta et al. 2008), shrimp pastes of Malaysia, Indonesia, Myanmar and the Philippines, respectively. In general, the taste and odor of salted shrimp paste were governed by amino acids (Hajeb and Jinap 2015). Some amino acids known as sweet compounds for fermented foods included Lys, Pro, Ala (Chung and Lee, 1976), Asp (Kim et al. 2005), Gly, Ser and Thr (Liu, 1989). Glu/Gln were associated with meaty or umami taste as reported by Chung and Lee (1976).
Based on the result, free amino acid compositions of final Kapi product was similar to those found in raw material used, inferring that different raw material with different amino acids more likely yielded Kapi with various tastes or flavors. However, those amino acids could be altered during processing, especially during fermentation.
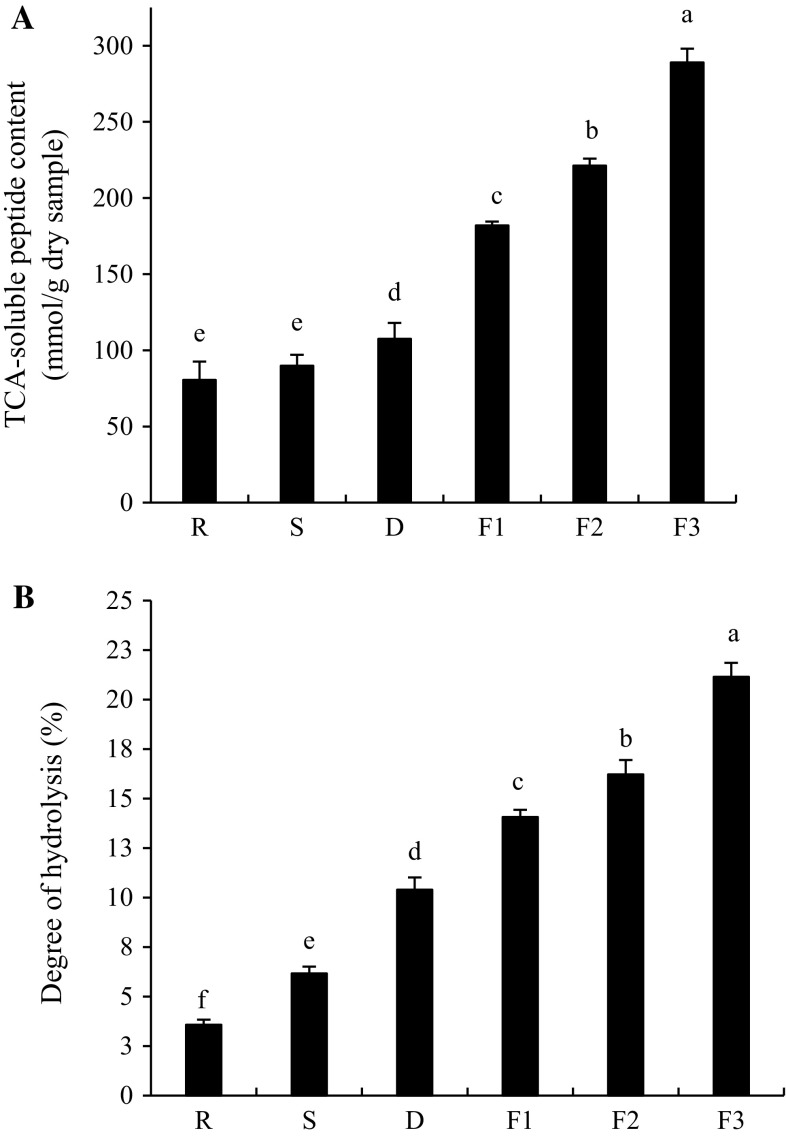
Changes in TCA-soluble peptide contents and degree of hydrolysis
As shown in Fig. 2a, fresh shrimp contained TCA-soluble peptides of 80.62 mmol/g dry weight sample. Pongsetkul et al. (2015) reported that fresh A. vulgaris contain some TCA-soluble peptides, which could be associated with rapid degradation after death, particularly during transportation. The initial level of TCA-soluble peptides might represented endogenous oligopeptides in sample and the degradation products generated during post-harvest handling (Benjakul and Morrissey 1997). The increase in the contents of TCA-soluble peptides during Kapi production suggested that the degradation mediated by either endogenous or microbial proteases in the presence of high salt still took place. Slight increase in TCA-soluble peptide contents was found after drying process, while the higher rate of increase was noticeable when dried shrimp were fermented at room temperature (P < 0.05). After 30 days of fermentation, TCA-soluble peptide content was 288.96 mmol/g dry weight sample, which was 3.58-fold