Abstract
The conditions for the solid–liquid extraction of the antioxidant polyphenol compounds from yellow passion fruit seeds were optimized by response surface methodology with the following variables as the extraction parameters: extraction time (12.8–147.2 min), ethanol concentration (13–97%), and temperature (16.4–83.6 °C). The polyphenol content and antioxidant capacity, which were assessed by the 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity, oxygen radical absorbance capacity, β-carotene bleaching assay, and ferric reducing antioxidant power assay, were considered dependent variables. The association of the dependent variables was effective for explaining the effect of the independent variables within a determination coefficient (R2) range of 0.88–0.96. A moderate-to-strong correlation for the polyphenol content and antioxidant capacity by the investigated methods was established, and optimized conditions were employed to maximize this response. Extraction was carried out at 80 °C using 70% ethanol concentration for 30 min, which was the most efficient condition to obtain an extract with high concentrations of polyphenolic compounds (3.12 g gallic acid equivalent/100 g seed dry basis) and a strong antioxidant capacity. The stilbene piceatannol was the major compound identified by liquid chromatography-electrospray ionization-tandem mass spectrometry (3.68 g/100 g seed dry basis). These results reinforce that agro-industrial waste demonstrates potential as a source of bioactive compounds, with implications in human health as well as in food and chemical industries.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2813-3) contains supplementary material, which is available to authorized users.
Keywords: Passiflora edulis Sims, Agro-industrial waste, Polyphenol extraction, Piceatannol, Response surface methodology
Introduction
Yellow passion fruit (Passiflora edulis Sims) is a tropical fruit, which has attracted considerable interest in international markets. Owing to its unique sensory characteristics, approximately half of the yellow passion fruit produced in Brazil is utilized for manufacturing juice (Brignani Neto 2002). Nearly 25% of the entire fruit is composed of pulp (Lopes et al. 2010), and the industrial juicing process generates a significant amount of by-products (seeds, aryl, and peel), which are typically considered as waste. To utilize the entire fruit, there is an increasing tendency to reuse agro-industrial waste to obtain new products and/or raw materials for industry.
In this context, the exploration of yellow passion fruits seeds (YPFS), albeit on a small scale, is an agro-industrial reality in Brazil. YPFS are rich in polyunsaturated fatty acids, mainly linoleic acid and other bioactive products, e.g. phytosterols, tocopherols, carotenoids, and polyphenols (Silva et al. 2015; Silva and Jorge 2014). Defatted flour obtained from oil extraction is used either in various industries, such as cosmetics (exfoliates) and food (ice cream and yogurts) or incorporated into animal feed. In addition, other components and antioxidant molecules in YPFS are of industrial importance (Jorge et al. 2009).
Bioactive compounds from fruits and vegetables have been attracting considerable attention because these compounds exert health benefits, such as protection against the development of chronic non-communicable diseases (Upadhyay and Dixit 2015), probably related to their high antioxidant content. Interestingly, fruits and vegetable by-products, compared to edible parts, contain higher concentrations of polyphenols and exert strong antioxidant capacities (Deng et al. 2012).
Bioactive compounds can be recovered by different extraction techniques, e.g., solvent extraction with or without ultrasonication or microwaves, the use of supercritical fluid or gas, and high-pressure techniques (Khoddami et al. 2013). Conventional solid–liquid solvent extraction is typically employed because it is simple and cost-effective.
Among the factors that possibly contribute to the high extraction efficiency, solvent, extraction time, and temperature are the commonly studied factors. It is crucial to consider that the role of each factor in extraction is not always evident (Sarkis et al. 2014) and that each food matrix interacts in a different manner with the solvent (Al Farsi and Lee 2008). Hence, it is imperative to optimize the extraction parameters for minimizing the production costs and maximizing product yields.
Response surface methodology (RSM) is a statistical modeling technique utilized for the optimization of complex processes, which has been extensively employed as it permits more efficient data arrangement and result interpretation. RSM is advantageous as it reduces the number of experimental trials to evaluate an outcome using different combinations of levels between independent variables and their interactions (Rodrigues and Iemma 2005).
Thus, this study aims to optimize the conditions for the extraction of the total polyphenolic compounds from YPFS.
Materials and methods
Raw material
YPFS from juice processing industries were provided by Extrair-Óleos Naturais (Rio de Janeiro, Brazil). Seeds were sanitized, lyophilized and milled for storage in polyethylene containers at −20 °C until further analysis.
Chemical characterization of raw material
The recommended methods from the Association of Official Analytical Chemists (AOAC 1995) were employed to determine the proximate composition, and the results were expressed as g/100 g of sample on dry basis (db). Inductively coupled plasma optical emission spectrometry (ICP-OES, iCAP 6500 Duo ICP model, Thermo Fisher Scientific, Cambridge, UK) was employed to analyze the mineral content. ICP-OES was operated under the following conditions: plasma gas, auxiliary flow, and nebulizer of 15, 1.50, and 0.68 L/min, respectively. Results were expressed as mg per 100 g of sample (db). The fatty acid profile was determined by the extraction and conversion of fatty acids into their corresponding fatty acid methyl esters (FAMEs) by acid hydrolysis according to method 996.06 (AOAC 2002). The FAMEs peaks were identified by comparing the retention times with those obtained from the standard mixture of FAMEs (C4–C24, Sigma Chemical Co, St. Louis, MO, USA) and the chromatograms viewed using the Ce 1h-05 methods (AOAC 1995). Results were expressed as g/100 g (%) of oil.
Extraction procedure
The antioxidant bioactive compounds were extracted according to the procedure proposed by Swain and Hillis (1959) using ethanol–water solutions, temperature, and time maintained constant as selected in the experimental design. The solid/liquid ratio was fixed at 1/10 (w/v) for all experiments, and extraction was carried out in a thermostatic bath under constant stirring. After the extraction was completed, the solution was subjected to centrifugation at 700 rpm for 15 min, and the supernatant was filtered under vacuum through a Whatman filter paper no. 1 and allowed to swell in a 10 mL flask with the extraction solution. The extraction yield was gravimetrically determined and expressed in g/100 g of sample (db).
Total polyphenol content
The total phenol content (TPC) was determined using the Folin–Ciocalteu’s reagent according to the method reported by Swain and Hillis (1959). The TPC was expressed as g of gallic acid equivalents (GAE)/100 g seed (db).
Total flavonoid content
The total flavonoid content (TFC) was estimated according to the method proposed by Zhishen et al. (1999), and the TFC was expressed as g of catechin equivalents (CAE) per 100 g of the seed (db).
Antioxidant activity assays
The β-carotene bleaching assay (BCB) was carried out according to the method reported by Moreira and Mancini-Filho (2003). The initial and final absorbance (Abs) was measured at 470 nm, and the results were expressed as the percentage of protection against oxidation.
The ferric reducing/antioxidant power (FRAP) assay was employed to evaluate the reductive capacity of the extracts according to the method reported by Oyaizu (1986), with minor modifications. A standard calibration curve of l-ascorbic acid (0.2–1.25 µg/µL) was prepared for calculating results.
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity was evaluated according to the method proposed by Brand-Williams et al. (1995).
The oxygen radical absorbance capacity (ORAC) assay was conducted on an automated microplate reader (Synergy HT, BIOTEK®, Vermont, USA) with 2,2′-azobios(2-amidinopropane) dihydrochloride (AAPH) used as the peroxyl radical generator, Trolox as the standard, and fluorescein as the fluorescent probe (Prior et al. 2003). Final ORAC values were calculated using the net area under the fluorescence decay curves.
In the optimization step, a single concentration of the extract was tested for antioxidant capacity, and the results were expressed as % scavenging, µg of acid ascorbic equivalent (AAE)/80 µg of extract db, % protection against oxidation, and μM of Trolox equivalent (TE)/80 µg of extract db for the DPPH, FRAP, BCB, and ORAC assays, respectively.
For the characterization of the optimized extract, the half maximal effective concentration (EC50) values were calculated to express the results obtained by DPPH (the concentration of extract required to scavenge 50% of the DPPH radical) and BCB (the concentration of extract required to prevent 50% of the total oxidation) assays. For ORAC and FRAP assays, the results were expressed as μmol of TE/g of the dry extract and µg of AAE/g of the dry extract, respectively.
HPLC–DAD and LC–ESI–MS/MS analysis of piceatannol
First, 10 mL of the extract was purified by solid-phase extraction in a column (Hypersep C18, Thermo Scientific) preconditioned with methanol (Arabbi et al. 2004).
Quantification was carried out by HPLC chromatography (Hewlett Packard 1100) equipped with an autosampler and a quaternary pump coupled to a diode array detector. Separation was performed on a C18 column (Luna, Phenomenex, Torrance, CA, USA). The mobile phase was composed of solvent A (98% water, 2% tetrahydrofuran, 0.1% trifluoroacetic acid) and solvent B (acetonitrile), and the following gradient was applied: 10% B (10 min), 15% B (2 min), 25% B (2 min), 80% B (3 min), and 10% B (3 min) at a flow rate of 1 mL/min at 25 °C. The runs were monitored at 270 and 370 nm. Quantification was performed using calibration curves of standard piceatannol (Sigma Chemicals Co., St. Louis, MO, USA), and the results were expressed as g of piceatannol/100 g of sample db.
Identification was conducted using a liquid chromatography (LC) apparatus (CBM 20A, Shimadzu, Japan) linked to an ion-trap mass spectrophotometer (Bruker Daltonics, Amazon speed, ETD, Germany) and an electrospray ionization interface (ESI). The separation conditions were the same as those employed for quantification (Arabbi et al. 2004). The mass detector was programmed to perform a full scan between m/z 100 and 1000, operated in the positive and negative ionization modes (4500 V). The piceatannol identification was carried out by comparing the mass spectrum and retention times obtained by the standard.
Experimental design and statistical analysis
The extraction conditions were optimized by RSM. A central composite design with three factors (23) was carried out, including eight experiments at factorial points (combination of −1 and +1 levels), six experiments at axial points (combination of levels −α and +α), and four experiments at the center point (0), totaling 18 tests. Temperature, time, and ethanol concentration were the independent variables. Table 1 summarizes the established levels, coded, and real values for each variable.
Table 1.
Independent variable levels in a complete 23 factorial experimental design for the optimization of extraction conditions
| Independent variables | Unit | Code | Level of coding variables | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |||
| Ethanol concentration | % v/v | X1 | 13 | 30 | 55 | 80 | 97 |
| Temperature | °C | X2 | 16.4 | 30 | 50 | 70 | 83.6 |
| Time | min | X3 | 12.8 | 40 | 80 | 120 | 147.2 |
The total content of extracted dry matter (EDM), TPC, and antioxidant capacity, determined by DPPH, FRAP, BCB, and ORAC, were used as dependent variables.