higher than that obtained in raw material. This suggested the continuous proteolysis during fermentation. Increasing TCA-soluble peptides throughout Kapi production process was coincidental with the increase in degree of hydrolysis (DH). DH of Kapi samples increased from 3.81% in raw material to 21.12% after 30 days of fermentation as shown in Fig. 2b. Rapid increase in DH was also found during fermentation process. With increasing fermentation time, short chain peptides were more produced, affecting the characteristics, bioactivities as well as acceptability of Kapi.
Fig. 2.
Changes in TCA-soluble peptide content (a) and degree of hydrolysis (b) of shrimp during Kapi production. Bars represent the standard deviation (n = 3). R raw material, S after salting, D after drying, F1, F2 and F3 after fermentation for 10, 20 and 30 days. Different letters on the bars indicate the significant differences (P < 0.05)
Changes in lipid oxidation products
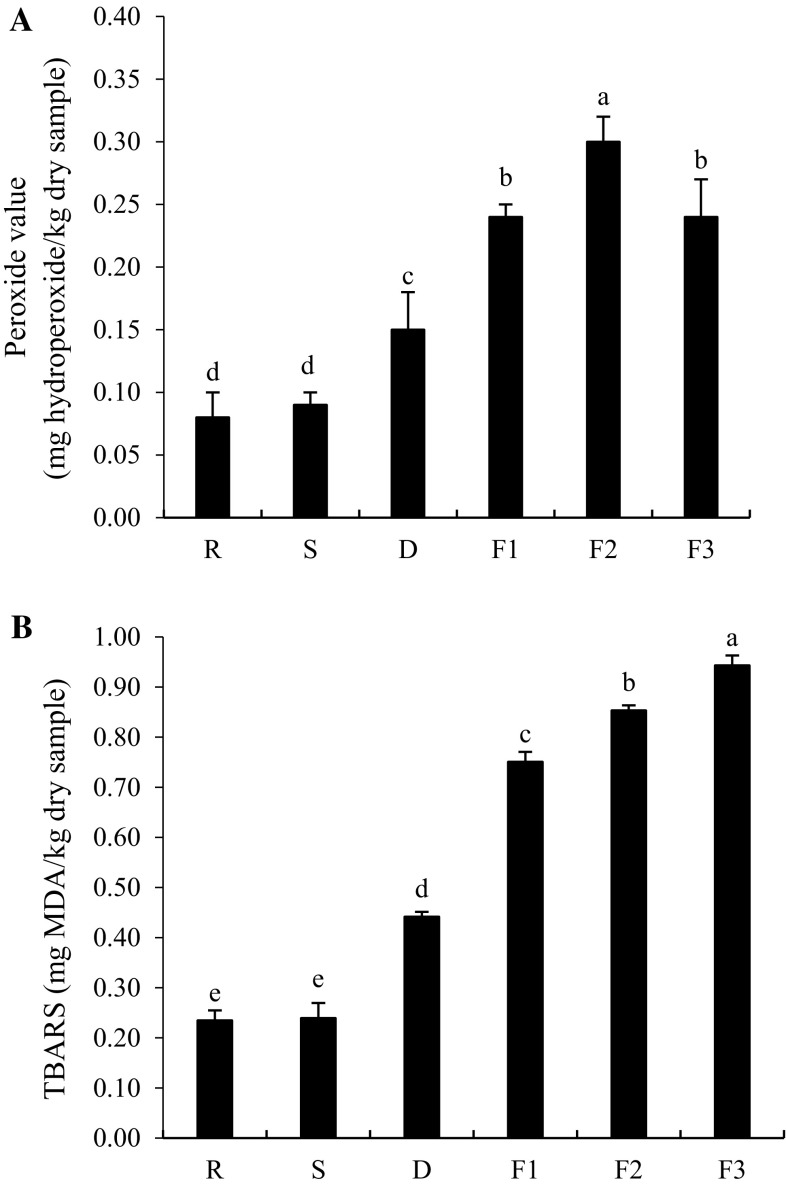
Lipid oxidation of shrimp during Kapi production expressed as PV and TBARS values was monitored (Fig. 3a, b). PV of fresh shrimp was 0.08 mg hydroperoxide/kg dry sample, suggesting that lipid oxidation occurred after harvest or during transportation in ice. There were no differences in PV between fresh sample and that after salting (P > 0.05). However, PV increased after drying with sunlight and continuously increased during fermentation up to 20 days. Subsequently, a decrease in PV was noticeable at day 30 of fermentation (P < 0.05). The increase in PV during processing and the first period of fermentation more likely resulted from the formation of hydroperoxide, a primary lipid oxidation products. Lipid hydroperoxides are formed by various pathways. Those include the reaction of singlet oxygen with unsaturated fatty acids or the lipoxygenase-catalyzed oxidation of PUFA (Sae-leaw et al. 2013). The decrease in PV after 30 days of fermentation was probably due to the decomposition of hydroperoxide to the secondary oxidation products. Shrimp oil was rich in polyunsaturated fatty acids (Takeungwongtrakul and Benjakul 2013). The result indicated that lipid oxidation occurred during Kapi production, probably owing to the high content of polyunsaturated fatty acids. Lipid oxidation products could affect odor and flavor characteristics of Kapi.
Fig. 3.
Changes in peroxide values (a) and TBARS values (b) of shrimp during Kapi production. Bars represent the standard deviation (n = 3). R raw material, S after salting, D after drying, F1, F2 and F3 after fermentation for 10, 20 and 30 days. Different letters on the bars indicate the significant differences (P < 0.05)
TBARS values of shrimp during Kapi production is shown in Fig. 3b. TBARS value of 0.23 mg MDA/kg dry sample was obtained in fresh shrimp before salting process, suggesting the presence of lipid oxidation products. No changes in TBARS value were noticeable after being salted (P > 0.05). Thereafter, a continuous increase in TBARS value during drying and fermentation was found (P < 0.05). TBARS value is the good index of concentration of relatively polar secondary reaction products, especially aldehydes, which are considered to be responsible for the off-flavors in meat (Cagdas and Kumcuoglu 2015). As fermentation time increased, lipid oxidation proceeded in sample as evidenced by the increases in TBARS value. At the end of process (30 days of fermentation), TBARS value was increased to 0.94 mg MDA/kg dry sample (fourfold higher than that of fresh sample). As fermentation time increased, shrimp matrix was loosened, caused by autolysis or microbial action. Thus, lipids bound with matrix were more released and exposed to oxidation, as indicated by the increases in both PV and TBARS values.