The factorial composite design generated mathematical models, which were adjusted to second-order polynomial equations. For each response variable, the predicted response function (Y) was divided into the quadratic and linear components of interaction, as described in the following equation:
where β0 is the constant, βi is the linear coefficient, βii is the quadratic quotient, βij is the coefficient of variable interaction of i and j, and Xi and Xj are independent variables.
The adequacy of the quadratic model generated by the interaction of independent variables was determined by assessing the lack of fit, Fisher test (F value), analysis of variance, and t test of the coefficients relating to its standard errors. The statistical significance of the model was determined at the 5% probability level (α = 0.05).
Response surface graphs were obtained using the predicted values for the adjusted models. To confirm the prediction of the mathematical model for all dependent variables, experiments were performed using the optimized parameters via RSM. The measurements were performed in triplicate, and the results were expressed as mean ± standard deviation. The Pearson correlation was used to assess the degree of association between variable responses.
All analyses were carried out in triplicate using Statistica 7.0 (Statsoft Inc., Tulsa, OK, USA).
Results and discussion
Chemical composition of YPFS
Carbohydrates and polyunsaturated fatty acids are the major components of the YPFS, with linoleic acid being its main type (Table 2). Our results are comparable to similar findings reported (67.4–77.2%) by other authors (Leão et al. 2014). Owing to the high concentrations of lipids and types of fatty acids, YPFS are commercially explored for the extraction of oil, which can be applied in the cosmetic, chemical, and pharmaceutical industries (Malacrida and Jorge 2012; Lopes et al. 2010).
Table 2.
Chemical characterization of Passiflora edulis Sims seeds
| Proximate composition (g/100 g seeds db) | |
| Moisture | 7.37 ± 0.57 |
| Lipids | 28.87 ± 0.29 |
| Ash | 1.69 ± 0.04 |
| Protein | 16.28 ± 0.29 |
| Carbohydrates | 45.80 ± 1.07 |
| Fatty acid profile (g/100 g oil) | |
| Myristic acid (C:14) | 0.10 ± 0.00 |
| Palmitic acid (C:16) | 11.00 ± 0.17 |
| Palmitoleic acid (C16:1) | 0.22 ± 0.01 |
| Stearic acid (C18:0) | 3.29 ± 0.31 |
| Oleic acid (C18:1, n-9) | 16.84 ± 0.36 |
| Vaccenic acid (C18:1, n-11) | 0.17 ± 0.00 |
| Linoleic acid (C18:2, n-6) | 67.39 ± 0.54 |
| α-Linolenic acid (C18:3, n-3) | 0.56 ± 0.03 |
| γ-Linolenic acid (C18:3) | 0.18 ± 0.02 |
| Docosanoic acid (C22:0) | 0.10 ± 0.00 |
| Unidentified | 0.20 ± 0.00 |
| Total saturated | 14.69 ± 0.12 |
| Total monounsaturated | 17.18 ± 0.47 |
| Total polyunsaturated | 68.12 ± 0.58 |
| Mineral profile (mg/100 g seeds db) | |
| Calcium (Ca) | 27.46 ± 2.66 |
| Iron (Fe) | 7.27 ± 0.27 |
| Barium (Ba) | 0.17 ± 0.01 |
| Cadmium (Cd) | 0.004 ± 0.00 |
| Chromium (Cr) | 0.28 ± 0.02 |
| Copper (Cu) | 0.89 ± 0.04 |
| Lithium (Li) | 0.25 ± 0.01 |
| Manganese (Mn) | 1.16 ± 0.02 |
| Molybdenum (Mo) | 0.03 ± 0.00 |
| Phosphorus (P) | 240.05 ± 7.78 |
| Lead (Pb) | 0.01 ± 0.00 |
| Vanadium (V) | 0.60 ± 0.01 |
| Zinc (Zn) | 3.72 ± 0.13 |
Data expressed as mean ± standard deviation (n = 3), n-3: omega 3, n-6: omega 6, n-9: omega 9, n-11: omega 11
With respect to the mineral content, YPFS comprise significant concentrations of phosphorus, calcium, and iron. Hence, YPFS can significantly contribute to the recommended daily intake (Institute of Medicine 2001) of these minerals. The daily consumption of 10 g of dried seed flour by an adult man (19–70 years) equates to 9.1, 9.9, and 80% of Fe, Co, and Cr, respectively.
Extraction optimization
Statistical methods, such as factorial design associated with RSM, have been extensively used as a tool to optimize the extraction of various chemical compounds in food matrices, with a high antioxidant capacity (Lai et al. 2014). The experimental and predicted results obtained for the extraction of YPFS according to the 23 factorial designs can be found in the supplementary material.
The experimentally determined values for the variables were analyzed by multiple regression to fit a second-order polynomial equation. The quality of this fit was verified by the coefficient of determination (R2) (Table 3). The obtained experimental data reveal a good fit for equations that are statistically acceptable at the 95% significance level (p < 0.05). This result indicated that the observed and predicted responses are in good agreement and that equations can adequately predict the experimental results. The use of predictive models permits the calculation of the theoretical optimal conditions, under which maximum values can be achieved.
Table 3.