Changes in microbial populations of shrimp during Kapi production
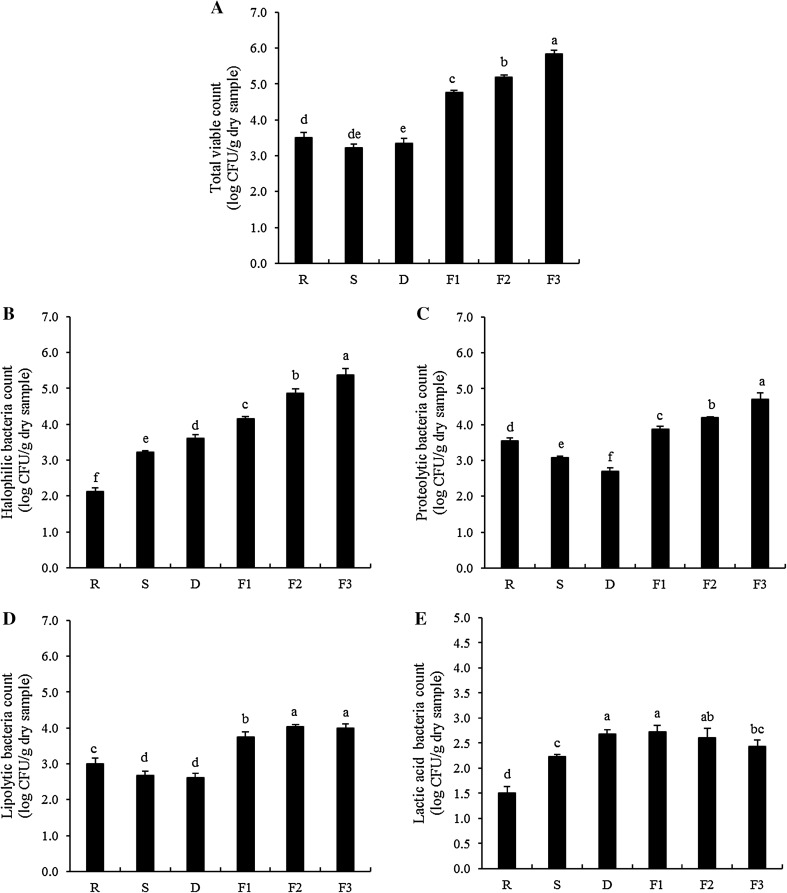
Initial total viable count (TVC) of shrimp used as raw material was 3.50 log CFU/g dry sample and slightly decreased after salting and drying process (P < 0.05) (Fig. 4a). Nevertheless, TVC continuously increased during fermentation at room temperature (P < 0.05) and reached 5.84 log CFU/g dry sample after 30 days of fermentation. Microorganisms found in fresh shrimp could be contaminated during handling or washing using seawater. In addition, sorting to remove foreign materials is commonly carried out, which is typically done by hand. This might introduce microorganisms to shrimp. The presence of microorganisms during fermentation contributes to the degradation of proteins and development of flavor and aroma (Majumdar et al. 2015). For the production of Kapi, salt plays a key role in preservation by inhibiting the growth of spoilage and pathogenic microorganisms. Phithakpol (1993) reported that putrefactive microorganisms are inhibited by salt at level higher than 6–8%. Salting and drying processes led to the lower moisture content and water activity (Aw) of shrimp as shown in Fig. 1a, b, respectively. These processing conditions could be both eradicate and select microorganisms. It was found that slight reduction of TVC after drying process was observed (P < 0.05). In contrast, TVC rapidly increased as fermentation increased (P < 0.05), especially within the first 10 days of fermentation. The selected strains might grow well under fermentation condition. The increase in microbial population was correlated well with the rapid increase in TCA-soluble peptides and DH (Fig. 2a, b) during fermentation. Latorre-Moratalla et al. (2011) revealed that free amino acids and other soluble non-nitrogenous substances derived from proteolysis could be considered as available nutrients for microorganisms. In general, the increase in TVC was in accordance with increasing free amino acids throughout the process (Table 1). Moreover, decomposition and oxidation of lipids during Kapi fermentation, as indicated by PV and TBARS (Fig. 3a, b), also occurred as induced by the increase in these microorganisms.
Fig. 4.
Changes in total viable count (a), halophilic bacteria (b), proteolytic bacteria (c), lipolytic bacteria (d) and lactic acid bacteria (e) of shrimp during Kapi production. Bars represent the standard deviation (n = 3). R raw material, S after salting, D after drying, F1, F2 and F3 after fermentation for 10, 20 and 30 days. Different letters on the bars indicate the significant differences (P < 0.05)
An increase in the number of halophilic or halo-tolerant bacteria was observed after salting and drying and also continuously increased during fermentation up to 30 days as shown in Fig. 4b. The high salt environment and microaerophilic or anaerobic conditions during Kapi processing and fermentation more likely resulted in the proliferation of halophilic bacteria. The increased halophilic bacteria count during Kapi production coincided well with the increasing TVC. This result suggested that dominant microorganisms in Kapi were halophilic bacteria. Chuon et al. (2014) revealed that halophilic and halotolerant bacteria genera, Staphylococcus and Tetragenococcus, were predominant microorganisms found in Kapi from Cambodia. However, Kobayashi et al. (2003) reported that Bacillus licheniformis and Bacillus sphaericus were found in Indonesian fermented shrimp (Terasi). Moreover, halophilic bacteria found in Kapi showing proteolytic activity included Oceanobacillus kapialis (Namwong et al. 2009), Lentibacillus kapialis (Pakdeeto et al. 2007) and Virgibacillus halodenitrificans (Tanasupawat et al. 2011), etc.
The increase in proteolytic bacteria count during fermentation was also observed in the present study (Fig. 4c). Furthermore, as shown in Fig. 4d, lipolytic bacteria count also increased during fermentation of Kapi. Halophilic bacteria might contain lipolytic enzymes. This resulted in the release of free fatty acids, which were susceptible to oxidation as indicated by the increases in PV and TBARS values (Fig. 3a, b). The initial proteolytic and lipolytic bacteria counts in fresh shrimp were 3.55 and 3.00 log CFU/g dry sample, respectively. Both types of bacteria showed similar changes throughout Kapi production. Both proteolytic and lipolytic bacteria counts of fresh shrimp were slightly decreased after drying process (P < 0.05). The presence of high salt as well as the lowered water activity after drying in this product could retard the growth of proteolytic or lipolytic microorganisms. However, both proteolytic and lipolytic bacteria counts increased during fermentation and reached the highest level at day 30 of fermentation (P < 0.05).
Figure 4e shows the changes in lactic acid bacteria (LAB) count of shrimp during Kapi production. The initial LAB count was quite low (about 1.50 log CFU/g dry sample). LAB count increased continuously until drying process was complete (P < 0.05). No further change in LAB count occurred during fermentation up to 20 days (P < 0.05). Conversely, slight decrease was found as fermentation was performed for 30 days (P < 0.05). In general, LAB species, especially belonging to Tetragenococcus, are known to be dominant microorganisms in many high-salt-containing fermented foods (Chuon et al. 2014). LAB plays a profound role in flavor of those products due to their fermentation of carbohydrates. The limited increase in LAB count after salting, drying as well as the first 10 days of fermentation was observed with the slight decrease in pH (Fig. 1c). No further growth of LAB was noticeable after 20 days of fermentation, while pH was slightly increased. It was suggested that LAB were not predominant or played an important role in Kapi fermentation with the extended fermentation time. Also, proteins in Kapi plausibly exhibited buffering capacity, thereby preventing the pH lowering. Furthermore, Kapi contained low amount of carbohydrate as reported by Pongsetkul et al. (2014) and Faithong et al. (2010). This could result in the reduction of LAB during fermentation because of insufficient substrates for their growth.