Polynomial equations and statistical parameters describing the effect of independent variables
| Response variable | Regression equation (second-order polynomial) | R2 | p value |
|---|---|---|---|
| EDM | 0.86 | <0.001 | |
| TPC | 0.96 | <0.001 | |
| DPPH | 0.88 | <0.001 | |
| BCB | 0.94 | <0.001 | |
| FRAP | 0.94 | <0.001 | |
| ORAC | 0.93 | <0.001 |
R2: coefficient of determination
EDM extracted dry matter, TPC total polyphenol content, DPPH 1,1-diphenyl-2-picryl-hydrazyl, FRAP ferric reducing antioxidant power, BCB β-carotene bleaching assay, ORAC oxygen radical absorbance capacity
Models are significant (p < 0.001) for all dependent variables analyzed with coefficients ranging between 0.86 and 0.96. These coefficients are positive, implying that the closer the maximum design level of each independent variable, the greater the observed values for the response variables.
The maximum and minimum values used for the independent variables were determined after conducting experimental pre-tests by applying the one-factor-at-a-time methodology according to the study reported by Liyana-Pathirana and Shahidi (2005). The effect of the three investigated independent variables on the total polyphenol content gave a regression model with good significance level (p < 0.01) and a high R2 of 0.96). In addition, the first-order linear effect for variables X1 and X2, the second-order quadratic effect for variables X1 and X2, and the interaction for X1X2 and X2X3 were significant (p < 0.001).
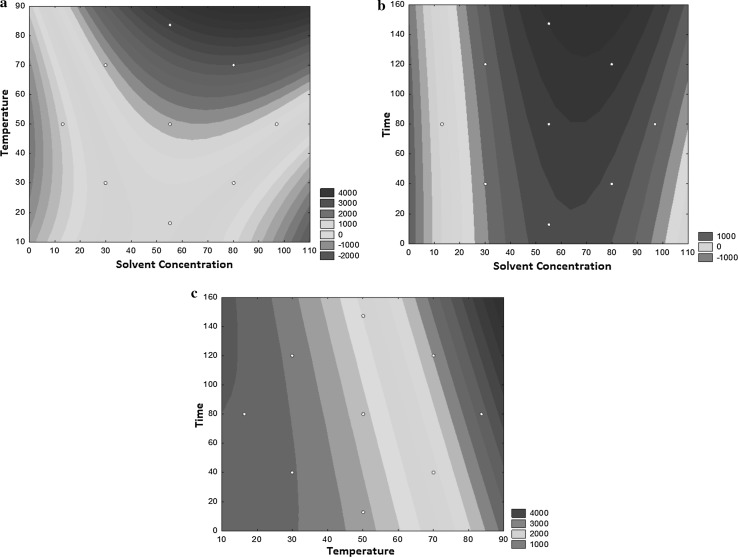
According to the results shown in the graph generated by RMS (Fig. 1a), ethanol concentrations between 40 and 90% are more efficient at temperatures greater than 70 °C. In this range of ethanol concentration, regardless of the amount of time employed for extraction, the optimal TPC is observed. However, this value increases after extraction for 30 min.
Fig. 1.
Response surface showing the combined effects of a temperature and solvent concentration, b time and solvent concentration, and c time and temperature on the total phenolic compounds in the extracts of the yellow passion fruit (Passiflora edulis Sims) seed
At temperatures greater than 70 °C, associated with extraction times greater than 80 min, a high extraction of TPC is observed (Fig. 1c). In combination with temperature, time exerts a greater effect on extraction.
Prasad et al. (2011) have postulated that solvent mixtures are more efficient for the extraction of polyphenols as this class of compounds includes several components with different polarities; therefore, a single solvent may not be effective for complete extraction. Notably, ethanol is used as it is a “generally recognized as a safe” solvent; therefore, it can be effectively used for applications in the food industry.
The use of high temperatures for extraction is considered contradictory. Some authors have reported that high temperatures might degrade the polyphenols that are mobilized at high temperatures; therefore, the antioxidant capacity of the final extract is possibly decreased. However, the opposite was observed for agro-industrial residues and by-products (Lai et al. 2014; Vázquez et al. 2012).
Increasing the temperature may benefit the extraction of phenols by reducing viscosity, thereby increasing the diffusion coefficient and solubility of phenols (Cacace and Mazza 2003). In addition, Al Farsi and Lee (2008) have suggested that heating could also lyse the cell wall of plants and facilitate the removal of conjugated phenolic compounds by the solvent.
Time is a crucial variable for the extraction of bioactive compounds as it regulates the equilibrium concentration during extraction (Spigno et al. 2007). Therefore, additional time used for extraction did not necessarily equate to higher efficiency. In this study, time did not affect the extraction of TPC. However, when combined with temperature, this parameter can be used for process optimization.
The contribution of phenolic compounds to the antioxidant activity of the extracts was demonstrated by the relationship between the TPC and antioxidant activity of each method, considering all 18 experiments. Generally, the phenolic compound content and antioxidant activity exhibit significant moderate-to-strong correlation (p < 0.05). TPC also exhibits moderate correlation with EDM (Table 4).
Table 4.
Pearson’s correlation coefficient between the total phenolic compounds, extracted dry matter, and antioxidant capacity
| ORAC | FRAP | EDM | TPC | DPPH | |
|---|---|---|---|---|---|
| FRAP | r = 0.964 (p < 0.01) | ||||
| EDM | r = 0.247 (p = 0.071) | r = 0.285 (p < 0.05) | |||
| TPC | r = 0.802 (p < 0.01) | r = 0.818 (p < 0.01) | r = 0.704 (p < 0.01) | ||
| DPPH | r = 0.782 (p < 0.01) | r = 0.836 (p < 0.01) | r = 0.055 (p = 0.691) | r = 0.563 (p < 0.01) | |
| BCB | r = 0.955 (p < 0.01) | r = 0.950 (p < 0.01) | r = 0.088 (p = 0.525) | r = 0.699 (p < 0.01) | R = 0.856 (p < 0.01) |
Correlation data (p value) = r(p)
EDM extracted dry matter, TPC total polyphenol content, DPPH 1,1-diphenyl-2-picryl-hydrazyl, FRAP ferric reducing antioxidant power, BCB β-carotene bleaching assay, ORAC oxygen radical absorbance capacity
This result is of importance as it permits the simplification of the extraction and the subsequent choice of the independent variables using only one response variable. It is of practical and biological interest to optimize the extraction of a substance with high polyphenolic compound concentrations without, however, compromising on the related antioxidant activity.
The correlation between the polyphenol content and different antioxidant activity assays is in agreement with the results obtained from similar studies using fruit pulp and by-products (Souza et al. 2012; Almeida et al. 2011).
It is important to emphasize that food matrices are complex, and the antioxidant activity quantified herein is the result of synergistic or antagonistic interactions between different chemical components. Babbar et al. (2011) have suggested that the other constituents, e.g., ascorbates, reducing carbohydrates, tocopherols, carotenoids, terpenes, and pigments, as well as the synergistic effect between these components, can contribute to antioxidant activity. With respect to the crude extracts, these claims should be carefully considered.
Finally, utilizing the results obtained from the response surface analysis, the combination of a temperature of 80 °C, an ethanol concentration of 70%, and an extraction time of 30 min is considered as the optimal experimental condition. To confirm the model’s predictive power, an experiment was carried out with the obtained optimized conditions to simultaneously maximize the polyphenols and antioxidant capacity. Because of the low absolute error values obtained by the comparison between the observed and predicted values for the variable TPC response, the proposed model could be used to predict the response value.
The analysis of response surfaces obtained for the dependent variable TPC permitted the selection of the best extraction conditions for obtaining YPFS extracts with high yield and antioxidant activity, as well as high concentration of phenolic compounds. This methodology has proven to be a useful tool to optimize experimental conditions (Liyana-Pathirana and Shahidi 2005).
Antioxidant capacity and the major component of the optimized extract
The amount of EDM utilizing the optimized experimental conditions (Table 5) was approximately 4.5 times greater (1.26 g/100 g seed) than that obtained by Jorge et al. (2009) for seeds of P. edulis (yellow passion fruit) subjected to extraction at room temperature with 95:5 ethanol:water. The value obtained by these authors for TPC (42.93 mg GAE/g extract) was also less than the quantified value for the optimized extract (549.61 mg GAE/g extract) obtained in this study. de Oliveira et al. (2009) have determined the concentration of phenolic compounds in methanol extracts from passion fruit waste (pulp, skin, and seeds) and found 41.2 mg GAE/g of the dry extract.