Overall, halophilic bacteria were the dominant microorganisms, which could grow and produce some proteolytic or lipolytic enzymes. These microbial enzymes, as well as endogenous enzymes play a profound role in degradation/decomposition of proteins and lipids in shrimp throughout the process. Those degradation products might contribute to the typical characteristics of final products, especially flavor or taste. As reported by Phithakpol (1993), the stronger aroma was obtained in Kapi with increasing fermentation time. Therefore, the development of flavor or taste of Kapi could be related with the increasing microbial load during Kapi fermentation.
Conclusion
Chemical compositions and microbial populations of shrimp underwent changes throughout the processes used for Kapi production. Salting and drying were mainly implemented to lower moisture content and Aw, in order to prolong the shelf-life of this product. Protein degradation and lipid oxidation occurred throughout Kapi production. For microorganisms, TVC increased continuously and halophilic bacteria were prevalent. Proteolytic and lipolytic bacteria were also increased throughout all processes. Therefore, proteolysis and lipolysis, mediated by both endogenous and microbial enzymes, were involved throughout the process and contributed to the characteristics of finished product. Isolation and identification of these bacteria isolated from Kapi production will be further studied. The use of the selected isolates as starter culture for production of Kapi with desired quality needs to be investigated.
Acknowledgements
Grant‐in‐Aid for dissertation from Graduate School, Prince of Songkla University, Thailand was acknowledged. Authors are grateful for the TRF Distinguished Research Professor Grant.
References
- Akintola SL. Effects of smoking and sun-drying on proximate, fatty and amino acids compositions of Southern pink shrimp (Penaeus notialis) Int J Food Sci Technol. 2015;52:2646–2656. doi: 10.1007/s13197-014-1303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K. Influence of the nutritive value of fish with special reference to fermented fish products of southeast Asia. In: Hee N, Kreuzer R, editors. Fish and nutrition. London: Fishing News; 1962. pp. 180–200. [Google Scholar]
- AOAC . Official methods of analysis. Washington: Association of Official Analytical Chemists; 2000. [Google Scholar]
- BAM . Aerobic plate count. In: Bryce J, editor. Bacteriological analytical manual. New York: U.S. Food and Drug Administration, E-con Publishing; 2001. pp. 53–67. [Google Scholar]
- Bellagha S, Sahli A, Farhat A, Kechaou N, Glenza A. Studies on salting and drying of sardine (Sardinella aurita): experimental kinetics and modeling. J Food Eng. 2007;78:947–952. doi: 10.1016/j.jfoodeng.2005.12.008. [DOI] [Google Scholar]
- Benjakul S, Morrissey MT. Protein hydrolysates from Pacific whiting solid wastes. J Agric Food Chem. 1997;45:3423–3430. doi: 10.1021/jf970294g. [DOI] [Google Scholar]
- Cagdas E, Kumcuoglu S. Effect of grape seed powder on oxidative stability of precooked chicken nuggets during frozen storage. J Food Sci Technol. 2015;52:2918–2925. doi: 10.1007/s13197-014-1333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaijan M. Physicochemical changes of tilapia (Oreochromis niloticus) muscle during salting. Food Chem. 2011;129:1201–1210. doi: 10.1016/j.foodchem.2011.05.110. [DOI] [PubMed] [Google Scholar]