Table 5.
Extracted dry matter, total polyphenol and flavonoid compounds, and antioxidant capacity of the optimized extract
| Parameters | Optimized extract |
|---|---|
| Extracted dry matter (g/100 g seed db) | 5.67 ± 0.17 |
| Total polyphenols (g GAE/100 g seed db) | 3.11 ± 0.07 |
| Piceatannol (g/100 g seed db) | 3.68 ± 0.09 |
| Total flavonoids (g CAE/100 g seed db) | 1.03 ± 0.02 |
| DPPH EC50 (µg/mL) | 26.96 ± 0.34 |
| BCB EC50 (mg/mL) | 1.26 ± 0.06 |
| FRAP (μg EAA/g dry extract) | 3.6 ± 0.29 |
| ORAC (μmol TE/g dry extract) | 6.2 ± 0.53 (103) |
Data expressed as medium ± standard deviation (n = 3)
FRAP ferric reducing antioxidant power, BCB β-carotene bleaching assay, ORAC oxygen radical absorbance capacity
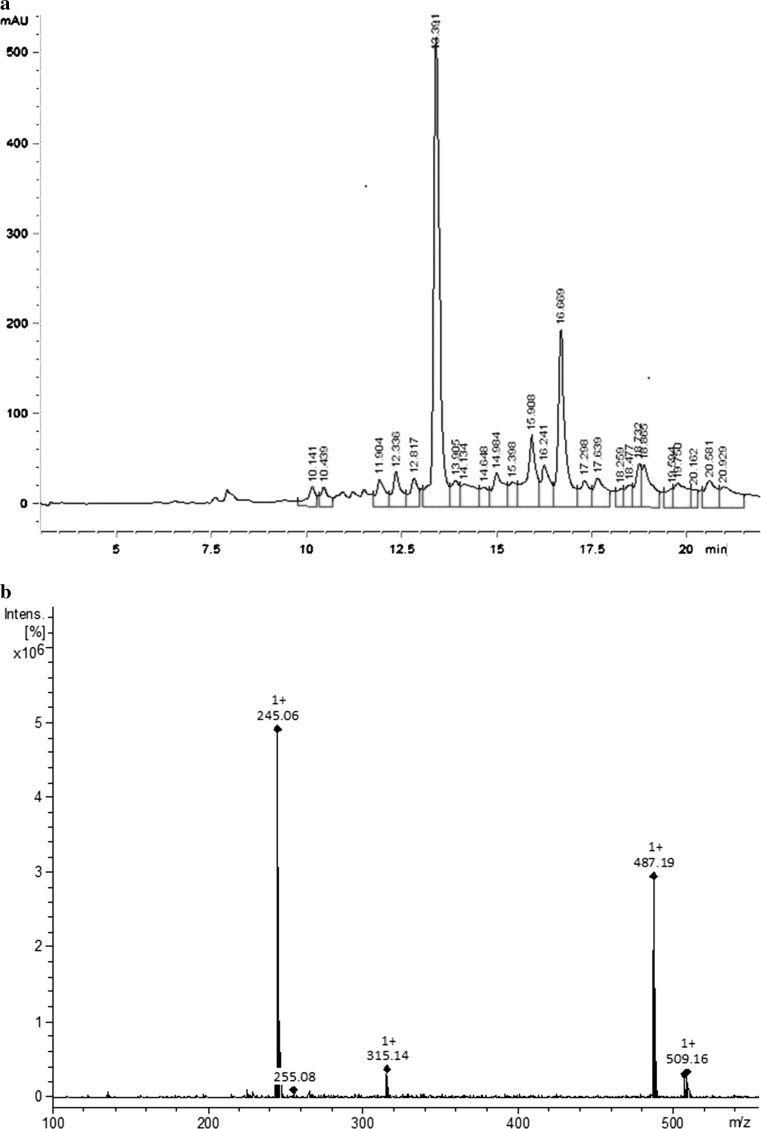
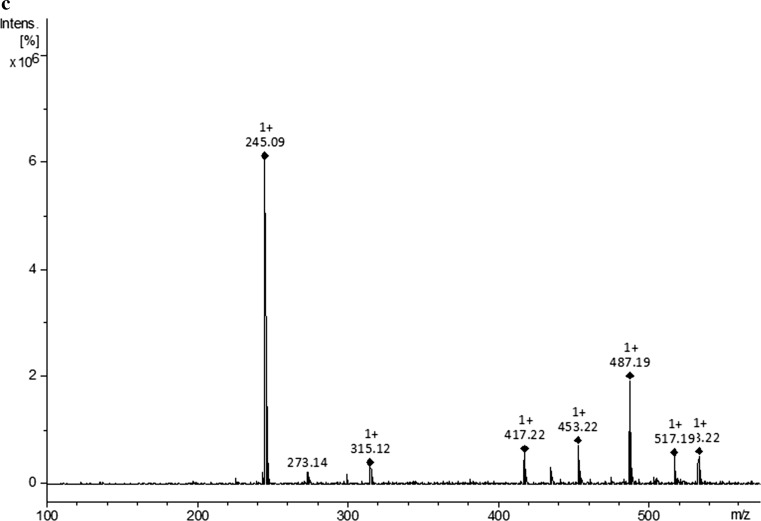
Figure 2a shows the polyphenol profile for the optimized YPFS extract. The major peak, with an approximate retention time of 13.4 min and a molecular ion m/z of 245.09, was identified as a stilbene compound known as piceatannol (3,4,3′,5′-tetrahydroxy-trans-stilbene) (Fig. 2b, c). Previously, this compound was identified by Matsui et al. (2010) as the major polyphenol component in purple passion fruit seed extracts (P. edulis), but to the best of our knowledge, this is the first report of this compound from the YPFS produced and marketed in Brazil.
Fig. 2.
CLAE-DAD chromatogram (270 nm) showing piceatannol as the major peak (a), mass spectra of standard piceatannol (b) and of piceatannol present in the yellow passion fruit (P. edulis Sims) seed extract (c)
Piceatannol exhibits various biological activities, including antioxidant, anticarcinogenic, antiatherogenic, and anti-inflammatory activities (Piotrowska et al. 2012). Recently, Lai et al. (2014) have reported an optimization study for the extraction of piceatannol from rose myrtle seeds (Rhodomyrtus tomentosa) and found that the optimum conditions for extraction are similar to those employed herein (78.8% ethanol, 85.3 °C, and 78.8 min). Considering previous studies and the results obtained herein, the extraction of piceatannol is thought to require high temperatures.
With respect to the complexity of interactions and diversity of action mechanisms between various compounds and antioxidant activity, this determination has been widely accepted to be carried out using two or more analytical methods (Moulehi et al. 2012). Thus, the antioxidant capacity of the optimized extract was evaluated by the methods employed during the optimization of extraction conditions (Table 5).
The ability of polyphenols to donate electrons or hydroxyl radicals to the DPPH radical was evaluated; hence, its ability to become a stable diamagnetic molecule was evaluated. The EC50 value observed for the optimized extract is lower, reflecting a higher antioxidant capacity, than those determined by Jorge et al. (2009) and Silva et al. (2015) for the ethanolic (113.41 g/mL) and methanolic (108 μg/mL) extracts of YPFS, respectively. Lourith and Kanlayavattanakul (2013) have reported that the DPPH radical scavenging potential of passion fruit seeds might be greater after the liquid–liquid partition with ethyl acetate of the crude extract.
The ability of the optimized extract to inhibit the peroxidation of linoleic acid and subsequent bleaching of β-carotene in the presence of oxidizing factors is superior (EC50 1.26 mg/mL) to those detected for other fruit seed extracts, e.g., mandarin (Citrus reticulata Blanco) (EC50 3.65 mg/ml) and bitter orange (C. aurantium L.) (EC50 1.80 mg/ml) (Moulehi et al. 2012).
With respect to the reducing capacity, a smaller amount of reducing agents are observed in the optimized extract (20.41 μg AAE/100 g seed db) compared to those reported by Chougui et al. (2013) for different varieties of Opuntia ficus-indica fruit seed extracts (2.3–51.3 mg AAE/100 g seed db). However, the optimized extract exhibits a higher capacity to absorb oxygen radicals than Rubus occidentalis (95.8 μmol TE/g) (Luther et al. 2007) and some fruit seed flour extracts (110.5–1076 μmol TE/g) (Parry et al. 2006).
Conclusion
Owing to high content of lipids, proteins, minerals, and phenolic compounds, yellow passion fruit seeds demonstrate immense potential for commercial exploitation. The extraction conditions to obtain crude extract include a high extraction yield for the total polyphenols achieved using 70% ethanol at 80 °C for 30 min, which was statistically correlated with the antioxidant capacity by most of the investigated methods. Under optimal conditions, stilbene piceatannol is the major polyphenol compound identified. In addition, the results suggested that the optimization of the extraction conditions is critical for accurately quantifying phenolic compounds and their antioxidant proprieties in YPFS in terms of yield and cost-effectiveness; therefore, this process can be scaled up to an industrial extraction procedure so as to harness the maximum potential of this fruit, including the use of the by-products by food, pharmaceutical, and chemical industries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-017-2813-3) contains supplementary material, which is available to authorized users.
References
- Al Farsi MA, Lee CY. Optimization of phenolics and dietary fiber extraction from date seeds. Food Chem. 2008;108:977–985. doi: 10.1016/j.foodchem.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Almeida MMB, Sousa PHM, Arriaga AMC, Prado GM, Magalhães CEC, Maia GA, Lemos TLG. Bioactive compounds and antioxidant activity of fresh exotic fruits from northeastern Brazil. Food Res Int. 2011;44:2155–2159. doi: 10.1016/j.foodres.2011.03.051. [DOI] [Google Scholar]
- AOAC (1995) Official methods of analysis of the association of official analytical chemists, 16th edn. AOAC International, Arlington
- AOAC (2002) Official methods of analysis of the association of official analytical chemists, 17th edn. AOAC International, Gaithersburg
- Arabbi PR, Genovese MI, Lajolo FM. Flavonoids in vegetable foods commonly consumed in Brazil and estimated ingestion by the Brazilian population. J Agric Food Chem. 2004;52:1124–1131. doi: 10.1021/jf0499525. [DOI] [PubMed] [Google Scholar]
- Babbar N, Oberoi HS, Uppal DS, Patil RT. Total phenolic content and antioxidant capacity of extracts obtained from six important fruit residues. Food Res Int. 2011;44:391–396. doi: 10.1016/j.foodres.2010.10.001. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method evaluate antioxidant activity. Lebensm-Wiss u-Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Brignani Neto F. Produção integrada de maracujá. Biológico. 2002;64:195–197. [Google Scholar]
- Cacace JE, Mazza G. Mass transfer process during extraction of phenolic compounds from milled berries. J Food Eng. 2003;59:379–389. doi: 10.1016/S0260-8774(02)00497-1. [DOI] [Google Scholar]
- Chougui N, Tamendjari A, Hamidj W, Hallal S, Barras A, Richard T, Larbart R. Oil composition and characterisation of phenolic compounds of Opuntia ficus-indica seeds. Food Chem. 2013;139:796–803. doi: 10.1016/j.foodchem.2013.01.054. [DOI] [PubMed] [Google Scholar]
- de Oliveira AC, Valentim IB, Silva CA, Bechara EJH, Barros MP, Mano CM, Goulart MOF. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit residues. Food Chem. 2009;115:469–475. doi: 10.1016/j.foodchem.2008.12.045. [DOI] [Google Scholar]
- Deng G, Shen C, Xu X, Kuang R, Guo Y, Zeng L, Gao L, Lin X, Xie J, Xia E, Li S, Wu S, Chen F, Ling W, Li B. Potential of fruit wastes as natural resources of bioactive compounds. Int J Mol Sci. 2012;137:8308–8323. doi: 10.3390/ijms13078308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (2001) Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. A report of the Panel on Micronutrients, Subcomittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, National Academy Press, Washington
- Jorge N, Malacrida CR, Angelo PM, Andreo D. Composição centesimal e atividade antioxidante do extrato de semente de maracujá (Passiflora edulis) em óleo de soja. Pesqui Agropecu Trop. 2009;39:380–385. [Google Scholar]
- Khoddami A, Wilkes MA, Robert TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai TNH, André CM, Chirinos R, Nguyen TBT, Larondelle Y, Rogez H. Optimisation of extraction of piceatannol from Rhodomyrtus tomentosa seeds using response surface methodology. Sep Purif Technol. 2014;134:139–146. doi: 10.1016/j.seppur.2014.07.032. [DOI] [Google Scholar]