- Chappe P, Mourey A, Kibertus G. Variataion of lipolytic activity in the genus Acinetobacter sp. J Gen Appl Microbiol. 1994;4:103–114. doi: 10.2323/jgam.40.103. [DOI] [Google Scholar]
- Chung SY, Lee EH. The taste compounds of fermented Acetes chinesis. B Korean Fish Soc. 1976;9:79–110. [Google Scholar]
- Chuon MR, Shiomoto M, Koyanagi T, Sasaki T, Michihata T, Chan S, Mao S, Enomoto T. Microbial and chemical properties of Cambodian traditional fermented fish products. J Sci Food Agric. 2014;94:1124–1131. doi: 10.1002/jsfa.6379. [DOI] [PubMed] [Google Scholar]
- Faithong N, Benjakul S. Changes in antioxidant activities and physicochemical properties of Kapi, a fermented shrimp paste, during fermentation. J Food Sci Technol. 2012;51:2463–2471. doi: 10.1007/s13197-012-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faithong N, Benjakul S, Phatcharat S, Binson W. Chemical composition and antioxidative activity of Thai traditional fermented shrimp and krill products. Food Chem. 2010;119:133–140. doi: 10.1016/j.foodchem.2009.06.056. [DOI] [Google Scholar]
- Fennema OR. Water and ice. In: Fennema OR, editor. Food chemistry. New York: Marcel Dekker; 1996. pp. 17–94. [Google Scholar]
- Hajeb P, Jinap S. Umami taste components and their sources in Asian foods. Crit Rev Food Sci Nutr. 2015;55:778–791. doi: 10.1080/10408398.2012.678422. [DOI] [PubMed] [Google Scholar]
- Kim JS, Shahidi F, Heu MS. Tenderization of meat by salt-fermented sauce from shrimp processing by-products. Food Chem. 2005;93:243–249. doi: 10.1016/j.foodchem.2004.09.022. [DOI] [Google Scholar]
- Kobayashi T, Kajiwara M, Wahyuni M, Kitakado T, Hamada SN, Imada C, Watanabe E. Isolation and characterization of halophilic lactic acid bacteria isolated from “terasi” shrimp paste: a traditional fermented seafood product in Indonesia. J Gen Appl Microbiol. 2003;49:279–286. doi: 10.2323/jgam.49.279. [DOI] [PubMed] [Google Scholar]
- Latorre-Moratalla ML, Bosch-Fuste L, Bover-Cid S, Aymerich T, Vidal-Carou MC. Contribution of enterococci to the volatile profile of slightly-fermented. LWT- Food Sci Technol. 2011;44:145–152. doi: 10.1016/j.lwt.2010.06.033. [DOI] [Google Scholar]
- Liu PZ. The umami of Yu-lu. Food Sci (Chinese) 1989;4:37–40. [Google Scholar]
- Lizaso G, Chasco J, Beriain MJ. Microbiological and biochemical changes during ripening of salchichon, a Spanish dry cured sausage. Food Microbiol. 1999;16:219–228. doi: 10.1006/fmic.1998.0238. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Majumdar RK, Bejjanki SK, Roy D, Shitole S, Saha A, Narayan B. Biochemical and microbial characterization of Ngari and Hentaak—traditional fermented fish products of India. J Food Sci Technol. 2015;52:8284–8291. doi: 10.1007/s13197-015-1978-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meland K, Willassen E. The disunity of “Mysidacea” (Crustacea) Mol Phylogenet Evol. 2007;44:1083–1104. doi: 10.1016/j.ympev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Minh-Thuy LT, Okazaki E, Osako K. Isolation and characterization of acid soluble collagen from the scales of marine fishes from Japan and Vietnam. Food Chem. 2014;149:264–270. doi: 10.1016/j.foodchem.2013.10.094. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Kimizuka A, Ruddle K, Ishige N. A chemical analysis of fermented fish products and discussion of fermented flavors in Asian cuisines. In: Atsushi N, editor. Bulletin of the national museum of ethnology. Japan: Osaka; 1987. pp. 810–864. [Google Scholar]
- Namwong S, Tanasupawat S, Lee KC, Lee JS. Oceanobacillus kapialis sp. nov., from fermented shrimp paste in Thailand. Int J Syst Evol Microbiol. 2009;59:2254–2259. doi: 10.1099/ijs.0.007161-0. [DOI] [PubMed] [Google Scholar]