- Leão KMM, Sampaio KL, Pagani AAC, Silva MAAP. Odor potency, aroma profile and volatiles composition of cold pressed oil from industrial passion fruit residues. Ind Crops Prod. 2014;58:280–286. doi: 10.1016/j.indcrop.2014.04.032. [DOI] [Google Scholar]
- Liyana-Pathirana C, Shahidi F. Optimization of extraction of phenolics compounds from wheat using response surface methodology. Food Chem. 2005;93:45–56. [Google Scholar]
- Lopes RM, Sevilha AC, Faleiro FG, Silva DB, Vieira RF, Agostini-Costa TS. Estudo comparativo do perfil de ácidos graxos em semente de passifloras nativas do cerrado brasileiro. Revis Bras Frutic. 2010;32:498–506. doi: 10.1590/S0100-29452010005000065. [DOI] [Google Scholar]
- Lourith N, Kanlayavattanakul M. Antioxidant activities and phenolics of Passiflora edulis seed recovered from juice production residue. J Oleo Sci. 2013;62:235–240. doi: 10.5650/jos.62.235. [DOI] [PubMed] [Google Scholar]
- Luther M, Parry J, Moore J, Meng J, Zhang Y, Cheng Z, Yu L. Inhibitory effect of chardonnay and black raspberry seed extracts on lipid oxidation in fish oil and their radical scavenging and antimicrobial properties. Food Chem. 2007;104:1065–1073. doi: 10.1016/j.foodchem.2007.01.034. [DOI] [Google Scholar]
- Malacrida CR, Jorge N. Yellow passion fruit seed oil (Passiflora edulis f. flavicarpa): physical and chemical characteristics. Braz Arch Biol Technol. 2012;55:127–134. doi: 10.1590/S1516-89132012000100016. [DOI] [Google Scholar]
- Matsui K, Sugiyama K, Kamei M, Takahashi T, Suzuki T, Katagata Y, Ito T. Extract of passion fruit (Passiflora edulis) seed containing high amounts of piceatannol inhibits melanogenesis and promotes collagen synthesis. J Agric Food Chem. 2010;58:11112–11118. doi: 10.1021/jf102650d. [DOI] [PubMed] [Google Scholar]
- Moreira AVB, Mancini-Filho J. Atividade antioxidante das especiarias mostarda, canela e erva-doce em sistemas aquoso e lipídico. Nutrire. 2003;39:31–46. [Google Scholar]
- Moulehi I, Bourgou S, Ourghemmi I, Tounsi MS. Variety and ripening impact on phenolic composition and antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind Crops Prod. 2012;39:74–80. doi: 10.1016/j.indcrop.2012.02.013. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of browning reaction: antioxidative activity of products of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Parry J, Su L, Moore J, Cheng Z, Luther M, Rao JN, Wang J, Yu LL. Chemical compositions, antioxidant capacities and antiproliferative activities of selected fruit seed flours. J Agric Food Chem. 2006;54:3773–3778. doi: 10.1021/jf060325k. [DOI] [PubMed] [Google Scholar]
- Piotrowska H, Kucinska M, Murias M. Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat Res. 2012;750:60–82. doi: 10.1016/j.mrrev.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Prasad KN, Hassan FA, Yang B, Kong KW, Ramanan RN, Azlan A, Ismail A. Response surface optimisation for the extraction of phenolic compounds and antioxidant capacities of underutilised Mangifera pajang Kosterm. peels. Food Chem. 2011;128:1121–1127. doi: 10.1016/j.foodchem.2011.03.105. [DOI] [Google Scholar]
- Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, Hampsch-Woodill M, Huang D, Ou B, Jacob R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J Agric Food Chem. 2003;51:3273–3279. doi: 10.1021/jf0262256. [DOI] [PubMed] [Google Scholar]
- Rodrigues MI, Iemma AF. Planejamento de experimentos e otimização de processos. 1. São Paulo: Casa do Pão; 2005. [Google Scholar]
- Sarkis JR, Michel I, Tessaro IC, Marczak LDF. Optimization of phenolics extraction from sesame seed cake. Sep Purif Technol. 2014;122:506–514. doi: 10.1016/j.seppur.2013.11.036. [DOI] [Google Scholar]
- Silva AC, Jorge N. Bioactive compounds of the lipid fractions of agro-industrial waste. Food Res Int. 2014;66:493–500. doi: 10.1016/j.foodres.2014.10.025. [DOI] [Google Scholar]
- Silva RM, Placido GR, Silva MAP, Castro CFS, Lima MS, Caliari M. Chemical characterization of passion fruit (Passiflora edulis f. flavicarpa) seeds. Afr J Biotechnol. 2015;14:1230–1233. doi: 10.5897/AJB2014.13945. [DOI] [Google Scholar]
- Souza VR, Pereira PAP, Queiroz F, Borges SV, Carneiro JDS. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012;134:381–386. doi: 10.1016/j.foodchem.2012.02.191. [DOI] [Google Scholar]
- Spigno G, Tramelli L, De Faveri DM. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J Food Eng. 2007;81:200–208. doi: 10.1016/j.jfoodeng.2006.10.021. [DOI] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents of Punnus domestica I. quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;19:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Upadhyay S, Dixit M. Role of polyphenols and other phytochemicals on molecular signaling. Oxid Med Cell Longev. 2015;2015:1–15. doi: 10.1155/2015/504253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez G, Fernández-Agulló A, Gómez-Castro C, Freire MS, Antorrena G, González-Álvarez J. Response surface optimization of antioxidants extraction from chestnut (Castanea sativa) bur. Ind Crops Prod. 2012;35:126–134. doi: 10.1016/j.indcrop.2011.06.022. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.