- Nirmal NP, Benjakul S. Effect of ferulic acid on inhibition of polyphenoloxidase and quality changes of Pacific white shrimp (Litopenaeus vannamei) during iced storage. Food Chem. 2009;116:323–331. doi: 10.1016/j.foodchem.2009.02.054. [DOI] [PubMed] [Google Scholar]
- Pakdeeto A, Tanasupawat S, Thawai C, Moonmangmee S, Kudo T, Itho T. Lentibacillus kapialis sp. nov., from fermented shrimp paste in Thailand. Int J Syst Evol Microbiol. 2007;57:364–369. doi: 10.1099/ijs.0.64315-0. [DOI] [PubMed] [Google Scholar]
- Peralta EM, Hatate H, Kawabe D, Kuwahara R, Wakamatsu S, Yuki T, Murata H. Improving antioxidant activity and nutritional components of Philippine salt-fermented shrimp paste through prolonged fermentation. Food Chem. 2008;111:72–77. doi: 10.1016/j.foodchem.2008.03.042. [DOI] [Google Scholar]
- Phithakpol B. Fish fermentation technology in Thailand. In: Steinkraus KH, Reilly PJ, editors. Fish fermentation technology. Tokyo: United Nation University Press; 1993. pp. 155–166. [Google Scholar]
- Pongsetkul J, Benjakul S, Sumpavapol P, Kazufumi O, Faithong N. Chemical composition and physical properties of salted shrimp paste (Kapi) produced in Thailand. Int Aquat Res. 2014;6:155–166. doi: 10.1007/s40071-014-0076-4. [DOI] [Google Scholar]
- Pongsetkul J, Benjakul S, Sumpavapol P, Kazufumi O, Faithong N. Properties of salted shrimp paste (Kapi) from Acetes vulgaris as affected be post-mortem storage prior to salting. J Food Process Pres. 2015;40:636–646. doi: 10.1111/jfpp.12643. [DOI] [Google Scholar]
- Pongsetkul J, Benjakul S, Sumpavapol P, Kazufumi O, Faithong N. Characterization of endogenous protease and the changes in proteolytic activity of Acetes vulgaris and Macrobrachium lanchesteri during Kapi production. J Food Biochem. 2016 [Google Scholar]
- Rodrigo J, Ros G, Periago MJ, Lopez C, Ortuno J. Proximate and mineral composition of dried salted roes of hake (Merltcccircs merluccius, L.) and ling (Molva molva, L.) Food Chem. 1998;63:221–225. doi: 10.1016/S0308-8146(98)00002-8. [DOI] [Google Scholar]
- Sae-leaw T, Benjakul S, Gokoglu N, Nalinanon S. Changes in lipids and fishy odour development in skin from Nile tilapia (Oreochromis niloticus) stored in ice. Food Chem. 2013;141:2466–2472. doi: 10.1016/j.foodchem.2013.05.049. [DOI] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH, Dickey DA. General statistics. In: Steel RGD, editor. Principle and procedure of statistics. New York: McGraw-Hill; 1980. pp. 457–490. [Google Scholar]
- Takeungwongtrakul S, Benjakul S. Oxidative stability of shrimp oil-in-water emulsions as affected by antioxidant incorporation. Int Aquat Res. 2013;5:1–12. doi: 10.1186/2008-6970-5-14. [DOI] [Google Scholar]
- Tanasupawat S, Taprig T, Akaracharanya A, Visessanguan W. Characterization of Virgibacillus strain TKNR13-3 from fermented shrimp paste (ka-pi) and its protease production. Afr J Microbiol Res. 2011;5:4714–4721. [Google Scholar]
- Tyn MT. Recent development in nutrition and food technology in Burma. In: Chu S, editor. Burmese encyclopedia. Rangoon: Sarpaybeikhman Publishers; 1983. pp. 228–253. [Google Scholar]
- Zlatanos S, Laskaridis K, Sagredos A. Determination of proximate composition, fatty acid content and amino acid profile of five lesser common sea organisms from the Mediterranean Sea. Int J Food Sci Technol. 2009;44:1590–1594. doi: 10.1111/j.1365-2621.2008.01870.x. [DOI] [Google Scholar]